Interaction of Oxygen with the Stable Ti5Si3 Surface
Abstract
:1. Introduction
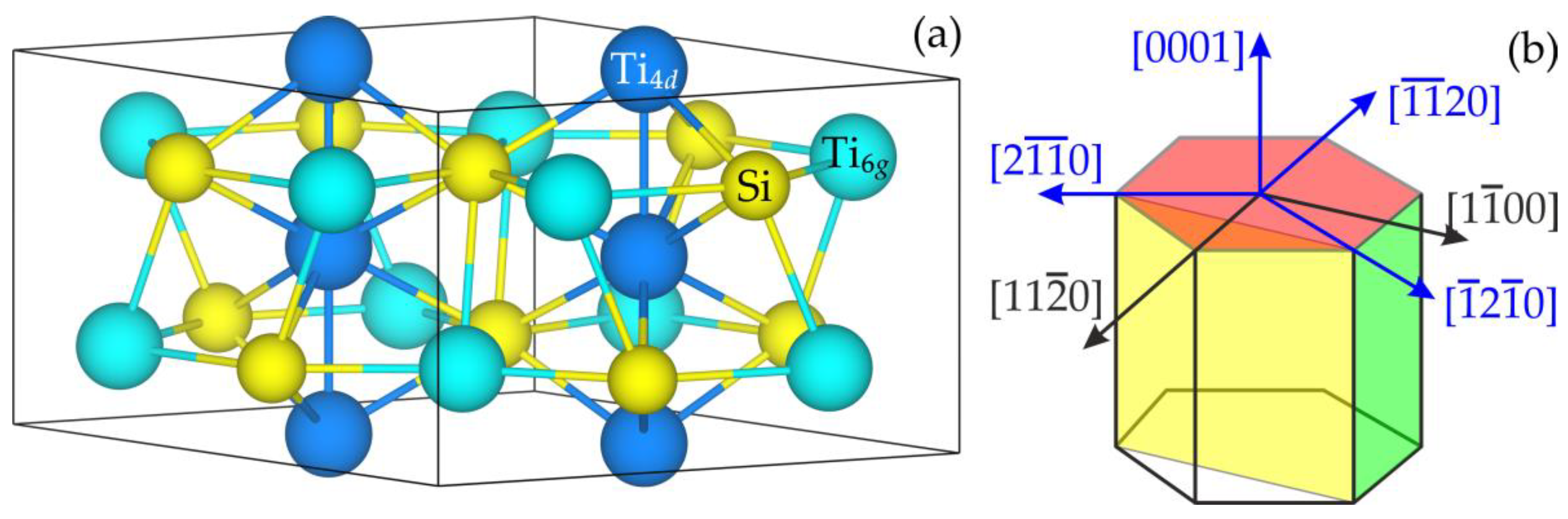
2. Computational Details
3. Results and Discussion
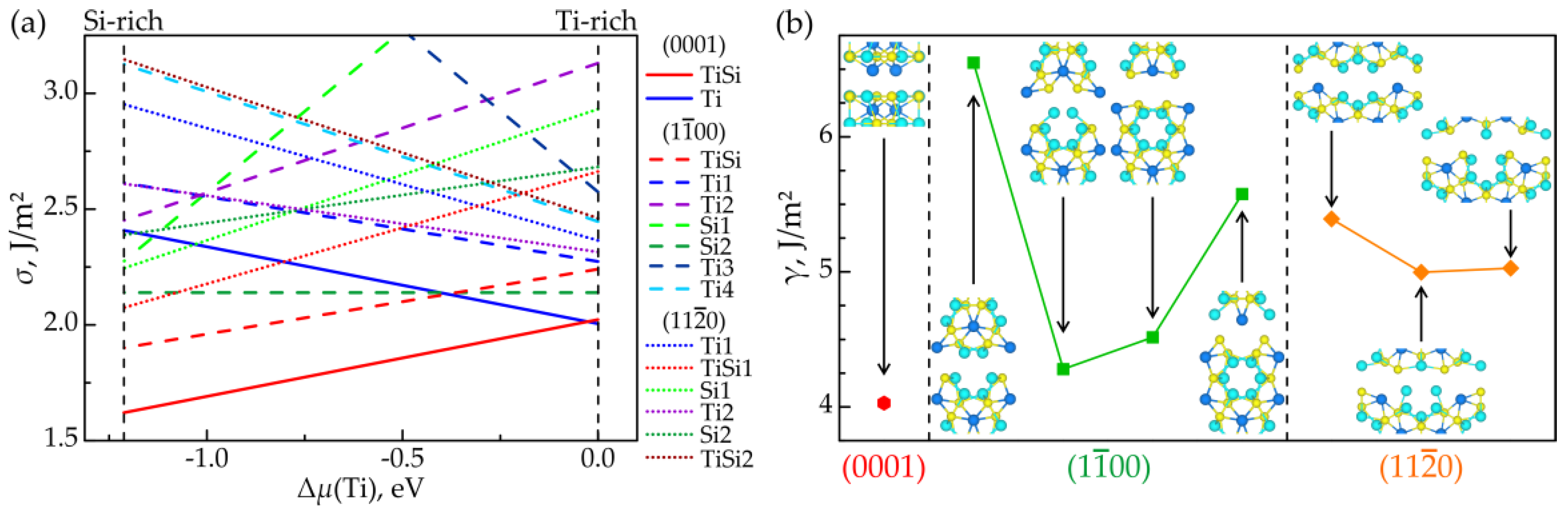
3.1. Surface Stability
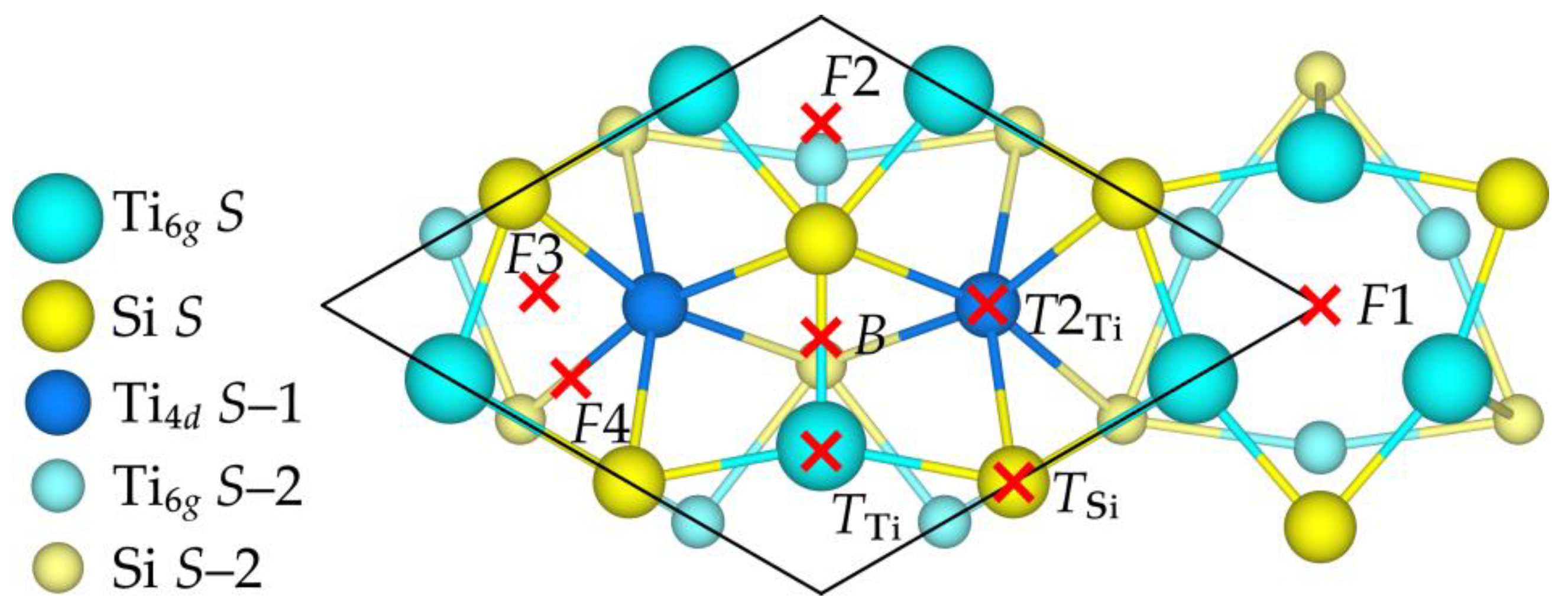
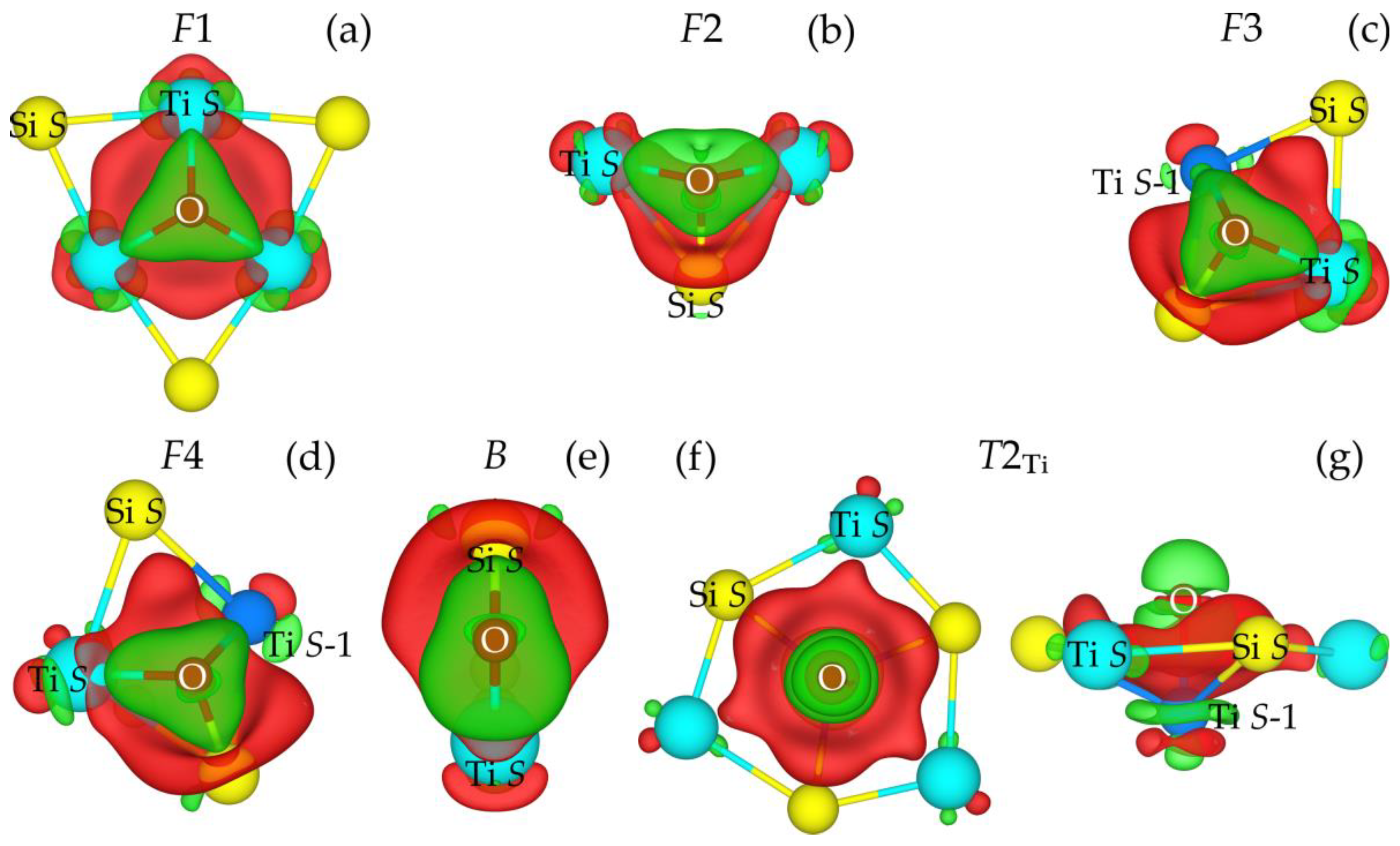
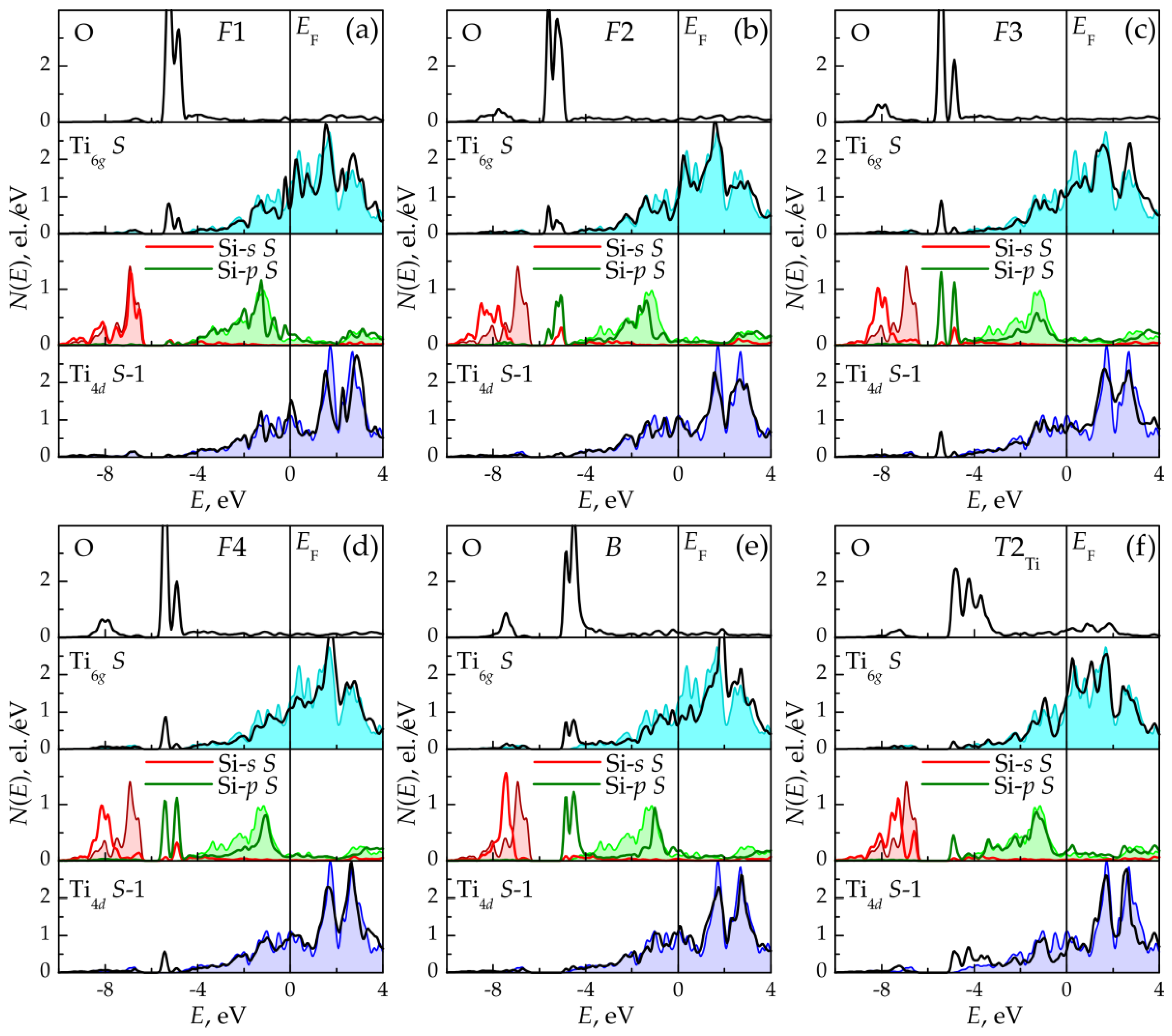
3.2. Oxygen Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gambino, J.P.; Colgan, E.G. Silicides and ohmic contacts. Mater. Chem. Phys. 1998, 52, 99–146. [Google Scholar] [CrossRef]
- Chen, L.J. Silicide Technology for Integrated Circuits; The Institute of Engineering and Technology: London, UK, 2004; pp. 1–279. [Google Scholar]
- Lie, L.N.; Tiller, W.A.; Saraswat, K.C. Thermal oxidation of silicides. J. Appl. Phys. 1984, 56, 2127–2132. [Google Scholar] [CrossRef]
- Jeon, H.; Sukow, C.A.; Honeycutt, J.W.; Rozgonyi, G.A.; Nemanich, R.J. Morphology and phase stability of TiSi2 on Si. J. Appl. Phys. 1992, 71, 4269–4276. [Google Scholar] [CrossRef]
- La Via, F.; Mammoliti, F.; Corallo, G.; Migas, D.B.; Miglio, L. Formation of the TiSi2 C40 as an intermediate phase during the reaction of the Si/Ta/Ti system. Appl. Phys. Lett. 2001, 78, 1864–1866. [Google Scholar] [CrossRef]
- Li, Z.; Gao, W. High temperature corrosion of intermetallics. In Intermetallics Research Progress; Berdovsky, N., Ed.; Nova Science: New York, NY, USA, 2008; pp. 1–64. [Google Scholar]
- Fleischer, R.L. High-strength, high-temperature intermetallic compounds. J. Mater. Sci. 1987, 22, 2281–2288. [Google Scholar] [CrossRef]
- Anton, D.L.; Shah, D.M.; Duhl, D.N.; Giamei, A.F. Selecting high-temperature structural intermetallic compounds: The engineering approach. JOM 1989, 9, 12–17. [Google Scholar] [CrossRef]
- Fleischer, R.L.; Dimiduk, D.M.; Lipsitt, H.A. Intermetallic compounds for strong high-temperature materials: Status and potential. Annu. Rev. Mater. Sci. 1989, 19, 231–263. [Google Scholar] [CrossRef]
- Shah, D.M.; Berczik, D.; Anton, D.L.; Hecht, R. Appraisal of other silicides as structural materials. Mater. Sci. Eng. 1992, A155, 45–57. [Google Scholar] [CrossRef]
- Skrzypek, J.J.; Ganczarski, A.W.; Rustichelli, F.; Egner, H. Material properties for high temperature applications. In Advanced Materials and Structures for Extreme Operating Conditions; Springer: Berlin, Germany, 2008; pp. 1–12. [Google Scholar] [CrossRef]
- Takasugi, T. Effects of alloying additions on intergranular fracture of ordered intermetallics. Mater. Res. Soc. Symp. Proc. 1991, 213, 403–416. [Google Scholar] [CrossRef]
- Grabke, H.J.; Meier, G.H. Accelerated oxidation, internal oxidation, intergranular oxidation, and pesting of intermetallic compounds. Oxid. Met. 1995, 44, 147–176. [Google Scholar] [CrossRef]
- Meyer, M.K.; Akinc, M. Oxidation behavior of boron-modified Mo5Si3 at 800°–1300°C. J. Am. Ceram. Soc. 1996, 79, 938–944. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, J.; Chen, C.; Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: A review. J. Alloys Compd. 2016, 685, 784–798. [Google Scholar] [CrossRef]
- Gui, W.; Liang, Y.; Hao, G.; Lin, J.; Sun, D.; Liu, M.; Liu, C.; Zhang, H. High Nb-TiAl-based porous composite with hierarchical micro-pore structure for high temperature applications. J. Alloys Compd. 2018, 744, 463–469. [Google Scholar] [CrossRef]
- Shanabarger, M.R. Comparative study of the initial oxidation behavior of a series of titanium–aluminum alloys. Appl. Surf. Sci. 1998, 134, 179–186. [Google Scholar] [CrossRef]
- Koizumi, Y.; Kishimoto, M.; Minamino, Y.; Nakajima, H. Oxygen diffusion in Ti3Al single crystals. Philos. Mag. 2008, 88, 2991–3010. [Google Scholar] [CrossRef]
- Song, Y.; Xing, F.J.; Dai, J.H.; Yang, R. First-principles study of influence of Ti vacancy and Nb dopant on the bonding of TiAl/TiO2 interface. Intermetallics 2014, 49, 1–6. [Google Scholar] [CrossRef]
- Wang, B.; Dai, J.; Wu, X.; Song, Y.; Yang, R. First-principles study of the bonding characteristics of TiAl(111)/Al2O3(0001) interface. Intermetallics 2015, 60, 58–65. [Google Scholar] [CrossRef]
- Li, Y.; Dai, J.; Song, Y. Research progress of first principles studies on oxidation behaviors of Ti-Al alloys and alloying influence. Metals 2021, 11, 985. [Google Scholar] [CrossRef]
- Li, Y.; Dai, J.H.; Song, Y. Enhancing adhesion of Al2O3 scale on Ti-Al intermetallics by alloying: A first principles study. Comput. Mater. Sci. 2020, 181, 109756. [Google Scholar] [CrossRef]
- Kulkova, S.E.; Bakulin, A.V.; Hu, Q.M.; Yang, R. Adsorption and diffusion of oxygen on γ-TiAl(001) and (100) surfaces. Comput. Mater. Sci. 2015, 97, 55–63. [Google Scholar] [CrossRef]
- Bakulin, A.V.; Latyshev, A.M.; Kulkova, S.E. Absorption and diffusion of oxygen in the Ti3Al alloy. J. Exp. Theor. Phys. 2017, 125, 138–147. [Google Scholar] [CrossRef]
- Bakulin, A.V.; Kulkov, S.S.; Kulkova, S.E. Diffusion properties of oxygen in the γ-TiAl alloy. J. Exp. Theor. Phys. 2020, 130, 579–590. [Google Scholar] [CrossRef]
- Bakulin, A.V.; Kulkov, S.S.; Kulkova, S.E. Adhesion properties of clean and doped Ti3Al/Al2O3 interface. Appl. Surf. Sci. 2021, 536, 147639. [Google Scholar] [CrossRef]
- Bakulin, A.V.; Kulkov, S.S.; Kulkova, S.E.; Hocker, S.; Schmauder, S. First principles study of bonding mechanisms at the TiAl/TiO2 interface. Metals 2020, 10, 1298. [Google Scholar] [CrossRef]
- Bakulin, A.V.; Kulkova, S.E. Effect of impurities on the formation energy of point defects in the γ-TiAl alloy. J. Exp. Theor. Phys. 2018, 127, 1046–1058. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Ma, W.; Feng, X.; Dong, Z.; Zhang, L.; Liu, Y. Effects of Nb and Si on high temperature oxidation of TiAl. Trans. Nonferrous Met. Soc. China 2008, 18, 512–517. [Google Scholar] [CrossRef]
- Li, X.Y.; Taniguchi, S.; Matsunaga, Y.; Nakagawa, K.; Fujita, K. Influence of siliconizing on the oxidation behavior of a γ-TiAl based alloy. Intermetallics 2003, 11, 143–150. [Google Scholar] [CrossRef]
- Riley, D.P.; Oliver, C.P.; Kisi, E.H. In-situ neutron diffraction of titanium silicide, Ti5Si3, during self-propagating high-temperature synthesis (SHS). Intermetallics 2006, 14, 33–38. [Google Scholar] [CrossRef]
- Shon, I.J.; Kim, H.C.; Rho, D.H.; Munir, Z.A. Simultaneous synthesis and densification of Ti5Si3 and Ti5Si3–20 vol% ZrO2 composites by field-activated and pressure-assisted combustion. Mater. Sci. Eng. A 1999, 269, 129–135. [Google Scholar] [CrossRef]
- Yan, W.; Dai, L.; Gui, C. In situ synthesis and hardness of TiC/Ti5Si3 composites on Ti-5Al-2.5Sn substrates by gas tungsten arc welding. Int. J. Miner. Metall. Mater. 2013, 20, 284–289. [Google Scholar] [CrossRef]
- Lee, D.B.; Kim, D.J. High-temperature oxidation of TiN-Ti5Si3 composites prepared by polymer pyrolysis. Oxid. Met. 2007, 67, 39–49. [Google Scholar] [CrossRef]
- Tang, Z.; Williams, J.J.; Thom, A.J.; Akinc, M. High temperature oxidation behavior of Ti5Si3-based intermetallics. Intermetallics 2008, 16, 1118–1124. [Google Scholar] [CrossRef]
- Swadźba, R.; Swadźba, L.; Mendala, B.; Witala, B.; Tracz, J.; Marugi, K.; Pyclik, Ł. Characterization of Si-aluminide coating and oxide scale microstructure formed on γ-TiAl alloy during long-term oxidation at 950 °C. Intermetallics 2017, 87, 81–89. [Google Scholar] [CrossRef]
- Palmero, P. Structural ceramic nanocomposites: A review of properties and powders’ synthesis methods. Nanomaterials 2015, 5, 656–696. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, F.; Cui, X.; Wang, J.; Xiong, T. Long-term oxidation behavior of silicon-aluminizing coating with an in-situ formed Ti5Si3 diffusion barrier on γ-TiAl alloy. Appl. Surf. Sci. 2022, 582, 152444. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, J. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Kematick, R.J.; Myers, C.E. Thermodynamics of the phase formation of the titanium silicides. Chem. Mater. 1996, 8, 287–291. [Google Scholar] [CrossRef]
- Meschel, S.V.; Kleppa, O.J. Standard enthalpies of formation of some 3d transition metal silicides by high temperature direct synthesis calorimetry. J. Alloys Compd. 1998, 267, 128–135. [Google Scholar] [CrossRef]
- Robins, D.A.; Jenkins, I. The heats of formation of some transition metal silicides. Acta Metall. 1955, 3, 598–604. [Google Scholar] [CrossRef]
- Colinet, C.; Tedenac, J.C. Structural stability of intermetallic phases in the Si–Ti system. Point defects and chemical potentials in D88–Si3Ti5 phase. Intermetallics 2010, 18, 1444–1454. [Google Scholar] [CrossRef]
- Poletaev, D.O.; Aksyonov, D.A.; Lipnitskii, A.G. Evolutionary search for new compounds in the Ti–Si system. Calphad 2020, 71, 102201. [Google Scholar] [CrossRef]
- Zhang, P.F.; Li, Y.X.; Bai, P.K. First principles study of Ti5Si3 intermetallic compounds with Cu additions: Elastic properties and electronic structure. IOP Conf. Ser. Mater. Sci. Eng. 2017, 284, 012013. [Google Scholar] [CrossRef]
- Bakulin, A.V.; Kulkova, S.E.; Hu, Q.M.; Yang, R. Theoretical study of oxygen sorption and diffusion in the volume and on the surface of a γ-TiAl alloy. J. Exp. Theor. Phys. 2015, 120, 257–267. [Google Scholar] [CrossRef]
- Latyshev, A.M.; Bakulin, A.V.; Kulkova, S.E. Adsorption of oxygen on low-index surfaces of Ti3Al alloy. Phys. Solid State 2017, 59, 1852–1866. [Google Scholar] [CrossRef]
- Villars, P.; Calvert, L.D. Pearson’s Handbook of Crystallographic Data for Intermetallic Phases; ASM: Metals Park, OH, USA, 1985. [Google Scholar]
- Yurov, V.M.; Eremin, E.N.; Guchenko, S.A.; Laurinas, V.C. Surface Energy and Thickness of Surface Layer of Atomic-Smooth Metals Silicides. AIP Conf. Proc. 2019, 2141, 040024. [Google Scholar] [CrossRef]
- Sunagawa, I. Crystals: Growth, Morphology and Perfection; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Wang, X.; Jie, J.; Liu, S.; Dong, Z.; Yin, G.; Li, T. Growth mechanism of primary Ti5Si3 phases in special brasses and their effect on wear resistance. J. Mater. Sci. Technol. 2021, 61, 138–146. [Google Scholar] [CrossRef]
- Abba, A.; Galerie, A.; Caillet, M. High-temperature oxidation of titanium silicide coatings on titanium. Oxid. Met. 1982, 17, 43–54. [Google Scholar] [CrossRef]
- Wei, L.J.; Guo, J.X.; Dai, X.H.; Wang, Y.L.; Liu, B.T. Adsorption and dissociation of O2 on Ti3Al(0001) studied by first-principles. Surf. Rev. Lett. 2015, 22, 1550053. [Google Scholar] [CrossRef]
- Loffreda, D. Theoretical insight of adsorption thermodynamics of multifunctional molecules on metal surfaces. Surf. Sci. 2006, 600, 2103–2122. [Google Scholar] [CrossRef]
- Manz, T.A.; Limas, N.G. Introducing DDEC6 atomic population analysis: Part 1. Charge partitioning theory and methodology. RSC Adv. 2016, 6, 47771–47801. [Google Scholar] [CrossRef] [Green Version]
- Manz, T.A. Introducing DDEC6 atomic population analysis: Part 3. Comprehensive method to compute bond orders. RSC Adv. 2017, 7, 45552–45581. [Google Scholar] [CrossRef] [Green Version]

| Surface | (0001) | (1–100) | ||
|---|---|---|---|---|
| Termination | TiSi | Ti | TiSi | Si2 |
| Δd12, % | −19.6 | −0.1 | −3.3 | −3.3 |
| Δd23, % | +13.7 | −5.9 | +0.5 | −4.7 |
| Δd34, % | −0.9 | +4.5 | +5.5 | +1.9 |
| ε, Å | 0.19 | – | 0.12 | – |
| Position | F1 | F2 | F3 | F4 | B | T2Ti |
|---|---|---|---|---|---|---|
| Eads, eV | 6.13 | 5.30 | 4.06 | 4.15 | 3.87 | 2.00 |
| d(O–Ti6giS), Å | 1.96 | 1.97 | 2.00 | 1.96 | 1.86 | 2.95 |
| d(O–Si S), Å | 3.15 | 1.90 | 1.71 | 1.71 | 1.71 | 2.52 |
| d(O–Ti4dS–1), Å | 4.74 | 3.80 | 2.13 | 2.17 | 3.28 | 1.86 |
| h, Å | 0.91 | 0.93 | 0.60 | 0.61 | 1.02 | 0.71 |
| QDDEC6, el. | 0.76 | 0.48 | 0.38 | 0.36 | 0.35 | 0.35 |
| Σθ, el. | 1.65 | 1.51 | 1.49 | 1.50 | 1.43 | 1.31 |
| θ(O–Ti6gS), el. | 0.48 | 0.43 | 0.37 | 0.41 | 0.52 | 0.05 |
| θ(O–Si S), el. | 0.07 | 0.65 | 0.86 | 0.85 | 0.87 | 0.23 |
| θ(O–Ti4dS–1), el. | 0.00 | 0.00 | 0.26 | 0.24 | 0.02 | 0.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chumakova, L.S.; Bakulin, A.V.; Hocker, S.; Schmauder, S.; Kulkova, S.E. Interaction of Oxygen with the Stable Ti5Si3 Surface. Metals 2022, 12, 492. https://doi.org/10.3390/met12030492
Chumakova LS, Bakulin AV, Hocker S, Schmauder S, Kulkova SE. Interaction of Oxygen with the Stable Ti5Si3 Surface. Metals. 2022; 12(3):492. https://doi.org/10.3390/met12030492
Chicago/Turabian StyleChumakova, Lora S., Alexander V. Bakulin, Stephen Hocker, Siegfried Schmauder, and Svetlana E. Kulkova. 2022. "Interaction of Oxygen with the Stable Ti5Si3 Surface" Metals 12, no. 3: 492. https://doi.org/10.3390/met12030492
APA StyleChumakova, L. S., Bakulin, A. V., Hocker, S., Schmauder, S., & Kulkova, S. E. (2022). Interaction of Oxygen with the Stable Ti5Si3 Surface. Metals, 12(3), 492. https://doi.org/10.3390/met12030492







