Progress and Perspective of Ultra-High-Strength Martensitic Steels for Automobile
Abstract
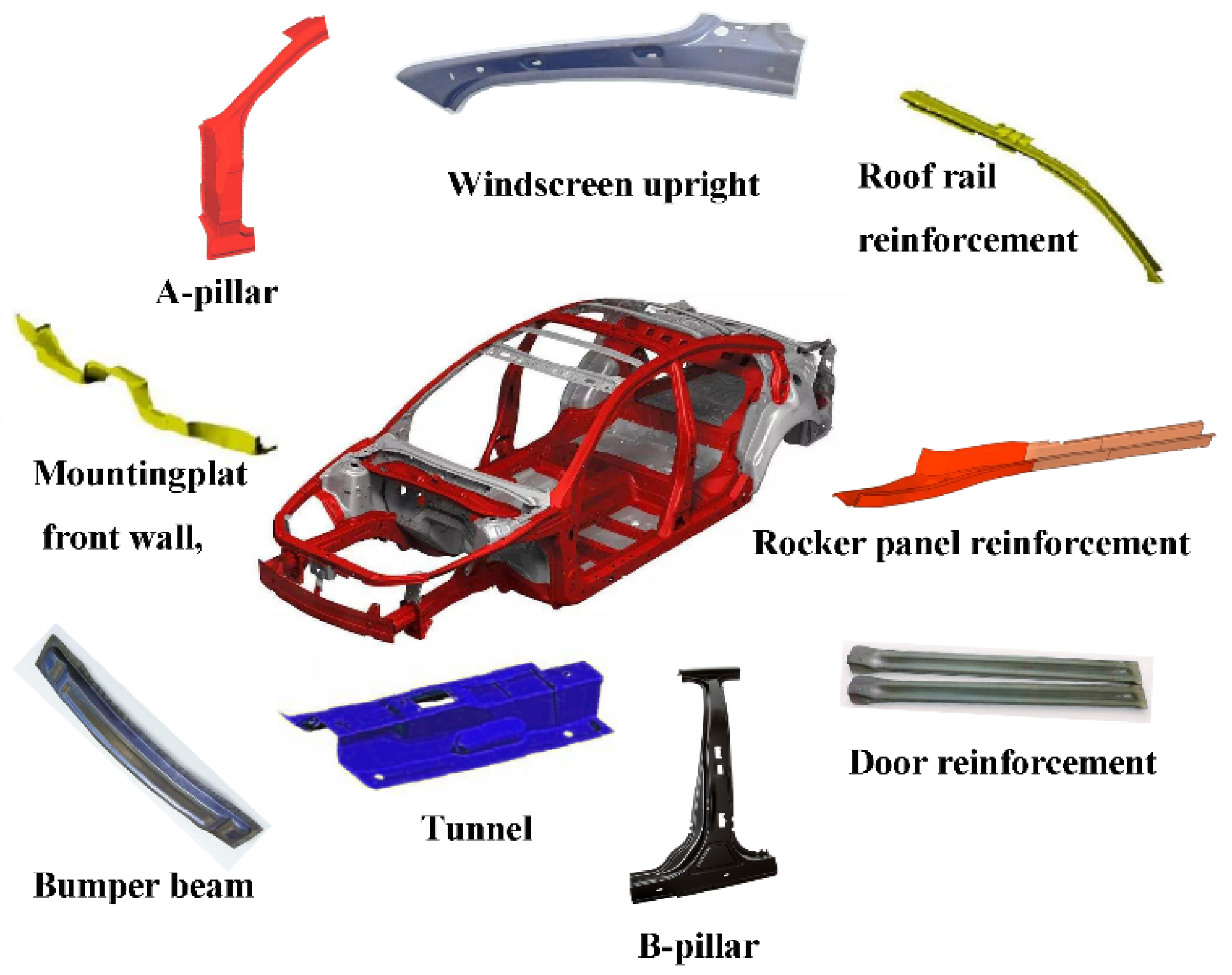
1. Introduction
2. Progress of Ultra-High-Strength Martensitic Steels
2.1. Ultra-High-Strength Cold-Formed Martensitic Steels
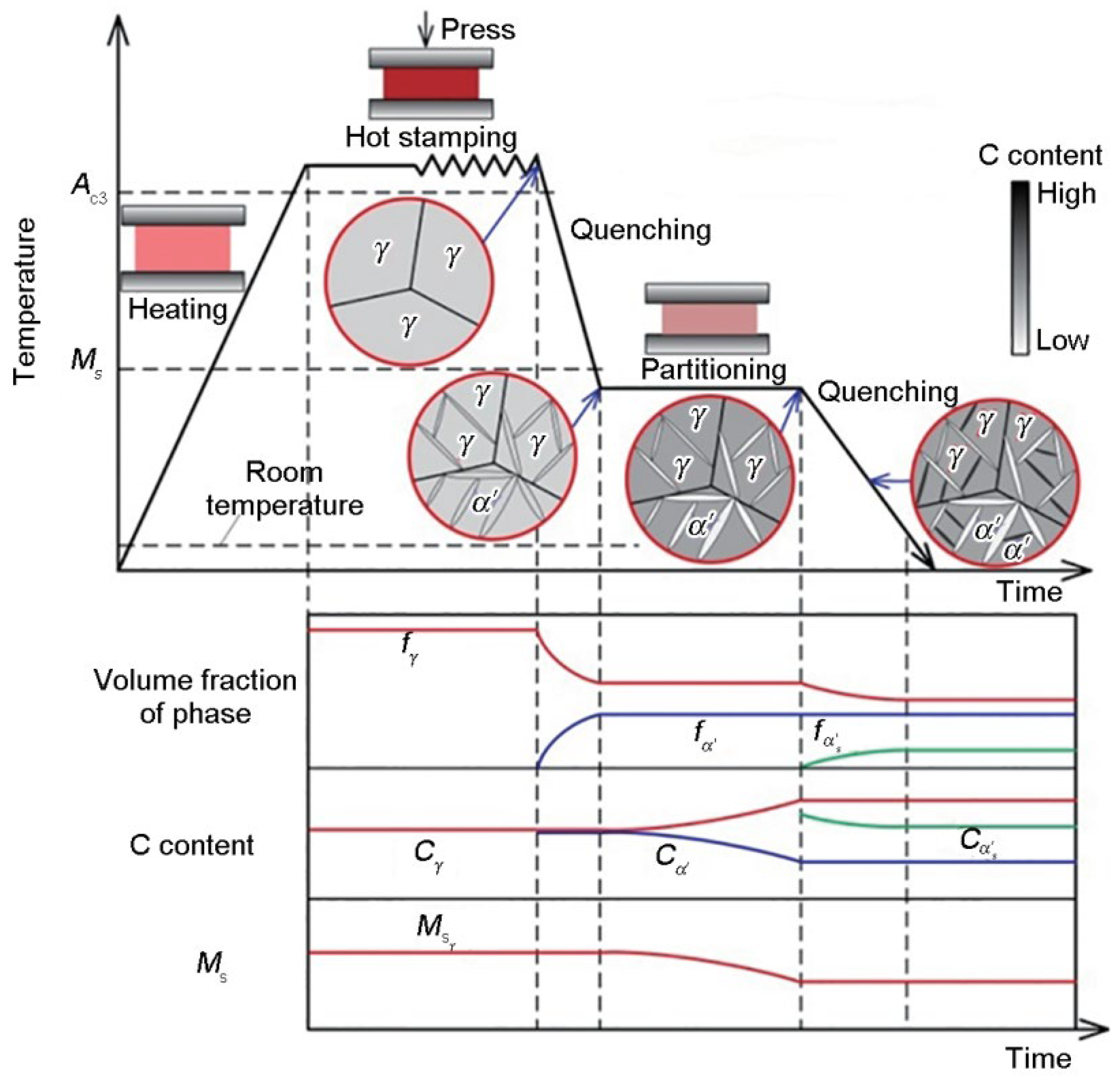
2.2. Ultra-High-Strength Press-Hardening Steels
3. Alloy Design and Strengthening
3.1. C Mn Si Alloying
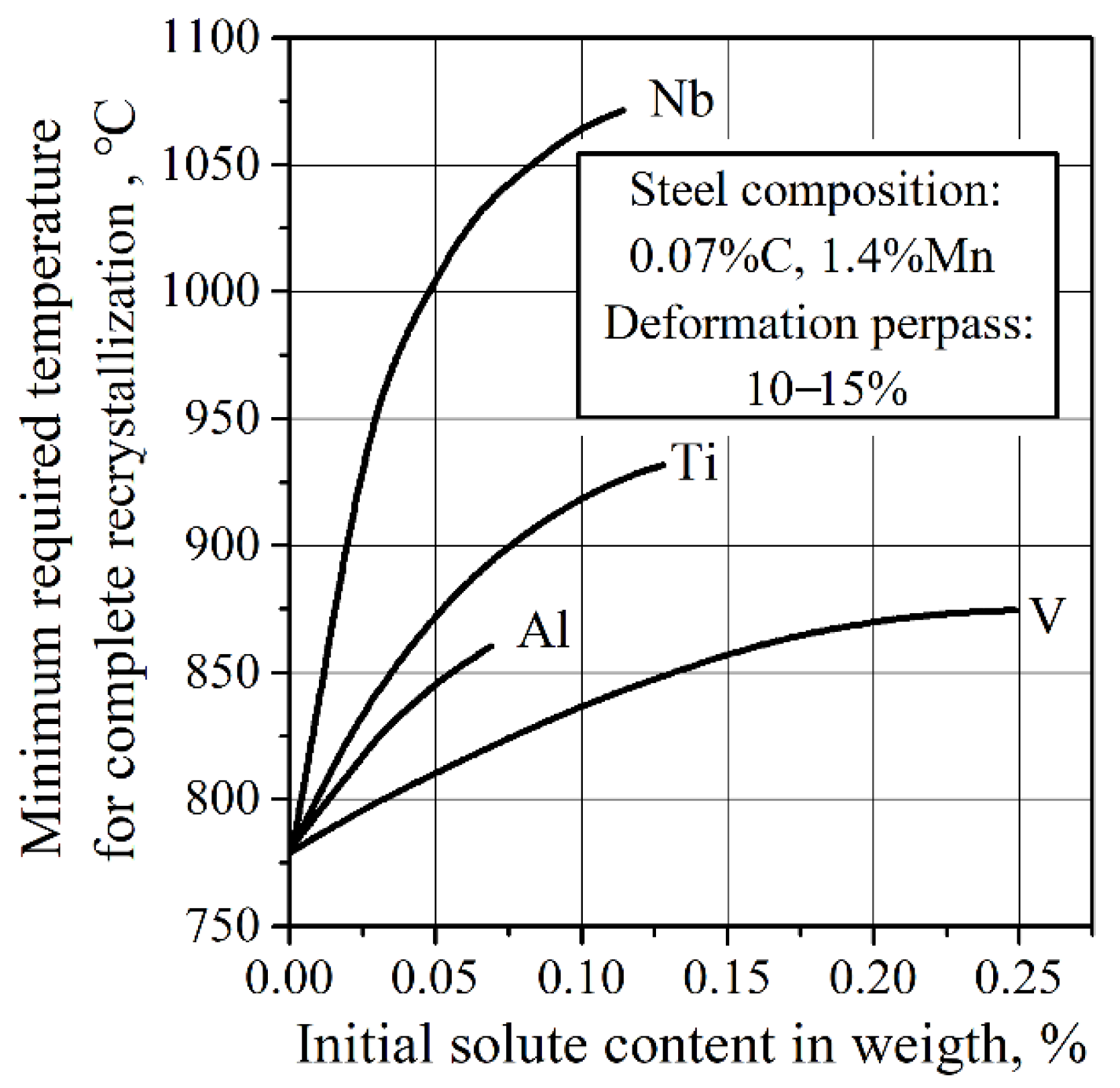
3.2. Nb V Ti Microalloying
3.3. B Microalloying
4. Hydrogen Embrittlement
4.1. Hydrogen Embrittlement Mechanism
4.2. Cold-Formed MS Hydrogen Embrittlement Study
4.3. PHS Hydrogen Embrittlement Study
4.4. Hydrogen Trap and Hydrogen Embrittlement Improvement
- (1)
- Grain refining: the crack source of UHSS hydrogen embrittlement fracture often appears at the original austenite grain boundary, and the crack extends along the grain boundary at the initial stage. With a decrease in intergranular fracture tendency, the delayed fracture resistance increases significantly [94]. Therefore, the addition of Al, Ti, Nb, V, and other elements to form dispersed carbonitrides to refine austenite grains can improve the strength and toughness as well as the delayed fracture property. However, some studies have shown that, when the grain size is less than 2 μ, refining the grain will increase the hydrogen embrittlement sensitivity [105,106].
- (2)
- Improve grain boundary strength: by reducing the segregation of P, S, and other impurities at the original austenite grain boundary, improving the grain boundary strength, delaying the initiation of delayed fracture cracks at the grain boundary, and thus improving the delayed fracture resistance of steel [14]. To this end, Mo and Ti can be added to form compounds with P, and Al and Ti can be added to form nitrides. Impurities can be trapped in the crystal to inhibit its grain boundary segregation [107].
- (3)
- Tempering precipitation: add elements with strong tempering softening resistance, such as Cr, Mo, V, etc., and precipitate carbides on the grain boundary during tempering, making the strength inside the grain closer to the grain boundary [108]. At the same time, precipitation of a large number of carbides in the grains reduces the solid solution strengthening of the matrix, and more three-dimensional reversible hydrogen traps are added to capture hydrogen [56,109].
- (4)
- Improve surface corrosion resistance: the corrosion pit on the metal surface will increase the contact area between hydrogen in the air and the matrix, thus increasing its delayed fracture sensitivity. The amount of hydrogen intrusion on the steel surface can be reduced by adding alloying elements, such as Cr and Mo, that inhibit formation of corrosion pits [110].
- (5)
- Harmless invading hydrogen: add an appropriate amount of microalloy elements V, Ti, Nb, etc., to form fine carbonitrides that can be used as hydrogen traps, inhibit the diffusion of hydrogen, and uniformly distribute hydrogen in steel [59].
5. Summary and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Zhang, J.; Huang, H.; Zhang, S. Research Status and Development Trend of High Strength Steel for Automotive Use. Mater. Rev. 2012, 26, 397–401. [Google Scholar]
- Kang, Y.; Zhu, G. Development trend of China’s automobile industry and the opportunities and challenges of steels for automobiles. Iron Steel 2014, 49, 1–7. [Google Scholar]
- Gupta, M.K.; Singhal, V. Review on materials for making lightweight vehicles. Mater. Today Proc. 2022, 56, 868–872. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Chang, Y.; Cao, W.; Dong, H. Development trend and challenge of advanced high strength automobile steels. Iron Steel 2019, 54, 1–6. [Google Scholar]
- Gong, Y.; Zhao, W.; Zhang, Z. New energy vehicles lightweight approach and its evaluation. Auto. App. Technol. 2017, 47, 5–6. [Google Scholar]
- Li, L. Development and Prospect of High Strength Steel for Automobile. Shanghai Met. 2022, 44, 1–8. [Google Scholar]
- Zhang, W.; Xu, J. Advanced lightweight materials for automobiles: A review. Mater. Des. 2022, 221, 110994. [Google Scholar] [CrossRef]
- Hilditch, T.B.; de Souza, T.; Hodgson, P.D. Properties and Automotive Applications of Advanced High-Strength Steels (AHSS). In Welding and Joining of Advanced High Strength Steels (AHSS); Woodhead Publishing: Sawston, UK, 2015; pp. 9–28. [Google Scholar]
- Keeler, S.; Kimchi, M. Advanced High-Strength Steels Application Guidelines V6; World Auto Steel: Brussels, Belgium, 2017. [Google Scholar]
- Gonçalves, M.; Monteiro, H.; Iten, M. Life cycle assessment studies on lightweight materials for automotive applications—An overview. Energy Rep. 2022, 8, 338–345. [Google Scholar] [CrossRef]
- Wang, S. Analysis workflows and methods of automobile competition. Auto. App. Technol. 2019, 44, 242–245. [Google Scholar]
- Wu, H.; Ju, B.; Tang, D.; Hu, R.; Guo, A.; Kang, Q.; Wang, D. Effect of Nb addition on the microstructure and mechanical properties of an 1800 MPa ultrahigh strength steel. Mater. Sci. Eng. A 2015, 622, 61–66. [Google Scholar] [CrossRef]
- Sato, M.; Utsumi, Y.; Watanabe, K. Hot rolled steel sheets for 1620MPa grade ultra high strength quench-type automobile door impact beams. Kobe Steel Technol. 2007, 57, 23–26. [Google Scholar]
- Machmeier, P.M.; Little, C.D.; Horowitz, M.H.; Oates, R.P. Development of a strong (1650MPa strength) martensitic steel having good fracture toughness. Met. Technol. 2013, 6, 291–296. [Google Scholar] [CrossRef]
- Jin, X.; Gong, Y.; Han, X.; Du, H.; Ding, W.; Zhu, B.; Zhang, Y.; Feng, Y.; Ma, M.; Liang, B. A review of current state and prospect of the manufacturing and application of advanced hot stamping automobile steels. Acta Metall. Sin. 2020, 56, 411–428. [Google Scholar]
- Qayyum, F.; Guk, S.; Kawalla, R.; Prahl, U. On Attempting to Create a Virtual Laboratory for Application-Oriented Microstructural Optimization of Multi-Phase Materials. Appl. Sci. 2021, 11, 1506. [Google Scholar] [CrossRef]
- Umar, M.; Qayyum, F.; Farooq, M.U.; Khan, L.A.; Guk, S.; Prahl, U. Investigating the Effect of Cementite Particle Size and Distribution on Local Stress and Strain Evolution in Spheroidized Medium Carbon Steels using Crystal Plasticity-Based Numerical Simulations. Steel Res. Int. 2021, 92, 2000407. [Google Scholar] [CrossRef]
- Han, F.; Shi, L.; Xiao, H.; Jiang, H. Development and key technologies on roll-formed automobile profiles with AHSS. J. Plast. Eng. 2013, 20, 65–69. [Google Scholar]
- Merklein, M.; Wieland, M.; Lechner, M.; Bruschi, S.; Ghiotti, A. Hot stamping of boron steel sheets with tailored properties: A review. J. Mater. Process. Tech. 2016, 228, 11–24. [Google Scholar] [CrossRef]
- Senuma, T. Hot Stamping Steel. In Encyclopedia of Materials: Metals and Alloys; Elsevier: Oxford, UK, 2022; pp. 26–36. [Google Scholar]
- Zuo, J.; Liu, Y.; Zhang, K.H.; Xiao-Yu, Y.E.; Wei-Ping, L.I.; Huang, X.J. The applications and practice of front ultra fast cooling technology in C-Mn steel. J. Iron Steel Res. Int. 2011, 18, 576–580. [Google Scholar]
- Wang, G.D.; Wang, Z.D.; Liu, Z.Y.; Wang, B.X.; Guo, Y.; Tian, Y. Development of TMCP technology based on ultra-fast cooling. Chin. Metall. 2016, 26, 9–17. [Google Scholar]
- Yuan, G.; Chen, D.; Kang, J.; Zhen-Lei, L.I.; Wang, G.D. Development and application of NG-TMCP technology based on ultra-fast cooling for large scale hot rolled strip lines. J. Iron Steel Res. 2019, 31, 150–158. [Google Scholar]
- Manoharan, K.A.; Quazi, M.M.; Bashir, M.N.; Salleh, M.; Zaifuddin, A.Q.; Linggamm, R. An overview of laser welding of high strength steels for automotive application. Int. J. Technol. Eng. Stud. 2020, 6, 23–40. [Google Scholar]
- Sun, B.; Wang, D.; Lu, X.; Wan, D.; Ponge, D.; Zhang, X. Current challenges and opportunities toward understanding hydrogen embrittlement mechanisms in advanced high-strength steels: A review. Acta Metall. Sin. Engl. 2021, 34, 741–754. [Google Scholar] [CrossRef]
- Dhara, S.; van Bohemen, S.M.C.; Santofimia, M.J. Isothermal decomposition of austenite in presence of martensite in advanced high strength steels: A review. Mater. Today Commun. 2022, 33, 104567. [Google Scholar] [CrossRef]
- Li, Y.; Martín, D.S.; Wang, J.; Wang, C.; Xu, W. A review of the thermal stability of metastable austenite in steels: Martensite formation. J. Mater. Sci. Technol. 2021, 91, 200–214. [Google Scholar] [CrossRef]
- Song, R.; Pottore, N.S. Martensitic Steels with 1700–2200 MPa Tensile Strength. EP2785888B1, 8 October 2014. [Google Scholar]
- ArcelorMittal Homepage. Available online: https://corporate.arcelormittal.com/ (accessed on 10 November 2022).
- SSAB Homepage. Available online: https://www.ssab.com/en (accessed on 10 November 2022).
- Shape Homepage. Available online: https://www.shapecorp.com/ (accessed on 10 November 2022).
- Vartanov, G. Lightweight steel on a (cold) roll. Auto. Eng. 2021, 8, 20–23. [Google Scholar]
- Hwang, E.H.; Jin, S.P.; Si, O.K.; Seong, H.G.; Kim, S.J. Study on the controlling factors for the quenching crack sensitivity of ultra-strong automotive steel. J. Mater. Sci. 2020, 55, 3605–3617. [Google Scholar] [CrossRef]
- Zhu, X.; Xue, P.; Li, W. Status of the development and application of Baosteel’s cold rolled martensitic steel sheets. Baosteel Technol. 2017, 35, 1–8. [Google Scholar]
- Zhu, X.; Xue, P.; Li, W. Effect of tempering on the mechanical properties of an ultra high strength martensitic steel. Baosteel Technol. 2019, 37, 1–4. [Google Scholar]
- Jiang, H.; He, Y.; Lin, L.; Liu, R.; Zhang, Y.; Zheng, W.; Li, L. Microstructures and properties of auto-tempering ultra-high strength automotive steel under different thermal-processing conditions. Metals 2021, 11, 1121. [Google Scholar] [CrossRef]
- Sugimoto, K.I.; Kobayashi, J.; Hojo, T. Microstructure and mechanical properties of ultrahigh-strength TRIP-aided steels. Tetsu Hagane 2017, 103, 1–11. [Google Scholar] [CrossRef]
- Junya, K.; Yuji, N.; Koh-ichi, S. Effects of cooling rate on impact toughness of an ultrahigh-strength TRIP-aided martensitic steel. Adv. Mater. Res. 2014, 922, 366–371. [Google Scholar]
- Koh-ichi, S.; Tomohiko, H.; Ashok, K.S. An Overview of Fatigue Strength of Case-Hardening TRIP-Aided Martensitic Steels. Metals 2018, 355, 355. [Google Scholar]
- Kobayashi, J.; Yoshikawa, N.; Sugimoto, K.I. Notch-fatigue strength of advanced TRIP-alded martensitic steels. ISIJ Int. 2013, 53, 1479–1486. [Google Scholar] [CrossRef]
- Speer, J.G.; Edmonds, D.V.; Rizzo, F.C.; Matlock, D.K. Partitioning of carbon from supersaturated plates of ferrite, with application to steel processing and fundamentals of the bainite transformation. Curr. Opin. Solid State Mater. 2004, 8, 219–237. [Google Scholar] [CrossRef]
- Hsu, T.Y.; Jin, X.J.; Rong, Y.H. Strengthening and toughening mechanisms of quenching-partitioning-tempering (Q-P-T) steels. J. Alloys Compd. 2013, 577, 568–571. [Google Scholar]
- Board, E. The State-of-the-Art Study in Global Car Body and Automotive Lightweight Technology; Beijing University of Technology Press: Beijing, China, 2019. [Google Scholar]
- Lu, H.; Zhao, Y.; Feng, Y.; Ma, M.; Side, A.; Liu, Y.; Guo, A. Progress and prospect for development and application of microalloying press-hardening steel. Mater. Mech. Eng. 2020, 44, 1–10. [Google Scholar]
- Karbasian, H.; Tekkaya, A.E. A review on hot stamping. J. Mater. Process. Tech. 2010, 210, 2103–2118. [Google Scholar] [CrossRef]
- Rana, R.; Singh, S.B. Automotive Steels: Design, Metallurgy, Processing and Applications; Woodhead Publishing: Cambridge, UK, 2017. [Google Scholar]
- Yi, H.; Chang, Z.; Cai, H.; Du, P.; Yang, D. Strength, Ductility and Fracture Strain of Press-Hardening Steels. Acta Metall. Sin. 2020, 56, 429–443. [Google Scholar]
- Speer, J.; Matlock, D.K.; De Cooman, B.C.; Schroth, J.G. Carbon partitioning into austenite after martensite transformation. Acta Mater. 2003, 51, 2611–2622. [Google Scholar] [CrossRef]
- Liu, H.; Jin, X.; Dong, H.; Shi, J. Martensitic microstructural transformations from the hot stamping, quenching and partitioning process. Mater. Charact. 2011, 62, 223–227. [Google Scholar] [CrossRef]
- Liu, H.; Lu, X.; Jin, X.; Dong, H.; Shi, J. Enhanced mechanical properties of a hot stamped advanced high-strength steel treated by quenching and partitioning process. Scr. Mater. 2011, 64, 749–752. [Google Scholar] [CrossRef]
- Cai, H.L.; Chen, P.; Oh, J.K.; Cho, Y.R.; Wu, D.; Yi, H.L. Quenching and flash-partitioning enables austenite stabilization during press-hardening processing. Scr. Mater. 2020, 178, 77–81. [Google Scholar] [CrossRef]
- Bernd, M.L.; Thomas, G.; Ansgar, H.; Ilaria, S.; Maribel, A. Impact of Si on microstructure and mechanical properties of 22MnB5 hot stamping steel treated by quenching & partitioning (Q&P). Metall. Mater. Trans. A 2018, 49, 54–65. [Google Scholar]
- Seo, E.J.; Cho, L.; De Cooman, B.C. Application of quenching and partitioning (Q&P) processing to press hardening steel. Metall. Mater. Trans. A 2014, 45, 4022–4037. [Google Scholar]
- Ariza, E.A.; Poplawsky, J.; Guo, W.; Tschiptschin, A.P. Hot straining and quenching and partitioning of a TRIP-assisted steel: Microstructural characterization and mechanical properties. Mater. Sci. Forum 2018, 941, 704–710. [Google Scholar] [CrossRef]
- Krauss, G. Deformation and fracture in martensitic carbon steels tempered at low temperatures. Metall. Mater. Trans. A 2011, 32, 861–877. [Google Scholar] [CrossRef]
- Jo, M.C.; Yoo, J.; Kim, S.; Kim, S.; Oh, J.; Bian, J.; Sohn, S.S.; Lee, S. Effects of Nb and Mo alloying on resistance to hydrogen embrittlement in 1.9 GPa-grade hot-stamping steels. Mater. Sci. Eng. A 2020, 789, 139656. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, W.; Gao, P.; Li, F.; Kuang, S.; Zou, Y.; Zhao, Z. Interaction between dislocations, precipitates and hydrogen atoms in a 2000 MPa grade hot-stamped steel. J. Mater. Res. Technol. 2022, 18, 4353–4366. [Google Scholar] [CrossRef]
- Bansal, G.K.; Rajinikanth, V.; Ghosh, C.; Srivastava, V.C.; Kundu, S.; Ghosh Chowdhury, S. Microstructure property correlation in low-Si steel processed through quenching and nonisothermal partitioning. Metall. Mater. Trans. A 2018, 49, 3501–3514. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, Q.; Qiao, W.; Chen, G.; Xu, G. Comparison of the strengthening effects of Nb, V, and Ti on the mechanical properties of 20MnSi low-alloy steel. Int. J. Mater. Res. 2020, 111, 504–510. [Google Scholar] [CrossRef]
- Najafi, H.; Rassizadehghani, J.; Halvaaee, A. Mechanical properties of as cast microalloyed steels containing V, Nb and Ti. Met. Sci. J. 2007, 23, 699–705. [Google Scholar] [CrossRef]
- Bellavoine, M.; Dumont, M.; Drillet, J.; Maugis, P.; Hebert, V. Influence of microalloying elements Ti and Nb on recrystallization during annealing of advanced high-strength steels. Mater. Sci. Forum 2017, 879, 217–223. [Google Scholar] [CrossRef]
- Xiao, F.R.; Cao, Y.B.; Qiao, G.Y.; Zhang, X.B.; Liao, B. Effect of Nb solute and NbC precipitates on dynamic or static recrystallization in Nb steels. J. Iron Steel Res. Int. 2012, 19, 52–56. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, D.; Liu, Y.; Li, H.; Xu, D. Effect of dissolution and precipitation of Nb on the formation of acicular ferrite/bainite ferrite in low-carbon HSLA steels. Mater. Charact. 2013, 84, 232–239. [Google Scholar] [CrossRef]
- Han, Y. Investigation on (Ti, Mo)C Precipitation Behavior and Mechanical Property in Low Carbon Martensitic Steels. Ph.D. Thesis, Central Iron and Steel Research Institute, Beijing, China, 2013. [Google Scholar]
- Roy, S.; Karmakar, A.; Kundu, S. Effect of microalloying elements on austenite grain growth in Nb-Ti and Nb-V steels. Mater. Sci. Technol. 2014, 30, 653–664. [Google Scholar]
- Yuan, S.; Xing, B.; Wu, X.; Yang, S.; He, X. Present study on strain-induced precipitation in Nb-bearing microalloyed steel. Mater. Rep. 2005, 19, 46–49. [Google Scholar]
- Yue, S. Research on Microstructure and Properties of Nb V Micro-Alloyed the Coiled Tubing. Ph.D. Thesis, Northeast University, Shengyang, China, 2013. [Google Scholar]
- Li, X.; Wang, Z.; Deng, X.; Zhang, Y.; Lei, C.; Wang, G. Effect of final temperature after ultrafast cooling on microstructural evolution and precipitation behavior of Nb-V-Ti bearing low alloy steel. Acta Metall. Sin. 2015, 51, 784–790. [Google Scholar]
- Ma, Y.; Liu, Y.; Zhang, L.; Zhou, L.; Liu, C. Effect of B content on morphology and properties of BN phase in martensite heat resistant steel. Chin. J. Mater. Res. 2017, 31, 345–350. [Google Scholar]
- Abe, F.; Tabuchi, M.; Tsukamoto, S. Mechanisms for boron effect on microstructure and creep strength of ferritic power plant steels. Energy Mater. 2012, 4, 166–174. [Google Scholar] [CrossRef]
- Abe, F. Effect of boron on microstructure and creep strength of advanced ferritic power plant steels. Procedia Eng. 2011, 10, 94–99. [Google Scholar] [CrossRef]
- Kapadia, B.M. Hardenability Concepts with Application to Steel; A.I.M.E.: Warrendale, PA, USA, 1978. [Google Scholar]
- Shi, C. Hardenability of Boron Steel, Boron Hardenability Factor and Boron Equilibrium Segregation. J. Iron Steel Res. 1998, 10, 43–47. [Google Scholar]
- Pradhan, A.; Vishwakarma, M.; Dwivedi, S.K. A review: The impact of hydrogen embrittlement on the fatigue strength of high strength steel. Mater. Today Proc. 2020, 26, 3015–3019. [Google Scholar] [CrossRef]
- Loidl, M.; Kolk, O.; Veith, S.; Gbel, T. Characterization of hydrogen embrittlement in automotive advanced high strength steels. Materialwiss. Werkst. 2011, 42, 1105–1110. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Q.; Jeffrey, V.; Zhang, M.; Andrej, A. Evaluation of the influence of hydrogen on some commercial DP, Q&P and TWIP advanced high-strength steels during automobile service. Eng. Fail. Anal. 2018, 94, 249–273. [Google Scholar]
- Sezgin, J.G.; Bosch, C.; Montouchet, A.; Perrin, G.; Wolski, K. Modelling of hydrogen induced pressurization of internal cavities. Int. J. Hydrog. Energy 2017, 42, 15403–15414. [Google Scholar] [CrossRef]
- Chen, Y.; Kuang, S.; Zhao, Z. Study status of hydrogen induced delayed fracture of advanced high strength automotive steel. J. Iron Steel Res. 2020, 32, 265–272. [Google Scholar]
- Huang, H.; Zhou, Q. Progress and perspectives of hydrogen induced delayed fracture of high strength steels. Baosteel Technol. 2015, 33, 11–16. [Google Scholar]
- Song, R.; Fonstein, N.; Pottore, N.; Jun, H.J.; Jansto, S. Effect of Nb on delayed fracture resistance of ultrahigh strength martensitic steels. In Proceedings of the HSLA Steels 2015, Microalloying 2015 & Offshore Engineering Steels 2015, Hangzhou, China, 11–13 November 2015; pp. 541–547. [Google Scholar]
- Martinez-Madrid, M.; Chan, S.L.I.; Charles, J.A. Hydrogen occlusivity and embrittlement in iron—Effect of grain structure and cold work. Met. Sci. J. 2013, 1, 454–460. [Google Scholar] [CrossRef]
- Fuchigami, H.; Minami§, H.; Nagumo, M. Effect of grain size on the susceptibility of martensitic steel to hydrogen-related failure. Phil. Mag. Lett. 2006, 86, 21–29. [Google Scholar] [CrossRef]
- Kimura, Y.; Takagi, S.; Hara, T.; Terasaki, S.; Tsuzaki, K. Hydrogen-induced delayed fracture of a martensitic steel with fine prior-austenite grain size. J. Phys. IV 2003, 112, 403–406. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, M.; Rong, L. Effect of grain size on the hydrogen embrittlement sensitivity of a precipitation strengthened Fe-Ni based alloy. Mater. Sci. Eng. A 2014, 594, 98–102. [Google Scholar] [CrossRef]
- Tsay, L.W.; Lu, H.L.; Chen, C. The effect of grain size and aging on hydrogen embrittlement of a maraging steel. Corros. Sci. 2008, 50, 2506–2511. [Google Scholar] [CrossRef]
- Takasawa, K.; Ikeda, R.; Ishikawa, N.; Ishigdki, R. Effects of grain size and dislocation density on the susceptibility to high-pressure hydrogen environment embrittlement of high-strength low-alloy steels. Int. J. Hydrog. Energy 2012, 37, 2669–2675. [Google Scholar] [CrossRef]
- Wei, F.-G.; Hara, T.; Tsuzaki, K. Nano-preciptates design with hydrogen trapping character in high strength steel. In Advanced Steels; Weng, Y., Dong, H., Gan, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 87–92. [Google Scholar]
- Venezuela, J.; Lim, F.Y.; Liu, L.; James, S.; Zhou, Q.; Knibbe, R.; Zhang, M.; Li, H.; Dong, F.; Dargusch, M.S.; et al. Hydrogen embrittlement of an automotive 1700 MPa martensitic advanced high-strength steel. Corros. Sci. 2020, 171, 108726. [Google Scholar] [CrossRef]
- Bian, J.; Mohrbacher, H.; Lu, H.; Wang, W. Development of Press Hardening Steel with High Resistance to Hydrogen Embrittlement. In Proceedings of the HSLA Steels 2015, Microalloying 2015 & Offshore Engineering Steels 2015, Hangzhou, China, 11–13 November 2015; pp. 571–576. [Google Scholar]
- Cho, L.; Sulistiyo, D.H.; Seo, E.J.; Jo, K.R.; Kim, S.W.; Oh, J.K.; Cho, Y.R.; Cooman, B.D. Hydrogen absorption and embrittlement of ultra-high strength aluminized press hardening steel. Mater. Sci. Eng. A 2018, 734, 416–426. [Google Scholar] [CrossRef]
- Jo, K.R.; Cho, L.; Sulistiyo, D.H.; Seo, E.J.; Kim, S.W.; De Cooman, B.C. Effects of Al-Si coating and Zn coating on the hydrogen uptake and embrittlement of ultra-high strength press-hardened steel. Surf. Coat. Tech. 2019, 374, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Jo, M.C.; Jo, M.C.; Kim, S.; Kim, S.-H.; Oh, J.; Sohn, S.S.; Lee, S. Effects of solid solution and grain-boundary segregation of Mo on hydrogen embrittlement in 32MnB5 hot-stamping steels. Acta Mater. 2021, 207, 116661. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jeon, S.-H.; Yang, W.-S.; Yoo, B.-G.; Chung, Y.-D.; Ha, H.-Y.; Chung, H.-Y. Effects of titanium content on hydrogen embrittlement susceptibility of hot-stamped boron steels. J. Alloys Compd. 2018, 735, 2067–2080. [Google Scholar] [CrossRef]
- Yoo, J.; Jo, M.C.; Jo, M.C.; Kim, S.; Oh, J.; Bian, J.; Sohn, S.S.; Lee, S. Effects of Ti alloying on resistance to hydrogen embrittlement in (Nb+Mo)-alloyed ultra-high-strength hot-stamping steels. Mater. Sci. Eng. A 2020, 791, 139763. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lu, H.; Liang, J.; Rosenthal, A.; Liu, H.; Sneddon, G.; Mccarroll, I.; Zhao, Z.; Li, W.; Guo, A. Observation of hydrogen trapping at dislocations, grain boundaries, and precipitates. Science 2020, 367, 171–175. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Y.; Sun, B.; Liao, Q.; Lu, H.; Jian, B.; Mohrbacher, H.; Zhang, W.; Guo, A.; Zhang, Y. Effect of Nb on hydrogen-induced delayed fracture in high strength hot stamping steels. Mater. Sci. Eng. A 2015, 626, 136–143. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, E.; Wan, J.; Liu, J.; Huang, Y.; Li, X. Effect of Nb on the hydrogen-induced cracking of high-strength low-alloy steel. Corros. Sci. 2018, 139, 83–96. [Google Scholar] [CrossRef]
- Darken, L.S.; Smith, R.P. Behavior of hydrogen in steel during and after immersion in acid. Corrosion 1949, 5, 1–16. [Google Scholar] [CrossRef]
- Pressouyre, G.M.; Bernstein, I.M. A quantitative analysis of hydrogen trapping. Metall. Trans. A 1978, 9, 1571–1580. [Google Scholar] [CrossRef]
- Michler, T.; Balogh, M.P. Hydrogen environment embrittlement of an ODS RAF steel-Role of irreversible hydrogen trap sites. Int. J. Hydrogen Energy 2010, 35, 9746–9754. [Google Scholar] [CrossRef]
- Grabke, H.J.; Gehrmann, F.; Riecke, E. Hydrogen in microalloyed steels. Steel Res. Int. 2001, 72, 225–235. [Google Scholar] [CrossRef]
- Thomas, R.; Li, D.; Gangloff, R.P.; Scully, J.R. Trap-governed hydrogen diffusivity and uptake capacity in ultrahigh-strength AERMET 100 steel. Metall. Mater. Trans. A 2002, 33, 1991–2004. [Google Scholar] [CrossRef]
- Venezuela, J.; Liu, Q.; Zhang, M.; Zhou, Q.; Atrens, A. A review of hydrogen embrittlement of martensitic advanced high-strength steels. Corros. Rev. 2016, 34, 153–186. [Google Scholar] [CrossRef]
- Nakatani, M.; Fujihara, H.; Sakihara, M.; Minoshima, K. Fatigue crack growth properties and hydrogen visualization under irreversible hydrogen charged condition in cold drawn high strength steel. T. Jpn. Soc. Mech. Eng. 2010, 76, 1214–1220. [Google Scholar] [CrossRef]
- Bai, Y.; Momotani, Y.; Chen, M.C.; Tsuji, N.; Shibata, A. Effect of grain refinement on hydrogen embrittlement behaviors of high-Mn TWIP steel. Mater. Sci. Eng. A 2016, 651, 935–944. [Google Scholar] [CrossRef]
- Koyama, M.; Ichii, K.; Tsuzaki, K. Grain refinement effect on hydrogen embrittlement resistance of an equiatomic CoCrFeMnNi high-entropy alloy. Int. J. Hydrog. Energy 2019, 44, 17163–17167. [Google Scholar] [CrossRef]
- Banerji, S.K.; Mcmahon, C.J.; Feng, H.C. Intergranular fracture in 4340-type steels: Effects of impurities and hydrogen. Metall. Trans. A 1978, 9, 237–247. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Z.; Liang, J.; Su, J.; Yang, Z.; Zhang, B.; Sheng, G. Correlation between the microstructure and hydrogen embrittlement resistance in a precipitation-hardened martensitic stainless steel. Corros. Sci. 2021, 182, 109260. [Google Scholar] [CrossRef]
- Tan, L.; Li, D.; Yan, L.; Pang, X.; Gao, K. A novel heat treatment for improving the hydrogen embrittlement resistance of a precipitation-hardened martensitic stainless steel. Corros. Sci. 2022, 206, 110530. [Google Scholar] [CrossRef]
- Depover, T.; Monbaliu, O.; Wallaert, E.; Verbeken, K. Effect of Ti, Mo and Cr based precipitates on the hydrogen trapping and embrittlement of Fe–C–X Q&T alloys. Int. J. Hydrogen Energy 2015, 40, 16977–16984. [Google Scholar]
- Sun, B.; Lu, W.; Gault, B.; Ding, R.; Makineni, S.K.; Wan, D.; Wu, C.H.; Chen, H.; Ponge, D.; Raabe, D. Chemical heterogeneity enhances hydrogen resistance in high-strength steels. Nat. Mater. 2021, 20, 1629–1634. [Google Scholar] [CrossRef]
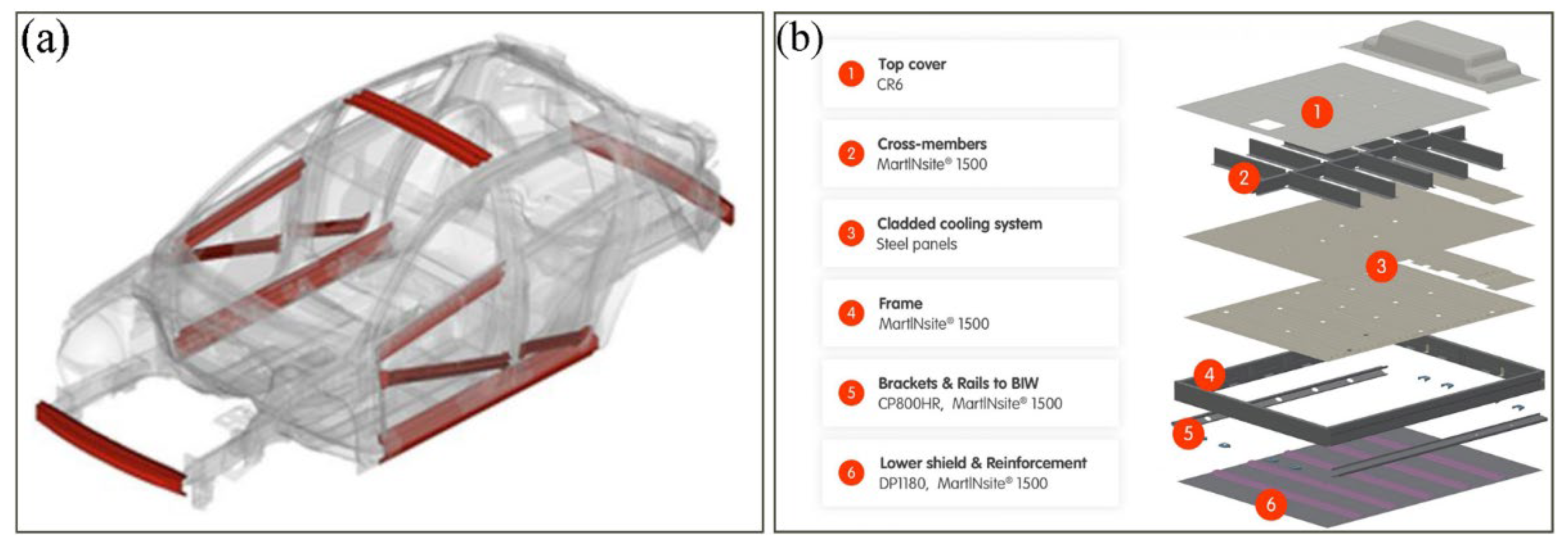
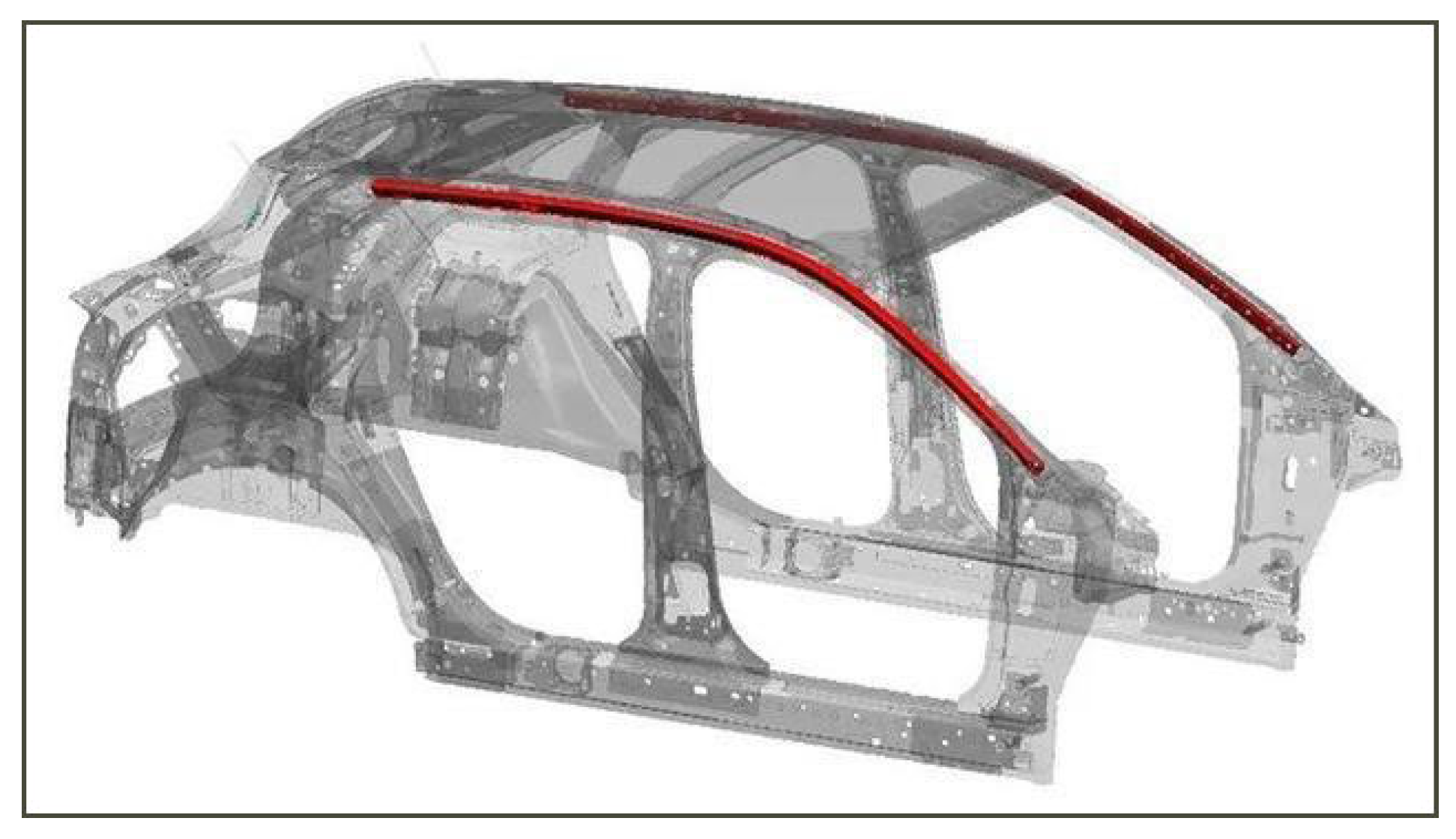
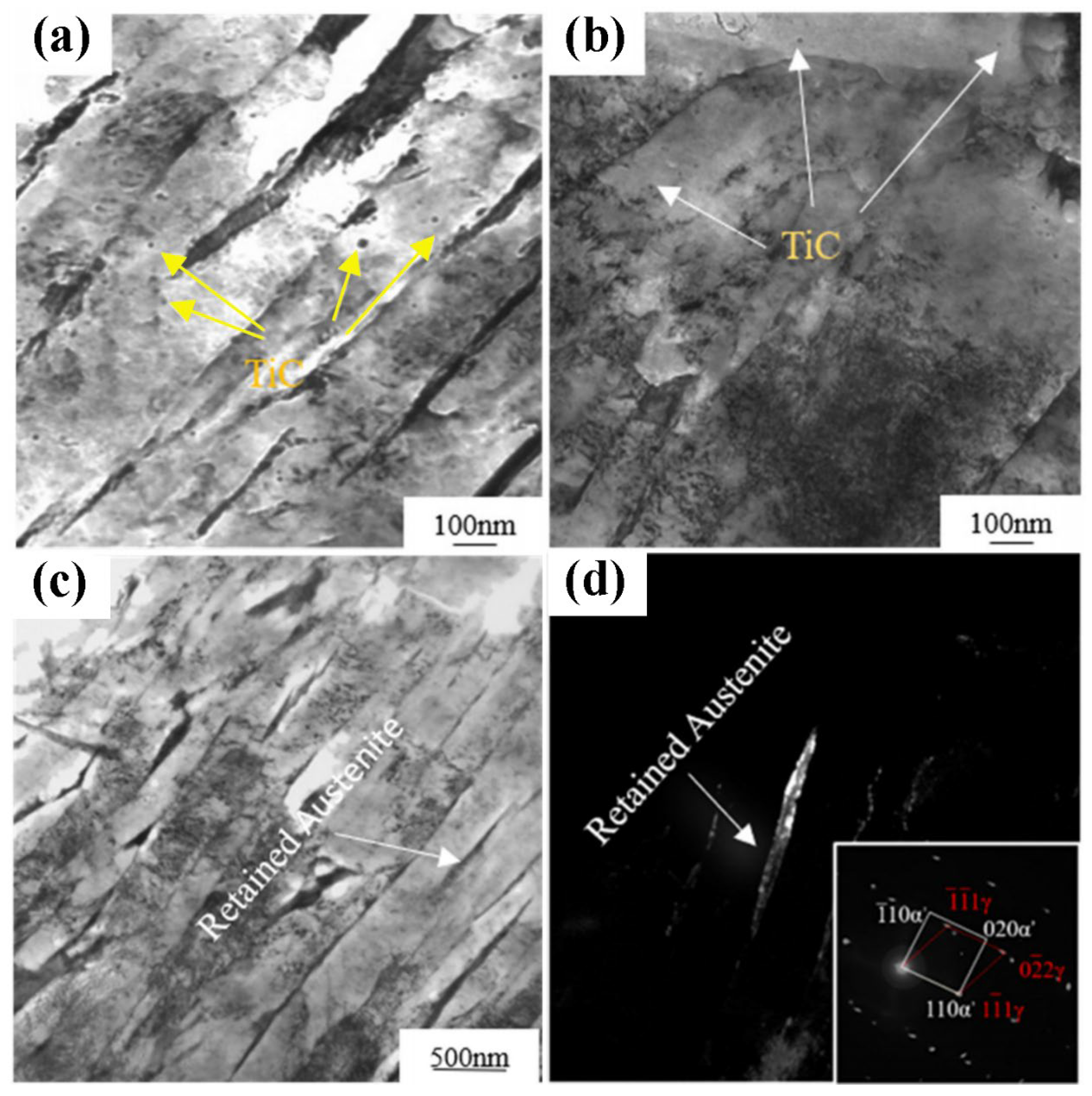
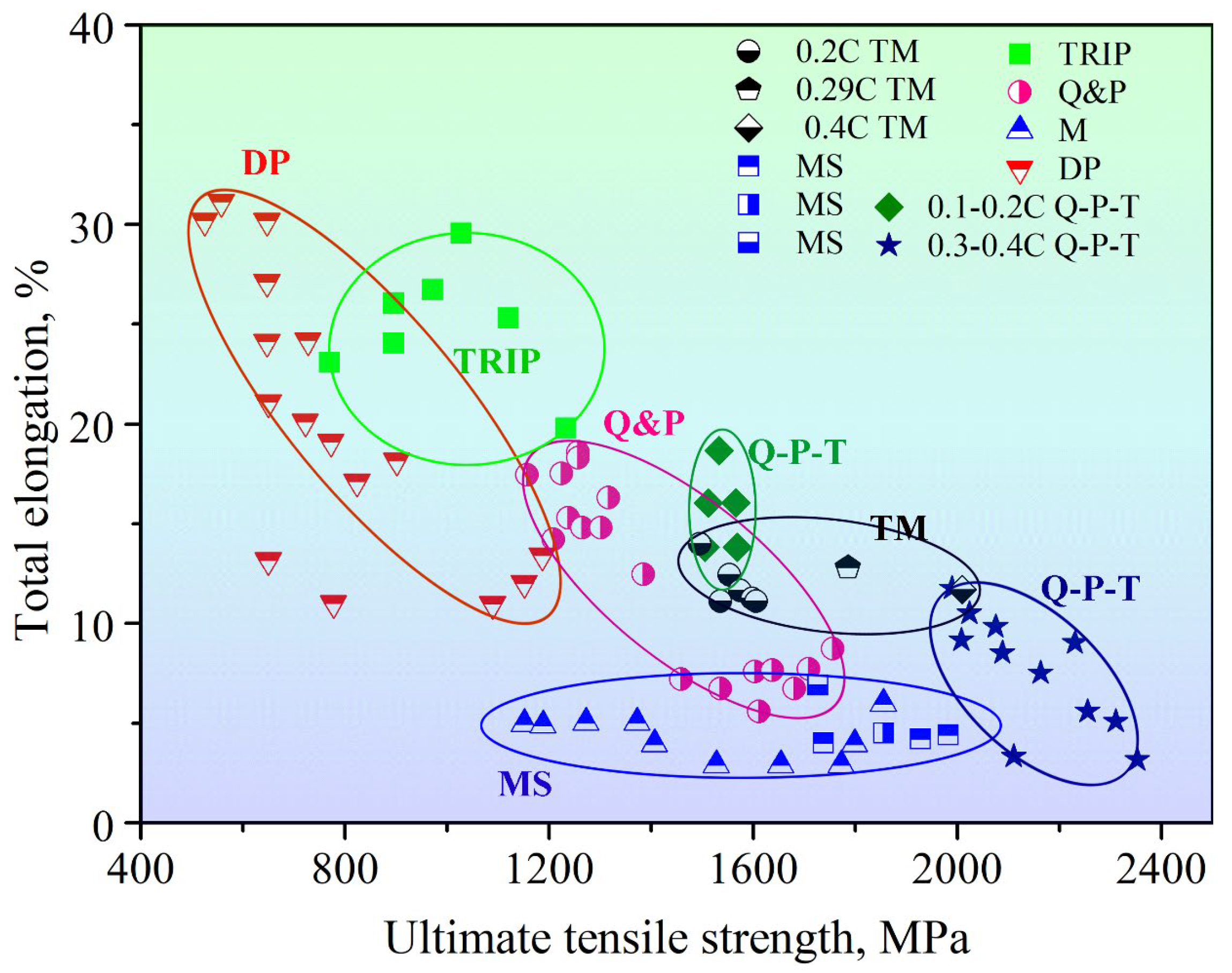


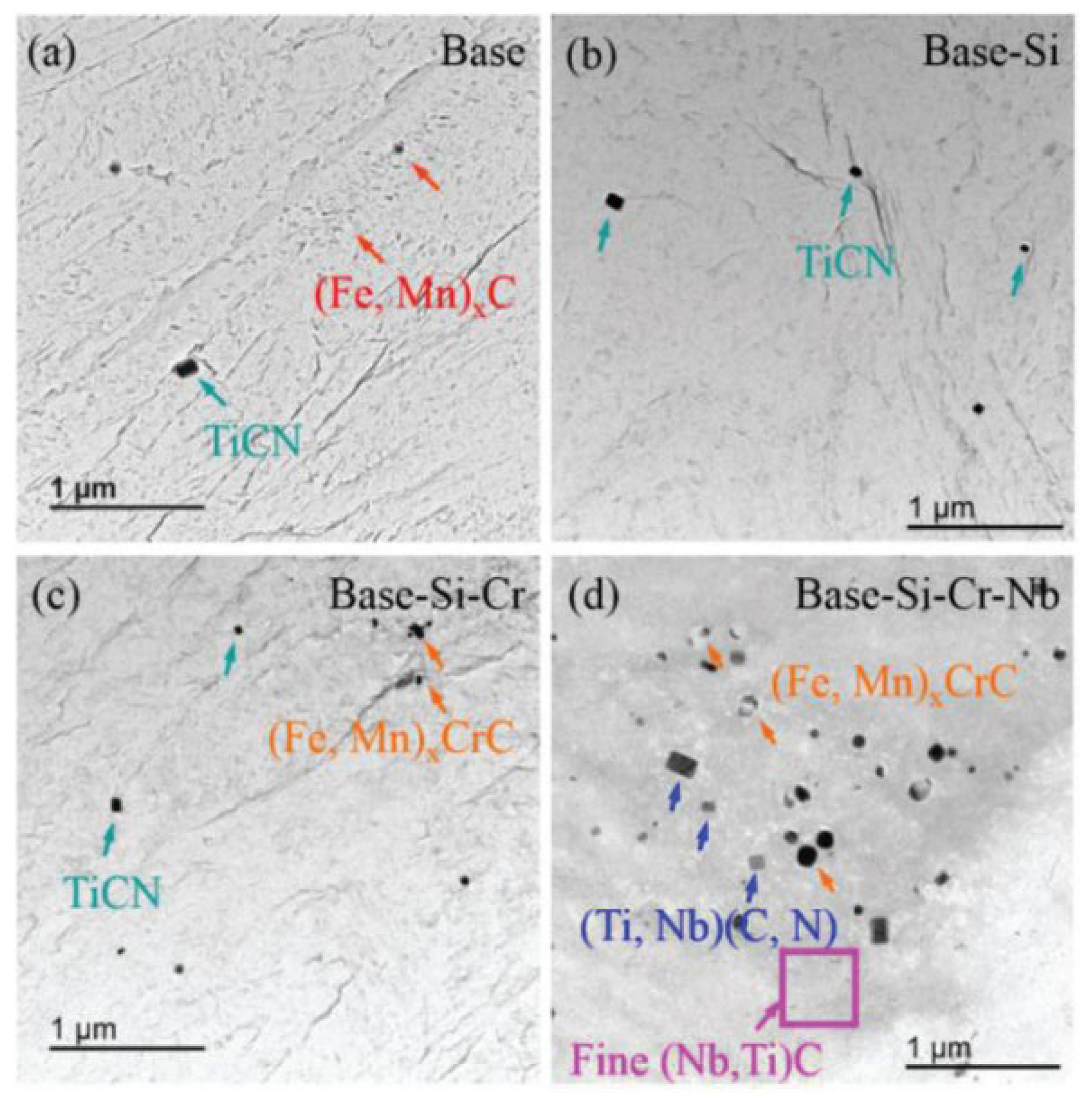



| C | Mn | Si | Other Alloys | Rm/MPa | CE | Ref. | |
|---|---|---|---|---|---|---|---|
| Cold-formed MS | 0.22 | 1.5 | 0.198 | 0.036 Al, 0.019 Nb | 1737 | 0.50 | [28] |
| 0.25 | 1.99 | 0.201 | 0.025 Al | 1858 | 0.62 | [28] | |
| 0.28 | 0.988 | 0.201 | 0.038 Al, 0.024 Ti, 0.001 B, 0.028 Nb | 1981 | 0.48 | [28] | |
| 0.28 | 2.01 | 0.202 | 0.032 Al | 1927 | 0.65 | [28] | |
| ≤0.35 | ≤3.0 | ≤1.00 | Nb + Ti ≤ 0.15, Cr + Mo ≤ 1.00, B ≤ 0.001, Cu ≤ 0.20 | ≥1700 | ≤1 | [29] | |
| 0.30 | 1.0 | 0.28 | 0.50 Cr, 0.012 Ti | 1752 | 0.51 | [12] | |
| 0.30 | 1.0 | 0.30 | 0.49 Cr, 0.015 Ti, 0.021 Nb | 1841 | 0.52 | [12] | |
| 0.275 | 1.52 | 0.40 | 0.22 Ti, 0.025 Nb, 0.0022 B | 1855 | 0.60 | [35] | |
| 0.18 | 2.13 | 1.40 | 1.00 Cr, 0.012 Ti | 1726 | 0.77 | [36] | |
| 0.23~0.25 | <1.0 | <1.0 | Cr, Ti | 1520~1620 | 0.98 | [32] | |
| 0.26~0.3 | <1.0 | <1.0 | Cr, Ti | 1520~1860 | 0.61 | [32] | |
| PHS | 0.23 | 1.18 | 0.22 | 0.16 Cr, 0.040 Ti, 0.002 B | 1478 | 0.46 | [46] |
| 0.25 | 1.24 | 0.21 | 0.34 Cr, 0.042 Ti, 0.002 B | 1611 | 0.49 | [46] | |
| 0.28 | 1.30 | 0.4 | 0.005 B | 1740 | 0.56 | [46] | |
| 0.34 | 1.30 | 0.4 | 0.005 B | 1919 | 0.62 | [46] | |
| 0.37 | 0.81 | 0.31 | 0.19 Cr, 0.046 Ti, 0.001 B | 2040 | 0.56 | [46] | |
| 0.32 | 1.2 | 0.25 | 0.12 Cr, 0.030 Ti, 0.002–0.003 B, 0.05 Nb | 1933 | 0.56 | [56] | |
| 0.32 | 1.2 | 0.25 | 0.12 Cr, 0.030 Ti, 0.002–0.003 B, 0.05 Nb, 0.1 Mo | 1917 | 0.56 | [56] | |
| 0.35 | 1.35 | 0.24 | 0.03–0.05 Ti, 0.0020 B, 0.25 Cr, 0.15–0.20 V | 1977 | 0.62 | [57] |
| YS, MPa | TS, MPa | Delayed Fracture Time, h | Average Martensitic Lath Size, μm | Average Misorientation, ° | |
|---|---|---|---|---|---|
| Base | 1611 | 1991 | <6 | 2.10 | 38.8 |
| Base–Si | 1647 | 2033 | 137 | 1.78 | 32.5 |
| Base–Si–Cr | 1610 | 1990 | 278 | 2.09 | 33.7 |
| Base–Si–Cr–Nb | 1684 | 2039 | >600 | 1.92 | 33.1 |
| Hydrogen Trap Type | Binding Energy (kJ∙mol−1) | Material |
|---|---|---|
| C atom | 3.0 | Pure iron |
| Mn atom | 11.0 | Pure iron |
| grain boundary | 17.2 | Pure iron |
| Ferrite/Fe3C interface | 18.4 | Medium-carbon steel |
| V and Cr atoms | 26.0~27.0 | Pure iron |
| dislocation | 26.8 | Pure iron |
| V4C3 (total grid) | 30.0 | Low-carbon-alloy steel |
| Micropore | 35.2 | Pure iron |
| TiC (total) | 46.0~59.0 | Mild steel |
| Single vacancy | 46.0–79.0 | Pure iron |
| retained austenite | 55.0 | Duplex steel |
| NbC | 63.0~68.0 | Mild steel |
| MnS | 72.3 | Low-carbon-alloy steel |
| Fe3C (incoherent) | 84.0 | Medium-carbon steel |
| TiC (incoherent) | 86.9 | Medium-carbon steel |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Zhao, L.; Lu, S.; Lin, Z.; Wen, T.; Chen, Z. Progress and Perspective of Ultra-High-Strength Martensitic Steels for Automobile. Metals 2022, 12, 2184. https://doi.org/10.3390/met12122184
Chen H, Zhao L, Lu S, Lin Z, Wen T, Chen Z. Progress and Perspective of Ultra-High-Strength Martensitic Steels for Automobile. Metals. 2022; 12(12):2184. https://doi.org/10.3390/met12122184
Chicago/Turabian StyleChen, Hao, Linlin Zhao, Shenghai Lu, Zhangguo Lin, Tong Wen, and Zejun Chen. 2022. "Progress and Perspective of Ultra-High-Strength Martensitic Steels for Automobile" Metals 12, no. 12: 2184. https://doi.org/10.3390/met12122184
APA StyleChen, H., Zhao, L., Lu, S., Lin, Z., Wen, T., & Chen, Z. (2022). Progress and Perspective of Ultra-High-Strength Martensitic Steels for Automobile. Metals, 12(12), 2184. https://doi.org/10.3390/met12122184







