Passive Film Properties of Martensitic Steels in Alkaline Environment: Influence of the Prior Austenite Grain Size
Abstract
:1. Introduction
2. Experimental
2.1. Material
2.2. Design of the Microstructure
- -
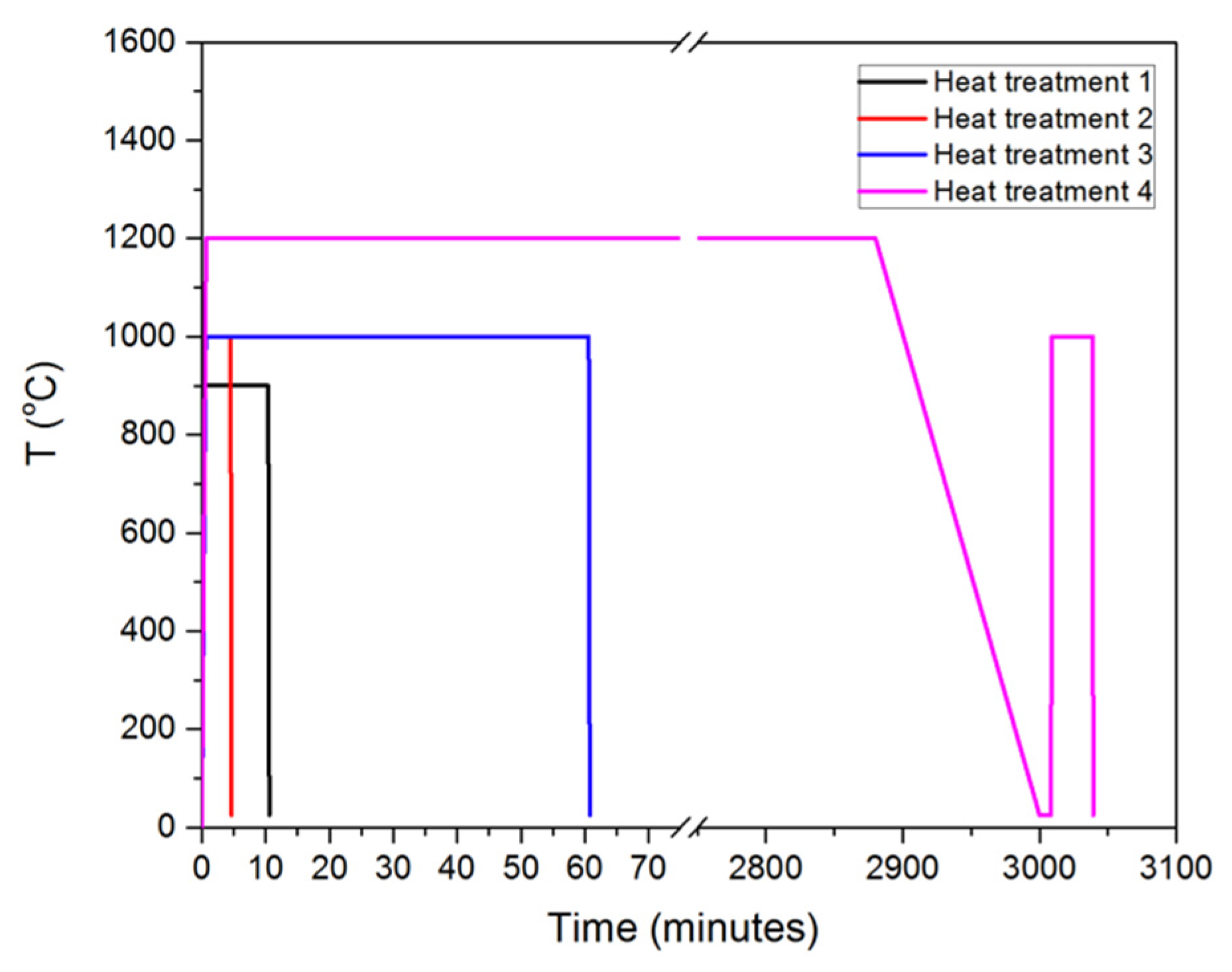
- Heat treatment 1 (HT-1)—Samples were heated to the austenitisation temperature of 900 °C, held for 10 min under vacuum and quenched to room temperature.
- -
- Heat treatment 2 (HT-2)—Higher austenitisation temperature of 1000 °C was chosen. Samples were held at austenitisation temperatures for 4 min. Subsequently, samples were quenched to room temperature.
- -
- Heat treatment 3 (HT-3)—Samples were heated to the austenitisation temperature of 1000 °C, held for 60 min under vacuum, then quenched to room temperature.
- -
- Heat Treatment 4 (HT-4)—Samples were heated to 1200 °C and held 48 h in a furnace and slowly cooled to room temperature. Subsequently, the samples were annealed at the austenitisation temperature of 1000 °C for 30 min under vacuum in the dilatometer, then quenched to room temperature.
2.3. Characterization of the Microstructure
2.4. Electrochemical Measurements
2.5. Passive Layer Analysis
3. Results and Discussion
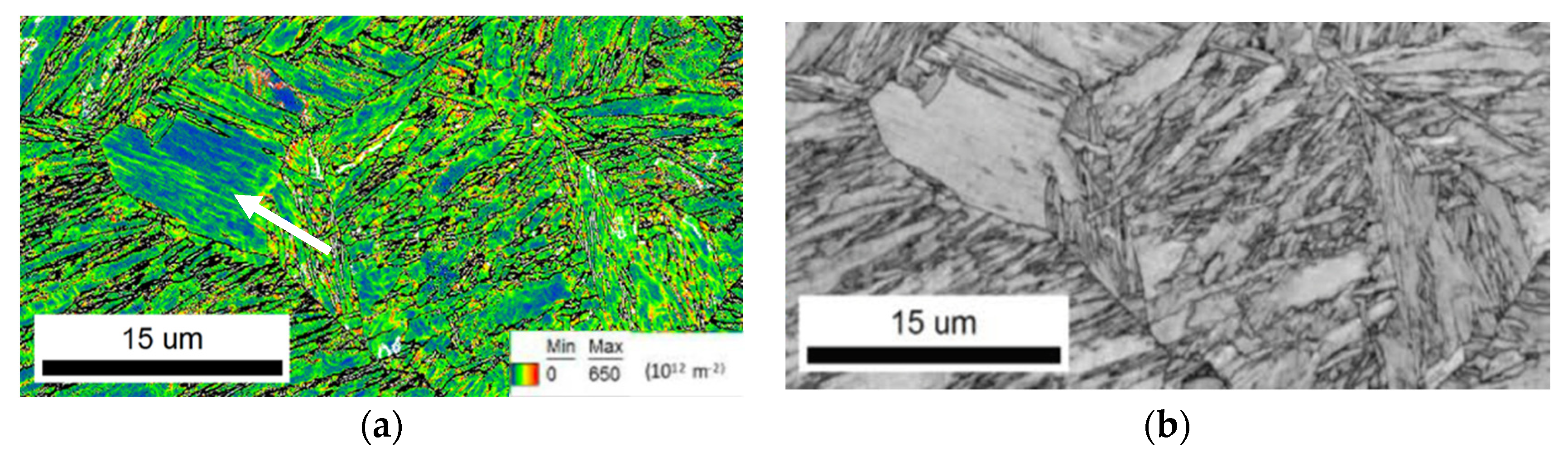
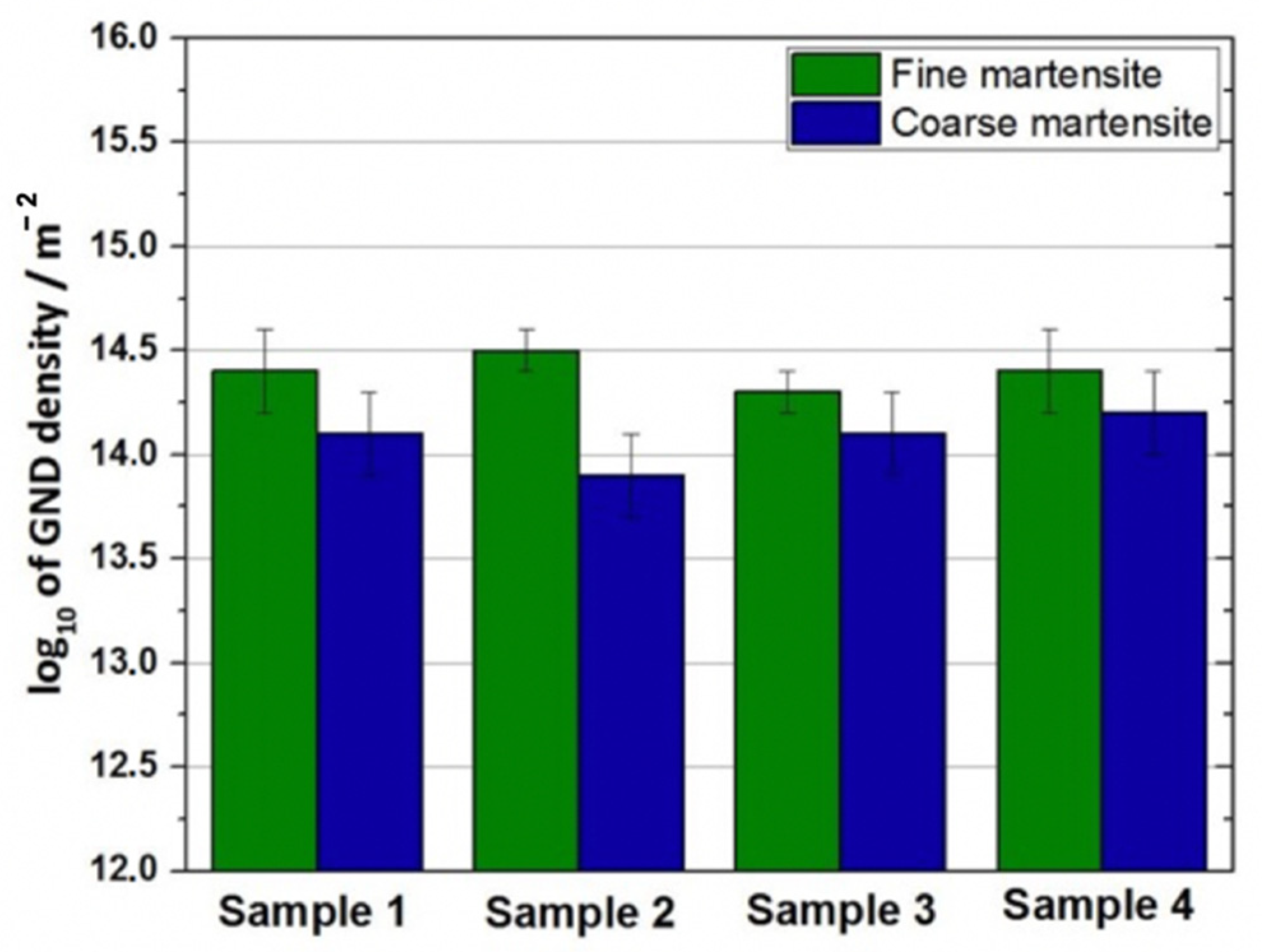
3.1. Microstructure Characterization of the Martensitic Steels
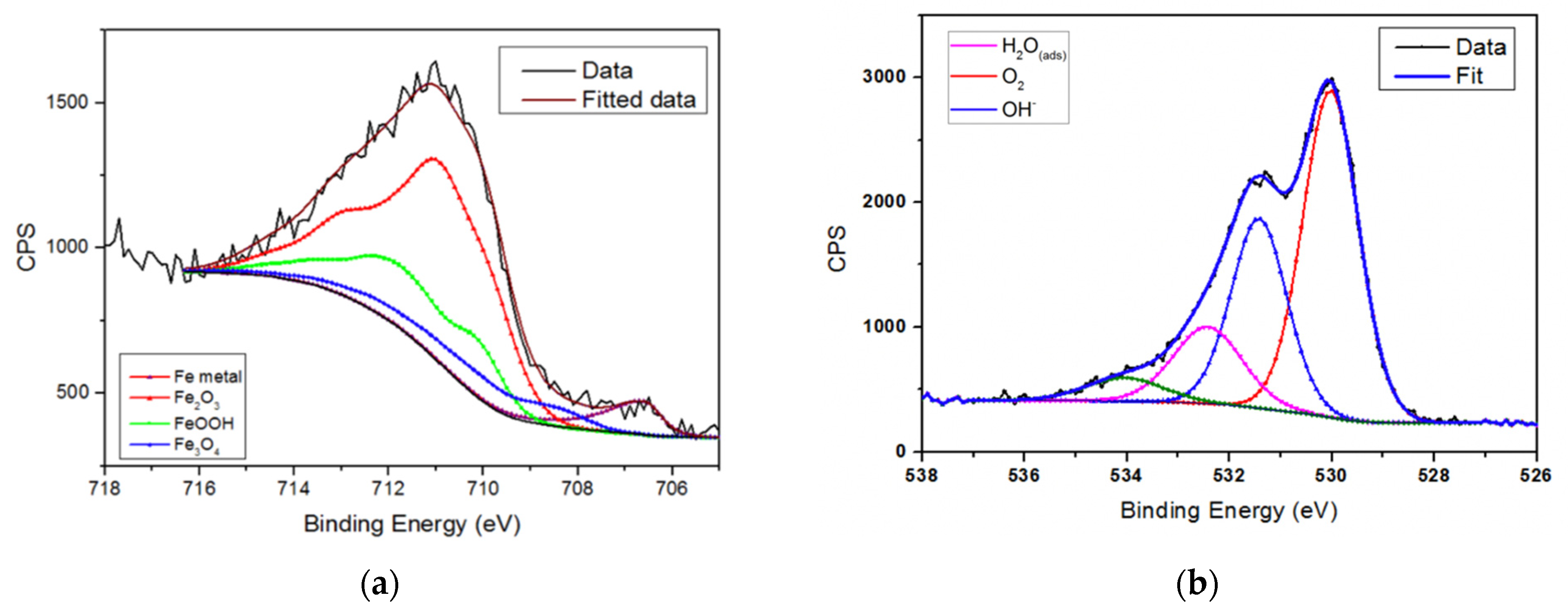
3.2. Passive Layer Analysis
3.3. Electrochemical Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tasan, C.; Diehl, M.; Yan, D.; Bechtold, M.; Roters, F.; Schemmann, L.; Zheng, C.; Peranio, N.; Ponge, D.; Koyama, M.; et al. An Overview of Dual-Phase Steels: Advances in Microstructure-Oriented Processing and Micromechanically Guided Design. Annu. Rev. Mater. Res. 2015, 45, 391–431. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of grain size on corrosion: A review. Corrosion 2010, 66, 0750051–07500513. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Vafaeian, S. Influence of grain refinement on the electrochemical behavior of AISI 430 ferritic stainless steel in an alkaline solution. Appl. Surf. Sci. 2016, 360, 921–928. [Google Scholar] [CrossRef]
- Pisarek, M.; Kedzierzawski, P.; Janik-Czachor, M.; Kurzydłowski, K.J. Effect of hydrostatic extrusion on passivity breakdown on 303 austenitic stainless steel in chloride solution. J. Solid State Electrochem. 2009, 13, 283–291. [Google Scholar] [CrossRef]
- Yilmaz, A.; Traka, K.; Pletincx, S.; Hauffman, T.; Sietsma, J.; Gonzalez-Garcia, Y. Effect of microstructural defects on passive layer properties of interstitial free (IF) ferritic steels in alkaline environment. Corros. Sci. 2021, 182, 109271. [Google Scholar] [CrossRef]
- Lv, J.; Luo, H. The effects of cold rolling temperature on corrosion resistance of pure iron. Appl. Surf. Sci. 2014, 317, 125–130. [Google Scholar]
- Adams, B.L.; Kacher, J. EBSD-based microscopy: Resolution of dislocation density. Comput. Mater. Contin. 2009, 14, 183–194. [Google Scholar]
- Hidalgo, J.; Santofimia, M.J. Effect of Prior Austenite Grain Size Refinement by Thermal Cycling on the Microstructural Features of As-Quenched Lath Martensite. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2016, 47, 5288–5301. [Google Scholar] [CrossRef] [Green Version]
- Celada-Casero, C.; Sietsma, J.; Santofimia, M.J. The role of the austenite grain size in the martensitic transformation in low carbon steels. Mater. Des. 2019, 167, 107625. [Google Scholar] [CrossRef]
- Gupta, R.P.; Sen, S.K. Calculation of multiplet structure of core p-vacancy levels. Phys. Rev. B 1974, 10, 71–77. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corp.: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Freire, L.X.R.N.; Nóvoa, X.R.; Montemor, M.F.; Carmezim, M.J. Study of passive films formed on mild steel in alkaline media by the application of anodic potentials. Mater. Chem. Phys. 2009, 114, 962–972. [Google Scholar] [CrossRef]
- Strehblow, H.; Marcus, P. X-ray photoelectron spectroscopy in corrosion research. In Analytical Methods in Corrosion Science and Engineering; Marcus, P., Mansfeld, F., Eds.; CRC Press Taylor Fr.: Boca Raton, FL, USA, 2006. [Google Scholar]
- Xu, W.; Daub, K.; Zhang, X.; Noel, J.; Shoesmith, D.; Wren, J. Oxide formation and conversion on carbon steel in mildly basic solutions. Electrochim. Acta 2009, 54, 5727–5738. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Y.F. Passive film growth on carbon steel and its nanoscale features at various passivating potentials. Appl. Surf. Sci. 2017, 396, 144–153. [Google Scholar] [CrossRef]
- Martin, J.E.; Herzing, A.A.; Yan, W.; Li, X.-Q.; Koel, B.; Kiely, C.; Zhang, W.-X. Determination of the oxide layer thickness in core-shell zerovalent iron nanoparticles. Langmui 2008, 24, 4329–4334. [Google Scholar] [CrossRef]
- Cumpson, P.J.; Seah, M.P. Elastic Scattering Corrections in AES and XPS. II. Estimating Attenuation Lengths and Conditions Required for their Valid Use in Overlayer/Substrate Experiments. Surf. Interface Anal. 1997, 25, 430–446. [Google Scholar] [CrossRef]
- Kim, J.J.; Young, Y.M. Study on the passive film of type 316 stainless steel. Int. J. Electrochem. Sci. 2013, 8, 11847–11859. [Google Scholar]
- Yanagisawa, K.; Nakanishi, T.; Hasegawa, Y.; Fushimi, K. Passivity of Dual-Phase Carbon Steel with Ferrite and Martensite Phases in pH 8.4 Boric Acid-Borate Buffer Solution. J. Electrochem. Soc. 2015, 162, C322–C326. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, M.; Gregori, J.; Alonso, C.; García-Jareño, J.; Takenouti, H.; Vicente, F. Electrochemical impedance spectroscopy for studying passive layers on steel rebars immersed in alkaline solutions simulating concrete pores. Electrochim. Acta 2007, 52, 7634–7641. [Google Scholar] [CrossRef]
- Sánchez, M.; Mahmoud, H.; Alonso, M.C. Electrochemical response of natural and induced passivation of high strength duplex stainless steels in alkaline media. J. Solid State Electrochem. 2012, 16, 1193–1202. [Google Scholar] [CrossRef]
- Joiret, S.; Keddam, M.; Nóvoa, X.R.; Pérez, M.C.; Rangel, C.; Takenouti, H. Use of EIS, ring-disk electrode, EQCM and Raman spectroscopy to study the film of oxides formed on iron in 1 M NaOH. Cem. Concr. Compos. 2002, 24, 7–15. [Google Scholar] [CrossRef]
- Andrade, C.; Keddam, M.; Nóvoa, X.R.; Pérez, M.C.; Rangel, C.M.; Takenouti, H. Electrochemical behaviour of steel rebars in concrete: Influence of environmental factors and cement chemistry. Electrochim. Acta 2001, 46, 3905–3912. [Google Scholar] [CrossRef]
- Hamadou, L.; Kadri, A.; Benbrahim, N. Characterisation of passive films formed on low carbon steel in borate buffer solution (pH 9.2) by electrochemical impedance spectroscopy. Appl. Surf. Sci. 2005, 252, 1510–1519. [Google Scholar] [CrossRef]
- Schoonman, J.; Vos, K.; Blasse, G. Donor Densities in TiO2 Photoelectrodes. J. Electrochem. Soc. 1981, 128, 1154. [Google Scholar] [CrossRef]
- Horowitz, G. Capacitance-voltage measurements and flat-band potential determination on Zr-doped α-Fe2O3 single-crystal electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1983, 159, 421–436. [Google Scholar] [CrossRef]
- Carmezim, M.; Simões, A.; Figueiredo, M.; Belo, M.D.C. Electrochemical behaviour of thermally treated Cr-oxide films deposited on stainless steel. Corros. Sci. 2002, 44, 451–465. [Google Scholar] [CrossRef]
- Kennedy, J.H.; Frese, K.W., Jr. Flatband Potentials and Donor Densities of Polycrystalline α -Fe2O3 Determined from Mott-Schottky Plots. J. Electrochem. Soc. 1978, 125, 723–726. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Huang, B.; Ma, Y.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y. Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl. Mater. Interfaces 2012, 4, 4024–4030. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Kalathil, S.; Lee, J.; Cho, M.H. Band gap engineering of CeO2 nanostructure using an electrochemically active biofilm for visible light applications. RSC Adv. 2014, 4, 16782–16791. [Google Scholar] [CrossRef]
- Sato, N.; Noda, T. Ion Migration in Anodic Barrier Oxide Films on Iron in Acidic Phosphate Solutions. Electrochim. Acta 1976, 22, 839–843. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides; Structure Properties, Reactions, Occurrences and Uses; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Stimming, U.; Schultze, J. A semiconductor model of the passive layer on iron electrodes and its application to electrochemical reactions. Electrochim. Acta 1979, 24, 859–869. [Google Scholar] [CrossRef]



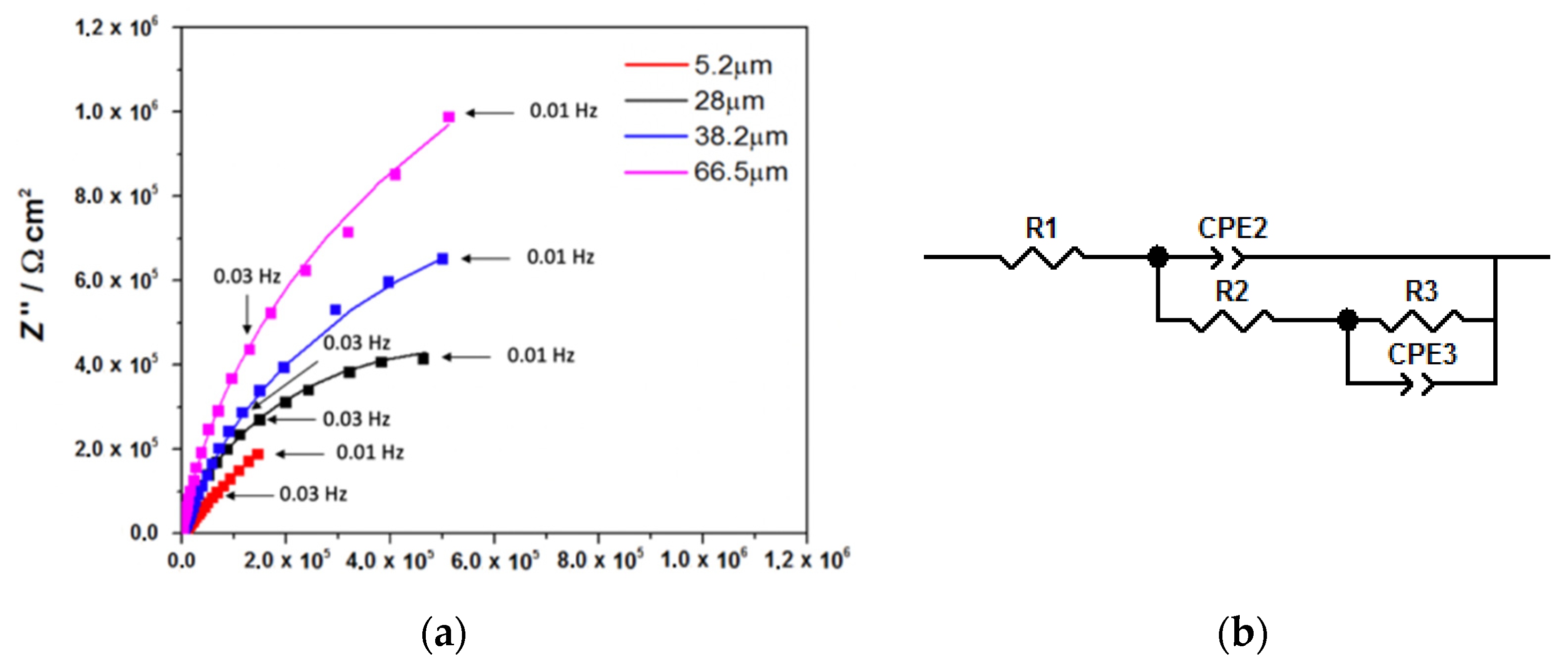
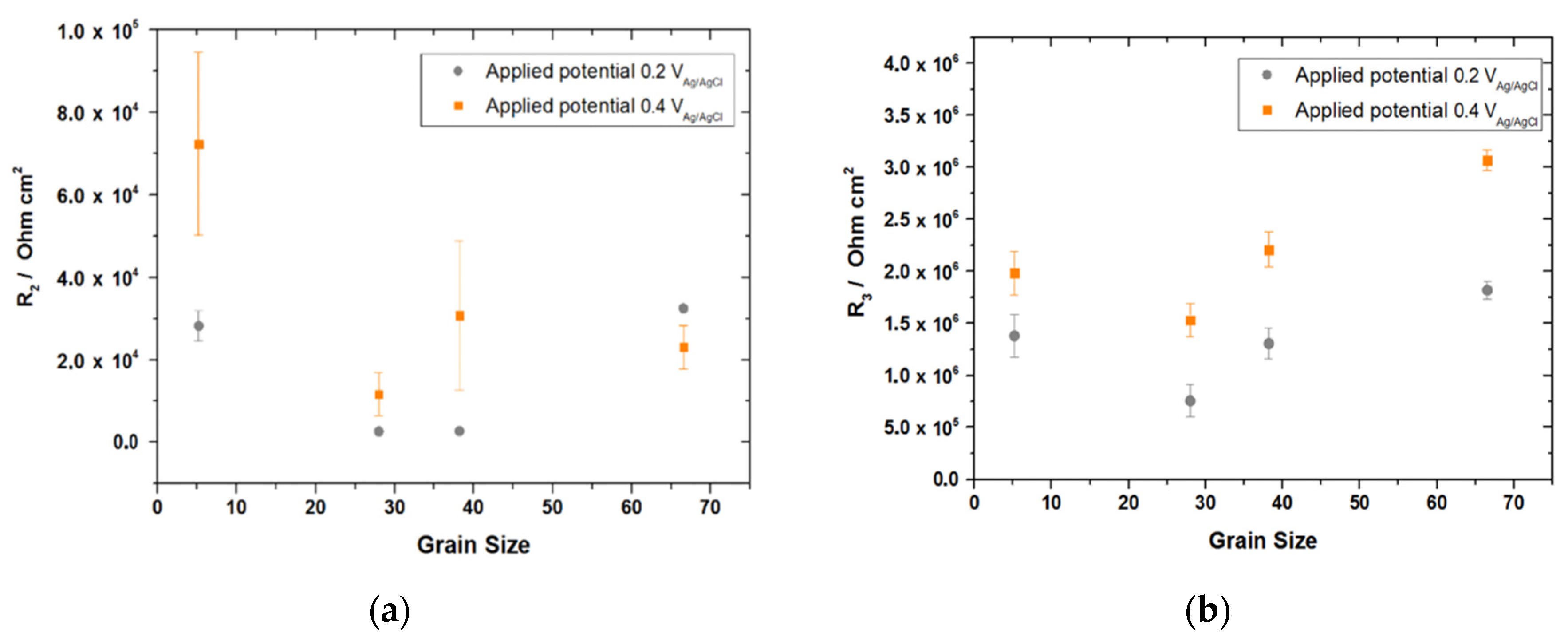

| Elements | Fe | C | Si | Mn | S | Al | Cr | N | Ni | Sn | Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | bal. | 0.095 | 0.248 | 1.776 | 0.002 | 0.032 | 0.574 | 0.008 | 0.026 | 0.002 | 0.003 |
| Heading | Sample 1 | Sample 2 | Sample 3 | Sample 4 |
|---|---|---|---|---|
| PAG Size | 5.2 ± 1.5 µm | 28 ± 3 µm | 38 ± 4 µm | 66 ± 6 µm |
| Passivation Potential/V | Passive Layer Thickness/nm | |||
|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | |
| 0.2 | 3.0 ± 0.2 | 2.9 ± 0.1 | 3.1 ± 0.2 | 3.1 ± 0.2 |
| 0.4 | 3.2 ± 0.2 | 3.3 ± 0.2 | 3.3 ± 0.2 | 3.3 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, A.; Li, X.; Pletincx, S.; Hauffman, T.; Sietsma, J.; Gonzalez-Garcia, Y. Passive Film Properties of Martensitic Steels in Alkaline Environment: Influence of the Prior Austenite Grain Size. Metals 2022, 12, 292. https://doi.org/10.3390/met12020292
Yilmaz A, Li X, Pletincx S, Hauffman T, Sietsma J, Gonzalez-Garcia Y. Passive Film Properties of Martensitic Steels in Alkaline Environment: Influence of the Prior Austenite Grain Size. Metals. 2022; 12(2):292. https://doi.org/10.3390/met12020292
Chicago/Turabian StyleYilmaz, Aytac, Xiaolin Li, Sven Pletincx, Tom Hauffman, Jilt Sietsma, and Yaiza Gonzalez-Garcia. 2022. "Passive Film Properties of Martensitic Steels in Alkaline Environment: Influence of the Prior Austenite Grain Size" Metals 12, no. 2: 292. https://doi.org/10.3390/met12020292
APA StyleYilmaz, A., Li, X., Pletincx, S., Hauffman, T., Sietsma, J., & Gonzalez-Garcia, Y. (2022). Passive Film Properties of Martensitic Steels in Alkaline Environment: Influence of the Prior Austenite Grain Size. Metals, 12(2), 292. https://doi.org/10.3390/met12020292







