Preheating Behaviors of Iron Ore Pellets with Humic Substance-Based Binder
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.1.1. Ore Materials
2.1.2. Humic Substance-Based Binder
2.2. Methods and Equipment
2.2.1. Preparation of Green Balls
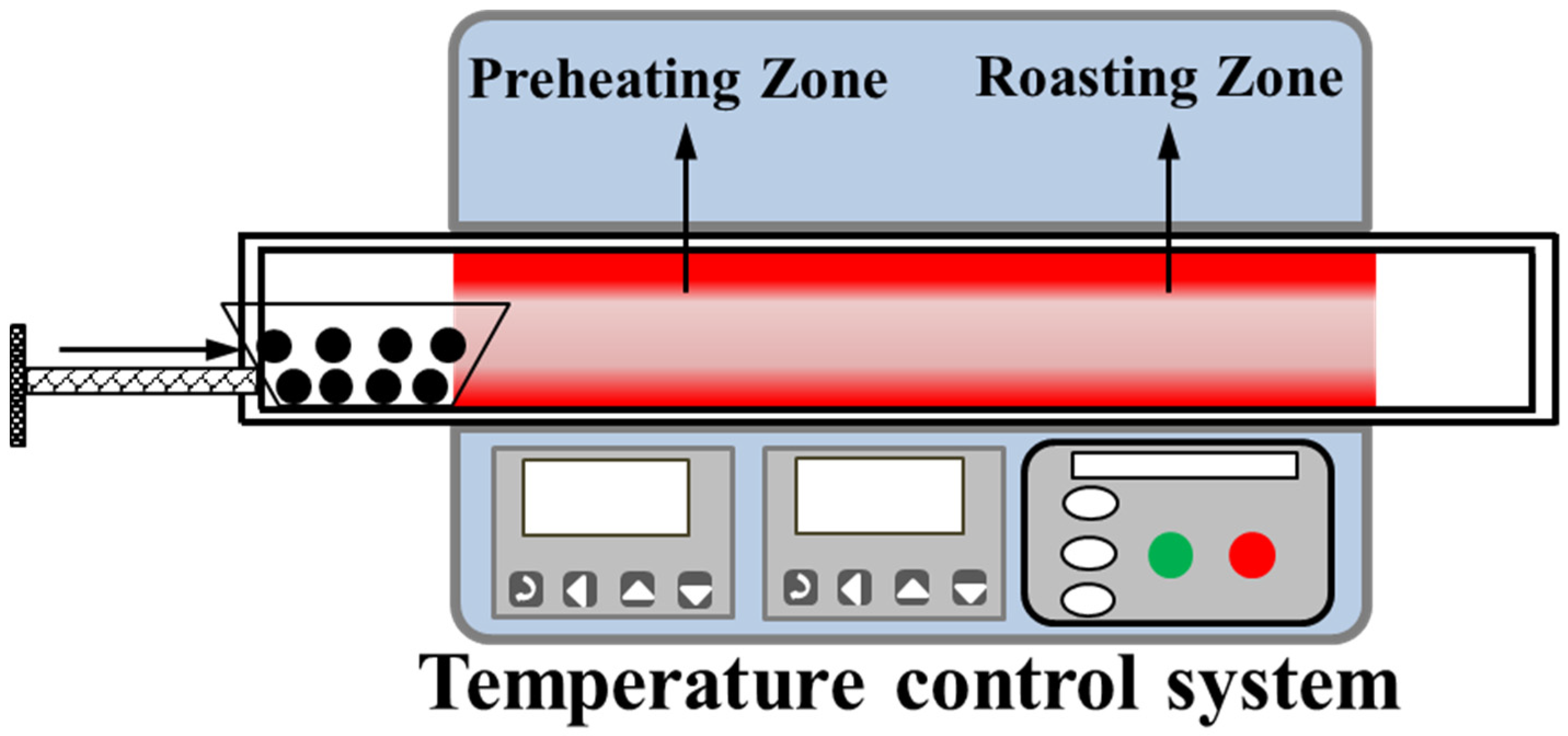
2.2.2. Preheating and Roasting Tests
2.2.3. Phase and Microstructure Analysis
3. Results and Discussion
3.1. Firing Conditions of Pellets with Humic Substance-Based Binder
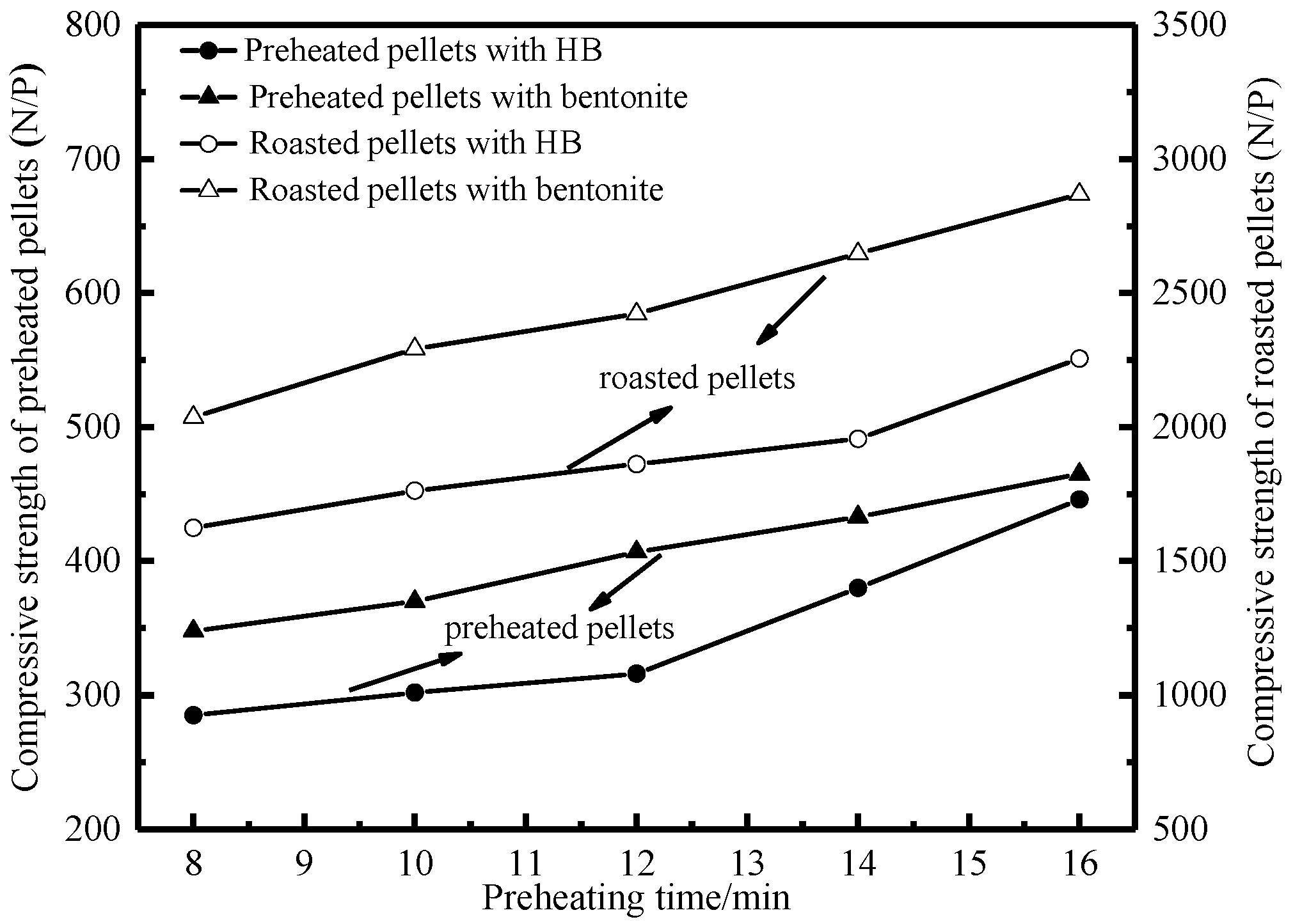
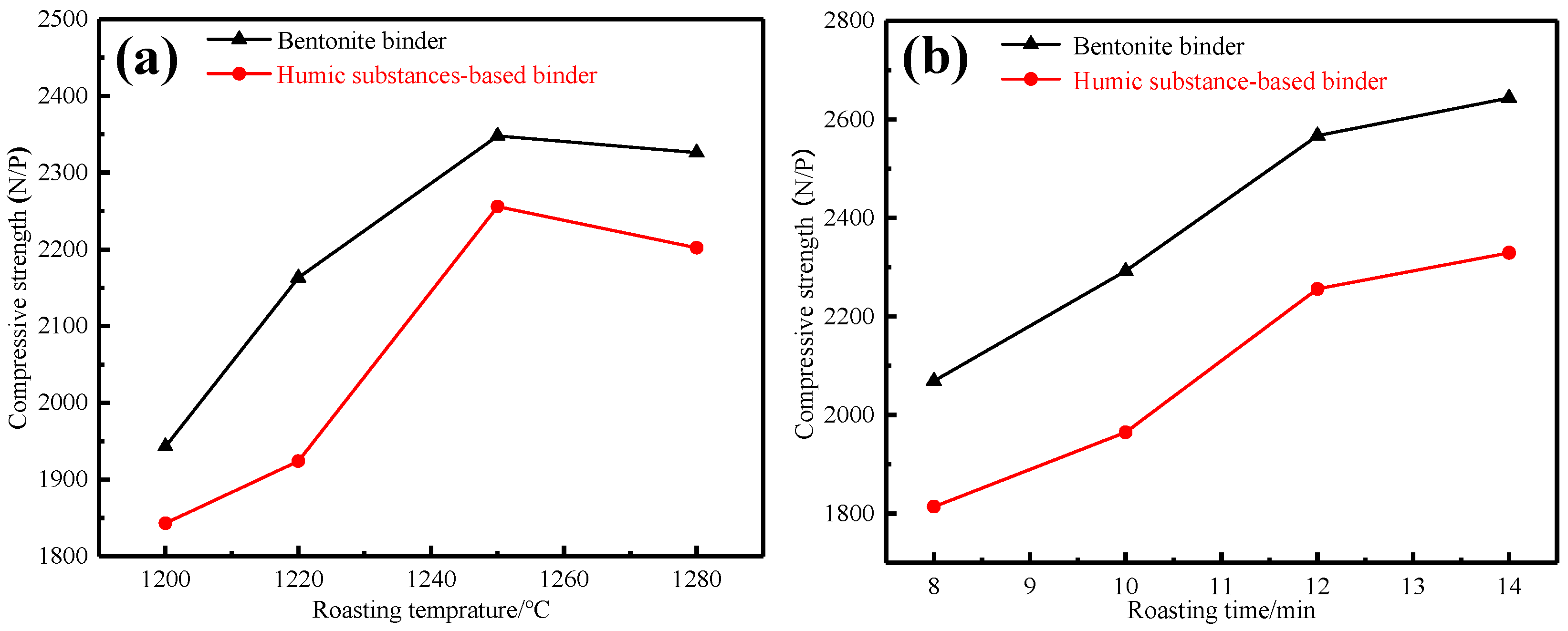
3.1.1. Effects of Preheating Conditions
3.1.2. Effects of Roasting Conditions
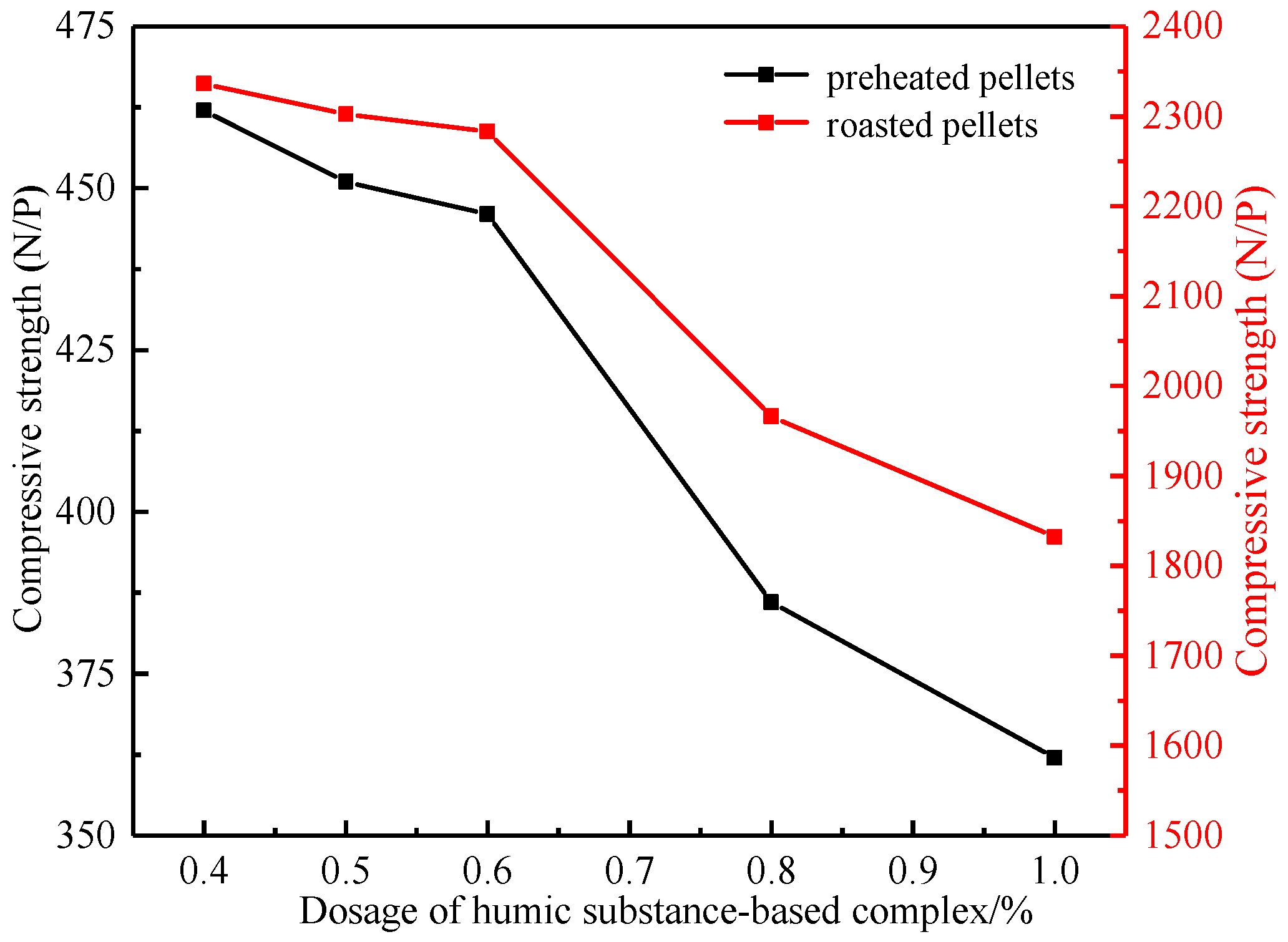
3.1.3. Effect of Humic Substance-Based Binder Dosage
3.2. Oxidation Behaviors of Pellets with HB during Preheating Process
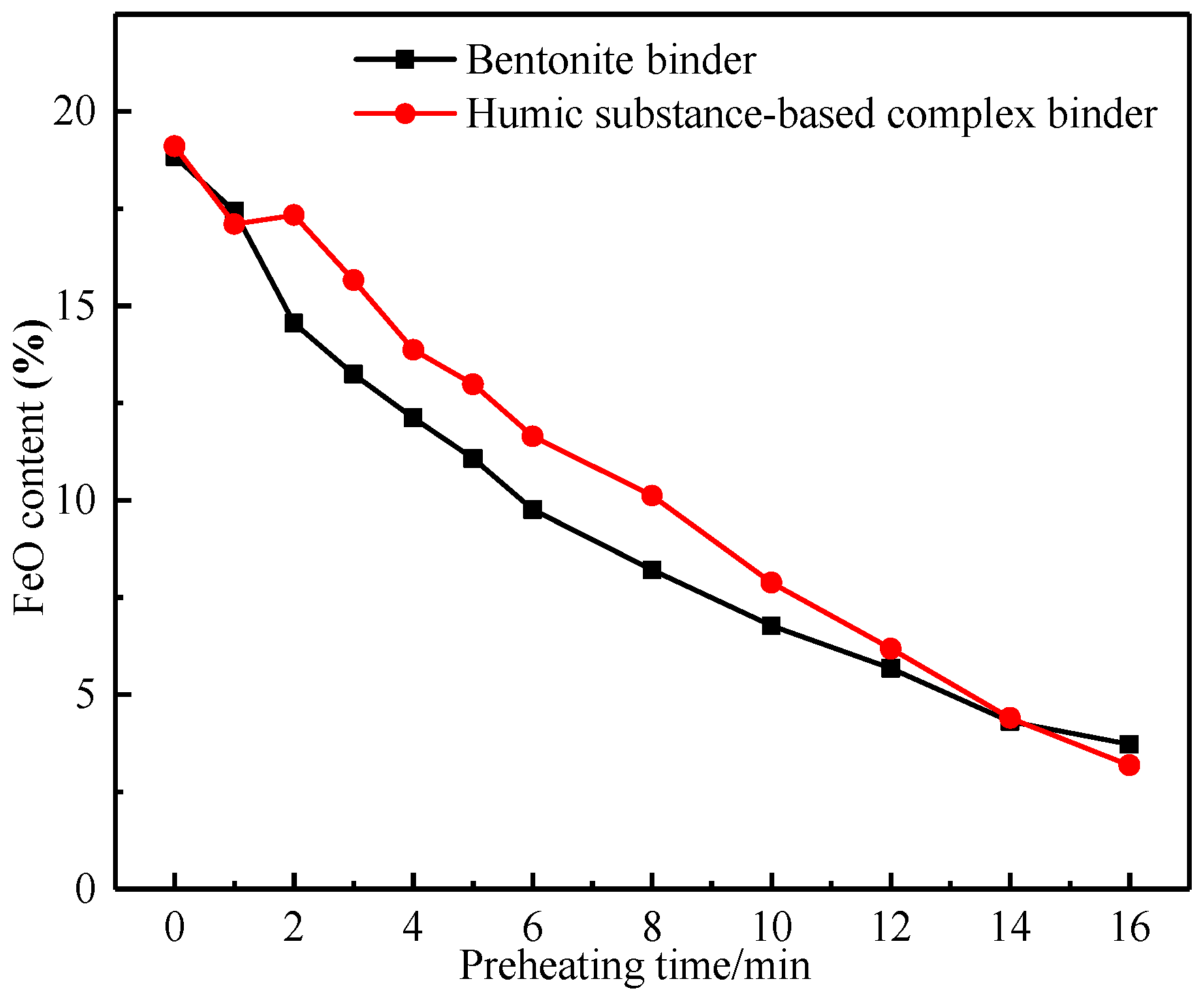
3.2.1. Oxidation Degree of Pellets at Different Preheating Times
3.2.2. Phase Compositions of Preheated Pellets at Different Preheating Times
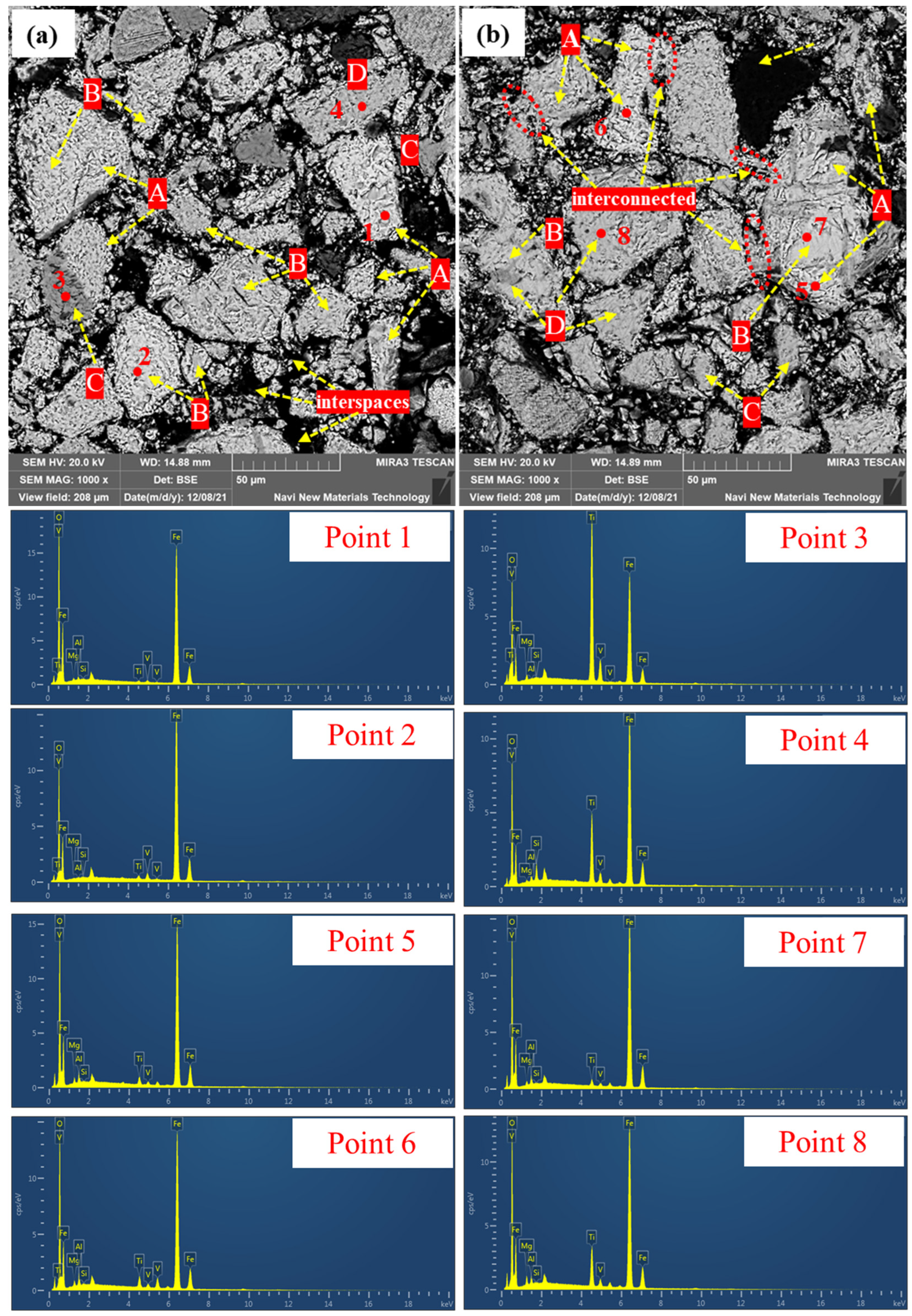
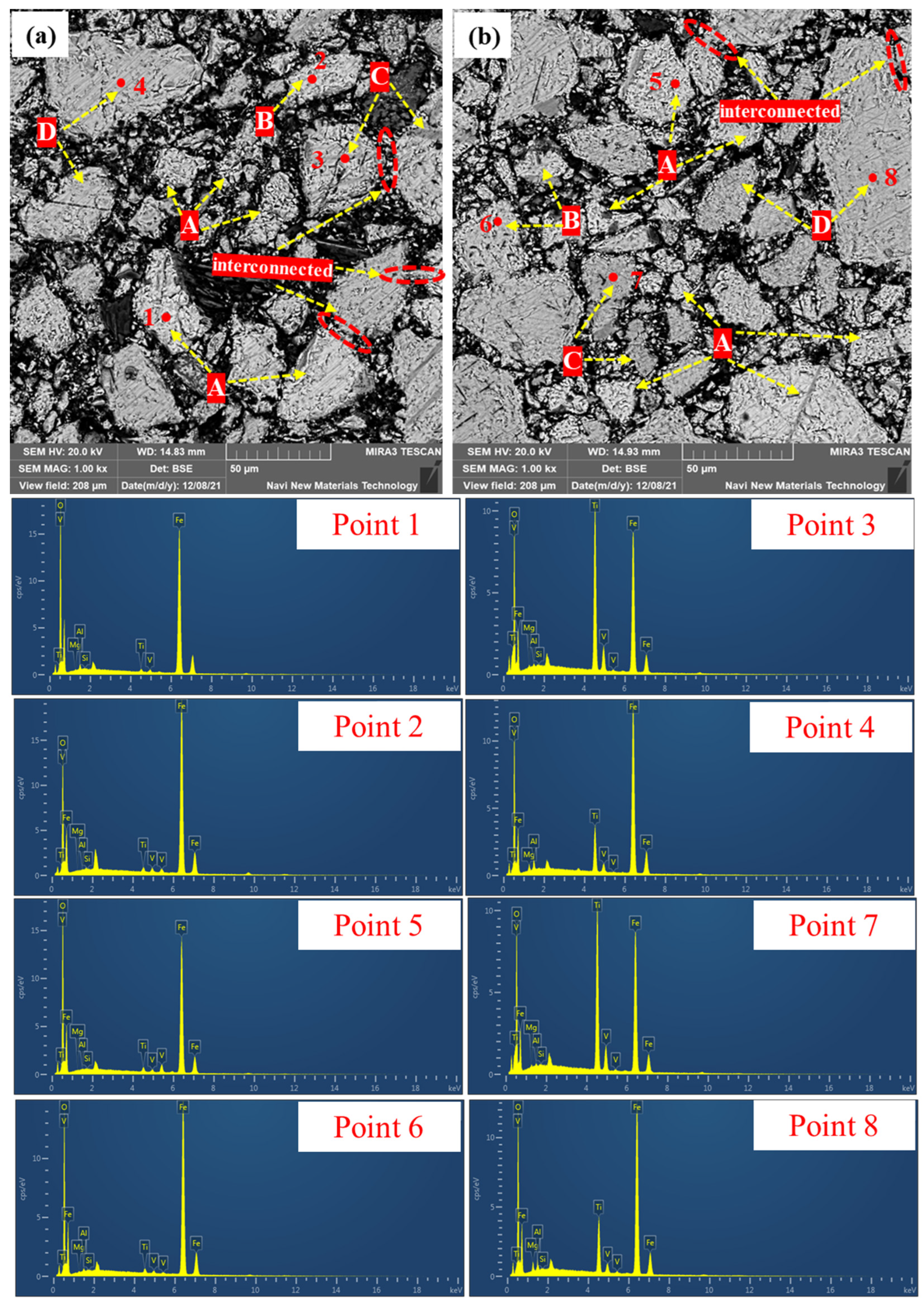
3.2.3. Microstructure of Preheated Pellets at Different Preheating Times
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sui, Y.; Guo, Y.; Jiang, T.; Qiu, G.-Z. Separation and recovery of iron and titanium from oxidized vanadium titano-magnetite by gas-based reduction roasting and magnetic separation. J. Mater. Res. Technol. 2019, 8, 3036–3043. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Y.; Jiang, T.; Yang, L.; Chen, F.; Zheng, F.; Xie, X.; Tang, M. Reduction Behaviors of Iron, Vanadium and Titanium Oxides in Smelting of Vanadium Titanomagnetite Metallized Pellets. JOM 2017, 69, 1646–1653. [Google Scholar] [CrossRef]
- Sui, Y.L.; Guo, Y.F.; Jiang, T.; Xie, X.L.; Wang, S.; Zheng, F.Q. Gas-based reduction of vanadium titano-magnetite concentrate: Behavior and mechanisms. Int. J. Miner. Metall. Mater. 2017, 24, 10–17. [Google Scholar] [CrossRef]
- Yu, J.; Hu, N.; Xiao, H.; Gao, P.; Sun, Y. Reduction behaviors of vanadium-titanium magnetite with H2 via a fluidized bed. Powder Technol. 2021, 385, 83–91. [Google Scholar] [CrossRef]
- Tan, P.; Hui-Ping, H.U.; Zhang, L. Effects of mechanical activation and oxidation-reduction on hydrochloric acid leaching of Panxi ilmenite concentration. Trans. Nonferrous Met. Soc. China 2011, 21, 1414–1421. [Google Scholar] [CrossRef]
- Xing, Z.; Cheng, G.; Gao, Z.; Yang, H.; Xue, X. Optimization of experimental conditions on preparation of oxidized pellets with New Zealand sea sand ore. Metall. Res. Technol. 2020, 117, 411. [Google Scholar] [CrossRef]
- Qiu, G.; Jiang, T.; Li, H.; Wang, D. Functions and molecular structure of organic binders for iron ore pelletization. Colloids Surf. A Physicochem. Eng. Asp. 2003, 224, 11–22. [Google Scholar] [CrossRef]
- Moraes, S.; Kawatra, S.K. Laboratory study of an organic binder for pelletization of a magnetite concentrate. Min. Met. Explor. 2010, 27, 148–153. [Google Scholar] [CrossRef]
- Srivastava, U.; Kawatra, K.; Eisele, T.C. Study of Organic and Inorganic Binders on Strength of Iron Oxide Pellets. Metall. Mater. Trans. B 2013, 44, 1000–1009. [Google Scholar] [CrossRef]
- Mohamed, O.A.; Shalabi, M.E.H.; El-Hussiny, N.A.; Khedr, M.H.; Mostafa, F. The role of normal and activated bentonite on the pelletization of barite iron ore concentrate and the quality of pellets. Powder Technol. 2003, 130, 277–282. [Google Scholar] [CrossRef]
- Sivrikaya, O.; Arol, A.I.; Eisele, T.; Kawatra, S.K. The Effect of Calcined Colemanite Addition on the Mechanical Strength of Magnetite Pellets Produced with Organic Binders. Miner. Process. Extr. Metall. Rev. 2013, 34, 210–222. [Google Scholar] [CrossRef]
- Kawatra, S.K.; Claremboux, V. Iron Ore Pelletization: Part I. Fundamentals. Miner. Process. Extr. Metall. Rev. 2021, 1–16. Available online: https://www.tandfonline.com/doi/abs/10.1080/08827508.2021.1897586 (accessed on 1 February 2022).
- Zhou, Y.L.; Kawatra, S.K. Humic Substance-based Binder In Iron Ore Pelletization: A Review. Miner. Process. Extr. Metall. Rev. 2017, 38, 321–337. [Google Scholar] [CrossRef]
- Tao, J.; Han, G.; Zhang, Y.; Li, G.; Huang, Y. A further study on the interaction between one of organic active fractions of the MHA binder and iron ore surface. Int. J. Miner. Process. 2011, 100, 172–178. [Google Scholar]
- Moraes, S.L.; de Lima, J.R.B.; Neto, J.B.F.; Fredericci, C.; Saccoccio, E.M. Binding Mechanism in Green Iron Ore Pellets with an Organic Binder. Miner. Process. Extr. Metall. Rev. 2020, 41, 247–254. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Liu, B.; Li, G.; Jiang, T. Roasting characteristics of specularite pellets with modified humic acid based (MHA) binder under different oxygen atmospheres. Powder Technol. 2014, 261, 279–287. [Google Scholar] [CrossRef]
- Bai, G.H.; Zhang, D.Y.; Zhang, Y.B.; Han, G.H.; Su, Z.J. Oxidized Pellet Preparation from Refractory Specularite Concentrates Using Modified Humic Acid (MHA) Binders. In Proceedings of the 2nd International Symposium on High-Temperature Metallurgical Processing, San Diego, CA, USA, 27 February–3 March 2011; pp. 299–305. [Google Scholar]
- Zhou, Y.; Kawatra, S.K. Pelletization Using Humic Substance-based Binder. Miner. Process. Extr. Metall. Rev. 2017, 38, 83–91. [Google Scholar] [CrossRef]
- Halt, J.A.; Kawatra, S.K. Review of organic binders for iron ore concentrate agglomeration. Miner. Metall. Process. 2014, 31, 73–94. [Google Scholar] [CrossRef]
- Kawatra, S.K.; Claremboux, V. Iron Ore Pelletization: Part II. Inorganic Binders. Miner. Process. Extr. Metall. Rev. 2021, 1–20. Available online: https://www.tandfonline.com/doi/abs/10.1080/08827508.2021.1947269 (accessed on 1 February 2022).
- Kawatra, S.K.; Ripke, S.J. Laboratory studies for improving green ball strength in bentonite-bonded magnetite concentrate pellets. Int. J. Miner. Process. 2003, 72, 429–441. [Google Scholar] [CrossRef]
- Kawatra, S.K.; Ripke, S.J. Developing and understanding the bentonite fiber bonding mechanism. Miner. Eng. 2001, 14, 647–659. [Google Scholar] [CrossRef]
- Qiu, G.; Jiang, T.; Fa, K.; Zhu, D.; Wang, D. Interfacial characterizations of iron ore concentrates affected by binders. Powder Technol. 2004, 139, 1–6. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, M.; Su, Z.; Wang, J.; Tu, Y.; Chen, X.; Cao, C.; Gu, F.; Liu, S.; Jiang, T. Interfacial reaction between humic acid and Ca-Montmorillonite: Application in the preparation of a novel pellet binder. Appl. Clay Sci. 2019, 180, 105177. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, Y.B.; Huang, Z.C.; Li, G.H.; Fan, X.H. Preheating and roasting characteristics of hematite-magnetite (H-M) concentrate pellets. Ironmak. Steelmak. 2008, 35, 21–26. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Zhang, Y.B.; Jiang, T.; Li, G.H.; Zhang, D.Y. Effects of MHA binder on roasting behaviors of oxidized pellets from specularite concentrate. In Proceedings of the 3rd International Symposium on High-Temperature Metallurgical Processing, Conference Proceeding, Hoboken, NJ, USA, 11–15 March 2012; pp. 507–514. [Google Scholar]
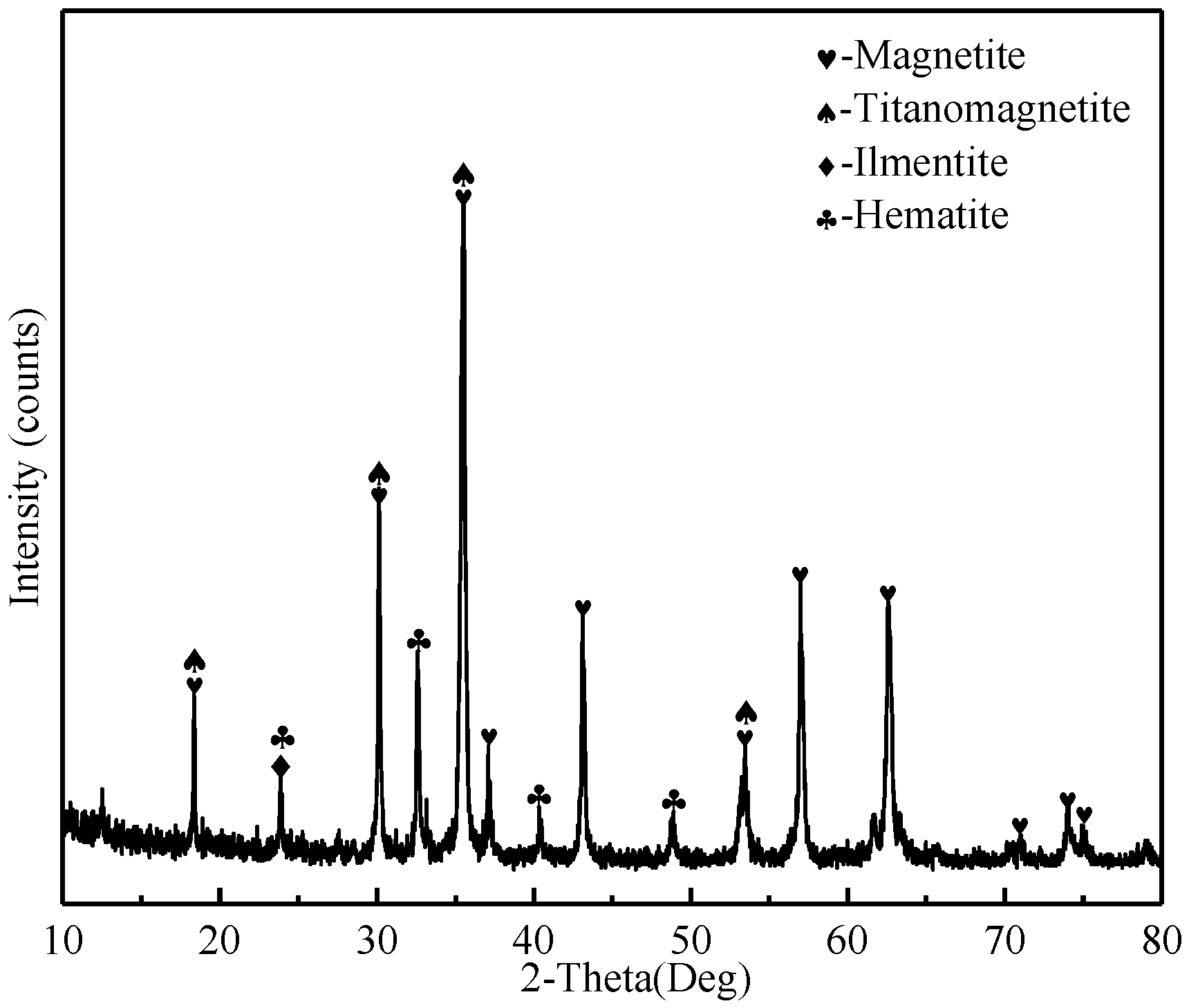
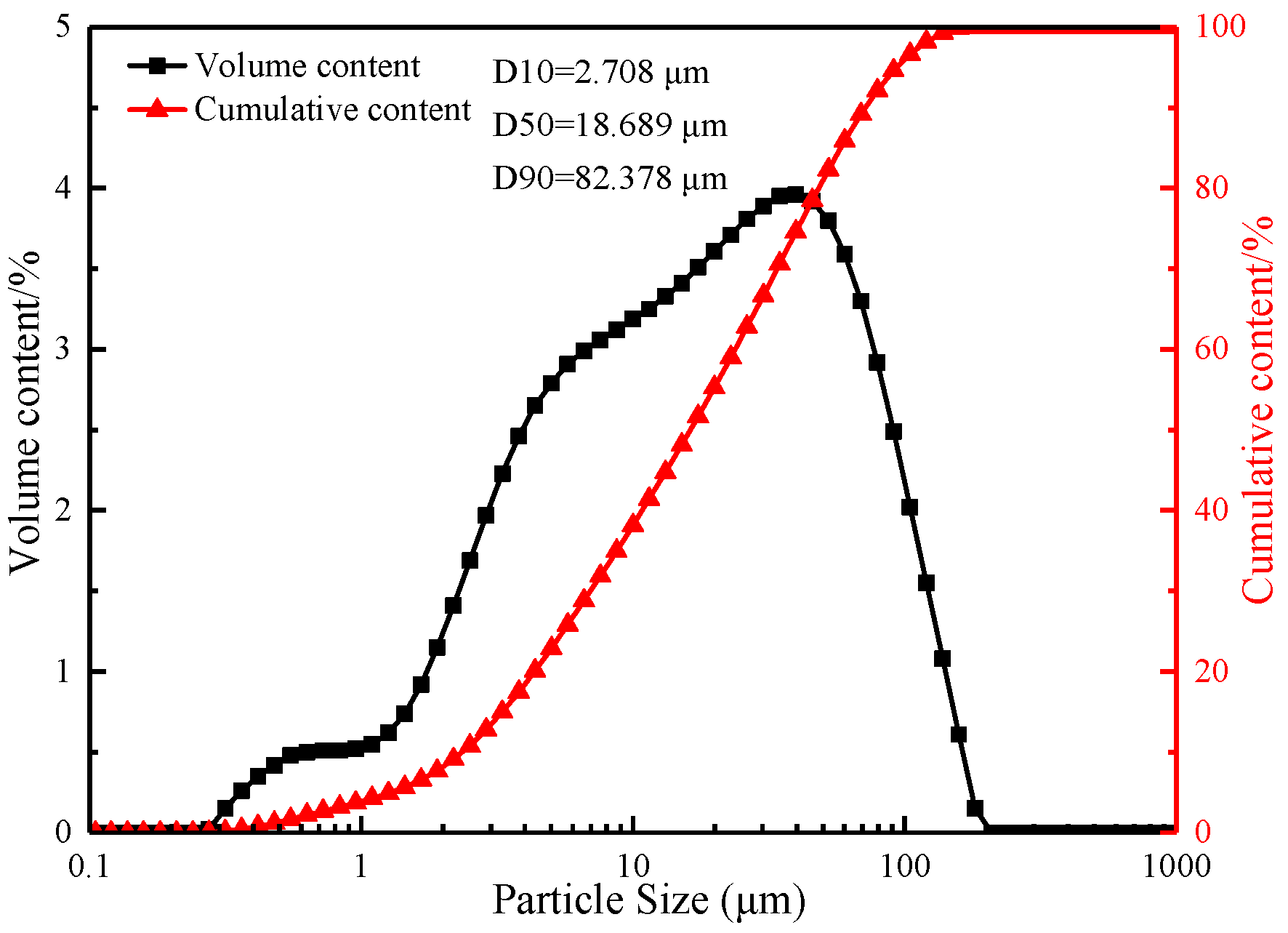
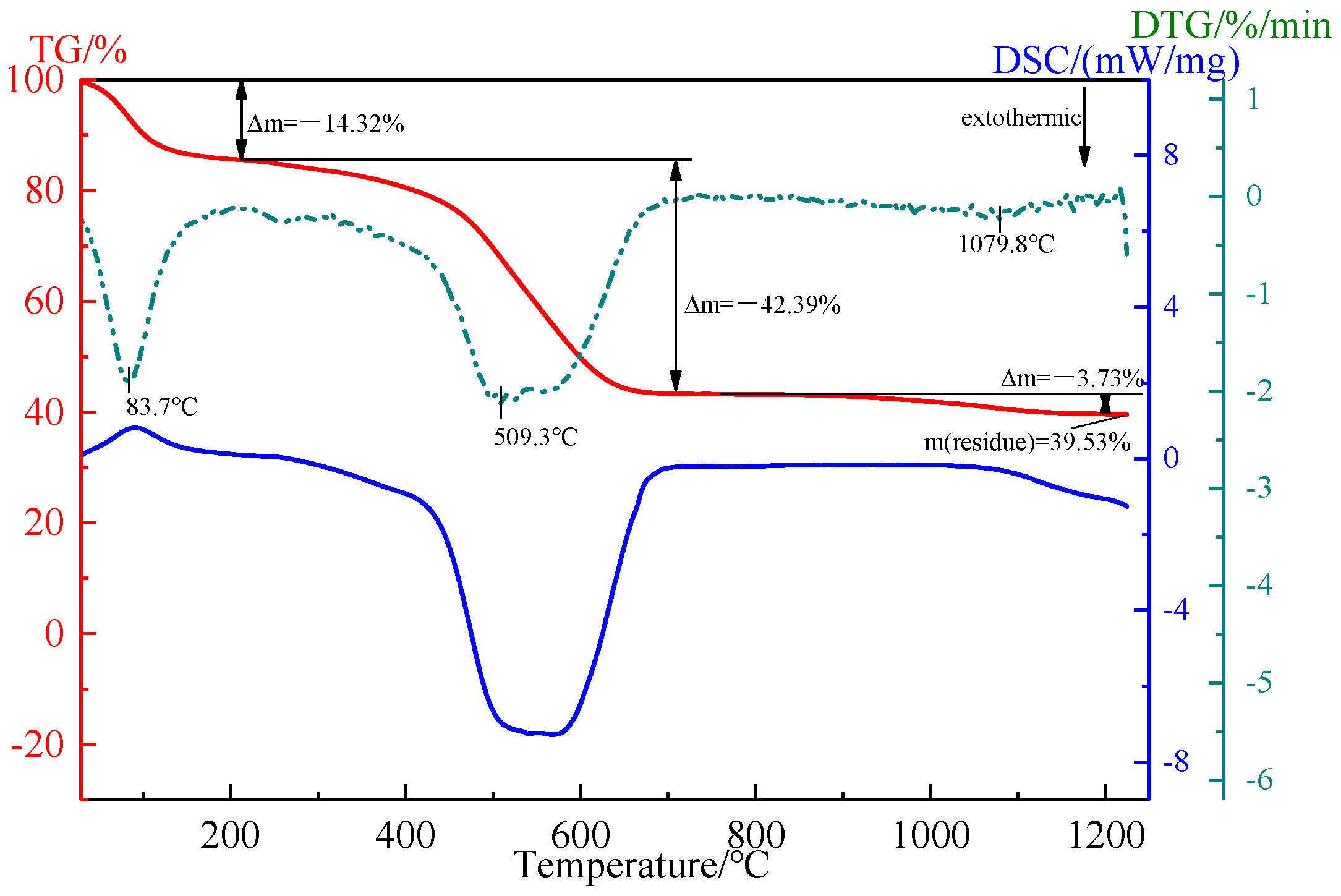
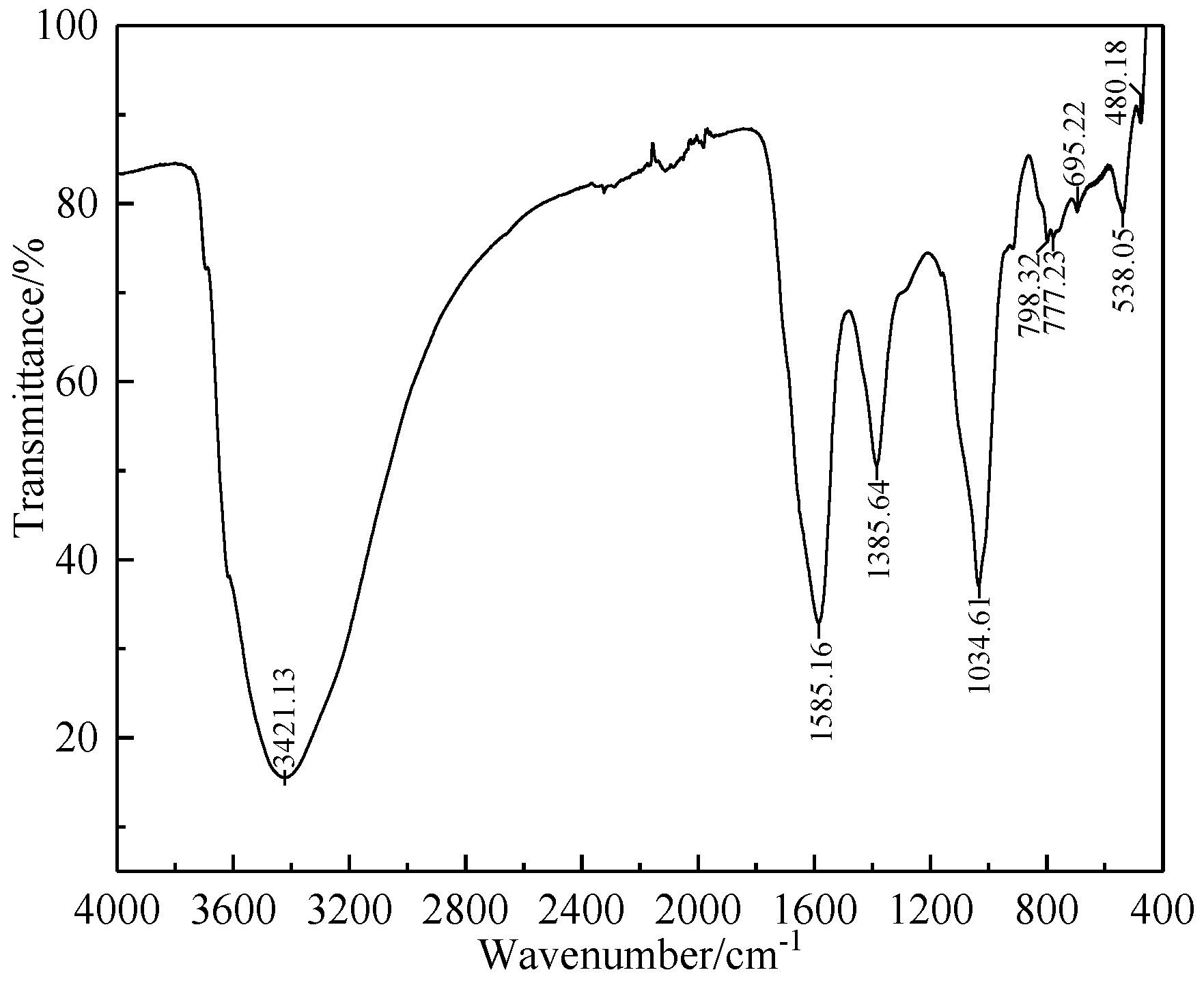


| Component | TFe | FeO | TiO2 | V2O5 | SiO2 | Al2O3 | CaO | MgO | K2O | Na2O |
|---|---|---|---|---|---|---|---|---|---|---|
| VTM | 54.47 | 28.57 | 7.84 | 0.43 | 4.48 | 3.09 | 1.27 | 0.43 | 0.03 | 0.07 |
| Bentonite | 1.76 | - | - | - | 64.01 | 13.47 | 2.26 | 1.81 | 0.45 | 0.85 |
| Component | Mad | Aad | Vad | FCad | Ash Chemical Composition/wt% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| TFe | SiO2 | Al2O3 | CaO | K2O | Na2O | |||||
| Content | 18.14 | 39.77 | 18.75 | 23.34 | 2.74 | 57.23 | 23.46 | 0.63 | 3.42 | 6.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, F.; Ou, Y.; Li, K.; Yang, Y.; Zhong, Q.; Li, Q.; Jiang, T. Preheating Behaviors of Iron Ore Pellets with Humic Substance-Based Binder. Metals 2022, 12, 570. https://doi.org/10.3390/met12040570
Meng F, Ou Y, Li K, Yang Y, Zhong Q, Li Q, Jiang T. Preheating Behaviors of Iron Ore Pellets with Humic Substance-Based Binder. Metals. 2022; 12(4):570. https://doi.org/10.3390/met12040570
Chicago/Turabian StyleMeng, Feiyu, Yang Ou, Ke Li, Yongbin Yang, Qiang Zhong, Qian Li, and Tao Jiang. 2022. "Preheating Behaviors of Iron Ore Pellets with Humic Substance-Based Binder" Metals 12, no. 4: 570. https://doi.org/10.3390/met12040570
APA StyleMeng, F., Ou, Y., Li, K., Yang, Y., Zhong, Q., Li, Q., & Jiang, T. (2022). Preheating Behaviors of Iron Ore Pellets with Humic Substance-Based Binder. Metals, 12(4), 570. https://doi.org/10.3390/met12040570






