Preparation of Ti-46Al-8Nb Alloy Ingots beyond Laboratory Scale Based on BaZrO3 Refractory Crucible
Abstract
:1. Introduction
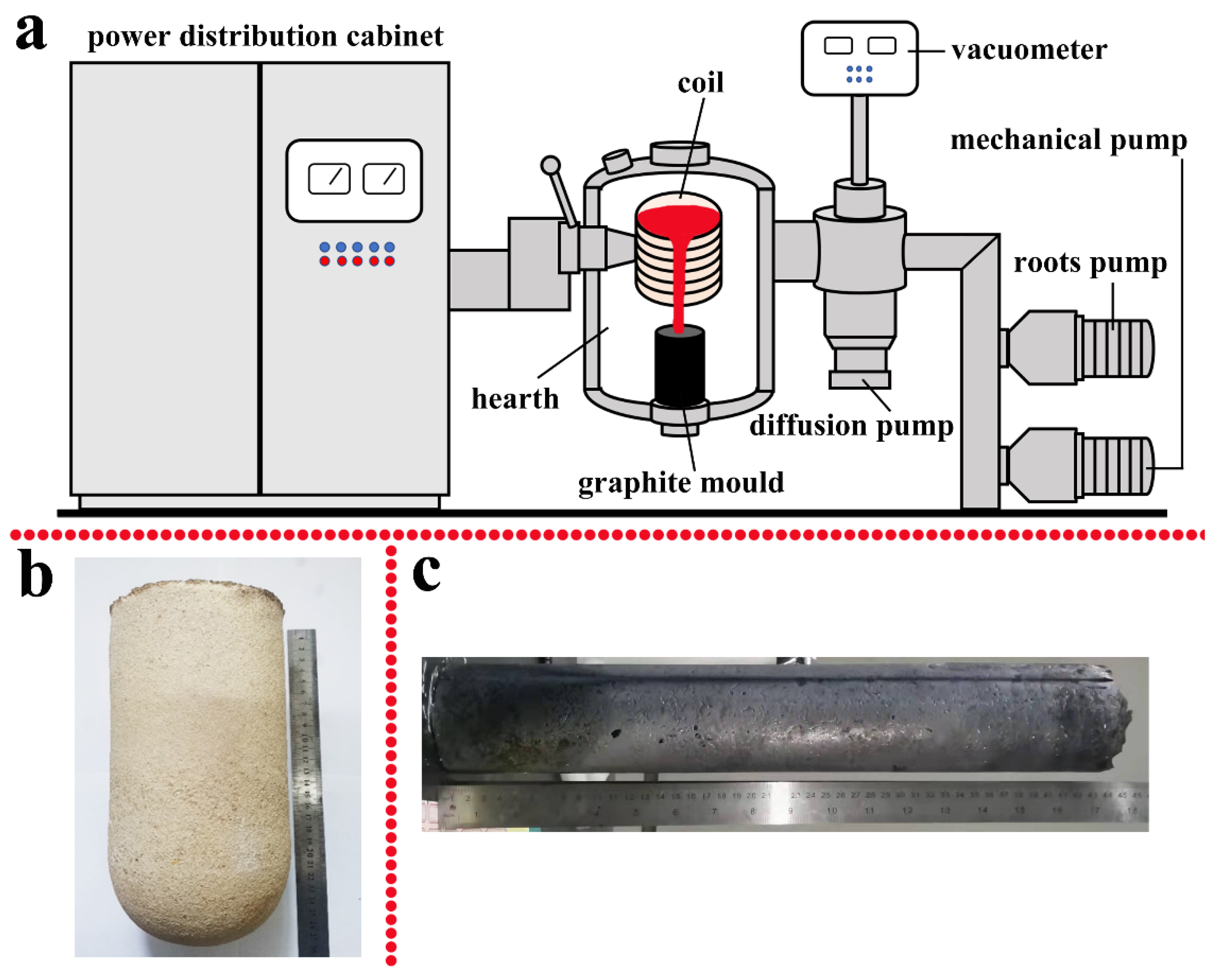
2. Experimental Method and Procedure
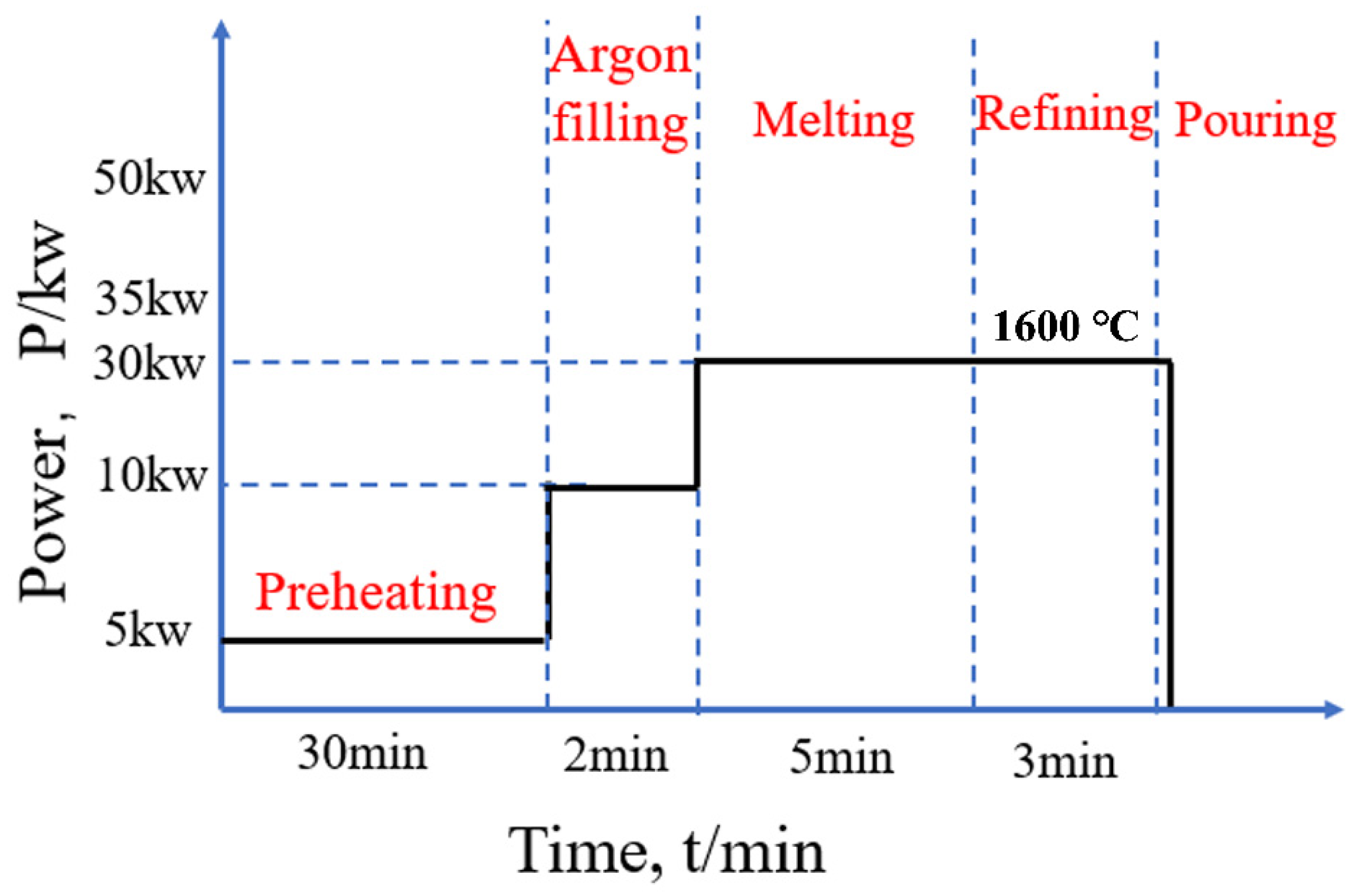
2.1. Melting Process
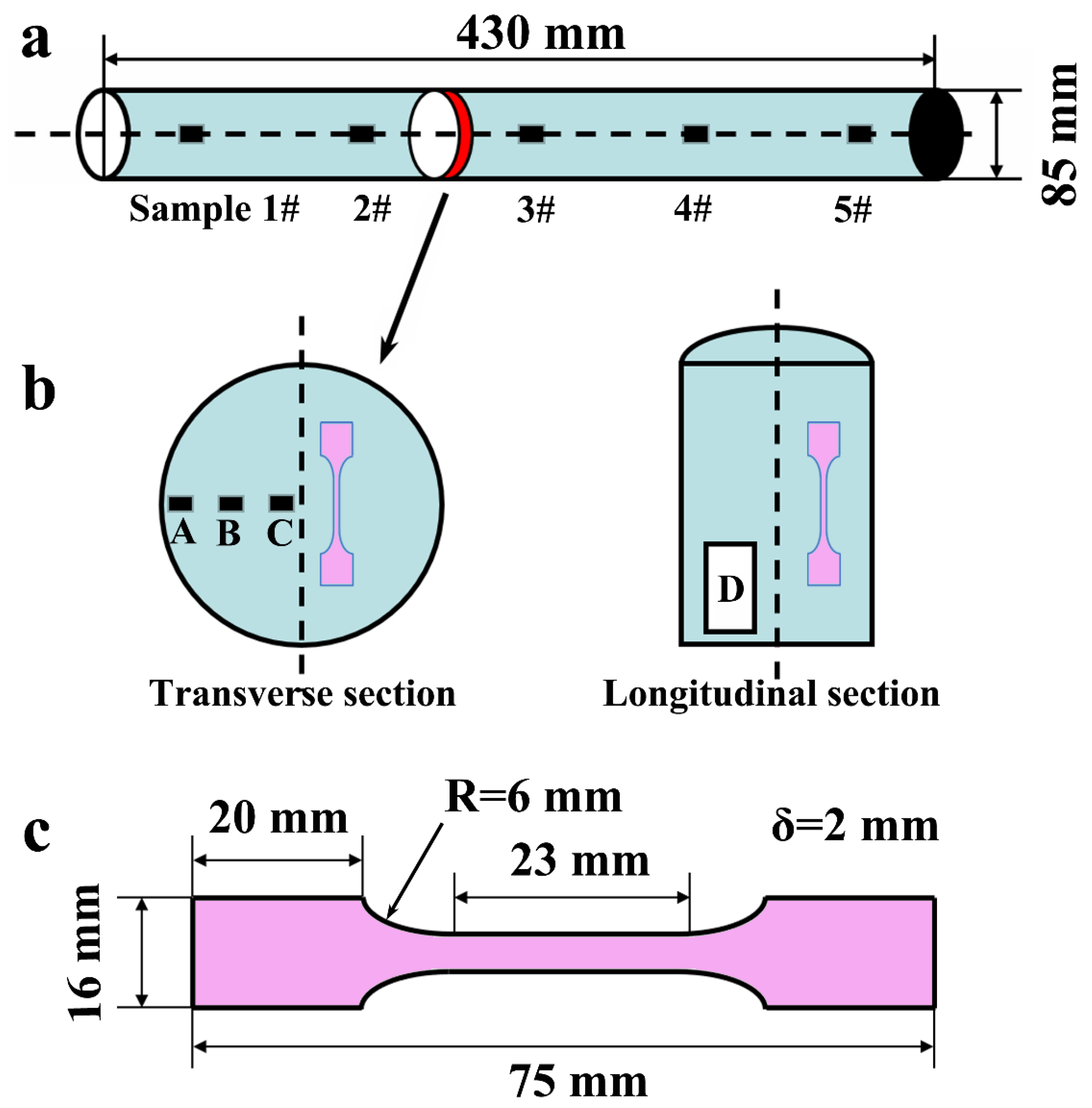
2.2. Sampling and Processing
2.3. Sample Characterization and Testing
3. Results and Discussions
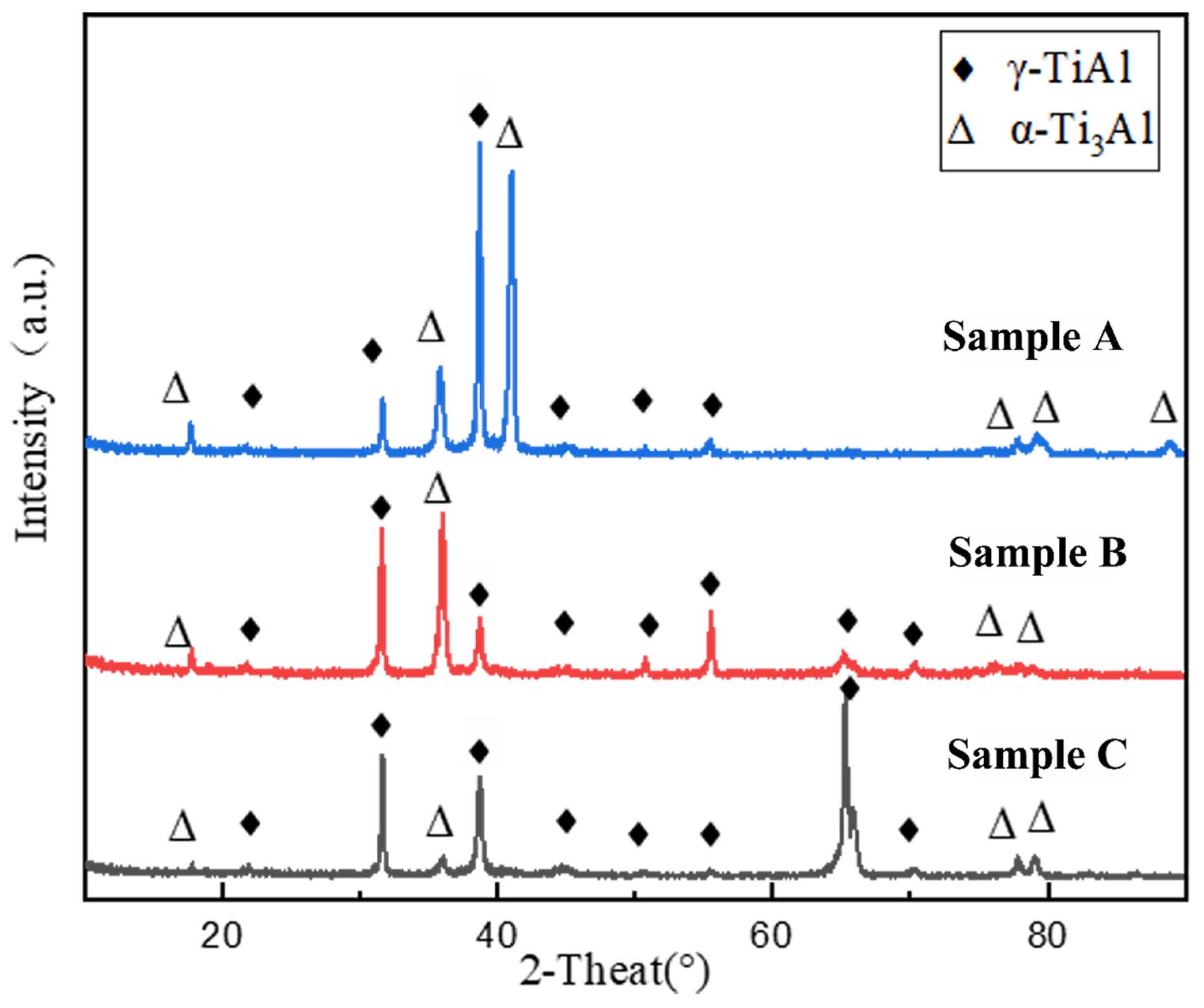
3.1. Chemical Composition and Phases
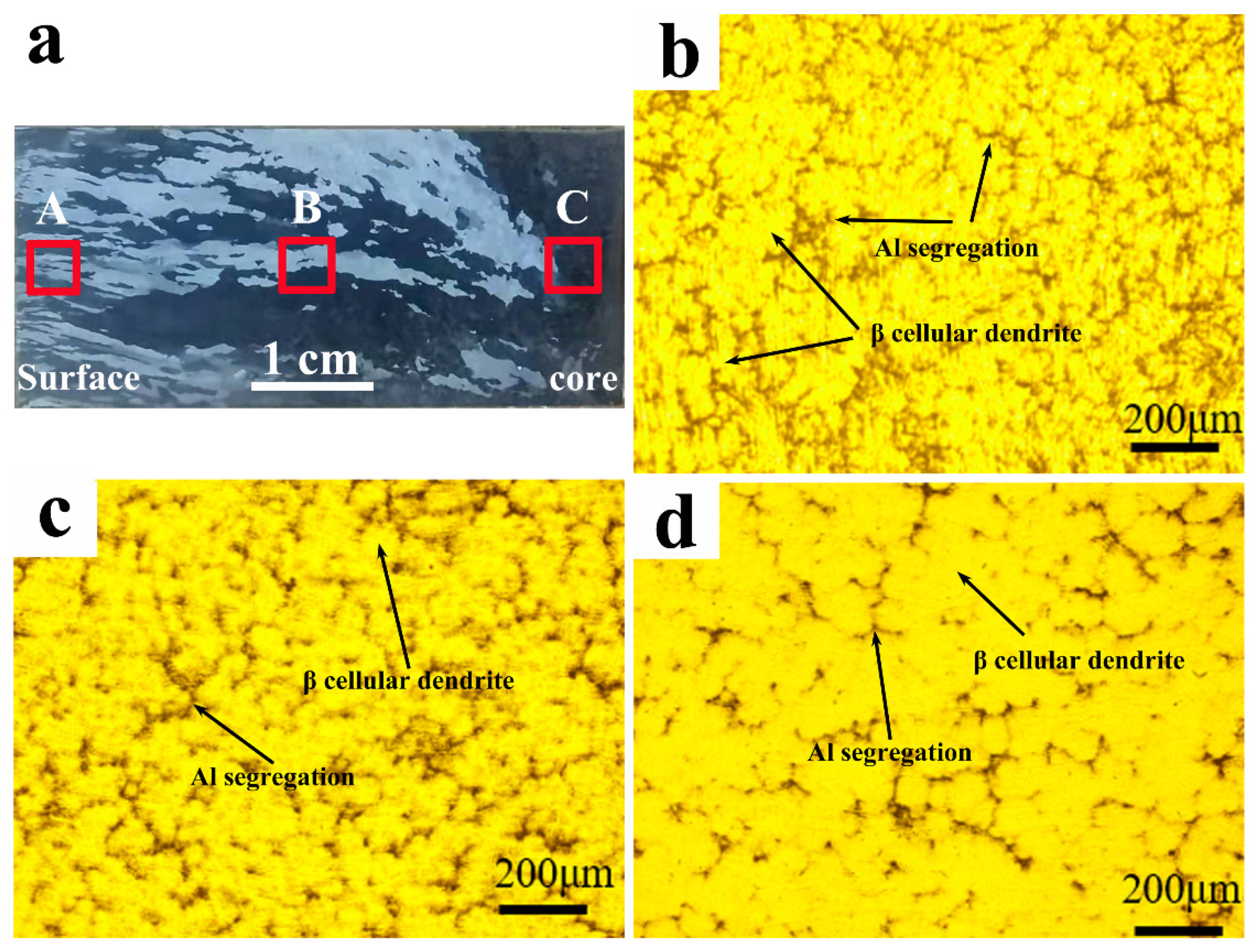
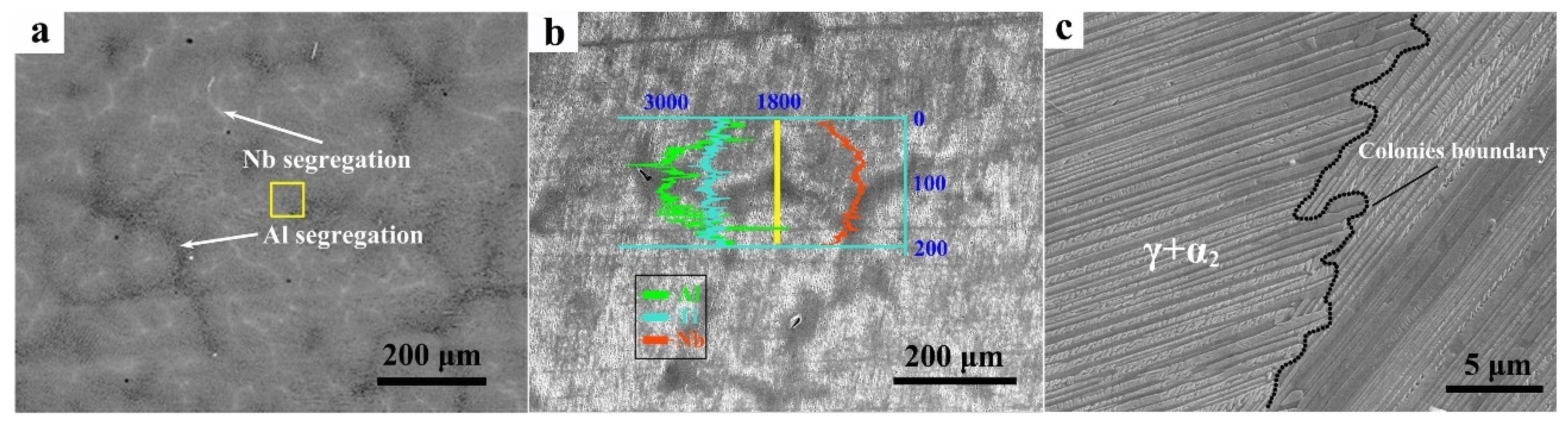
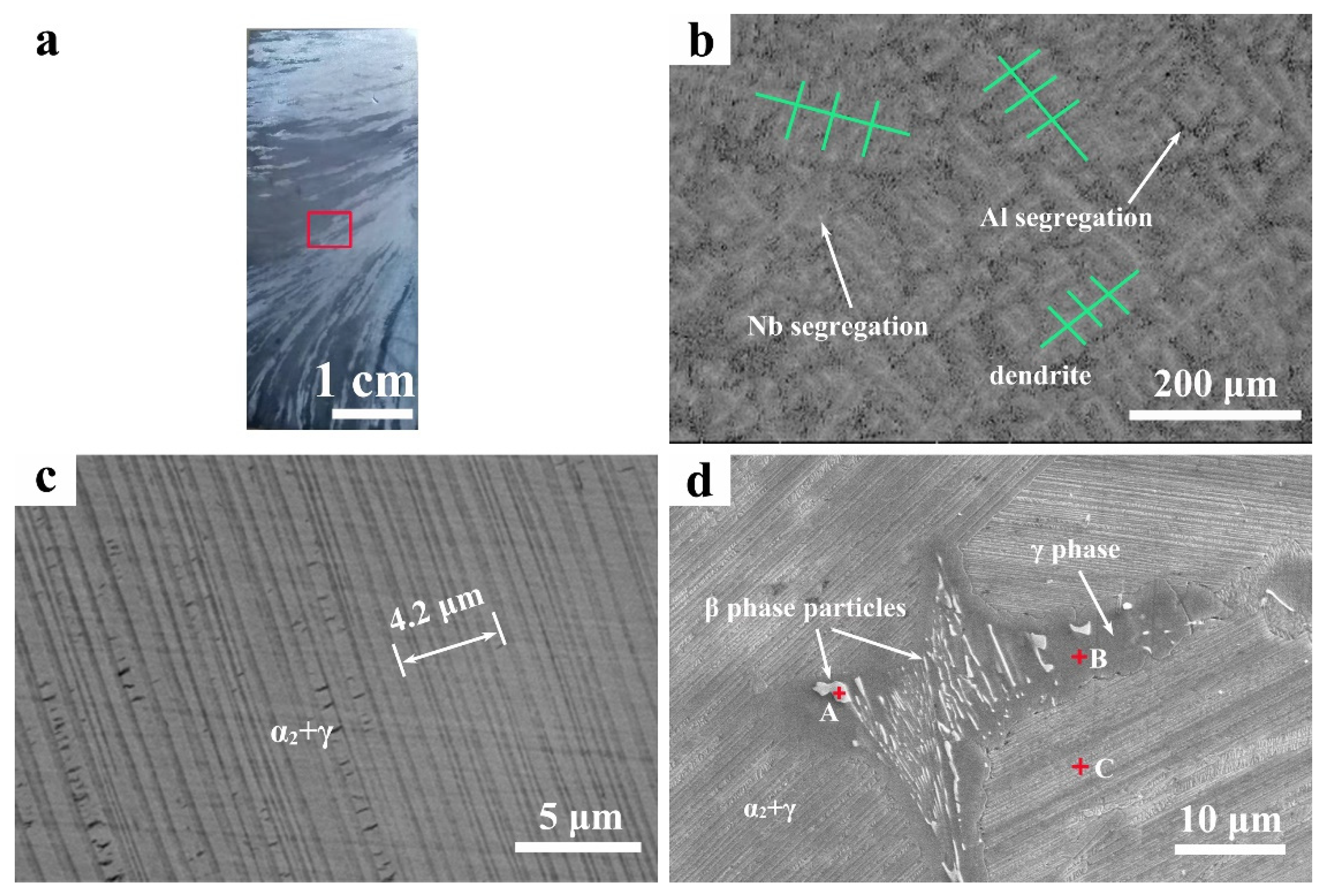
3.2. Structure and Microsegregation
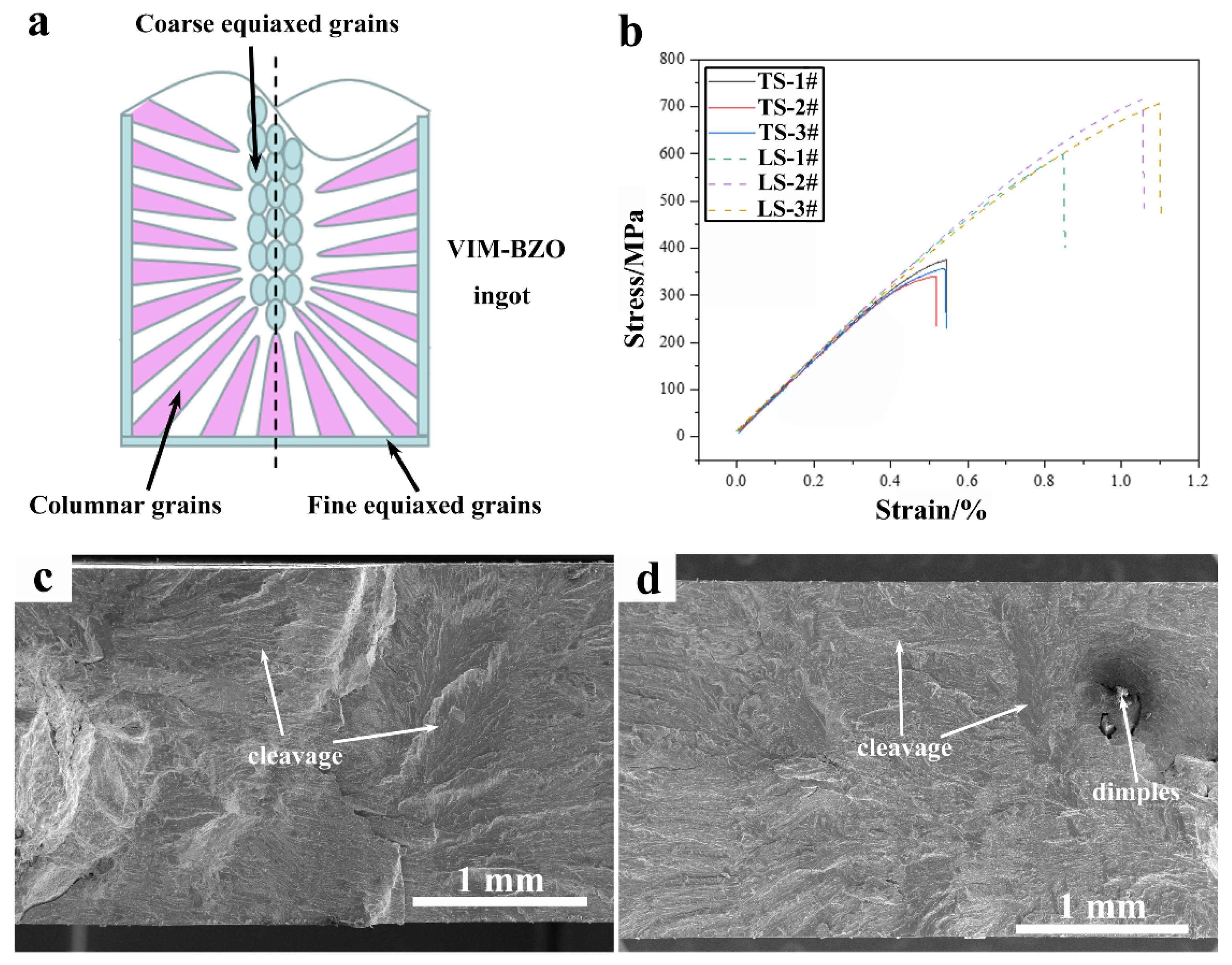
3.3. Mechanical Properties
4. Conclusions
- (1)
- By refining at 1600 °C for 3 min, a high quality and high Nb-containing TiAl-based alloy round bar ingot with a diameter of 85 mm and a length of 430 mm was finally obtained.
- (2)
- The deviations of Al and Nb content along a 430 mm long central part of the ingot are approximately ±0.39 at.% and ±0.14 at.%, and the oxygen content in the ingot can be controlled at around 1000 ppm.
- (3)
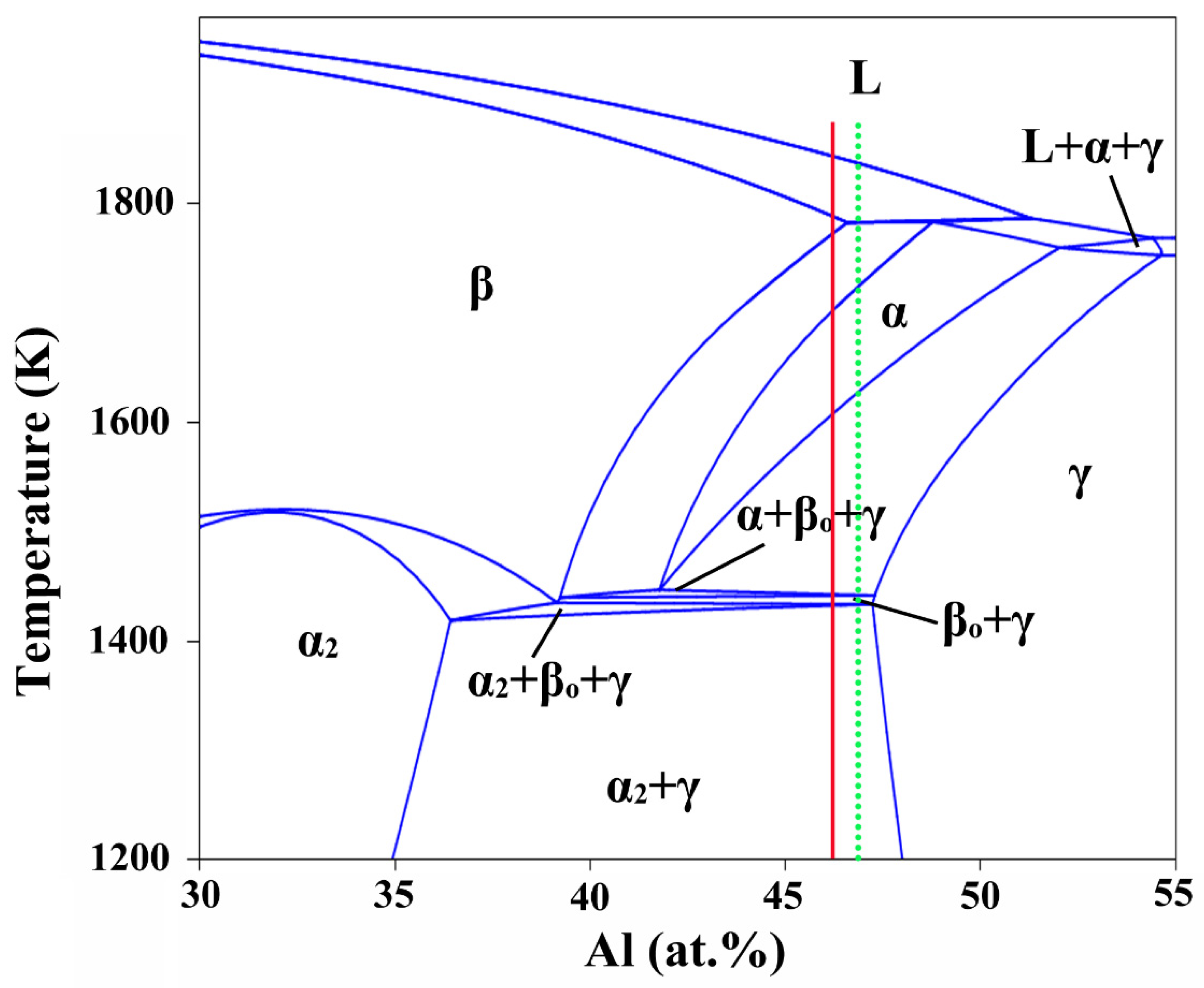
- During the solidification process of the alloy ingot, the primary phase is the β phase, which subsequently undergoes a peritectic reaction via the α phase instead of solidified via the β phase.
- (4)
- The structure of the alloy ingot is a full lamellar structure composed of γ and α2 phases, and the thickness of the lamellae is approximately 0.53 μm. The content of the α2 phase near the surface of the ingot is higher than that in the middle region and the core region, and it shows a decreasing relationship. In addition, there are some Nb-rich β phase particles surrounded by γ-phase particles, and these Nb-rich β-phase particles aggregat at the triple conjunction of colonies.
- (5)
- The tensile properties of transverse-cut samples are significantly better than those of longitudinal-cut samples, with a tensile strength of up to 700 MPa and a corresponding fracture elongation of 1.1%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, X. Review of alloy and process development of TiAl alloys. Intermetallics 2006, 14, 1114–1122. [Google Scholar] [CrossRef]
- Dimiduk, D.M. Gamma titanium aluminide alloys—An assessment within the competition of aerospace structural materials. Mater. Sci. Eng. A 1999, 263, 281–288. [Google Scholar] [CrossRef]
- Clemens, H.; Wallgram, W.; Kremmer, S.; Güther, V.; Otto, A.; Bartels, A. Design of Novel β-Solidifying TiAl Alloys with Adjustable β/B2-Phase Fraction and Excellent Hot-Workability. Adv. Eng. Mater. 2008, 10, 707–713. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S. Design, Processing, Microstructure, Properties, and Applications of Advanced Intermetallic TiAl Alloys. Adv. Eng. Mater. 2013, 15, 191–215. [Google Scholar] [CrossRef]
- Hu, D. Effect of composition on grain refinement in TiAl-based alloys. Intermetallics 2001, 9, 1037–1043. [Google Scholar] [CrossRef]
- Shida, Y.; Anada, H. The effect of various ternary additives on the oxidation behavior of TiAl in high-temperature air. Oxid. Met. 1996, 45, 197–219. [Google Scholar] [CrossRef]
- Yoshihara, M.; Miura, K. Effects of Nb addition on oxidation behavior of TiAl. Intermetallics 1995, 3, 357–363. [Google Scholar] [CrossRef]
- Güther, V.; Allen, M.; Klose, J.; Clemens, H. Metallurgical processing of titanium aluminides on industrial scale. Intermetallics 2018, 103, 12–22. [Google Scholar] [CrossRef]
- Foroozmehr, A.; Kermanpur, A.; Ashrafizadeh, F.; Kabiri, Y. Effects of thermo-mechanical parameters on microstructure and mechanical properties of Ti-50 at %Ni shape memory alloy produced by VAR method. Mater. Sci. Eng. A 2012, 535, 164–169. [Google Scholar] [CrossRef]
- Mi, J.; Harding, R.A.; Wickins, M.; Campbell, J. Entrained oxide films in TiAl castings. Intermetallics 2003, 11, 377–385. [Google Scholar] [CrossRef]
- Kim, Y.W.; Rosenberger, A.; Dimiduk, D.M. Microstructural changes and estimated strengthening contributions in a gamma alloy Ti–45Al–5Nb pack-rolled sheet. Intermetallics 2009, 17, 1017–1027. [Google Scholar] [CrossRef]
- Gomes, F.; Barbosa, J.; Ribeiro, C.S. Induction melting of γ-TiAl in CaO crucibles. Intermetallics 2008, 16, 1292–1297. [Google Scholar] [CrossRef]
- Lapin, J.; Gabalcová, Z.; Pelachová, T. Effect of Y2O3 crucible on contamination of directionally solidified intermetallic Ti-46Al-8Nb alloy. Intermetallics 2011, 19, 396–403. [Google Scholar] [CrossRef]
- Subbarao, E.C.; Sutter, P.H.; Hrizo, J. Defect Structure and Electrical Conductivity of ThO2-Y2O3 Solid Solutions. J. Am. Ceram. Soc. 1965, 48, 443–446. [Google Scholar] [CrossRef]
- Li, C.H.; He, J.; Wei, C.; Wang, H.B.; Lu, X.G. Solidification and Interface Reaction of Titanium Alloys in the BaZrO3 Shell-Mould. Mater. Sci. Forum 2015, 828–829, 106–111. [Google Scholar] [CrossRef]
- Li, C.H.; Gao, Y.H.; Lu, X.G.; Ding, W.Z.; Ren, Z.M.; Deng, K. Interaction between the Ceramic CaZrO3 and the Melt of Titanium Alloys. Adv. Sci. Technol. 2011, 70, 136–140. [Google Scholar]
- Tetsui, T.; Kobayashi, T.; Ueno, T.; Harada, H. Consideration of the influence of contamination from oxide crucibles on TiAl cast material, and the possibility of achieving low-purity TiAl precision cast turbine wheels. Intermetallics 2012, 31, 274–281. [Google Scholar] [CrossRef]
- Barbosa, J.J.; Ribeiro, C.S. Influence of crucible material on the level of contamination in TiAl using induction melting. Int. J. Cast Met. Res. 2000, 12, 293–301. [Google Scholar] [CrossRef]
- Čegan, T.; Szurman, I.; Kursa, M.; Holesinsky, J.; Vontorova, J. Preparation of TiAl-based alloys by induction melting in graphite crucibles. Kov. Mater. 2015, 53, 69–78. [Google Scholar]
- Chapin, E.; Friske, W. A Metallurgical Evaluation of Refractory Compounds for Containing Molten Titanium: I. Oxides. Nav. Res. Lab. Rept. 2015. Available online: https://www.osti.gov/biblio/4400730 (accessed on 29 January 2022).
- Kuang, J.P.; Harding, R.A.; Campbell, J. A study of refractories as crucible and mould materials for melting and casting γ-TiAl alloys. Int. J. Cast Met. Res. 2001, 13, 277–292. [Google Scholar] [CrossRef]
- Gomes, F.; Puga, H.; Barbosa, J.; Ribeiro, C.S. Effect of melting pressure and superheating on chemical composition and contamination of yttria-coated ceramic crucible induction melted titanium alloys. J. Mater. Sci. 2011, 46, 4922–4936. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.R.; Tang, X.X.; Zhou, L.; Gao, M.; Zhou, C.G.; Zhang, H. Interactions between Ni-44Ti-5Al-2Nb-Mo alloy and oxide ceramics during directional solidification process. J. Mater. Sci. 2012, 47, 6451–6458. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, X.; Zhou, C.; Zhang, H.; Zhang, S. Comparison of directional solidification of γ-TiAl alloys in conventional Al2O3 and novel Y2O3-coated Al2O3 crucibles. J. Eur. Ceram. Soc. 2013, 33, 925–934. [Google Scholar] [CrossRef]
- Kostov, A.; Friedrich, B. Predicting thermodynamic stability of crucible oxides in molten titanium and titanium alloys. Comput. Mater. Sci. 2006, 38, 374–385. [Google Scholar] [CrossRef]
- Lapin, J.; Gabalcová, Z. Solidification behaviour of TiAl-based alloys studied by directional solidification technique. Intermetallics 2011, 19, 797–804. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, H.; Wang, Q.; Chen, R.; Guo, J.; Fu, H. Effect of Y2O3 particles on the fracture toughness of directionally solidified TiAl-based alloys. Mater. Sci. Eng. A 2017, 703, 108–115. [Google Scholar] [CrossRef]
- Trivedi, P.; Patankar, S.; Baburaj, E.G. Formation of Al-Y Oxide during Processing of γ-TiAl. J. Alloys Compd. 2002, 340, 231–235. [Google Scholar] [CrossRef]
- Wu, Y.; Hwang, S.K. The effect of yttrium on microstructure and dislocation behavior of elemental powder metallurgy processed TiAl-based intermetallics. Mater. Lett. 2004, 58, 2067–2072. [Google Scholar] [CrossRef]
- Chen, G.; Gao, P.; Kang, J.; Li, B.; Ali, W.; Qin, Z.; Lu, X.; Li, C. Improved stability of BaZrO3 refractory with Y2O3 additive and its interaction with titanium melts. J. Alloys Compd. 2017, 726, 403–409. [Google Scholar] [CrossRef]
- Chen, G.; Lan, B.; Xiong, F.; Gao, P.; Zhang, H.; Lu, X.; Li, C. Pilot-scale experimental evaluation of induction melting of Ti-46Al-8Nb alloy in the fused BaZrO3 crucible. Vacuum 2019, 159, 293–298. [Google Scholar] [CrossRef]
- Li, K.; Chen, G.; Hao, Z.; Ali, W.; Li, C. Microstructure evolution of directionally solidified Ti-46Al-8Nb alloy in the BaZrO3-based mould. Mater. Res. Express 2018, 5, 116529. [Google Scholar] [CrossRef]
- Chen, G.; Kang, J.; Lan, B.; Gao, P.; Lu, X.; Li, C. Evaluation of Ca-doped BaZrO3 as the crucible refractory for melting TiAl alloys. Ceram. Int. 2018, 44, 12627–12633. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, K.; Liu, L.; Wu, G.; Chen, G. Preparation of BaZrO3 Crucible and Its Interfacial Reaction with Molten Titanium Alloys. J. Chin. Ceram. Soc. 2013, 41, 1278–1283. [Google Scholar]
- Khalid, F.; Edmonds, D. The Processing, Properties and Applications of Metallic Ceramic Materials. Proc. IRC Birm. 1992, 14, 5. [Google Scholar]
- Blackburn, M.J.; Malley, D.R. Plasma arc melting of titanium alloys. Mater. Des. 1993, 14, 19–27. [Google Scholar] [CrossRef]
- Hu, D.; Godfrey, A.; Blenkinsop, P.A.; Loretto, M.H. Processing-property-microstructure relationships in TiAl-based alloys. Metall. Mater. Trans. A 1998, 29, 919–925. [Google Scholar] [CrossRef]
- Yanqing, S.; Jingjie, G.; Jun, J.; Guizhong, L.; Yuan, L. Composition control of a TiAl melt during the induction skull melting (ISM) process. J. Alloys Compd. 2002, 334, 261–266. [Google Scholar] [CrossRef]
- Zollinger, J.; Lapin, J.; Daloz, D.; Combeau, H. Influence of oxygen on solidification behaviour of cast TiAl-based alloys. Intermetallics 2007, 15, 1343–1350. [Google Scholar] [CrossRef]
- Friedrich, B.; Morscheiser, J.; Lochbichler, C. Potential of ceramic crucibles for melting of titanium-alloys and gamma-titaniumaluminide. Int. Feuerfestkolloquium 2008, 15, 16. [Google Scholar]
- Liu, K.; Ma, Y.C.; Gao, M.; Rao, G.B.; Li, Y.Y.; Wei, K.; Wu, X.; Loretto, M.H. Single step centrifugal casting TiAl automotive valves. Intermetallics 2005, 13, 925–928. [Google Scholar] [CrossRef]
- Appel, H.F.; Paul, J.; Oehring, M. Gamma Titanium Aluminide Alloys: Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Fashu, S.; Lototskyy, M.; Davids, M.W.; Pickering, L.; Linkov, V.; Tai, S.; Renheng, T.; Fangming, X.; Fursikov, P.V.; Tarasov, B.P. A review on crucibles for induction melting of titanium alloys. Mater. Des. 2020, 186, 108295. [Google Scholar] [CrossRef]
- Witusiewicz, V.T.; Bondar, A.A.; Hecht, U.; Velikanova, T.Y. The Al-B-Nb-Ti system: IV. Experimental study and thermodynamic re-evaluation of the binary Al-Nb and ternary Al-Nb-Ti systems. J. Alloys Compd. 2009, 472, 133–161. [Google Scholar] [CrossRef]
- McCullough, C.; Valencia, J.J.; Levi, C.G.; Mehrabian, R. Phase equilibria and solidification in Ti-Al alloys. Acta Metall. 1989, 37, 1321–1336. [Google Scholar] [CrossRef]
- Larsen, D.E.; Kampe, S.; Christodoulou, L. Effect of XDTM TiB2 Volume Fraction on the Microstructure of a Cast Near-gamma titanium aluminide alloy. MRS Online Proc. Libr. 1990, 194, 285–292. [Google Scholar] [CrossRef]
- Imayev, R.M.; Imayev, V.M.; Oehring, M.; Appel, F. Alloy design concepts for refined gamma titanium aluminide based alloys. Intermetallics 2007, 15, 451–460. [Google Scholar] [CrossRef]
- Bonetti, E.; Pasquini, L.; Sampaolesi, E. The influence of grain size on the mechanical properties of nanocrystalline aluminium. Nanostruct. Mater. 1997, 9, 611–614. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, X.; Lu, L.; Zhu, T. Fracture in a thin film of nanotwinned copper. Acta Mater. 2015, 98, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Hertzberg, R.W.; Vinci, R.P.; Hertzberg, J.L. Deformation and Fracture Mechanics of Engineering Materials; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Saage, H.; Huang, A.J.; Hu, D.; Loretto, M.H.; Wu, X. Microstructures and tensile properties of massively transformed and aged Ti46Al8Nb and Ti46Al8Ta alloys. Intermetallics 2009, 17, 32–38. [Google Scholar] [CrossRef]
- Xiao, S.; Guo, Y.; Liang, Z.; Wang, X.; Yang, J.; Wang, X.; Xu, L.; Tian, J.; Chen, Y. The effect of nano-Y2O3 addition on tensile properties and creep behavior of as-cast TiAl alloy. J. Alloys Compd. 2020, 825, 153852. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Liu, B.; He, W.; Li, J.; Gan, Z.; He, Y. Investigations on processing powder metallurgical high-Nb TiAl alloy sheets. Intermetallics 2014, 55, 80–89. [Google Scholar] [CrossRef]
| Elements | Al (at.%) | Nb (at.%) | O (ppm) | Ti (at.%) | ||
|---|---|---|---|---|---|---|
| Sample 1# | 45.01 | 7.97 | 1053 | Bal. | ||
| Sample 2# | 45.40 | 7.90 | 991 | Bal. | ||
| Sample 3# | 45.32 | 7.75 | 975 | Bal. | ||
| Sample 4# | 45.53 | 7.84 | 1021 | Bal. | ||
| Sample 5# | 45.76 | 7.98 | 1047 | Bal. | ||
| Deviation | − xmin | − xmax | − xmin | − xmax | / | / |
| +0.39 | −0.36 | +0.14 | −0.09 | |||
| Target | 46 | 8 | / | Bal. | ||
| Point | Unit | Element | ||||
|---|---|---|---|---|---|---|
| Ti | Al | Nb | O | Zr | ||
| A | wt.% | 50.07 | 22.71 | 24.37 | 0.45 | 2.39 |
| at.% | 46.71 | 37.62 | 11.72 | 1.27 | 1.17 | |
| B | wt.% | 47.86 | 33.50 | 16.13 | 0.30 | 2.20 |
| at.% | 38.91 | 48.35 | 6.76 | 0.74 | 0.94 | |
| C | wt.% | 50.97 | 29.47 | 17.09 | 0.41 | 1.90 |
| at.% | 44.38 | 45.55 | 7.67 | 1.08 | 0.87 | |
| Alloys | Melting Techniques | Processing Techniques | Tensile Strength (MPa) | Elongation (%) | References |
|---|---|---|---|---|---|
| Ti-46Al-8Nb | DM-PAM | SBQ | 571 ± 18 | 0.43 | [51] |
| Ti-45Al-6Nb-2.5V-0.15Y2O3 | ISM | Casting | 666 ± 31 | 0.56 ± 0.15 | [52] |
| Ti-45Al-7Nb-0.3W | VAR-PM | HIP/HR | 875 | 0.76 | [53] |
| Ti-46Al-8Nb (longitudinal section) | VIM-BZO | Casting | 700 | 1.1 | This work |
| Ti-46Al-8Nb (transverse section) | VIM-BZO | Casting | 375 | 0.52 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, B.; Mao, L.; Yang, Y.; Feng, Q.; Zhang, X.; Li, H.; Jiao, L.; Zhang, R.; Lu, X.; Chen, G.; et al. Preparation of Ti-46Al-8Nb Alloy Ingots beyond Laboratory Scale Based on BaZrO3 Refractory Crucible. Metals 2022, 12, 524. https://doi.org/10.3390/met12030524
Duan B, Mao L, Yang Y, Feng Q, Zhang X, Li H, Jiao L, Zhang R, Lu X, Chen G, et al. Preparation of Ti-46Al-8Nb Alloy Ingots beyond Laboratory Scale Based on BaZrO3 Refractory Crucible. Metals. 2022; 12(3):524. https://doi.org/10.3390/met12030524
Chicago/Turabian StyleDuan, Baohua, Lu Mao, Yuchen Yang, Qisheng Feng, Xuexian Zhang, Haitao Li, Lina Jiao, Rulin Zhang, Xionggang Lu, Guangyao Chen, and et al. 2022. "Preparation of Ti-46Al-8Nb Alloy Ingots beyond Laboratory Scale Based on BaZrO3 Refractory Crucible" Metals 12, no. 3: 524. https://doi.org/10.3390/met12030524
APA StyleDuan, B., Mao, L., Yang, Y., Feng, Q., Zhang, X., Li, H., Jiao, L., Zhang, R., Lu, X., Chen, G., & Li, C. (2022). Preparation of Ti-46Al-8Nb Alloy Ingots beyond Laboratory Scale Based on BaZrO3 Refractory Crucible. Metals, 12(3), 524. https://doi.org/10.3390/met12030524








