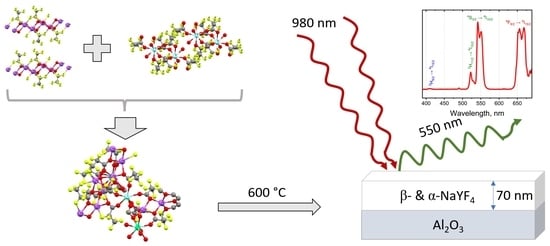
Single-Source Precursors for Chemical Solution Deposition of Up-Converting NaLnF4 Thin Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Metal Pentafluoropropionates
2.2. NaLnF4 Powders Preparation and VT-PXRD Study
2.3. Precursor Solution Preparation and Thin Film Deposition
2.4. Methods and Instrumentation
2.5. X-ray Crystallography
3. Results and Discussion
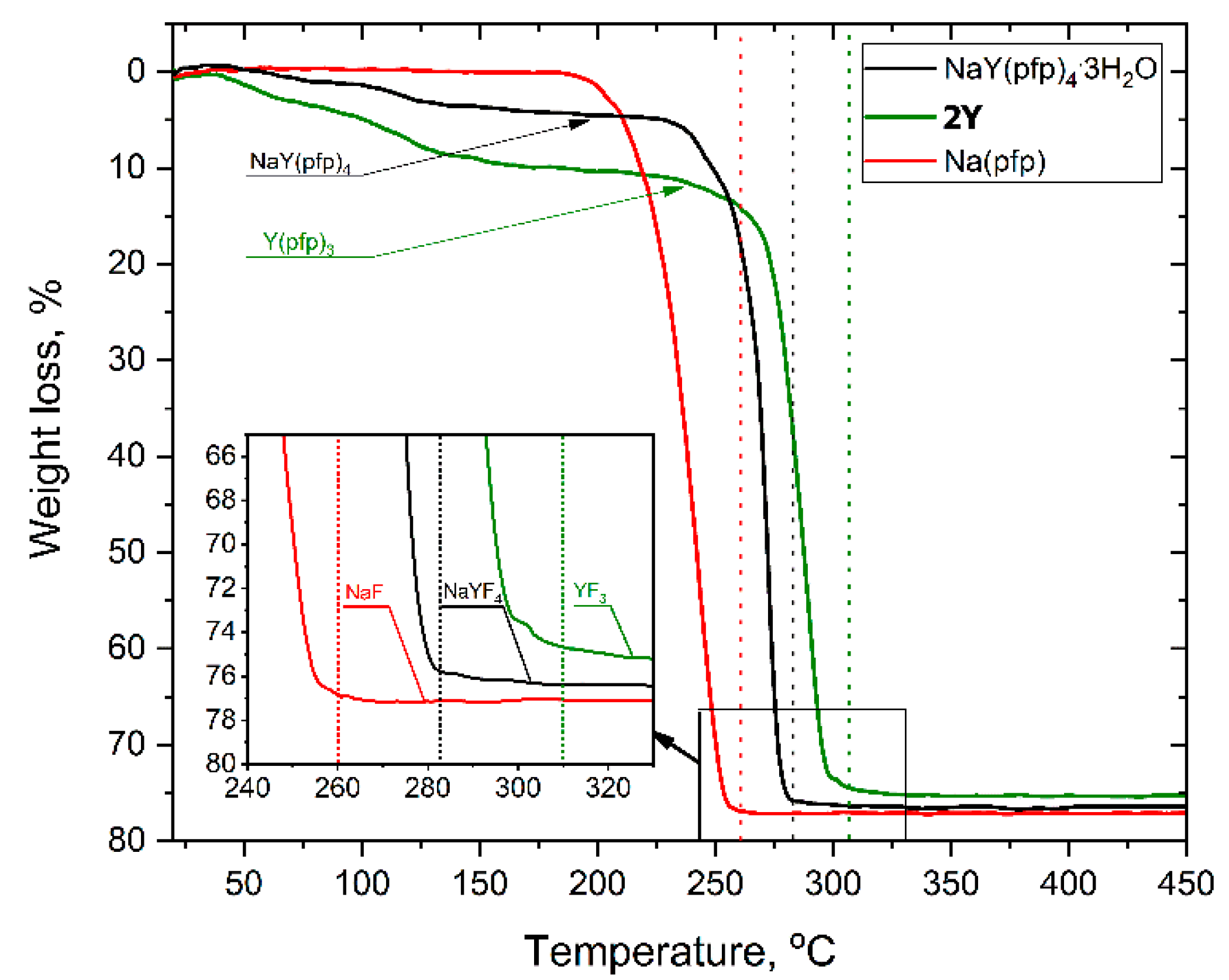
3.1. Synthesis and Thermal Behavior of Single-Source Precursors
3.2. Crystal Structure
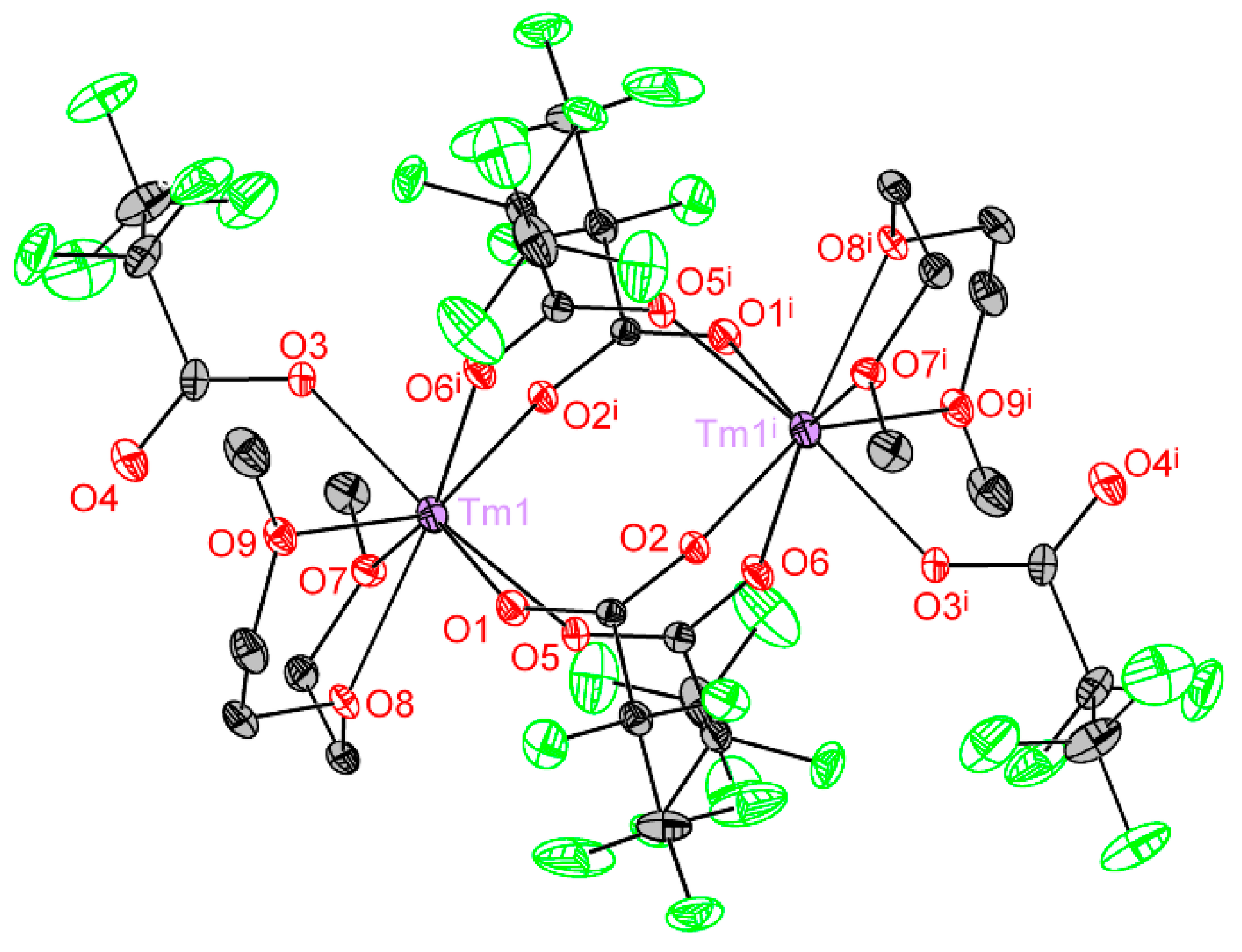
3.2.1. [Ln2(pfp)6(H2O)6] (Ln = Y, Er, Tm, Yb) (1Ln)
3.2.2. [Ln2(pfp)6(diglyme)2], (Ln = Y, Er, Tm, Yb) (2Ln)
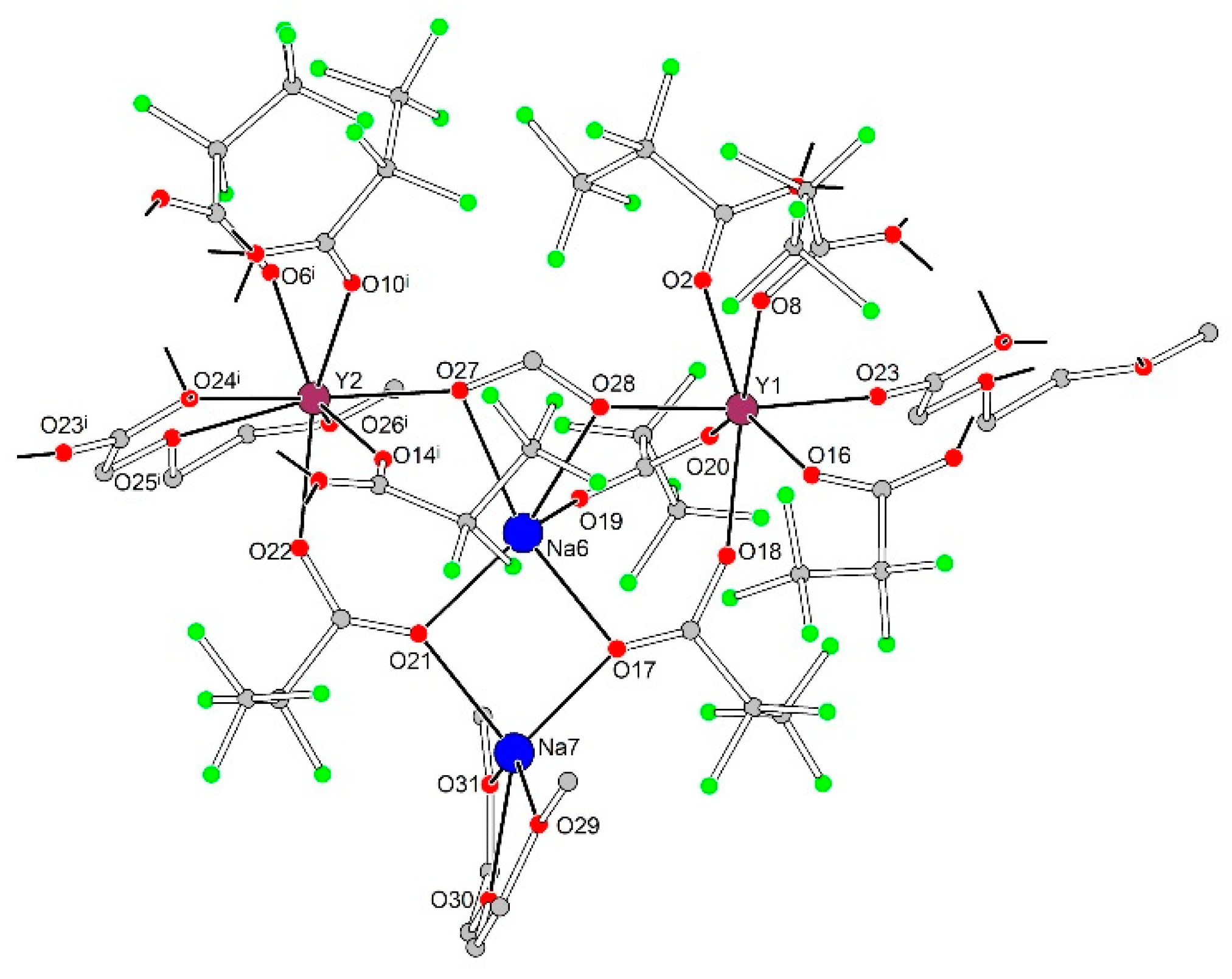
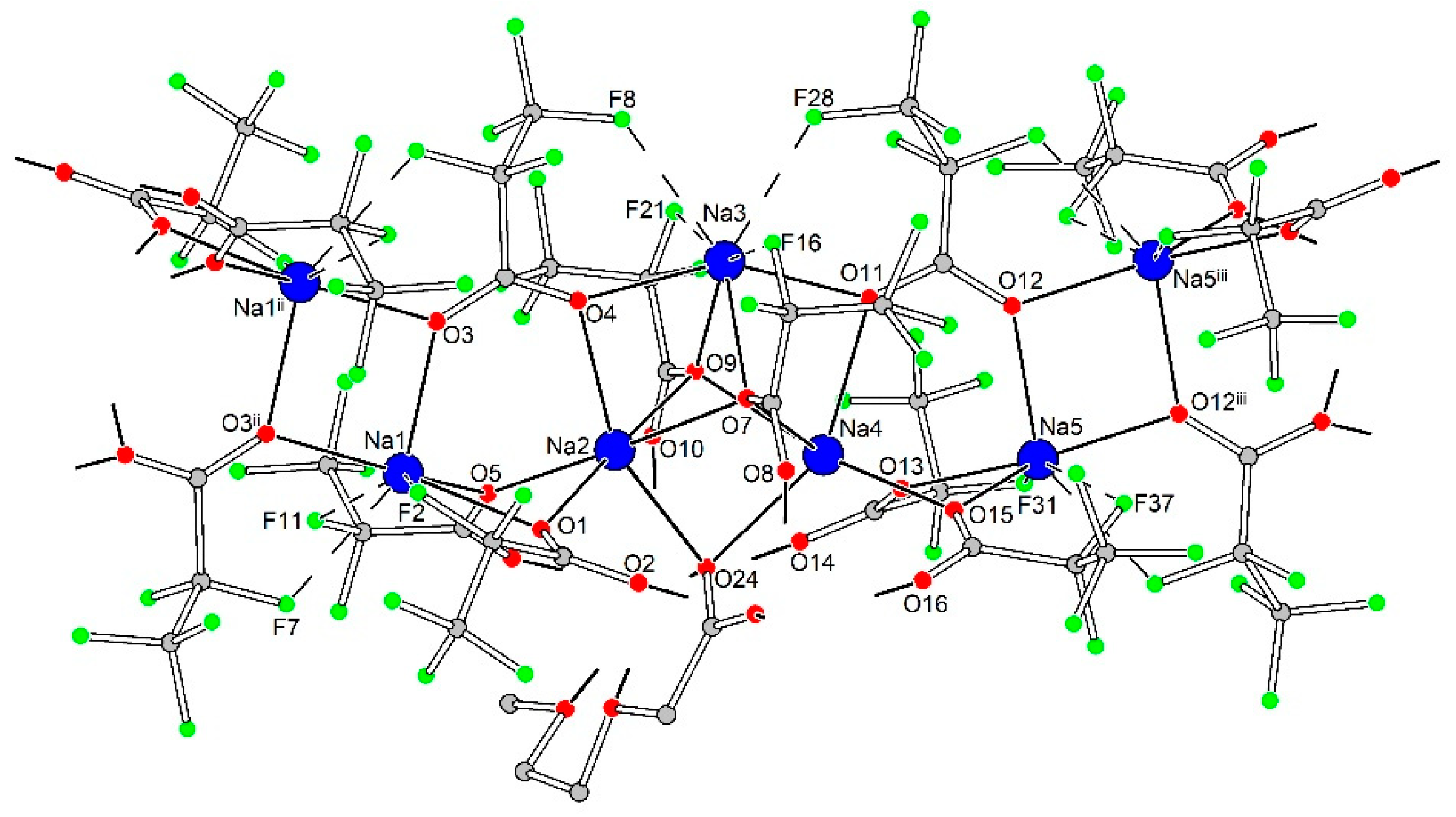
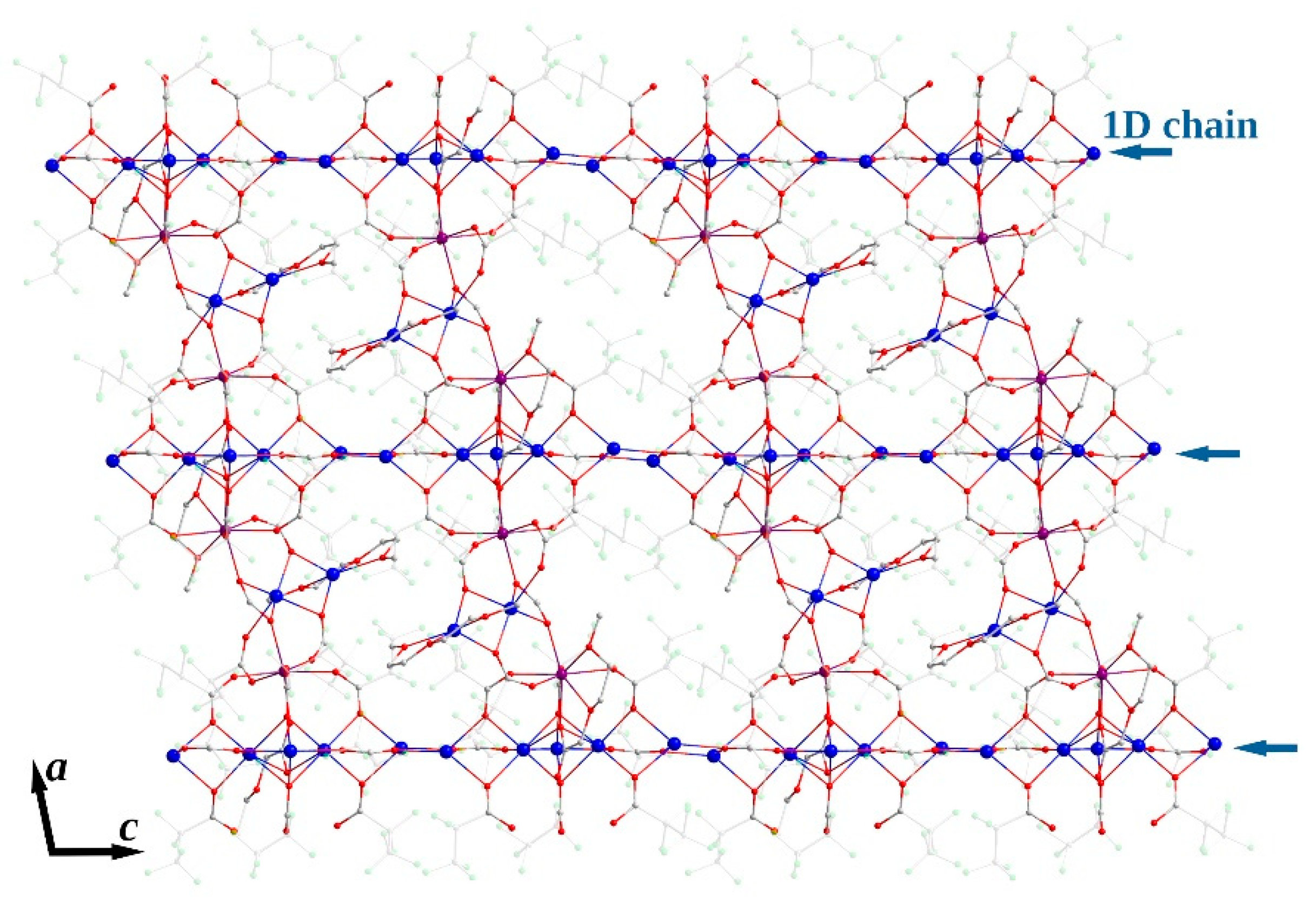
3.2.3. [Na7Ln2(pfp)11(form)(diglyme)(Meac)]∞ (Ln = Y, Er, Yb) (3Ln)
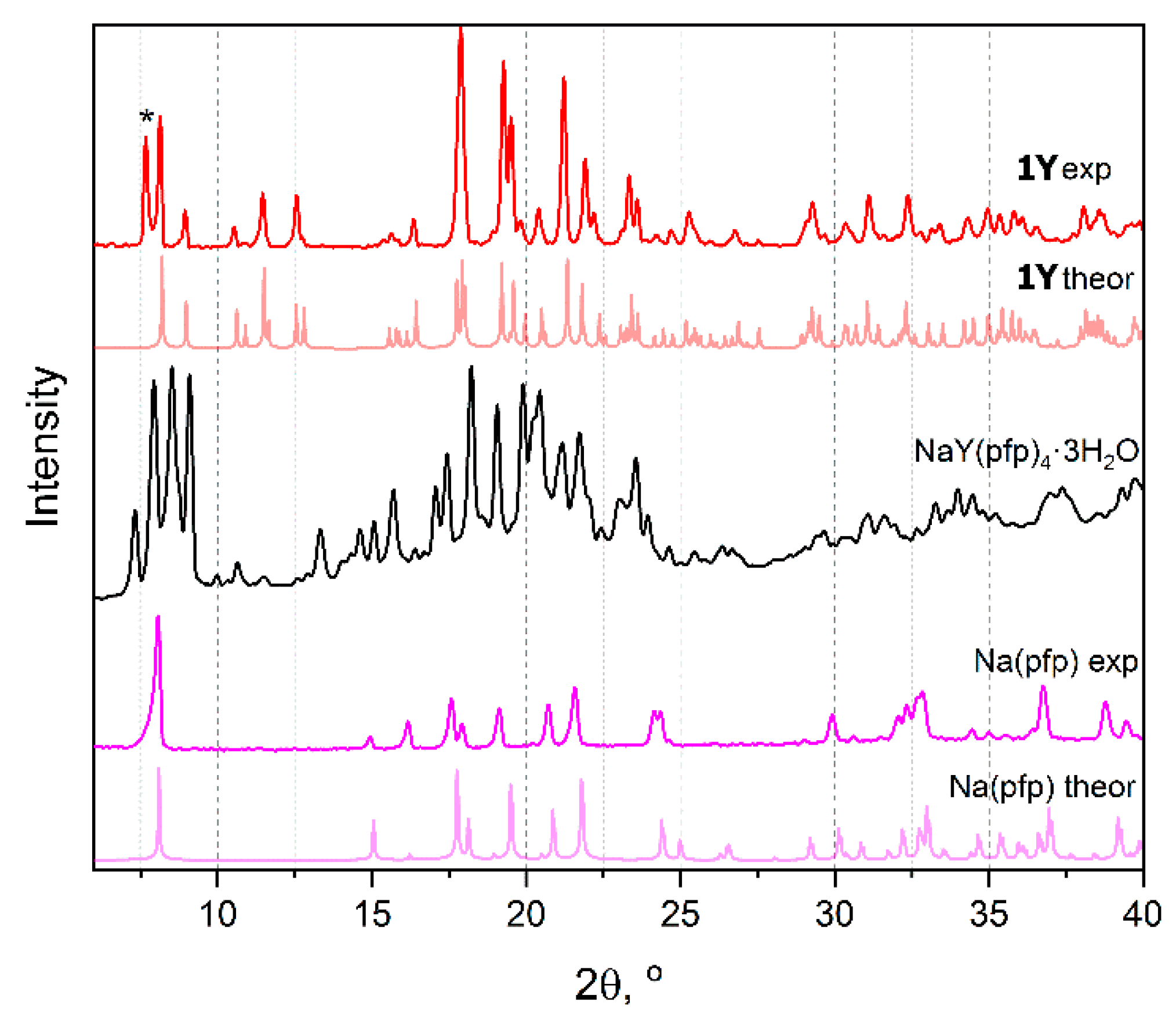
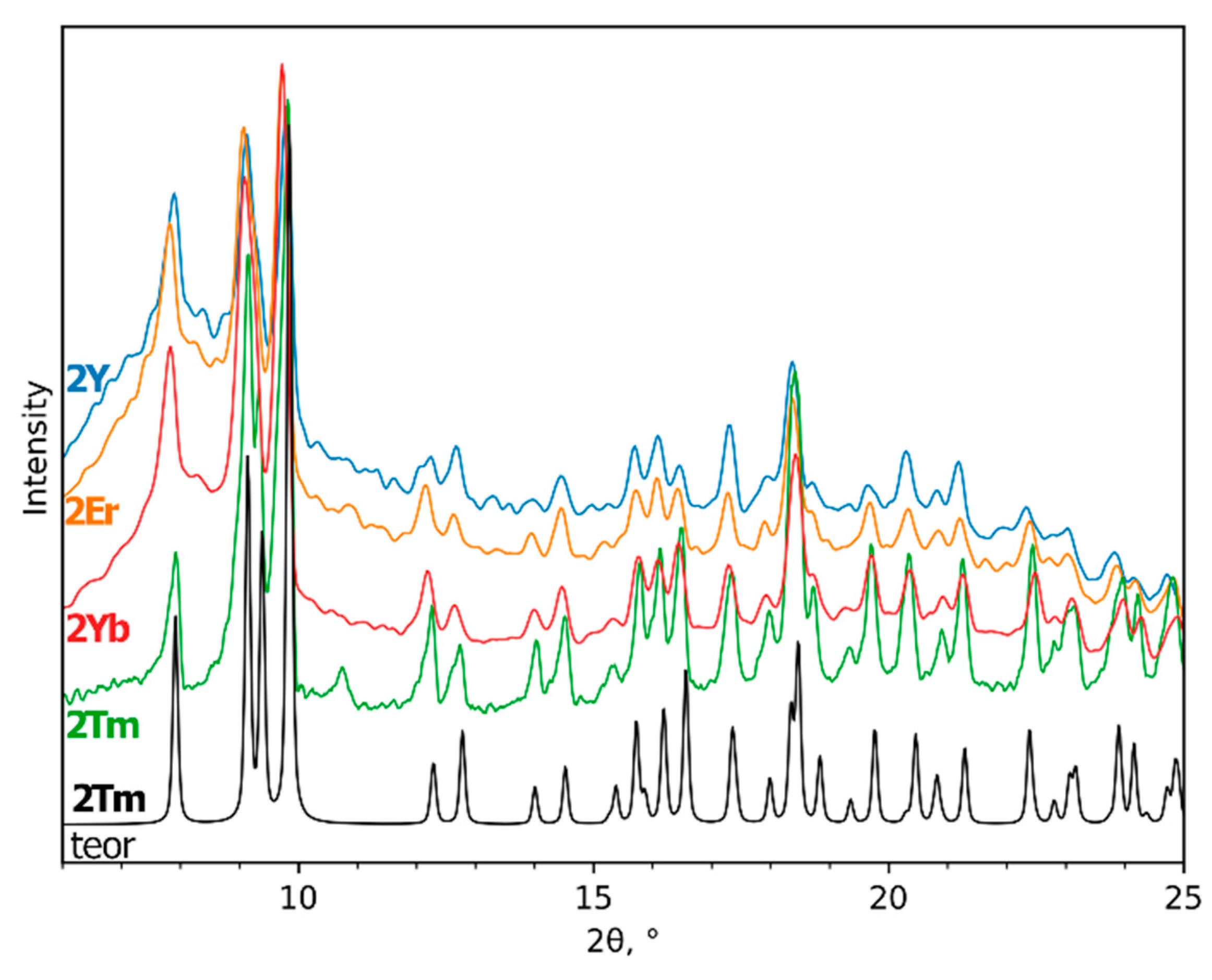
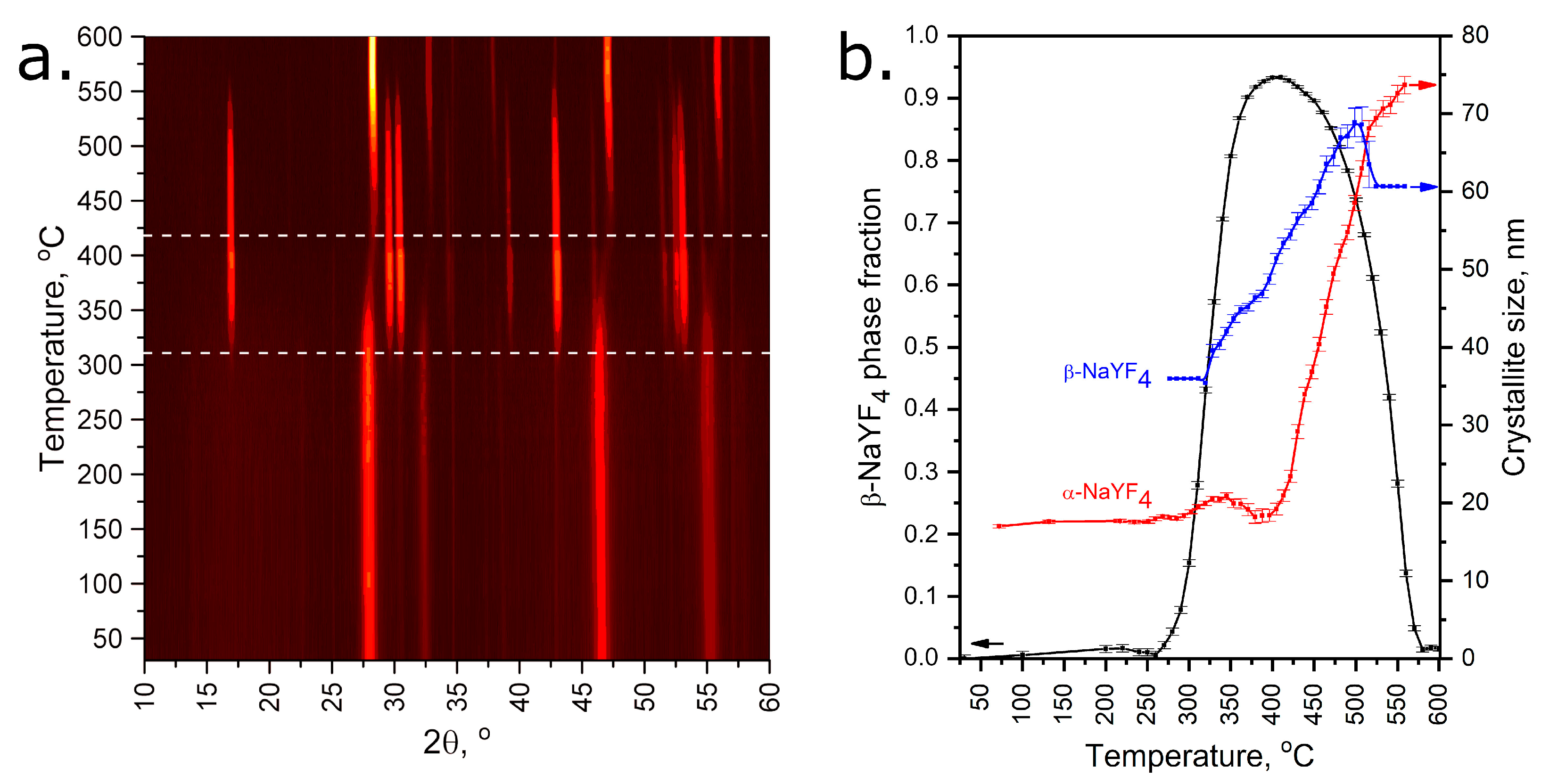
3.3. NaLnF4 Powders Preparation and Characterization
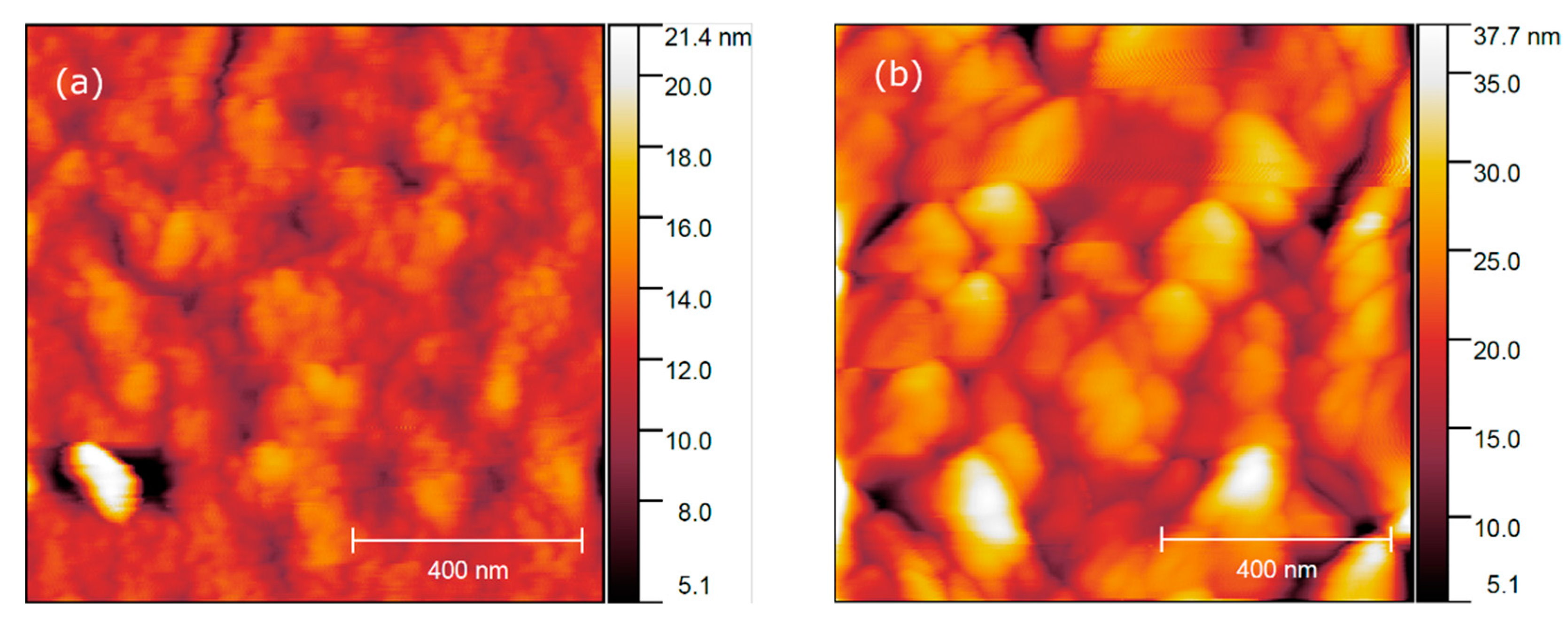
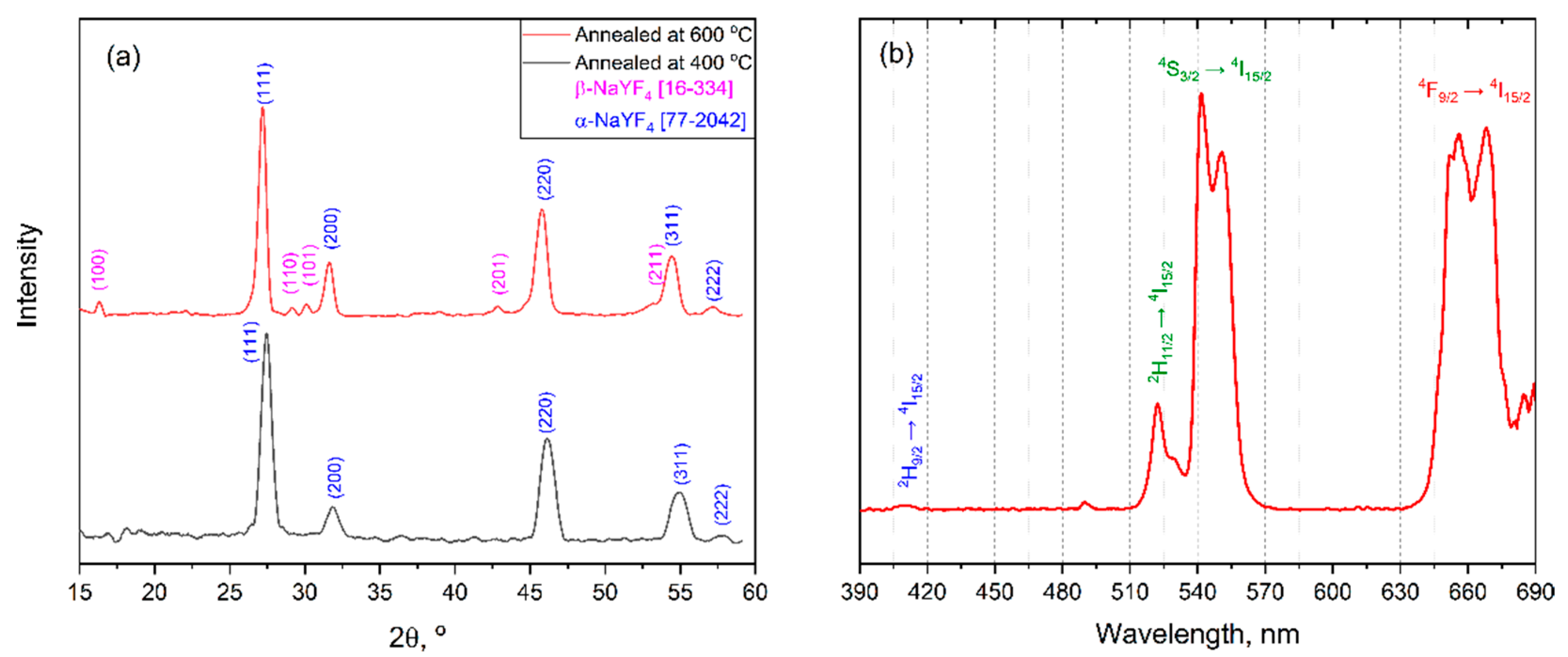
3.4. NaLnF4 Thin Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joubert, M.-F. Photon avalanche upconversion in rare earth laser materials. Opt. Mater. 1999, 11, 181–203. [Google Scholar] [CrossRef]
- Lui, Y.; Chem, W.; Wang, S.; Joly, A.G. X-ray luminescence of LaF3:Tb3+ and LaF3: Ce3+,Tb3+ water-soluble nanoparticles. J. Appl. Phys. 2008, 103, 063105. [Google Scholar]
- Yang, L.W.; Han, H.L.; Zhang, Y.Y.; Zhong, J.X. White Emission by Frequency Up-Conversion in Yb3+-Ho3+-Tm3+ Triply Doped Hexagonal NaYF4 Nanorods. J. Phys. Chem. C 2009, 113, 18995–18999. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, H.; Yang, Y.; Zhao, D.; Jia, Z.; Zheng, K.; Qin, G.; Qin, W. 3D up-conversion display of NaYF4-PMMA co-valent-linking nanocomposites. J. Alloys Compd. 2018, 753, 725–730. [Google Scholar] [CrossRef]
- Menyuk, N.; Dwight, K.; Pierce, J.W. NaYF4: Yb, Er—An efficient upconversion phosphor. Appl. Phys. Lett. 1972, 21, 159–161. [Google Scholar] [CrossRef]
- Tsuyoshi, K.; Yamamoto, H.; Otomo, Y. NaLnF4:Yb3+, Er3+ (Ln: Y, Gd, La): Efficient Green-Emitting Infrared-Excited Phos-phors. J. Electrochem. Soc. 1972, 119, 1561. [Google Scholar]
- Pinol, L.; Rebello, K.; Caruso, K.; Francomacaro, A.S.; Coles, G.L. Physical vapor deposition and patterning of calcium fluoride films. J. Vac. Sci. Technol. A 2011, 29, 21001. [Google Scholar] [CrossRef]
- Ylilammi, M.; Ranta-aho, T. Metal Fluoride Thin Films Prepared by Atomic Layer Deposition. J. Electrochem. Soc. 1994, 141, 1278. [Google Scholar] [CrossRef]
- Jia, H.; Xu, C.; Wang, J.; Chen, P.; Liu, X.; Qui, J. Synthesis of NaYF4:Yb–Tm thin film with strong NIR photon up-conversion photoluminescence using electro-deposition method. CrystEngComm 2014, 16, 4023–4028. [Google Scholar] [CrossRef]
- Battiato, S.; Rossi, P.; Paoli, P.; Malandrino, G. Heterobimetallic Sodium Rare-Earth Complexes: “Third-Generation” MOCVD Precursors for the Deposition of NaREF4 (RE = Y, Gd) Films. Inorg. Chem. 2018, 57, 15035–15039. [Google Scholar] [CrossRef]
- Barranco, J.; Méndez-Blas, A.; Calixto, M.E. Structural, morphology and optical properties of NaYF4 thin films doped with trivalent lanthanide ions. J. Mater. Sci. Mater. Electron. 2019, 30, 4855–4866. [Google Scholar] [CrossRef]
- Rillings, K.; Roberts, J. A thermal study of the trifluoroacetates and pentafluoropropionates of praseodymium, samarium and erbium. Thermochim. Acta 1974, 10, 269–277. [Google Scholar] [CrossRef]
- Hercules, D.A.; Parrish, C.A.; Sayler, T.S.; Tice, K.T.; Williams, S.M.; Lowery, L.E.; Brady, M.E.; Coward, R.B.; Murphy, J.A.; Hey, T.A.; et al. Preparation of tetrafluoroethylene from the pyrolysis of pentafluoropropionate salts. J. Fluor. Chem. 2017, 196, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Peng, D.; Ju, Q.; Wang, F. Photon upconversion in core–shell nanoparticles. Chem. Soc. Rev. 2015, 44, 1318–1330. [Google Scholar] [CrossRef]
- Ayadi, H.; Fang, W.; Mishra, S.; Jeanneau, E.; Ledoux, G.; Zhang, J.; Daniele, S. Influence of Na+ion doping on the phase change and upconversion emissions of the GdF3: Yb3+, Tm3+nanocrystals obtained from the designed molecular precursors. RSC Adv. 2015, 5, 100535–100545. [Google Scholar] [CrossRef]
- Mishra, S.; Jeanneau, E.; Bulin, A.-L.; Ledoux, G.; Jouguet, B.; Amans, D.; Belsky, A.; Daniele, S.; Dujardin, C. A molecular precursor approach to monodisperse scintillating CeF3 nanocrystals. Dalton Trans. 2013, 42, 12633–12643. [Google Scholar] [CrossRef]
- Grebenyuk, D.; Ryzhkov, N.; Tsymbarenko, D. Novel mononuclear mixed ligand complexes of heavy lanthanide trifluoro-acetates with diethylenetriamine. J. Fluor. Chem. 2017, 202, 82–90. [Google Scholar] [CrossRef]
- Chen, Y.; Mishra, S.; Ledoux, G.; Jeanneau, E.; Daniel, M.; Zhang, J.; Daniele, S. Direct synthesis of hexagonal NaGdF4 nano-crystals from a single-source precursor: Upconverting NaGdF4:Yb3+,Tm3+ and its composites with TiO2 for near-IR-driven photocatalysis. Chem. Asian J. 2014, 9, 2415–2421. [Google Scholar] [CrossRef]
- Mishra, S.; Daniele, S.; Ledoux, G.; Jeanneau, E.; Joubert, M.-F. Heterometallic−complexes as novel single-source precursors for upconverting NaYF4 co-doped with Yb and Er/Tm ions. Chem. Commun. 2010, 46, 3756–3758. [Google Scholar] [CrossRef]
- Mishra, S.; Ledoux, G.; Jeanneau, E.; Daniele, S.; Joubert, M.-F. Novel heterometalorganic complexes as first single source precursors for up-converting NaY(Ln)F4 (Ln = Yb, Er Tm) nanomaterials. Dalton Trans. 2012, 41, 1490–1502. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Krist.-Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS V. 2.01, Bruker/Siemens Area Detector Absorption Correction Program; Bruker AXS: Madison, WI, USA, 1998. [Google Scholar]
- Sheldrick, G.M. CELL_NOW; Georg-August-Universität: Göttingen, Germany, 2008. [Google Scholar]
- Sheldrick, G.M. TWINABS 2012/1; Bruker AXS: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. SHELXTL, Ver. 5.10. In Structure Determination Software Suite; Bruker AXS: Madison, WI, USA, 1998. [Google Scholar]
- Tang, S.; Zhao, H. Glymes as versatile solvents for chemical reactions and processes: From the laboratory to industry. RSC Adv. 2014, 4, 11251–11287. [Google Scholar] [CrossRef] [PubMed]
- Rohde, A.; Urland, W. Synthese, Kristallstruktur und magnetisches Verhalten von Gd(CF3CF2COO)3(H2O)3. Z. Anorg. Allg. Chem. 2006, 632, 1141–1144. [Google Scholar] [CrossRef]
- Tsymbarenko, D.M.; Korsakov, I.E.; Lyssenko, K.A.; Troyanov, S.I. One-dimensional coordination polymers in the crystal structures of sodium and potassium acetylacetonates. Polyhedron 2015, 92, 68–76. [Google Scholar] [CrossRef]
- Kendin, M.; Nikiforov, A.; Svetogorov, R.; Degtyarenko, P.; Tsymbarenko, D. A 3D-coordination polymer assembled from copper propionate paddlewheels and potassium propionate 1D-polymeric rods possessing a temperature-driven sin-gle-crystal-to-single-crystal phase transition. Cryst. Growth Des. 2021, 21, 6183–6194. [Google Scholar] [CrossRef]
- Tsymbarenko, D.; Martynova, I.; Grebenyuk, D.; Shegolev, V.; Kuzmina, N. One-dimensional coordination polymers of whole row rare earth tris-pivalates. J. Solid State Chem. 2018, 258, 876–884. [Google Scholar] [CrossRef]
- Grebenyuk, D.; Zobel, M.; Polentarutti, M.; Ungur, L.; Kendin, M.; Zakharov, K.; Degtyarenko, P.; Vasiliev, A.; Tsymbarenko, D. A Family of Lanthanide Hydroxo Carboxylates with 1D Polymeric Topology and Ln4 Butterfly Core Exhibits Switchable Supramolecular Arrangement. Inorg. Chem. 2021, 60, 8049–8061. [Google Scholar] [CrossRef]
- Grebenyuk, D.; Tsymbarenko, D.; D. Synthesis and crystal structure of polymeric erbium cyclohexanecarboxylate with an unusual geometry of the polymer chain. J. Struct. Chem. 2022, 63, 3. [Google Scholar]
- Thoma, R.E.; Hebert, G.M.; Insley, H.; Weaver, C.F. Phase Equilibria in the System Sodium Fluoride-Yttrium Fluoride. Inorg. Chem. 1963, 2, 1005–1012. [Google Scholar] [CrossRef]
- Krämer, K.W.; Biner, D.; Frei, G.; Güdel, H.U.; Hehlen, M.P.; Lüthi, S.R. Hexagonal Sodium Yttrium Fluoride Based Green and Blue Emitting Upconversion Phosphors. Chem. Mater. 2004, 16, 1244–1251. [Google Scholar] [CrossRef]
- Van Santen, R.A. The Ostwald step rule. J. Phys. Chem. 1984, 88, 5768–5769. [Google Scholar] [CrossRef]
- Park, H.; Yoo, G.Y.; Kim, M.-S.; Kim, K.; Lee, C.; Park, S.; Kim, W. Thin film fabrication of upconversion lanthanide-doped NaYF4 by a sol-gel method and soft lithographical nanopatterning. J. Alloys Compd. 2017, 728, 927–935. [Google Scholar] [CrossRef]
- Bünzli, J.C.G.; Eliseeva, S.V. Basics of Lanthanide Photophysics. In Lanthanide Luminescence. Springer Series on Fluorescence (Methods and Applications); Hänninen, P., Härmä, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 7. [Google Scholar] [CrossRef] [Green Version]



| Compound | 1Y | 2Tm | 3Ln |
|---|---|---|---|
| Formula | C18H12F30O18Y2 | C30H28F30O18Tm2 | C45H24F55Na7O31Y1.56Yb0.40Er0.04 |
| Formula weight | 1264.10 | 1584.38 | 2481.18 |
| Diffractometer | Bruker D8 QUEST | Bruker Smart APEX DUO | Bruker D8 QUEST |
| Data collection method | ω-scans | ω-scans | ω-scans |
| Temperature (K) | 100(2) | 120(2) | 150(2) |
| Crystal system | Triclinic | Monoclinic | Triclinic |
| Space group | P1− | P21/c | P1− |
| a (Å) | 9.062(4) | 12.036(2) | 12.8526(12) |
| b (Å) | 10.202(5) | 18.553(3) | 14.7852(13) |
| c (Å) | 11.113(6) | 12.084(2) | 23.055(2) |
| α (°) | 90.78(2) | 90 | 100.753(2) |
| β (°) | 110.03(2) | 113.849(3) | 101.520(2) |
| γ (°) | 106.05(2) | 90 | 91.705(2) |
| V (Å3) | 921.0(8) | 2468.1(7) | 4207.3(7) |
| Z | 1 | 2 | 2 |
| Color, habit | Colorless, plate | Colorless, plate | Colorless, block |
| Crystal dimensions (mm) | 0.24 × 0.17 × 0.06 | 0.31 × 0.30 × 0.12 | 0.269 × 0.172 × 0.097 |
| Dcalc (g·cm−3) | 2.279 | 2.132 | 1.959 |
| μ (mm−1) | 3.360 | 3.751 | 1.785 |
| Unique reflections (Rint) | 4731 (0.1108) | 5948 (0.0499) | 18,172 (0.1015) |
| Observed reflections [I > 2σ(I)] | 3327 | 4324 | 9391 |
| Parameters, restrains | 307, 0 | 363, 0 | 1262, 179 |
| R1[I > 2σ(I)], ωR2 | 0.0704, 0.1700 | 0.0349, 0.0790 | 0.0901, 0.2755 |
| Goodness-of-fit on F2 | 1.039 | 1.066 | 1.020 |
| Absorption correction | SADABS | SADABS | TWINABS |
| Tmin, Tmax | 0.499, 0.824 | 0.2543, 0.6593 | 0.5359, 0.7455 |
| ρmin, ρmax (eÅ−3) | −1.529, 1.861 | −1.923, 1.996 | −0.860, 1.808 |
| Parameter | Distance |
|---|---|
| Ln1–O1 | 2.293(4) |
| Ln1–O2 i | 2.306(4) |
| Ln1–O3 | 2.322(4) |
| Ln1–O5 | 2.312(4) |
| Ln1–O4 i | 2.383(4) |
| Ln1–O7 | 2.339(4) |
| Ln1–O8 | 2.411(4) |
| Ln1–O9 | 2.339(4) |
| Ln1···Ln1 i | 4.483(2) |
| D···A distance in hydrogen bonds | |
| O7–H1···O6 ii | 2.721(5) |
| O8–H3···O4 iii | 2.873(7) |
| O9–H6···O6 | 2.629(6) |
| Parameter | 2Tm |
|---|---|
| Tm1–O1 | 2.343(4) |
| Tm1–O2 i | 2.278(4) |
| Tm1–O3 | 2.245(3) |
| Tm1–O5 | 2.304(3) |
| Tm1–O6 i | 2.279(3) |
| Tm1–O7 | 2.432(3) |
| Tm1–O8 | 2.399(3) |
| Tm1–O9 | 2.405(3) |
| Tm1···Tm1 i | 4.5199(6) |
| Parameter | Distance | Parameter | Distance | Parameter | Distance | Parameter | Distance | Parameter | Distance |
|---|---|---|---|---|---|---|---|---|---|
| Distances in 1D polymeric {Na5(pfp)5}∞species | |||||||||
| Na1–O1 | 2.352(9) | Na2–O1 | 2.621(9) | Na3–O4 | 2.338(7) | Na4–O7 | 2.381(8) | Na5–O12 | 2.324(11) |
| Na1–O3 | 2.345(10) | Na2–O4 | 2.352(9) | Na3–O7 | 2.408(9) | Na4–O9 | 2.455(8) | Na5–O13 | 2.315(12) |
| Na1–O3 ii | 2.284(8) | Na2–O5 | 2.307(10) | Na3–O9 | 2.326(9) | Na4–O11 | 2.429(10) | Na5–O12 iii | 2.291(8) |
| Na1–O5 | 2.373(9) | Na2–O7 | 2.409(9) | Na3–O11 | 2.318(8) | Na4–O13 | 2.287(10) | Na5–O15 | 2.352(10) |
| Na1–F2 | 2.699(11) | Na2–O9 | 2.370(8) | Na3–F8 | 2.602(9) | Na4–O15 | 2.319(9) | Na5–F26 iii | 2.617(10) |
| Na1–F7 ii | 2.639(10) | Na2–O24 | 2.287(9) | Na3–F16 | 2.478(11) | Na4–O23 | 2.871(9) | Na5–F31 | 2.879(15) |
| Na1–F11 | 2.678(12) | - | - | Na3–F21 | 2.767(10) | Na4–O24 | 2.498(9) | Na5–F37 | 2.817(14) |
| - | - | - | - | Na3–F28 | 2.558(11) | - | - | - | - |
| Distances in tetranuclear heterometallic {Na2Ln2(diglyme)(Meac)(pfp)6(form)} species | |||||||||
| Na6–O17 | 2.288(11) | Na7–O17 | 2.233(12) | Y1–O2 | 2.266(8) | Y2–O6 i | 2.300(7) | - | - |
| Na6–O19 | 2.34(2) | Na7–O21 | 2.318(11) | Y1–O8 | 2.311(11) | Y2–O10 i | 2.319(9) | - | - |
| Na6–O21 | 2.262(10) | Na7–O29 | 2.31(2) | Y1–O16 | 2.267(9) | Y2–O14 i | 2.282(10) | - | - |
| Na6–O27 | 2.402(12) | Na7–O30 | 2.289(18) | Y1–O18 | 2.252(11) | Y2–O22 | 2.291(9) | - | - |
| Na6–O28 | 2.374(12) | Na7–O31 | 2.39(2) | Y1–O20 | 2.334(12) | Y2–O24 i | 2.325(8) | - | - |
| - | - | Na7–F51 | 2.938(12) | Y1–O23 | 2.226(8) | Y2–O26 i | 2.429(12) | - | - |
| - | - | - | - | Y1–O28 | 2.229(8) | Y2–O27 | 2.259(8) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shevchenko, A.; Anosov, A.; Blinnikova, D.; Grebenyuk, D.; Tsymbarenko, D. Single-Source Precursors for Chemical Solution Deposition of Up-Converting NaLnF4 Thin Films. Metals 2022, 12, 488. https://doi.org/10.3390/met12030488
Shevchenko A, Anosov A, Blinnikova D, Grebenyuk D, Tsymbarenko D. Single-Source Precursors for Chemical Solution Deposition of Up-Converting NaLnF4 Thin Films. Metals. 2022; 12(3):488. https://doi.org/10.3390/met12030488
Chicago/Turabian StyleShevchenko, Artem, Andrei Anosov, Daria Blinnikova, Dimitry Grebenyuk, and Dmitry Tsymbarenko. 2022. "Single-Source Precursors for Chemical Solution Deposition of Up-Converting NaLnF4 Thin Films" Metals 12, no. 3: 488. https://doi.org/10.3390/met12030488
APA StyleShevchenko, A., Anosov, A., Blinnikova, D., Grebenyuk, D., & Tsymbarenko, D. (2022). Single-Source Precursors for Chemical Solution Deposition of Up-Converting NaLnF4 Thin Films. Metals, 12(3), 488. https://doi.org/10.3390/met12030488