A Review of Recovery of Palladium from the Spent Automobile Catalysts
Abstract
:1. Introduction
2. Palladium and Its Spent Catalysts
2.1. Palladium and Its Components
2.2. The Spent Automobile Catalysts and Their Deactivation Reasons
3. The Development of Recovering Palladium from the Spent Automobile Catalysts
3.1. Pretreatment
3.2. Hydrometallurgy
3.2.1. Leaching or Extraction
Traditional Cyanide Leaching
HCl(aq) + Oxidant Leaching
Bio-Leaching
Supercritical Fluid Extraction
Other Innovative Methods
3.2.2. Separation or Recovery of Palladium from Leachate
Precipitation
Solvent Extraction
Ion Exchange
3.3. Pyrometallurgy
3.3.1. Chlorination Volatilization Method
3.3.2. Metal Capture Method
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jha, M.K.; Lee, J.C.; Kim, M.S.; Jeong, J.; Kim, B.S.; Kumar, V. Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: A review. Hydrometallurgy 2013, 133, 23–32. [Google Scholar] [CrossRef]
- Ding, Y.J.; Zhang, S.E.; Liu, B.; Zheng, H.D.; Chang, C.C.; Ekberg, C. Recovery of precious metals from electronic waste and spent catalysts: A review. Resour. Conserv. Recycl. 2019, 141, 284–298. [Google Scholar] [CrossRef]
- Zheng, H.; Ding, Y.; Wen, Q.; Liu, B.; Zhang, S. Separation and purification of platinum group metals from aqueous solution: Recent developments and industrial applications. Resour. Conserv. Recycl. 2021, 167, 105417–105433. [Google Scholar] [CrossRef]
- Yakoumis, I.; Panou, M.; Moschovi, A.M.; Panias, D. Recovery of platinum group metals from spent automotive catalysts: A review. Clean. Eng. Technol. 2021, 3, 100112–100122. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, J.; Chen, J.; Wu, Y.; Li, B. Recovery of platinum group metals from spent catalysts: A review. Int. J. Miner. Processing 2015, 145, 108–113. [Google Scholar] [CrossRef]
- Xu, A.Y.; Ye, T.; Zhao, S.H. Recovery of Valuable Metals from Spent Hydrogenation Catalysts. In Advanced Materials & Sports Equipment Design; Applied Mechanics and Materials; Yang, D., Zhang, T.B., Luo, Q., Eds.; Trans Tech Publications Ltd.: Bäch SZ, Switzerland, 2014; Volume 440, pp. 97–103. [Google Scholar]
- Fajar, A.T.N.; Hanada, T.; Firmansyah, M.L.; Kubota, F.; Goto, M. Selective Separation of Platinum Group Metals via Sequential Transport through Polymer Inclusion Membranes Containing an Ionic Liquid Carrier. ACS Sustain. Chem. Eng. 2020, 8, 11283–11291. [Google Scholar] [CrossRef]
- Yu, W.L.; Chen, Z.; Yu, S.T.; Ding, J.W.; Shan, Y.L.; Liu, F.S.; Li, M. Highly dispersed Pt catalyst supported on nanoporous carbon derived from waste PET bottles for reductive alkylation. Rsc. Adv. 2019, 9, 31092–31101. [Google Scholar]
- Barakat, M.A.; Mahmoud, M.H.H.; Mahrous, Y.S. Recovery and separation of palladium from spent catalyst. Appl. Catal. A-Gen. 2006, 301, 182–186. [Google Scholar] [CrossRef]
- Minerals Yearbook of PGMs. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/platinum/myb1-2021-plati.pdf (accessed on 15 January 2022).
- USGS. ADVANCE RELAEASE. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/platinum/ar-2021-plati.pdf (accessed on 15 January 2022).
- KITCO. Available online: https://www.kitco.com/charts/livepalladium.html (accessed on 15 January 2022).
- Peng, Z.; Li, Z.; Lin, X.; Tang, H.; Ye, L.; Ma, Y.; Rao, M.; Zhang, Y.; Li, G.; Jiang, T. Pyrometallurgical Recovery of Platinum Group Metals from Spent Catalysts. JOM 2017, 69, 1047–4838. [Google Scholar] [CrossRef]
- Alekseeva, T.Y.; Karpov, Y.A.; Dal‘nova, O.A.; Es‘kina, V.V.; Baranovskaya, V.B.; Gorbatova, L.D. Current State and Problems of Analytical Control of Spent Automobile Catalysts (Review). Inorg. Mater. 2018, 54, 1421–1429. [Google Scholar] [CrossRef]
- Rzelewska, M.; Regel-Rosocka, M. Wastes generated by automotive industry—Spent automotive catalysts. Phys. Sci. Rev. 2018, 3, 2365–2381. [Google Scholar]
- Al-Sheeha, H.; Marafi, M.; Raghavan, V.; Rana, M.S. Recycling and Recovery Routes for Spent Hydroprocessing Catalyst Waste. Ind. Eng. Chem. Res. 2013, 52, 12794–12801. [Google Scholar] [CrossRef]
- A Threefold Increase in Two Years, the Myth of Palladiun. Available online: https://news.smm.cn/news/100877360. (accessed on 15 January 2022).
- Chauhan, G.; Pant, K.K.; Nigam, K.D.P. Metal Recovery from Hydroprocessing Spent Catalyst: A Green Chemical Engineering Approach. Ind. Eng. Chem. Res. 2013, 52, 16724–16736. [Google Scholar] [CrossRef]
- Liu, C.; Sun, S.; Zhu, X.; Tu, G. Metals smelting-collection method for recycling of platinum group metals from waste catalysts: A mini review. Waste Manag. Res. 2021, 39, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.; Ting, Y.-P. Recycling pathways for platinum group metals from spent automotive catalyst: A review on conventional approaches and bio-processes. Resour. Conserv. Recycl. 2021, 170, 113–116. [Google Scholar] [CrossRef]
- Sun, S.; Jin, C.; He, W.; Li, G.; Zhu, H.; Huang, J. A review on management of waste three-way catalysts and strategies for recovery of platinum group metals from them. J. Environ. Manag. 2020, 305, 114383. [Google Scholar] [CrossRef]
- Liu, S.J. Metallurgy of Platinum Group Metals; Central South University Press: Changsha, China, 2013; pp. 441–454. [Google Scholar]
- Deng, J.; Zhou, Y.; Li, S.; Xiong, L.; Wang, J.; Yuan, S.; Chen, Y. Designed synthesis and characterization of nanostructured ceria-zirconia based material with enhanced thermal stability and its application in three-way catalysis. J. Ind. Eng. Chem. 2018, 64, 219–229. [Google Scholar] [CrossRef]
- Zheng, T.; He, J.; Zhao, Y.; Xia, W.; He, J. Precious metal-support interaction in automotive exhaust catalysts. J. Rare Earths 2014, 32, 97–107. [Google Scholar] [CrossRef]
- Penner, S.; Wang, D.; Su, D.S.; Rupprechter, G.; Podloucky, R.; Schlögl, R.; Hayek, K. Platinum nanocrystals supported by silica, alumina and ceria: Metal–support interaction due to high-temperature reduction in hydrogen. Surf. Sci. 2003, 13, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, A.S.; Slavinskaya, E.M.; Gulyaev, R.V.; Zaikovskii, V.I.; Stonkus, О.А.; Danilova, I.G.; Plyasova, L.M.; Polukhina, I.A.; Boronin, A.I. Metal–support interactions in Pt/Al2O3 and Pd/Al2O3 catalysts for CO oxidation. Appl. Catal. B Environ. 2010, 97, 57–71. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, B.; Lv, J.; Wang, H.; Li, Z.; Ma, X.; Qin, S.; Sun, Q. Effect of sulfidation temperature on the catalytic activity of MoO3/CeO2–Al2O3 toward sulfur-resistant methanation. Appl. Catal. A Gen. 2013, 466, 224–232. [Google Scholar] [CrossRef]
- Yao, H.C. Surface interaction in the Pt/γ-Al2O3 system IV. Additive effects of CeO2 and MoO3. Appl. Surf. Sci. 1984, 19, 398–406. [Google Scholar] [CrossRef]
- Li, W.-J.; Wey, M.-Y. Sintering-resistant, highly thermally stable and well-dispersed Pd@CeO2/halloysite as an advanced three-way catalyst. Sci. Total Environ. 2020, 707, 136137. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Hirabayashi, T.; Dohmae, K.; Takagi, N.; Minami, T.; Shinjoh, H.; Matsumoto, S.I. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide–support interaction. J. Catal. 2006, 242, 103–109. [Google Scholar] [CrossRef]
- Nunan, J.G.; Robota, H.J.; Cohn, M.J.; Bradley, S.A. Physico-Chemical Properties of Ce-Containing Three-Way-Catalysts and the Effect of Ce on Catalyst Activity. Surf. Sci. Catal. 1991, 71, 221–238. [Google Scholar]
- Luo, M.-F.; Zheng, X.-M. Redox behaviour and catalytic properties of Ce0.5Zr0.5O2-supported palladium catalysts. Appl. Catal. A Gen. 1999, 189, 15–21. [Google Scholar] [CrossRef]
- Ordóñez, S.; Hurtado, P.; Sastre, H.; Díez, F.V. Methane catalytic combustion over Pd/Al2O3 in presence of sulphur dioxide: Development of a deactivation model. Appl. Catal. A Gen. 2004, 259, 41–48. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Scofield, C.F.; Neto, A.A.; Cardoso, M.J.B.; Zotin, F.M.Z. The influence of temperature on the deactivation of commercial Pd/Rh automotive catalysts. Process Saf. Environ. Prot. 2009, 87, 315–322. [Google Scholar] [CrossRef]
- Martin-Martinez, M.; Álvarez-Montero, A.; Gómez-Sainero, L.M.; Baker, R.T.; Palomar, J.; Omar, S.; Eser, S.; Rodriguez, J.J. Deactivation behavior of Pd/C and Pt/C catalysts in the gas-phase hydrodechlorination of chloromethanes: Structure–reactivity relationship. Appl. Catal. B Environ. 2015, 162, 532–543. [Google Scholar] [CrossRef] [Green Version]
- Nieto-Sandoval, J.; Ortiz, D.; Munoz, M.; de Pedro, Z.M.; Casas, J.A. On the deactivation and regeneration of Pd/Al2O3 catalyst for aqueous-phase hydrodechlorination of diluted chlorpromazine solution. Catal. Today 2019, 14, 53–59. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, J.C.; Jeong, J.; Yang, D.H.; Shin, D.; Lee, K.I. A Novel Process for Extracting Precious Metals from Spent Mobile Phone PCBs and Automobile Catalysts. Mater. Trans. 2013, 54, 1045–1048. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Song, Q.; Liu, Y.; Xu, Z. Novel approach for recovery of palladium in spent catalyst from automobile by a capture technology of eutectic copper. J. Clean. Prod. 2019, 239, 50–59. [Google Scholar] [CrossRef]
- Jimenez de Aberasturi, D.; Pinedo, R.; Ruiz de Larramendi, I.; Ruiz de Larramendi, J.I.; Rojo, T. Recovery by hydrometallurgical extraction of the platinum-group metals from car catalytic converters. Miner. Eng. 2011, 24, 505–513. [Google Scholar] [CrossRef]
- Angelidis, T.N. Development of a laboratory scale hydrometallurgical procedure for the recovery of Pt and Rh from spent automotive catalysts. Top. Catal. 2001, 16, 419–423. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Z.P.; Song, Z.W.; Ding, K.; Deng, A.D. Modification and regeneration of HZSM-5 catalyst in microwave assisted catalytic fast pyrolysis of mushroom waste. Energy Convers. Manag. 2016, 123, 29–34. [Google Scholar] [CrossRef]
- Suoranta, T.; Zugazua, O.; Niemelä, M.; Perämäki, P. Recovery of palladium, platinum, rhodium and ruthenium from catalyst materials using microwave-assisted leaching and cloud point extraction. Hydrometallurgy 2015, 154, 56–62. [Google Scholar] [CrossRef]
- Lin, G.; Cheng, S.; Wang, S.X.; Hu, T.; Peng, J.H.; Xia, H.Y.; Jiang, F.; Li, S.W.; Zhang, L.B. Process optimization of spent catalyst regeneration under microwave and ultrasonic spray-assisted. Catal. Today 2018, 318, 191–198. [Google Scholar] [CrossRef]
- Ye, X.L.; Koppala, S.; Qu, W.W.; Xu, S.M.; Zhang, L.B.; Liu, B.G.; Guo, S.H.; Wang, L. New approach to the utilization of microwave thermal energy: Desulfurization and decarburization of spent catalyst via microwave treatment. Powder Technol. 2018, 338, 764–773. [Google Scholar] [CrossRef]
- Spooren, J.; Atia, T.A. Combined microwave assisted roasting and leaching to recover platinum group metals from spent automotive catalysts. Miner. Eng. 2020, 146, 0892–0895. [Google Scholar] [CrossRef]
- Jun, R. Recovery of spent catalysts. Rare Met. Separately 1994, 118, 0926–0928. [Google Scholar]
- Saily, A.; Khurana, U.; Yadav, S.K.; Tandon, S.N. Thiophosphinic acids as selective extractants for molybdenum recovery from a low grade ore and spent catalysts. Hydrometallurgy 1996, 41, 0304–0308. [Google Scholar] [CrossRef]
- Paiva, A.P.; Ortet, O.; Carvalho, G.I.; Nogueira, C.A. Recovery of palladium from a spent industrial catalyst through leaching and solvent extraction. Hydrometallurgy 2017, 171, 394–401. [Google Scholar] [CrossRef]
- Nogueira, C.A.; Paiva, A.P.; Oliveira, P.C.; Costa, M.C.; da Costa, A.M. Oxidative leaching process with cupric ion in hydrochloric acid media for recovery of Pd and Rh from spent catalytic converters. J. Hazard. Mater. 2014, 278, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Huang, K. A new technique for extraction of platinum group metals by pressure cyanidation. Hydrometallurgy 2006, 82, 164–171. [Google Scholar] [CrossRef]
- Naghavi, Z.; Ghoreishi, S.M.; Rahimi, A.; Hadadzadeh, H. Kinetic Study for Platinum Extraction from Spent Catalyst in Cyanide Solution at High Temperatures. Int. J. Chem. React. Eng. 2016, 14, 143–154. [Google Scholar] [CrossRef]
- Wong, F.F.; Lin, C.M.; Chang, C.P.; Huang, J.R.; Yeh, M.Y.; Huang, J.J. Recovery and reduction of spent nickel oxide catalyst via plasma sintering technique. Plasma Chem. Plasma Process. 2006, 26, 585–595. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Muir, D.M. Thiosulfate leaching of gold—A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Reddy, B.R.; Raju, B.; Lee, J.Y.; Park, H.K. Process for the separation and recovery of palladium and platinum from spent automobile catalyst leach liquor using LIX 84I and Alamine 336. J. Hazard. Mater. 2010, 180, 253–258. [Google Scholar] [CrossRef]
- Sarioglan, S. Recovery of Palladium from Spent Activated Carbon-Supported Palladium Catalysts. Platin. Met. Rev. 2013, 57, 289–296. [Google Scholar] [CrossRef]
- Firmansyah, M.L.; Kubota, F.; Goto, M. Selective Recovery of Platinum Group Metals from Spent Automotive Catalysts by Leaching and Solvent Extraction. J. Chem. Eng. Jpn. 2019, 52, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Paiva, A.P. Recycling of Palladium from Spent Catalysts Using Solvent Extraction-Some Critical Points. Metals 2017, 7, 505. [Google Scholar] [CrossRef] [Green Version]
- Mishra, D.; Kim, D.J.; Ralph, D.E.; Alm, J.G.; Rhee, Y.H. Bioleaching of spent hydro-proces sing catalyst using acidophilic bacteria and its kinetics aspect. J. Hazard. Mater. 2008, 152, 1082–1091. [Google Scholar] [CrossRef]
- Ting, Y.P.; Aung, K.M.M. Bacterial and fungal bioleaching of spent hydroprocessing catalysts for metal recovery. J. Biotechnol. 2008, 136, S649. [Google Scholar] [CrossRef]
- Pathak, A.; Srichandan, H.; Kim, D.J. Column bioleaching of metals from refinery spent catalyst by Acidithiobacillus thiooxidans: Effect of operational modifications on metal extraction, metal precipitation, and bacterial attachment. J. Environ. Manag. 2019, 242, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Nanusha, M.Y.; Carlier, J.D.; Carvalho, G.I.; Costa, M.C.; Paiva, A.P. Separation and recovery of Pd and Fe as nanosized metal sulphides by combining solvent extraction with biological strategies based on the use of sulphate-reducing bacteria. Sep. Purif. Technol. 2019, 212, 747–756. [Google Scholar] [CrossRef]
- Faisal, M.; Atsuta, Y.; Daimon, H.; Fujie, K. Recovery of precious metals from spent automobile catalytic converters using supercritical carbon dioxide. Asia-Pac. J. Chem. Eng. 2008, 3, 364–367. [Google Scholar] [CrossRef]
- Iwao, S.; El-Fatah, S.A.; Furukawa, K.; Seki, T.; Sasaki, M.; Goto, M. Recovery of palladium from spent catalyst with supercritical CO2 and chelating agent. J. Supercrit. Fluids 2007, 42, 200–204. [Google Scholar] [CrossRef]
- Faisal, M.; Atsuta, Y.; Daimon, H. Effect of Agitation on the Extraction of Palladium from Spent Industrial Catalyst with Supercritical Carbon Dioxide. Asian J. Chem. 2010, 22, 1693–1699. [Google Scholar]
- Zhang, X.X.; Zong, B.N.; Qiao, M.H. Reactivation of Spent Pd/AC Catalyst by Supercritical CO2 Fluid Extraction. AlChE J. 2009, 55, 2382–2388. [Google Scholar] [CrossRef]
- Latsuzbaia, R.; Negro, E.; Koper, G.J.M. Environmentally Friendly Carbon-Preserving Recovery of Noble Metals From Supported Fuel Cell Catalysts. ChemSusChem 2015, 8, 1926–1934. [Google Scholar] [CrossRef]
- Hodnik, N.; Baldizzone, C.; Polymeros, G.; Geiger, S.; Grote, J.P.; Cherevko, S.; Mingers, A.; Zeradjanin, A.; Mayrhofer, K.J. Platinum recycling going green via induced surface potential alteration enabling fast and efficient dissolution. Nat. Commun. 2016, 7, 13164. [Google Scholar] [CrossRef] [PubMed]
- Lanaridi, O.; Platzer, S.; Nischkauer, W.; Limbeck, A.; Schnurch, M.; Bica-Schroder, K. A Combined Deep Eutectic Solvent-Ionic Liquid Process for the Extraction and Separation of Platinum Group Metals (Pt, Pd, Rh). Molecules 2021, 26, 7204. [Google Scholar] [CrossRef] [PubMed]
- Vasile, E.; Ciocanea, A.; Ionescu, V.; Lepadatu, I.; Diac, C.; Stamatin, S.N. Making precious metals cheap: A sonoelectrochemical—Hydrodynamic cavitation method to recycle platinum group metals from spent automotive catalysts. Ultrason. Sonochem. 2021, 72, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- Sobral, L.G.S.; Granato, M. Palladium: Extraction and refining. Miner. Eng. 1992, 5, 17–25. [Google Scholar] [CrossRef]
- Chao, Y. Research on Recovery of Palladium from the Spent Pd/Al2O3 Catalyst. Master’s Dissertation, Northeastern University, Shenyang, China, 2016. [Google Scholar]
- Zhang, Z.H. The separation and purification of palladium from the spent catalyst. Miner. Processing 2002, 54, 60–62. [Google Scholar]
- Singh, R.; Khwaja, A.R.; Gupta, B.; Tandon, S.N. Extraction and separation of nickel(II) using bis(2,4,4-trimethylpentyl) dithiophosphinic acid (Cyanex 301) and its recovery from spent catalyst and electroplating bath residue. Solvent Extr. Ion Exch. 1999, 17, 367–390. [Google Scholar] [CrossRef]
- Swain, B.; Jeong, J.; Kim, S.-K.; Lee, J.-C. Separation of platinum and palladium from chloride solution by solvent extraction using Alamine 300. Hydrometallurgy 2010, 104, 1–7. [Google Scholar] [CrossRef]
- Traeger, J.; König, J.; Städtke, A.; Holdt, H.-J. Development of a solvent extraction system with 1,2-bis(2-methoxyethylthio)benzene for the selective separation of palladium(II) from secondary raw materials. Hydrometallurgy 2012, 127–128, 30–38. [Google Scholar] [CrossRef]
- Lee, J.Y.; Raju, B.; Kumar, B.N.; Kumar, J.R.; Park, H.K.; Reddy, B.R. Solvent extraction separation and recovery of palladium and platinum from chloride leach liquors of spent automobile catalyst. Sep. Purif. Technol. 2010, 73, 213–218. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Sonu, C.H.; Lee, M.S. Separation of Pt(IV), Pd(II), Rh(III) and Ir(IV) from concentrated hydrochloric acid solutions by solvent extraction. Hydrometallurgy 2016, 164, 71–77. [Google Scholar] [CrossRef]
- Costa, M.C.; Assunção, A.; Almeida, R.; da Costa, A.M.R.; Nogueira, C.; Paiva, A.P. N,N′-dimethyl-N,N′-dicyclohexylsuccinamide: A novel molecule for the separation and recovery of Pd(II) by liquid-liquid extraction. Sep. Purif. Technol. 2018, 201, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Rajesh, N. Augmenting the adsorption of palladium from spent catalyst using a thiazole ligand tethered on an amine functionalized polymeric resin. Chem. Eng. J. 2016, 283, 999–1008. [Google Scholar] [CrossRef]
- Dewan, A.; Sarmah, M.; Bora, U.; Thakur, A.J. In situ generation of palladium nanoparticles using agro waste and their use as catalyst for copper-, amine- and ligand-free Sonogashira reaction. Appl. Organomet. Chem. 2017, 31, 0268–0272. [Google Scholar] [CrossRef]
- Maeda, M.; Narita, H.; Tokoro, C.; Tanaka, M.; Motokawa, R.; Shiwaku, H.; Yaita, T. Selective extraction of Pt(IV) over Fe(III) from HCl with an amide-containing tertiary amine compound. Sep. Purif. Technol. 2017, 177, 176–181. [Google Scholar] [CrossRef]
- Mhaske, A.A.; Dhadke, P.M. Extraction separation studies of Rh, Pt and Pd using Cyanex 921 in toluene—A possible application to recovery from spent catalysts. Hydrometallurgy 2001, 61, 143–150. [Google Scholar] [CrossRef]
- Das, A.; Ruhela, R.; Singh, A.K.; Hubli, R.C. Evaluation of novel ligand dithiodiglycolamide (DTDGA) for separation and recovery of palladium from simulated spent catalyst dissolver solution. Sep. Purif. Technol. 2014, 125, 151–155. [Google Scholar] [CrossRef]
- Anpilogova, G.R.; Khisamutdinov, R.A.; Golubyatnikova, L.G.; Murinov, Y.I. Propiconazole and Penconazole as Effective Extractants for Selective recovery and concentration of platinum(IV) and palladium(II) from hydrochloric acid solutions formed in leaching of spent aluminoplatinum and aluminopalladium catalysts. Russ. J. Appl. Chem. 2016, 89, 206–211. [Google Scholar] [CrossRef]
- Yao, Z.H. The synthesis of the amido oximime chelate fiber and its adsorption behavior of palladium. Chin. Polym. Mater. Sci. Eng. 1999, 15, 10–13. [Google Scholar]
- Gan, S.C. Separation and enrichment of Gold, platinum and palladium by dt-1016 anion exchange resin. Chin. Rock Miner. Anal. 2002, 2, 113–116. [Google Scholar]
- Turanov, A.N.; Karandashev, V.К.; Artyushin, O.I.; Sharova, E.V.; Genkina, G.K. Adsorption of palladium(II) from hydrochloric acid solutions using polymeric resins impregnated with novel N-substituted 2-(diphenylthiophosphoryl)acetamides. Sep. Purif. Technol. 2017, 187, 355–364. [Google Scholar] [CrossRef]
- Hageluken, C. Recycling of spent noble metal catalysts with emphasis on pyrometallurgical processing. Oil Gas-Eur. Mag. 1999, 25, 36–39. [Google Scholar]
- Liu, C.; Sun, S.C.; Zhu, X.P.; Tu, G.F.; Zhang, J.Y. Recovery of platinum from the spent auto-catalysts by pyrometallurgy. In Proceedings of the 3rd International Conference on New Material and Chemical Industry, Sanya, China, 17–19 November 2018. [Google Scholar]
- Varley, T. The chloride volatilization process. J. Frankl. Inst. 1923, 195, 715–716. [Google Scholar] [CrossRef]
- Benson, M.; Bennett, C.R.; Harry, J.E.; Patel, M.K.; Cross, M. The recovery mechanism of platinum group metals from catalytic converters in spent automotive exhaust systems. Resour. Conserv. Recycl. 2000, 31, 1–7. [Google Scholar] [CrossRef]
- Zheng, H.; Ding, Y.; Wen, Q.; Zhao, S.; He, X.; Zhang, S.; Dong, C. Slag design and iron capture mechanism for recovering low-grade Pt, Pd, and Rh from leaching residue of spent auto-exhaust catalysts. Sci. Total Environ. 2022, 802, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
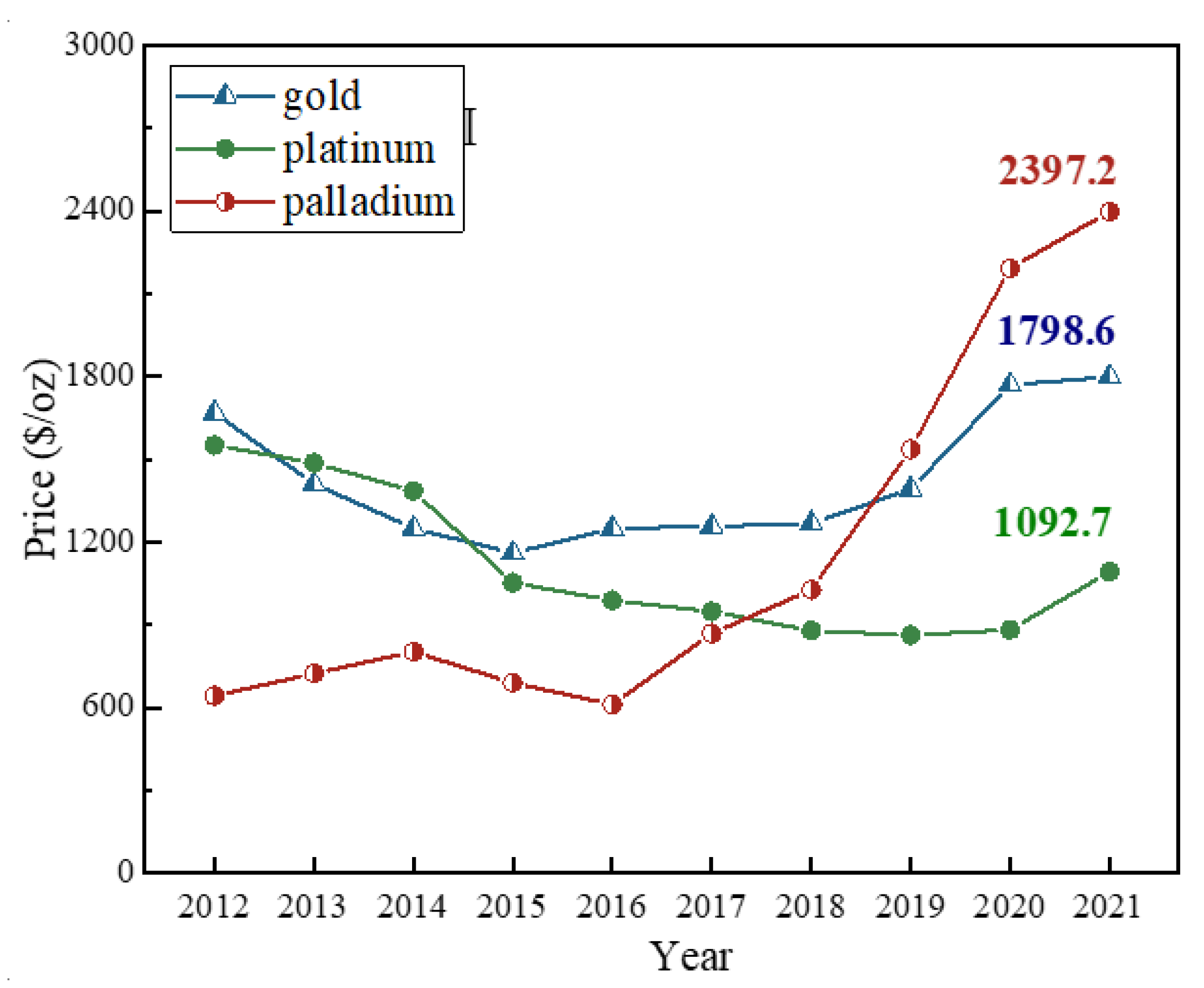
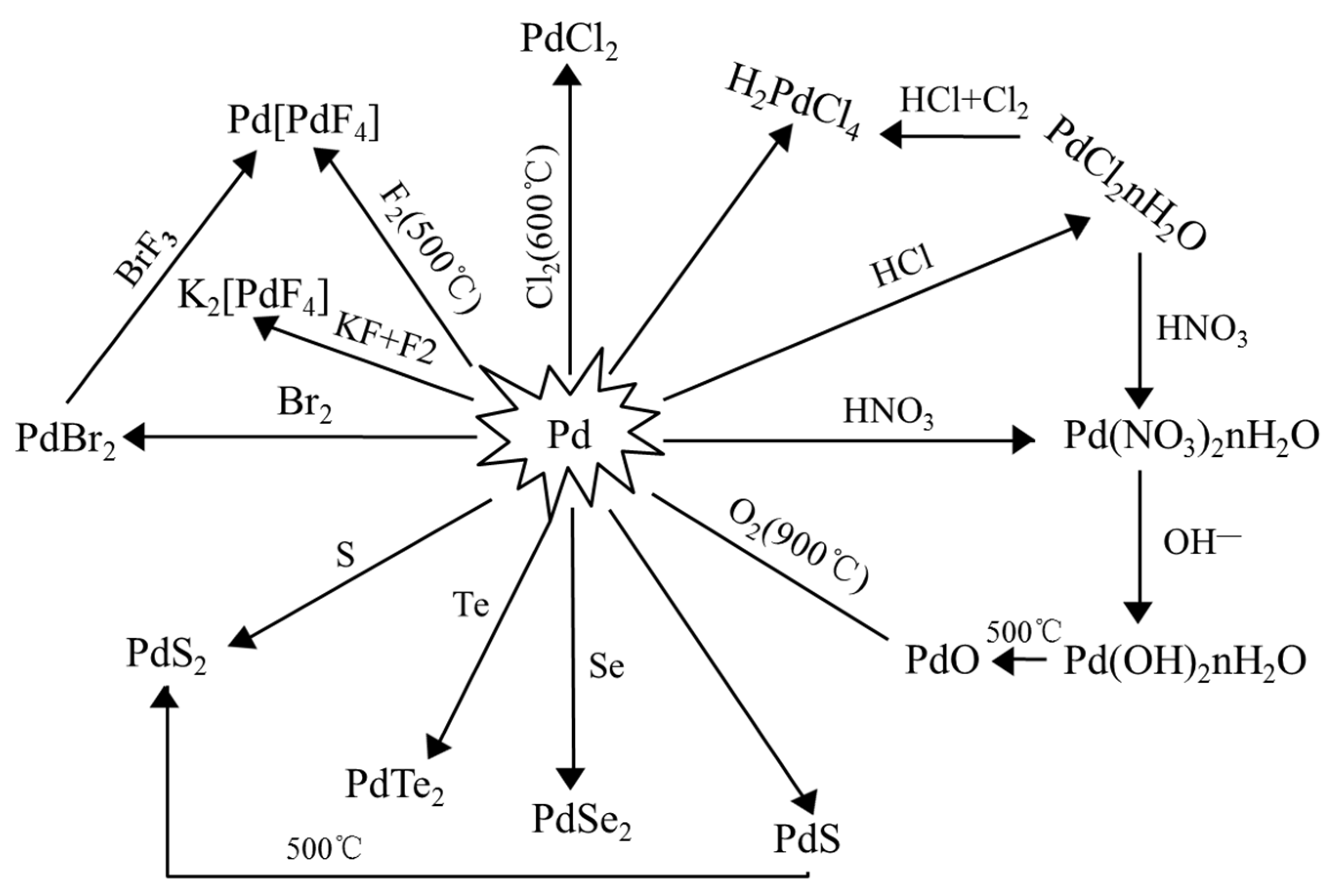
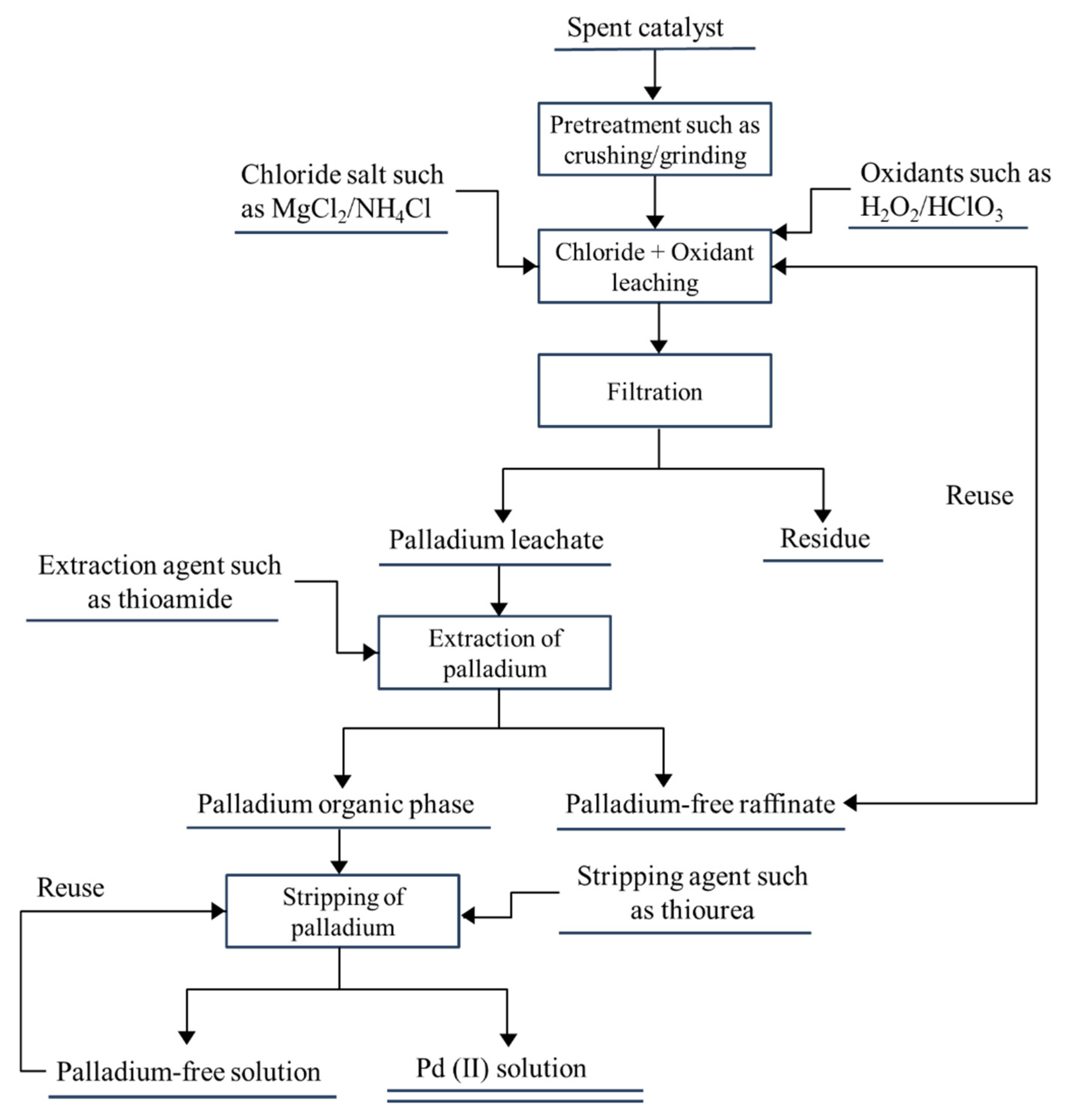
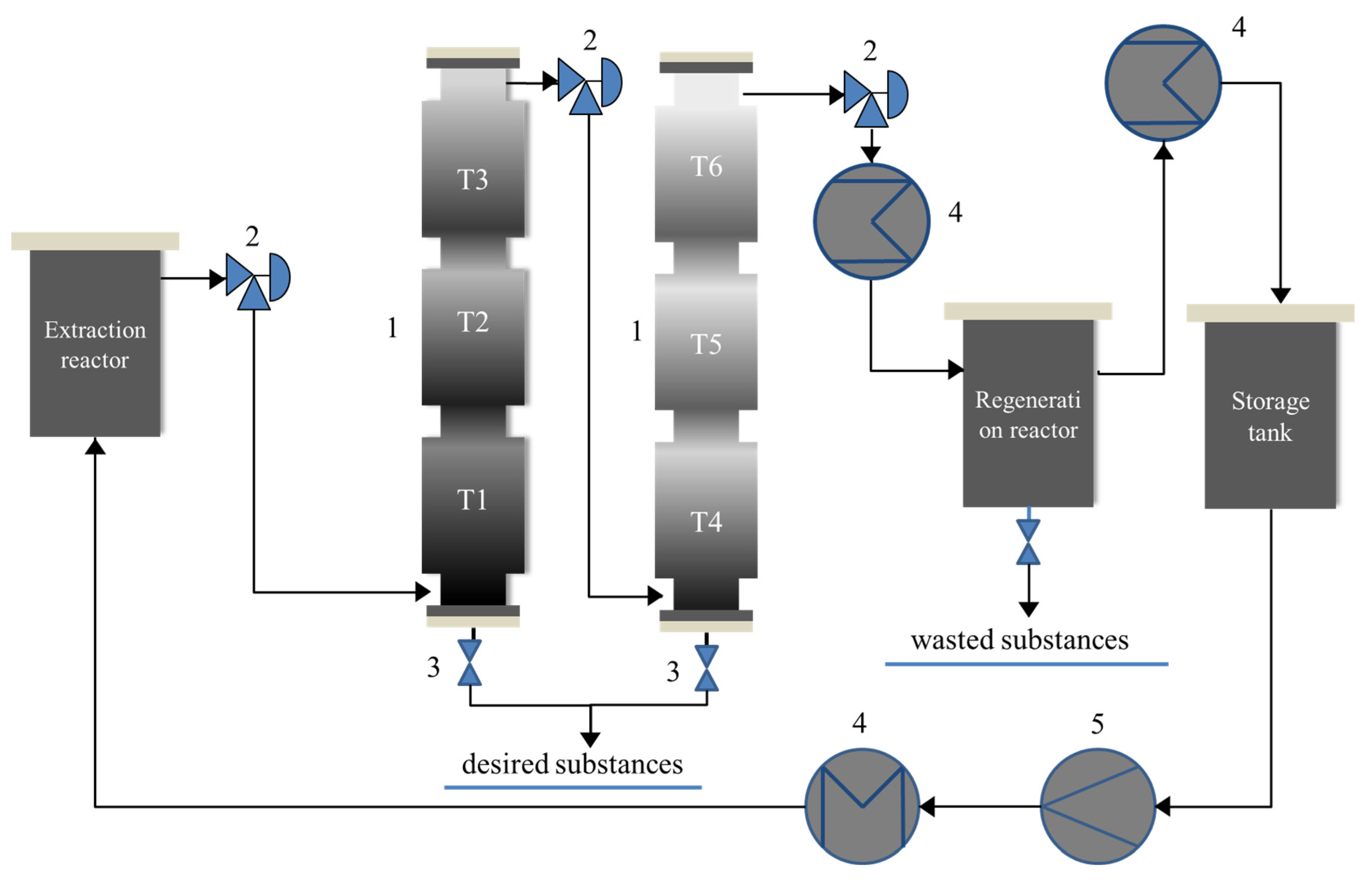

| Country | Production of Palladium | Production of Platinum | PGM Reserve (2020) | ||
|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | ||
| South Africa | 80,700 | 70,000 | 133,000 | 120,000 | 63,000,000 |
| Russia | 98,000 | 91,000 | 24,000 | 21,000 | 3,900,000 |
| Zimbabwe | 11,400 | 12,000 | 13,500 | 14,000 | 1,200,000 |
| United States | 14,300 | 14,000 | 4150 | 4000 | 900,000 |
| Canada | 20,000 | 20,000 | 7800 | 7800 | 310,000 |
| Other Countries | 2420 | 2600 | 3730 | 3800 | NA |
| World total | 227,000 | 210,000 | 186,000 | 170,000 | 69,000,000 |
| Physical Properties | Palladium | Physical Properties | Palladium |
|---|---|---|---|
| Atomic number | 46 | Melting point/°C | 1550 |
| The electron configuration | 4d10 | Heat of fusion/(kJ/mol) | 16.9 |
| Atomic weight | 106.4 | Boiling point/°C | 2900 |
| Atomic volume/10−6 m3 | 8.859 | Sublimation heat kJ/mol | 372 |
| Atomic radius/nm | 0.137 | Density (20 °C)/(g/cm3) | 12.02 |
| Electronegativity | 2.1 | Specific heat (25 °C)/(kJ/mol) | 26.0 |
| The crystal structure | Face-centered cubic | Thermal conductivity (10–100 °C) | 0.18 |
| Lattice constant/Å | 0.38895 | Resistivity (0 °C)/(mu Ω cm) | 9.93 |
| The first ionization potential/mV | 8.33 | Temperature coefficient of Resistance (0~100 °C) | 0.0038 |
| Corrosive Medium | Temperature/°C | Erosion Resistance | Corrosive Medium | Temperature/°C | Erosion Resistance |
|---|---|---|---|---|---|
| Concentrated H2SO4 | 25 | A | NaOH(aq) | 25 | A |
| 100 | B | KOH(aq) | 25 | A | |
| 250 | C | NH4OH | 25 | A | |
| HNO3 (0.1 mol/L) | 25 | A | HgCl2(aq) | 100 | A |
| (1 mol/L) | 25 | B | KCN(aq) | 25 | C |
| (2 mol/L) | 25 | C | 100 | D | |
| (70%) | 25 | D | CaCl2(aq) | 100 | C |
| HCl (36%) | 25 | A | FeCl3(aq) | 25 | C |
| (36%) | 100 | B | 100 | D | |
| HBr (density 1.7) | 25 | B | NaClO3(aq) | 25 | C |
| 100 | D | Molten caustic soda | - | B | |
| HI (density 1.6) | 25 | D | Molten potash | - | B |
| HClO4 (density 1.6) | 100 | A | Molten sodium peroxide | - | D |
| H3PO4 | 25 | A | Molten sodium sulfate | - | C |
| Aqua regia | 25 | D | Molten sodium carbonate | - | B |
| CH3COOH | 100 | A | Molten sodium nitrate | - | C |
| Physical Properties | Density/(g/cm3) | Viscosity/(g/cm·s) | Diffusion Coefficient/(cm2/s) |
|---|---|---|---|
| Gas (25 °C, 101.325 Pa) | (0.6~2) × 10−3 | (1~3) × 10−4 | 0.1~0.4 |
| Liquid (25 °C) | 0.6~1.6 | 0.2~3 × 10−2 | (0.2~2) × 10−5 |
| SCF | 0.2~0.9 | (1~3) × 10−4 | 10−3~10−4 |
| Solvent | Boiling Point/(°C) | Critical Temperature/(°C) | Critical Pressure/(MPa) |
|---|---|---|---|
| Ammonia | −33.4 | 132.3 | 11.28 |
| Carbon dioxide | −78.5 | 31.06 | 7.39 |
| Chlorotrifluoromethane | 23.7 | 196.6 | 4.22 |
| Dichlorodifluoromethane | −29.8 | 111.7 | 3.99 |
| Diethyl ether | 34.6 | 193.6 | 3.68 |
| Ethane | −88.0 | 32.4 | 4.89 |
| Ethyl alcohol | 78.2 | 243.4 | 6.38 |
| Ethylene | −103.7 | 9.5 | 5.04 |
| Methane | −164.0 | −83.0 | 4.6 |
| Methyl alcohol | 64.7 | 240.5 | 7.99 |
| Methylbenzene | 110.6 | 318 | 4.11 |
| Propane | −44.5 | 97 | 4.26 |
| Propylene | −47.7 | 92 | 4.62 |
| Water | 100 | 374.2 | 22.00 |
| Extractants | Industrial Applications | Conditions | Evaluations |
|---|---|---|---|
| LIX84I [55] | YES | leachate at pH = 2, A/O = 3. | Pd recovery ratio >99%; Strong acid corrosion. |
| Cyanex921 [82] | YES | 6 M HCl, A/O = 2. | High selectivity for Pd; Mild reaction conditions. |
| Dithiodiglycolamide(DTDGA) [83] | NO | 3 M HCl, A/O = 1, back extraction of palladium using 0.01 M thiourea in 0.1 M HCl. | High selectivity for Pd; Pd recovery ratio 98.8%. |
| BSO [48] | NO | >4 M HCl, A/O = 3. | Pd extraction ratio >99%; Solution of high acidity |
| Cyphos 101IL [78] | NO | 6 M HCl, all extractants in toluene, A/O = 1. | Pd extraction ratio 100%; Real SACs solutions were not involved. |
| Propiconzole and penconazole [84] | NO | 3~4 M HCl, A/O = 2, using ammonia solution to extract Pd. | High selectivity for Pd; High acidity solution. |
| Methods | Advantages | Disadvantages | |
|---|---|---|---|
| Hydrometallurgical methods | Cyanide leaching | Conventional technique and yields high recovery ratios >99% of Pd and Pt; low cost of reagents. | Cyano-compounds are highly toxic to living organisms; wastewater needs to be properly treated. |
| HCl+oxidants leaching | High leaching ratio >99% of Pd; low investment of equipment. | Proper pretreatment is needed; extractants should possess high selectivity of Pd; equipment corrosion of high concentrated HCl. | |
| Bio-recovery | Low cost of reagents; no pollution to the environment. | Needs a long reaction period; main leaching mechanisms are unclear. | |
| Supercritical fluid process | High reaction speed of Pd recovery; no pollution to air and water. | Low recovery ratio of Pd; high temperature and pressure conditions. | |
| Electrochemical transient dissolution | Milder and safer. | Application is currently limited and needs further study. | |
| Pyrometallurgical methods | Chlorination volatilization | Low melting temperature (1000~1200 °C); reusable carrier; high recovery ratios of PGMs. | Gases generated (Cl2 and COCl2) are toxic and corrosive. |
| Iron collection | Low cost of collection iron; high recovery ratios of PGMs. | High energy consumption of plasma smelting (1500~1600 °C); Short service of the plasma gun. High investment for equipment. | |
| Copper collection | Collection copper can be reused; high recovery ratios of PGMs; moderate melting temperature. | A long production cycle; high energy consumption of smelting (1300~1400 °C). | |
| Lead collection | Simple operation; relatively low melting temperature. | Serious lead dust pollution, which is harmful to workers’ health. | |
| Matte collection | Can be directly combined with smelting equipment of copper and nickel. | Generated sulfur and its corresponding oxides pose an environmental risk. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Chen, Y.; Zhou, Y.; Zhang, B.; Liu, G.; Li, Q.; Yang, Y.; Jiang, T. A Review of Recovery of Palladium from the Spent Automobile Catalysts. Metals 2022, 12, 533. https://doi.org/10.3390/met12040533
Xu B, Chen Y, Zhou Y, Zhang B, Liu G, Li Q, Yang Y, Jiang T. A Review of Recovery of Palladium from the Spent Automobile Catalysts. Metals. 2022; 12(4):533. https://doi.org/10.3390/met12040533
Chicago/Turabian StyleXu, Bin, Yufeng Chen, Yujuan Zhou, Bangsheng Zhang, Guiqing Liu, Qian Li, Yongbin Yang, and Tao Jiang. 2022. "A Review of Recovery of Palladium from the Spent Automobile Catalysts" Metals 12, no. 4: 533. https://doi.org/10.3390/met12040533
APA StyleXu, B., Chen, Y., Zhou, Y., Zhang, B., Liu, G., Li, Q., Yang, Y., & Jiang, T. (2022). A Review of Recovery of Palladium from the Spent Automobile Catalysts. Metals, 12(4), 533. https://doi.org/10.3390/met12040533





