Structural Characterization of Ion Nitrided 316L Austenitic Stainless Steel: Influence of Treatment Temperature and Time
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
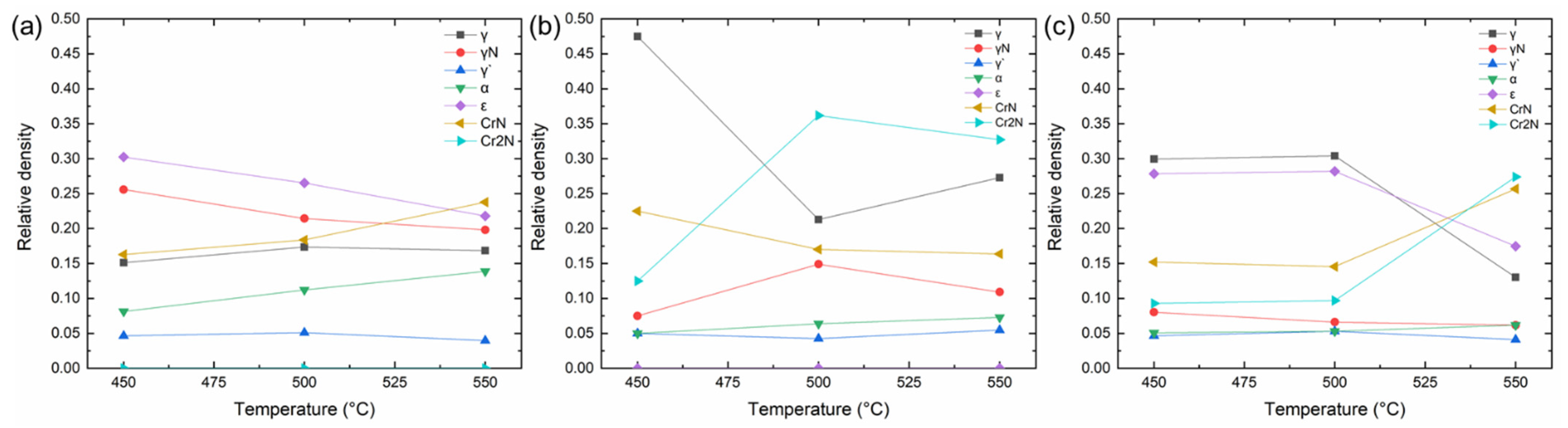
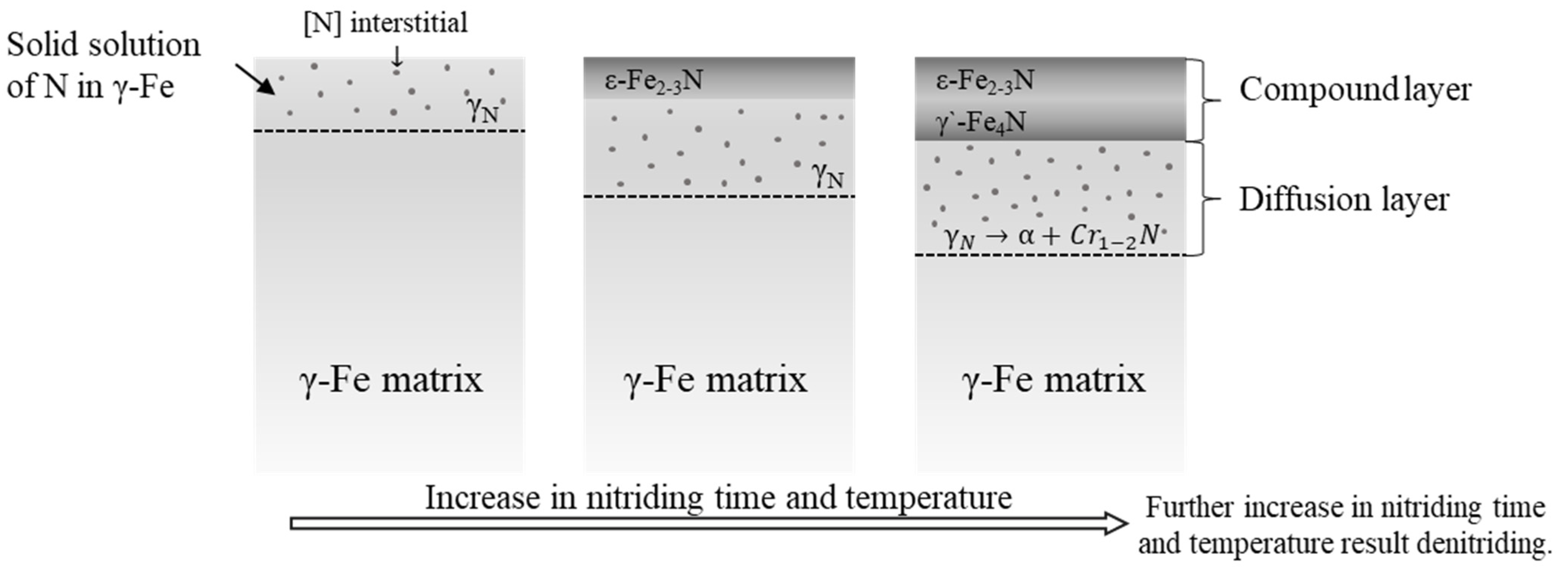
3.1. Phase Formation Characteristics
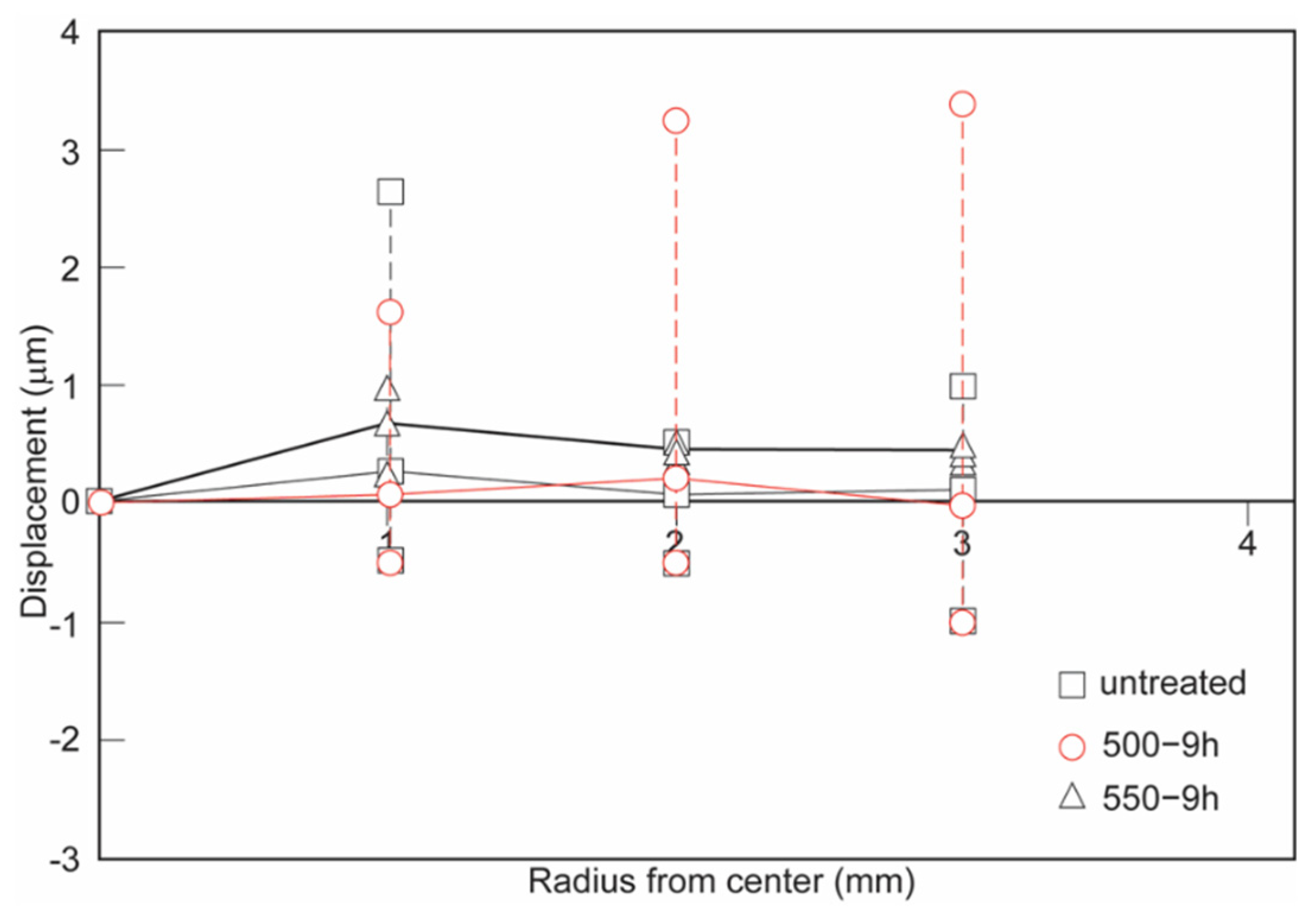
3.2. Measurements of Residual-Stress-Induced Displacements
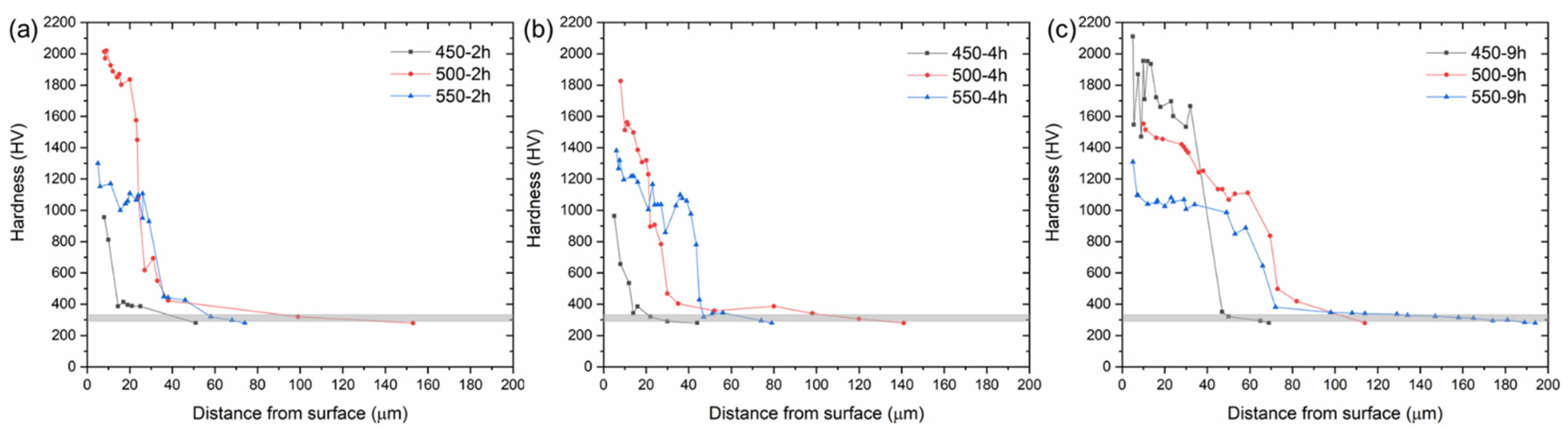
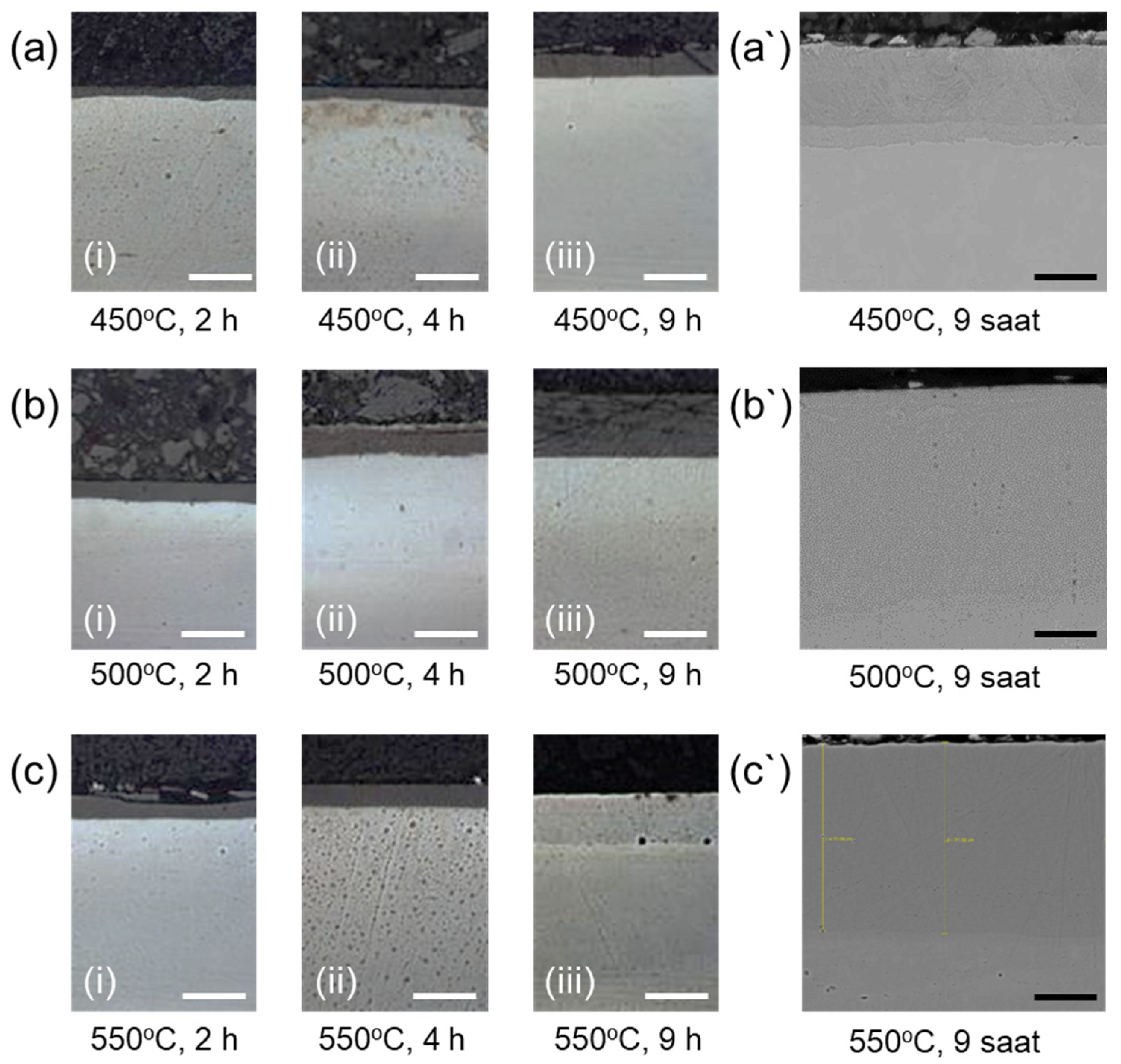
3.3. Microhardness and Microstructural Analysis
4. Conclusions
- The γN phase existed in the diffusion layer in all different nitriding conditions. This phase was responsible for the hardness increase upon the ion nitriding process.
- The compound layer thickness consisting of hard and brittle ε phase increased significantly at 9 h nitriding time. Increasing nitriding temperature caused a decrease in the amount of ε and γN phases due to the denitriding and resulting CrN precipitation.
- The γ’ phase was also existent in all nitriding conditions. However, the amount of the phase did not show a significant difference with changing nitriding parameters.
- The samples nitrided at 450 °C for 9 h exhibited the highest surface hardness values. However, they suffered from cracks and/or delamination, most probably due to the sharp concentration gradient of the diffusing nitrogen and consequent residual stresses in the nitride layer.
- The sample nitrided at 500 °C for 9 h exhibited the best nitride layer thickness and hardness combination without any cracks and/or delamination.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Michler, T. Austenitic Stainless Steels. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-0-12-803581-8. [Google Scholar]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface modification of stainless steel for biomedical applications: Revisiting a century-old material. Mater. Sci. Eng. C 2018, 93, 1073–1089. [Google Scholar] [CrossRef]
- Mateescu, A.O.; Mateescu, G.; Balan, A.; Ceaus, C.; Stamatin, I.; Cristea, D.; Samoila, C.; Ursutiu, D. Stainless Steel Surface Nitriding in Open Atmosphere Cold Plasma: Improved Mechanical, Corrosion and Wear Resistance Properties. Materials 2021, 14, 4836. [Google Scholar] [CrossRef] [PubMed]
- Gokcekaya, O.; Webster, T.J.; Ueda, K.; Narushima, T.; Ergun, C. In vitro performance of Ag-incorporated hydroxyapatite and its adhesive porous coatings deposited by electrostatic spraying. Mater. Sci. Eng. C 2017, 77, 556–564. [Google Scholar] [CrossRef]
- Ueda, T.; Kondo, N.; Sado, S.; Gokcekaya, O.; Ueda, K.; Ogasawara, K.; Narushima, T. Ceramic coating of Ti and its alloys using dry processes for biomedical applications BT—Interface Oral Health Science 2016. In Proceedings of the Interface Oral Health Science 2016; Sasaki, K., Suzuki, O., Takahashi, N., Eds.; Springer: Singapore, 2017; pp. 23–34. [Google Scholar]
- Borgioli, F. From Austenitic stainless steel to expanded austenite-S phase: Formation, characteristics and properties of an elusive metastable phase. Metals 2020, 10, 187. [Google Scholar] [CrossRef]
- Alsaran, A.; Çelik, A. Structural characterization of ion-nitrided AISI 5140 low-alloy steel. Mater. Charact. 2001, 47, 207–213. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Bell, T. Structure and corrosion resistance of plasma nitrided stainless steel. Surf. Eng. 1985, 1, 131–136. [Google Scholar] [CrossRef]
- Borgioli, F.; Fossati, A.; Galvanetto, E.; Bacci, T. Glow-discharge nitriding of AISI 316L austenitic stainless steel: Influence of treatment temperature. Surf. Coat. Technol. 2005, 200, 2474–2480. [Google Scholar] [CrossRef]
- Sun, Y.; Bell, T.; Wood, G. Wear behaviour of plasma-nitrided martensitic stainless steel. Wear 1994, 178, 131–138. [Google Scholar] [CrossRef]
- Borowski, T. Enhancing the corrosion resistance of austenitic steel using active screen plasma nitriding and nitrocarburising. Materials 2021, 14, 3320. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiong, J.; Peng, Q.; Fan, H.; Wang, Y.; Li, G.; Shen, B. Effects of DC plasma nitriding parameters on microstructure and properties of 304L stainless steel. Mater. Charact. 2009, 60, 197–203. [Google Scholar] [CrossRef]
- Christiansen, T.; Somers, M.A.J. Decomposition kinetics of expanded austenite with high nitrogen contents. Int. J. Mater. Res. 2006, 97, 79–88. [Google Scholar] [CrossRef]
- Tschiptschin, A.P.; Nishikawa, A.S.; Varela, L.B.; Pinedo, C.E. Thermal stability of expanded austenite formed on a DC plasma nitrided 316L austenitic stainless steel. Thin Solid Films 2017, 644, 156–165. [Google Scholar] [CrossRef]
- Fraczek, T.; Ogorek, M.; Skuza, Z.; Prusak, R. Mechanism of ion nitriding of 316L austenitic steel by active screen method in a hydrogen-nitrogen atmosphere. Int. J. Adv. Manuf. Technol. 2020, 109, 1357–1368. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Yilmaz, S.; Ergun, C.; Kaya, B.; Yücel, O. Plasma nitrided austenitic stainless steel for biomedical applications. In Proceedings of the Ceramic Engineering and Science Proceedings, Daytona Beach, FL, USA, 24–29 January 2010; Volume 31. [Google Scholar]
- Li, G.Y.; Lei, M.K. Microstructure and properties of plasma source nitrided AISI 316 austenitic stainless steel. J. Mater. Eng. Perform. 2017, 26, 418–423. [Google Scholar] [CrossRef]
- Shen, H.; Wang, L.; Sun, J. Characteristics and properties of CrN compound layer produced by plasma nitriding of Cr-electroplated of AISI 304 stainless steel. Surf. Coat. Technol. 2020, 385, 125450. [Google Scholar] [CrossRef]
- Kurny, A.S.W.; Mallya, R.M.; Mohan Rao, M. A study on the nature of the compound layer formed during the ion nitriding of En40B steel. Mater. Sci. Eng. 1986, 78, 95–100. [Google Scholar] [CrossRef]
- Wang, L.; Ji, S.; Sun, J. Effect of nitriding time on the nitrided layer of AISI 304 austenitic stainless steel. Surf. Coat. Technol. 2006, 200, 5067–5070. [Google Scholar] [CrossRef]
- Aizawa, T.; Yoshino, T.; Morikawa, K.; Yoshihara, S.-I. Microstructure of plasma nitrided AISI420 martensitic stainless steel at 673 K. Crystals 2019, 9, 60. [Google Scholar] [CrossRef]
- Qin, X.; Guo, X.; Lu, J.; Chen, L.; Qin, J.; Lu, W. Erosion-wear and intergranular corrosion resistance properties of AISI 304L austenitic stainless steel after low-temperature plasma nitriding. J. Alloys Compd. 2017, 698, 1094–1101. [Google Scholar] [CrossRef]
- Spies, H.-J. 6—Corrosion Behaviour of Nitrided, Nitrocarburised and Carburised Steels. In Thermochemical Surface Engineering of Steels; Mittemeijer, E.J., Somers, M.A.J., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 267–309. ISBN 978-0-85709-592-3. [Google Scholar]
- García Molleja, J.; Milanese, M.; Piccoli, M.; Moroso, R.; Niedbalski, J.; Nosei, L.; Bürgi, J.; Bemporad, E.; Feugeas, J. Stability of expanded austenite, generated by ion carburizing and ion nitriding of AISI 316L SS, under high temperature and high energy pulsed ion beam irradiation. Surf. Coat. Technol. 2013, 218, 142–151. [Google Scholar] [CrossRef]
- Peng, T.; Chen, Y.; Liu, X.; Wu, M.; Lu, Y.; Hu, J. Phase constitution control of plasma nitrided layer and its effect on wear behavior under different loads. Surf. Coat. Technol. 2020, 403, 126403. [Google Scholar] [CrossRef]
- Yasumaru, N. Low-Temperature Ion Nitriding of Austenitic Stainless Steels. Mater. Trans. JIM 1998, 39, 1046–1052. [Google Scholar] [CrossRef][Green Version]

| C | Si | Mn | P | S | Cr | Mo | Ni | Cu |
|---|---|---|---|---|---|---|---|---|
| 0.022 | 0.79 | 1.6 | 0.25 | 0.002 | 15.3 | 2.63 | 14.09 | 0.05 |
| Nitriding Time | Nitride Layer Thickness (µm) | ||
|---|---|---|---|
| 450 °C | 500 °C | 550 °C | |
| 2 h | 15 | 40 | 45 |
| 4 h | 15 | 45 | 50 |
| 9 h | 50 | 95 | 75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gokcekaya, O.; Ergun, C.; Gulmez, T.; Nakano, T.; Yilmaz, S. Structural Characterization of Ion Nitrided 316L Austenitic Stainless Steel: Influence of Treatment Temperature and Time. Metals 2022, 12, 306. https://doi.org/10.3390/met12020306
Gokcekaya O, Ergun C, Gulmez T, Nakano T, Yilmaz S. Structural Characterization of Ion Nitrided 316L Austenitic Stainless Steel: Influence of Treatment Temperature and Time. Metals. 2022; 12(2):306. https://doi.org/10.3390/met12020306
Chicago/Turabian StyleGokcekaya, Ozkan, Celaletdin Ergun, Turgut Gulmez, Takayoshi Nakano, and Safak Yilmaz. 2022. "Structural Characterization of Ion Nitrided 316L Austenitic Stainless Steel: Influence of Treatment Temperature and Time" Metals 12, no. 2: 306. https://doi.org/10.3390/met12020306
APA StyleGokcekaya, O., Ergun, C., Gulmez, T., Nakano, T., & Yilmaz, S. (2022). Structural Characterization of Ion Nitrided 316L Austenitic Stainless Steel: Influence of Treatment Temperature and Time. Metals, 12(2), 306. https://doi.org/10.3390/met12020306







