Reduction Behavior and Characteristics of Metal Oxides in the Nanoscale
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Reduction Techniques
3. Results
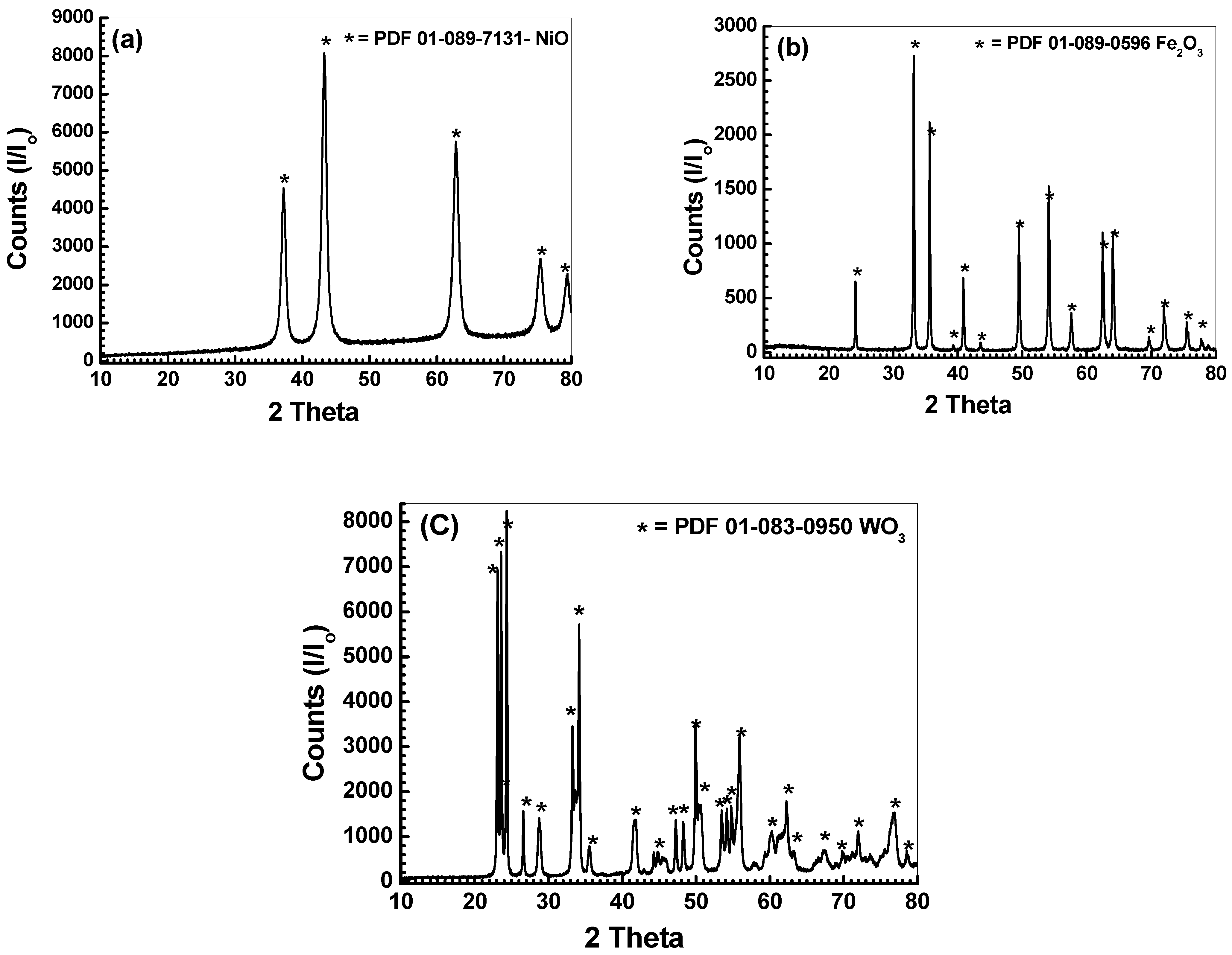
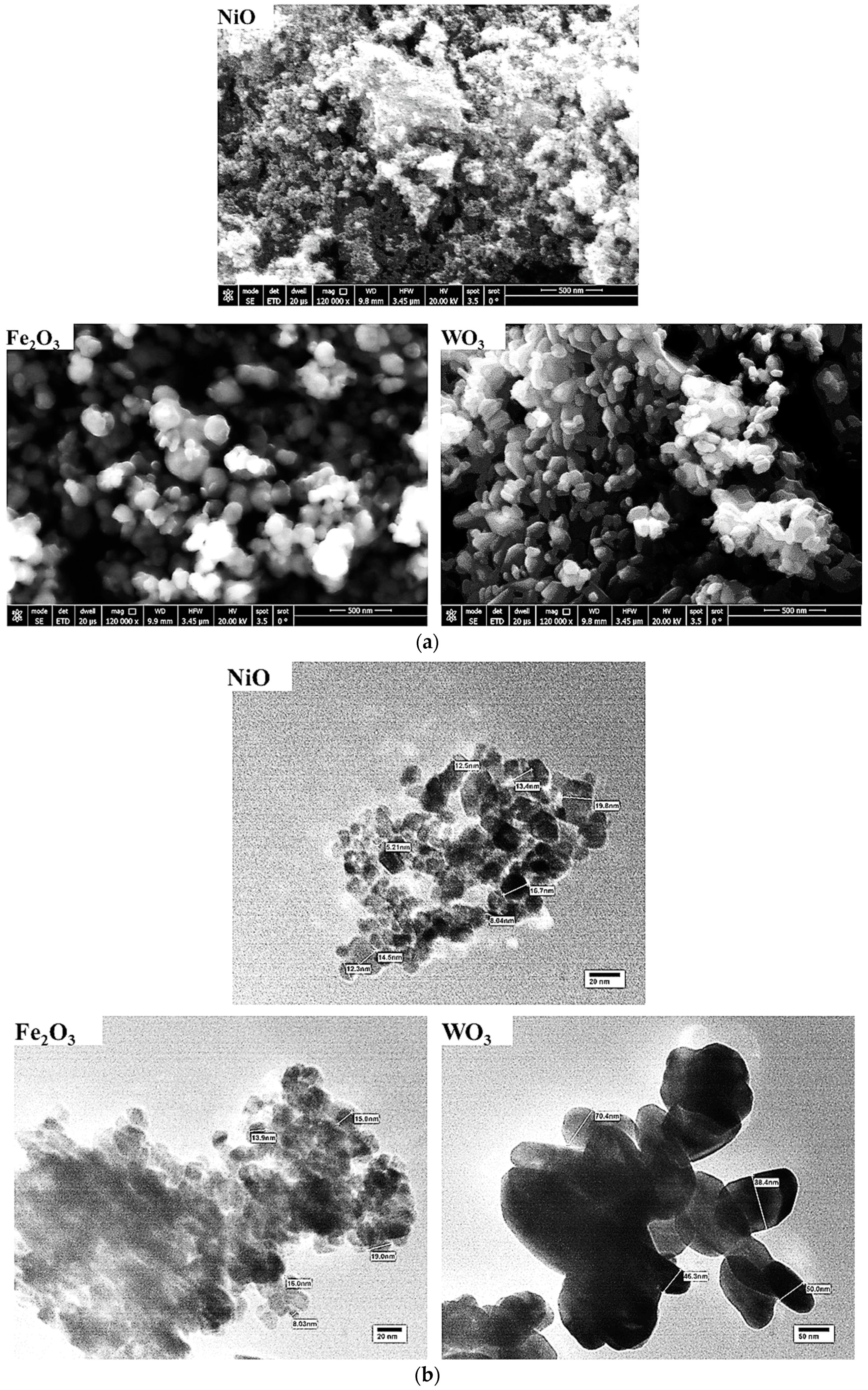
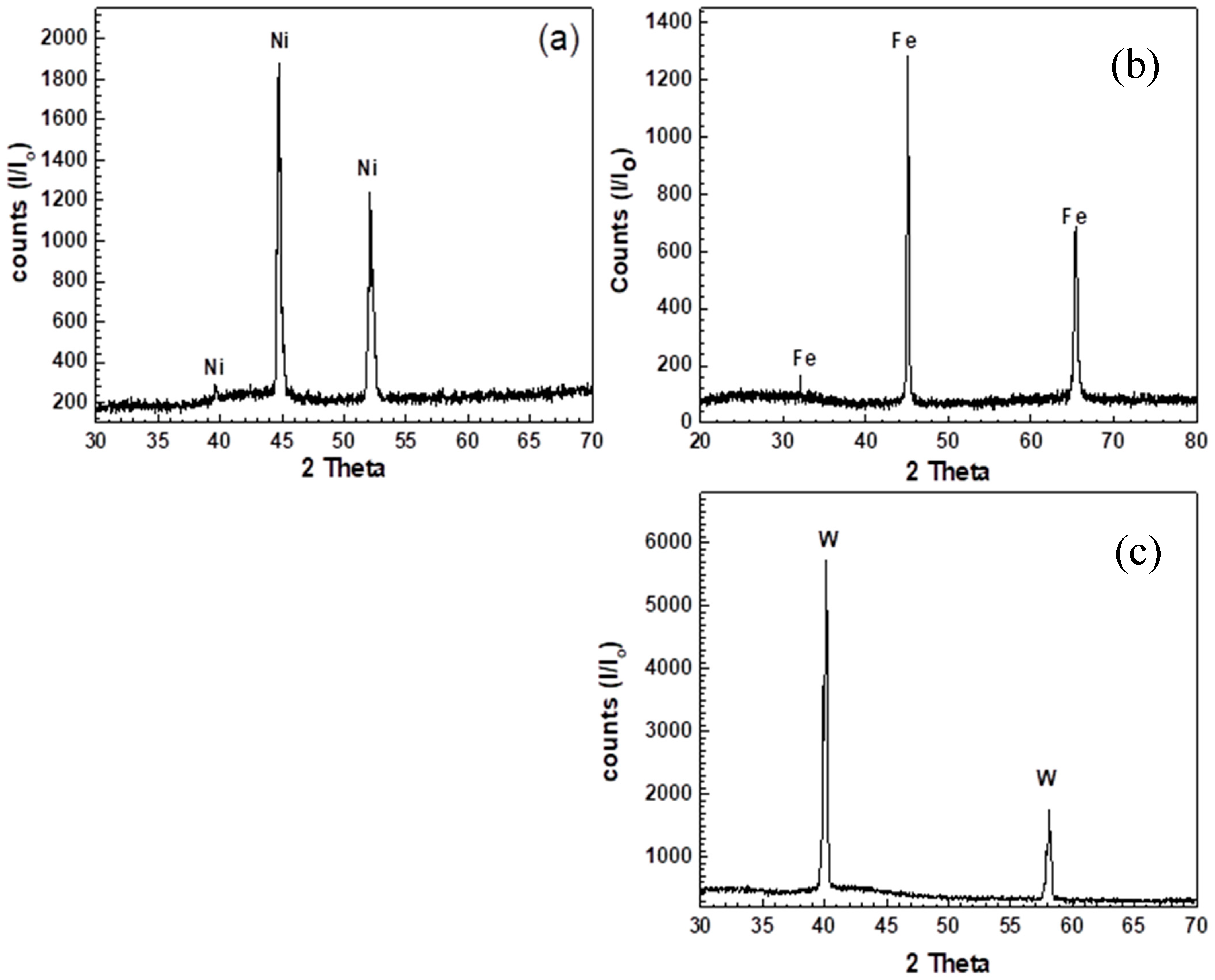
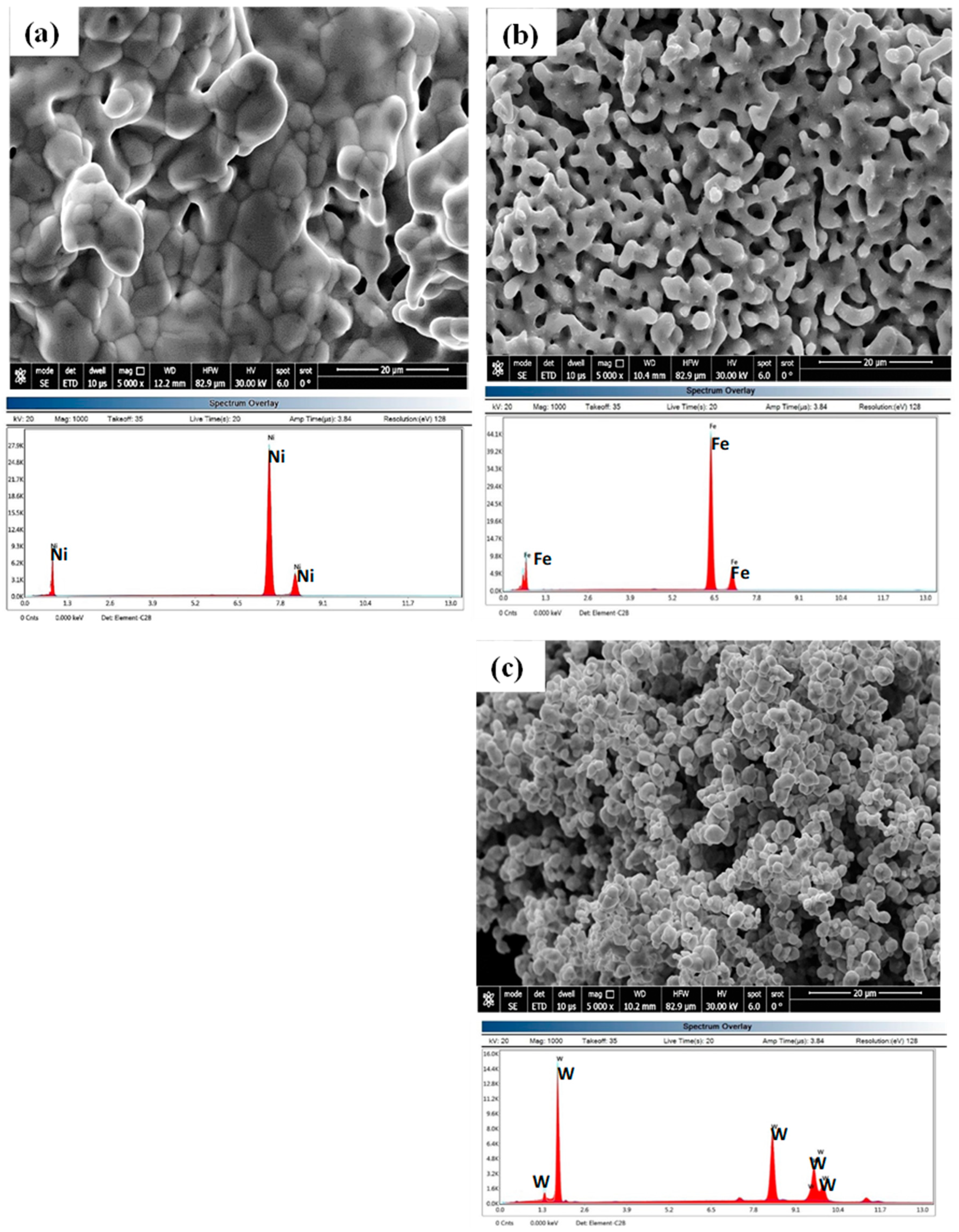
3.1. Characterization of Nanosized Metal Oxides
3.2. Reduction Behavior of Metal Oxides
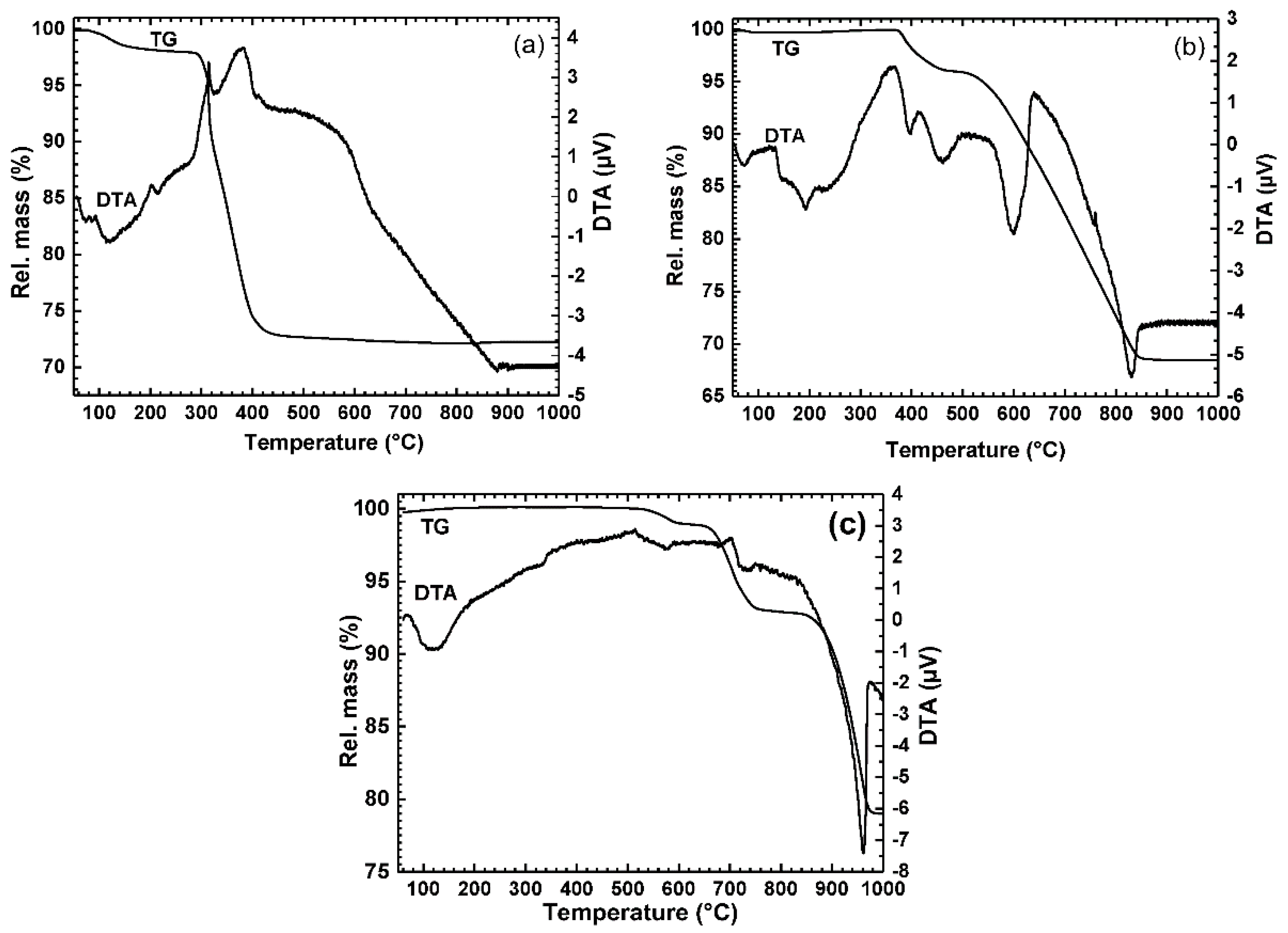
3.2.1. Non-Isothermal Reduction of Nanosized Metal Oxide Powder
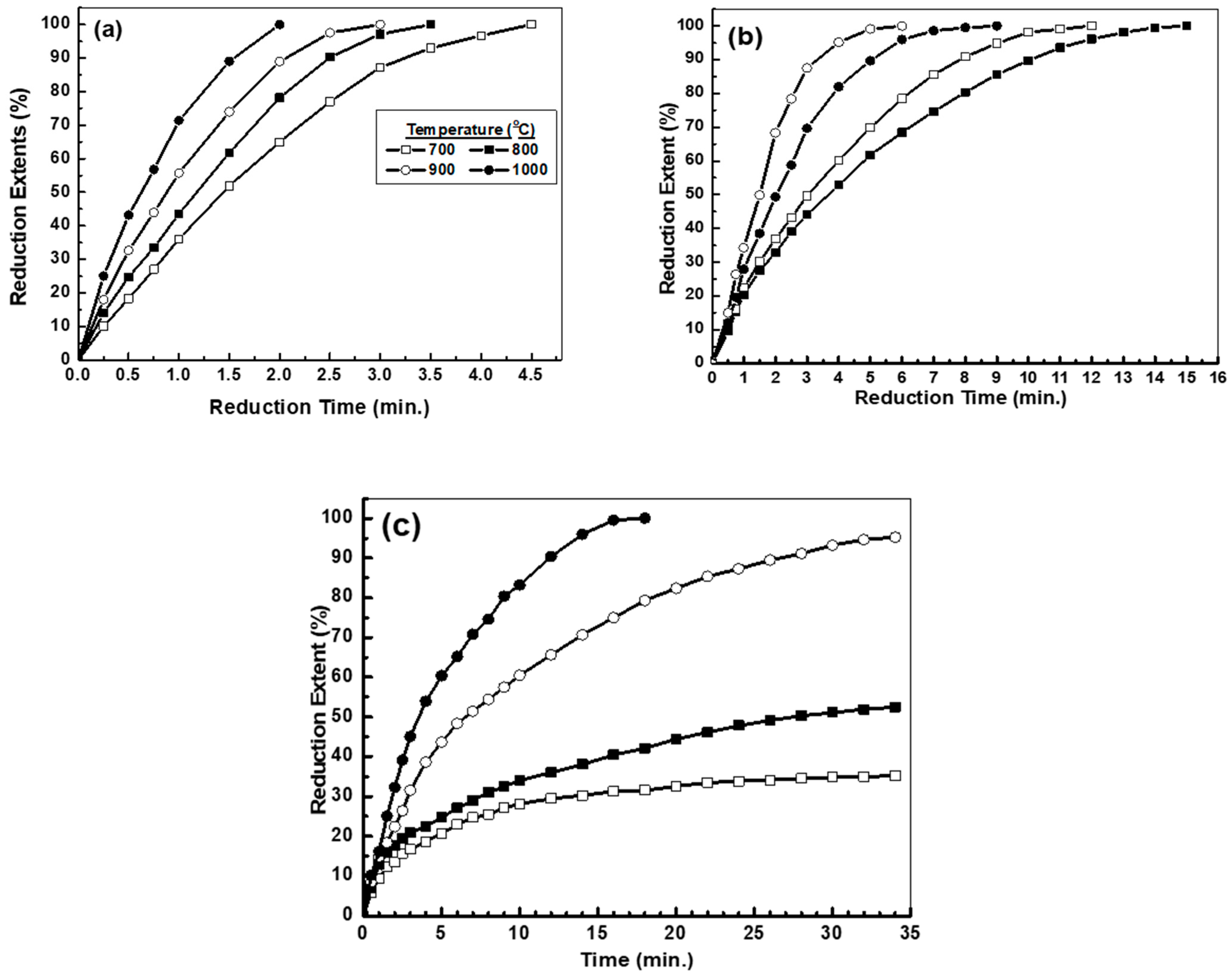
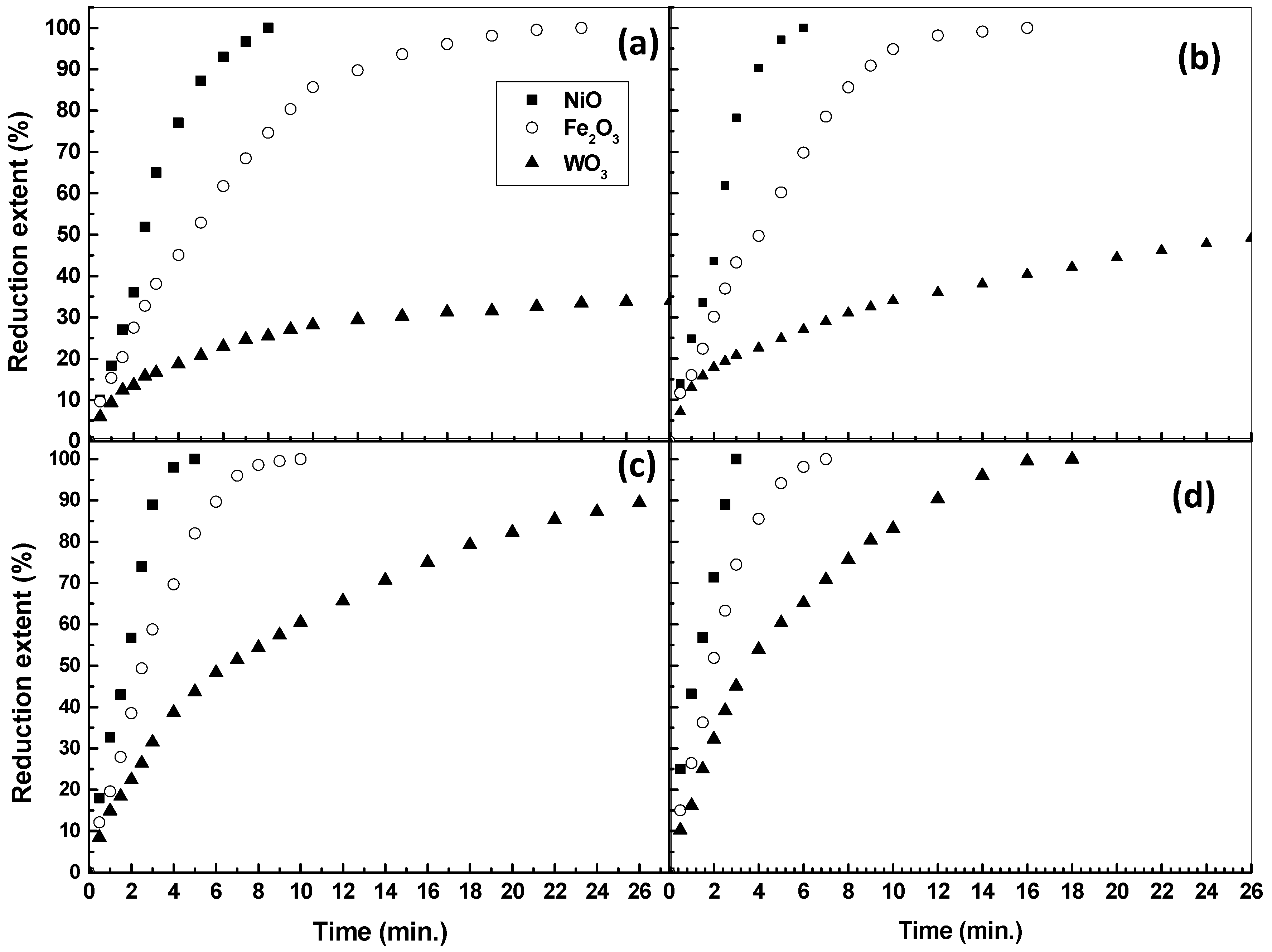
3.2.2. Isothermal Reduction of Metal Oxide Compacts
3.3. Reduction Kinetics and Mechanism
- In the initial stages (5–10% extent), the Ea values obtained from the reduction of NiO and Fe2O3 are 9.6 and 12.43 kJ/mole, indicating that the reduction is controlled by a gaseous diffusion mechanism. The Ea value for WO3 is relatively high (23.68 kJ/mole) but is still in the range of the gas diffusion mechanism. The considerable difference in the Ea values between NiO, Fe2O3, and WO3 is in accordance with the slower reduction rate of WO3 compared to other oxides, as was previously shown in Figure 4a–c. This finding is in agreement with the principles of gas–solid reactions in which the rate-determining step is considered the slowest step in the whole reactions [11,12,13,14,15]. In the present study, nanosized metal oxides were reduced in a H2 atmosphere, and the chemical reaction easily proceeded at the grain surface. The produced metallic phase at this stage could not be able to surround the whole oxide grain surface. Under these conditions, the gas diffusion inside the oxide grains is relatively the slowest process in the gas–solid reaction and is considered the determining step.
- In the later stages (80–85 % extent), the Ea values for the reduction of NiO and Fe2O3 are 13.24 and 21.65 kJ/mole, indicating that the reduction reactions are still also controlled by the gaseous diffusion mechanism. On the other hand, the Ea value for the reduction of WO3 could not be calculated due to the stopping of reduction at 700–900 °C at lower extents depending on the applied reduction temperature, and the reduction is completed only at 1000 °C.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdel Halim, K.S. Novel synthesis of porous Fe-Ni ferroalloy powder for energy applications. Mater. Lett. 2012, 68, 478. [Google Scholar] [CrossRef]
- Abdel Halim, K.S.; Bahgat, M.; Fouad, O.A. Thermal Synthesis of Nanocrystalline FCC Fe-Ni Alloy by Gaseous Reduction of Co-precipitated NiFe2O4 from Secondary Resources. Mater. Sci. Technol. 2006, 22, 1396–1400. [Google Scholar] [CrossRef]
- Abdel Halim, K.S.; Bram, M.; Buchkremer, H.P.; Bahgat, M. Synthesis of heavy tungsten alloy by thermal technique. Ind. Eng. Chem. Res. 2012, 51, 16354–16360. [Google Scholar] [CrossRef]
- Upadhyaya, A. Processing strategy for consolidating tungsten heavy alloys for ordnance applications. Mater. Chem. Phys. 2001, 67, 101. [Google Scholar] [CrossRef]
- Crowson, A.; Chen, E.S. (Eds.) Tungsten and Tungsten Alloys—Recent Advances; Minerals, Metals and Materials Society: Warrendale, PA, USA, 1991. [Google Scholar]
- German, R.M. Critical developments in tungsten heavy alloys. In Tungsten and Tungsten Alloys; Bose, A., Dowding, R.J., Eds.; MPIF: Princeton, NJ, USA, 1992; pp. 3–13. [Google Scholar]
- Fan, J.L.; Liu, T.; Cheng, H.; Wang, D. Preparation of fine grain tungsten heavy alloy with high properties by mechanical alloying and yttrium oxide addition. J. Mater. Process. Technol. 2008, 208, 463. [Google Scholar]
- Mikolaj, D.; Henrikas, C.; Zbigniew, S. Electrodeposition and properties of Ni-W, Fe-W and Fe-Ni-W amorphous alloys. Electrochim. Acta 2000, 45, 3389. [Google Scholar]
- He, F.; Yang, J.; Lei, T.; Gu, C. Structure and properties of electrodeposited Fe-Ni-W alloy with different levels of tungsten content: A comparative study. Appl. Surf. Sci. 2007, 253, 7591. [Google Scholar] [CrossRef]
- Cai, W.D.; Li, Y.; Dowding, R.J.; Mohamed, F.A.; Lavernia, E.J. A review of tungsten-based alloys as kinetic energy penetrator materials. Rev. Part. Mater. 1995, 3, 71. [Google Scholar]
- Unal, A.; Bradshow, A.V. Rate processes and structural changes in gaseous reduction of hematite particles to magnetite. Metall. Trans. 1983, 14, 743–752. [Google Scholar] [CrossRef]
- El-Geassy, A.A. Gaseous reduction of Fe2O3 compacts at 600 to 1050 °C. J. Mater. Sci. 1986, 21, 3889–3900. [Google Scholar] [CrossRef]
- El-Geassy, A.A.; Rajakumar, V. Influence of Particle Size on the Gaseous Reduction of Wustite at 900–1100 °C. Trans. ISIJ 1985, 25, 1202–1211. [Google Scholar] [CrossRef]
- Mousa, E.A.; Omar, A.A.; Nasr, M.I.; El-Geassy, A.A. Volume Changes of Iron Oxide Compacts under Isothermal Reduction Conditions. Steel Res. Int. 2007, 78, 579. [Google Scholar] [CrossRef]
- El-Geassy, A.A. Influence of doping with CaO and/or MgO on stepwise reduction of pure hematite compacts. J. Ironmak. Steelmak. 1999, 26, 41–52. [Google Scholar] [CrossRef]
- El-Geassy, A.A.; Nasr, M.I.; Mousa, E.A. Influence of Manganese Oxide and Silica on the Morphological Structure of Hematite Compacts. Steel Res. Int. 2010, 81, 2010. [Google Scholar] [CrossRef]
- El-Geassy, A.A.; Abdel Halim, K.S.; Bahgat, M.; Mousa, E.A.; El-Sherify, E.E.; El-Tawil, A.A. Carbothermic reduction of Fe2O3/C compacts: Comparative approach to kinetics and mechanism. Ironmak. Steelmak. 2013, 40, 534–544. [Google Scholar] [CrossRef]
- El-Geassy, A.A.; Nasr, M.I.; El-Raghy, S.M.; Hammam, A.E. Comparative studies on isothermal and non-isothermal reduction of haematite in carbon monoxide atmosphere. Ironmak. Steelmak. 2020, 47, 948–957. [Google Scholar] [CrossRef]
- Sun, G.; Li, B.; Guo, H.; Yang, W.; Li, S.; Guo, J. Thermodynamic Study of Energy Consumption and Carbon Dioxide Emission in Ironmaking Process of the Reduction of Iron Oxides by Carbon. Energies 2021, 14, 1999. [Google Scholar] [CrossRef]
- Abdel Halim, K.S.; Khedr, M.H.; Nasr, M.I.; Abdel Wahab, M.S. Carbothermic reduction kinetics of nanocrystallite Fe2O3/NiO composites for the production of Fe/Ni alloy. J. Alloys Compd. 2008, 463, 585–590. [Google Scholar] [CrossRef]
- Bandrowski, J.; Bickling, R.; Yang, K.H.; Hougen, O.A. Kinetics of the reduction of nickel oxide by hydrogen. Chem. Eng. Sci. 1962, 17, 379–390. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Avetisyan, A.G.; Shuck, C.E.; Chatilyan, H.A.; Rouvimov, S.; Kharatyan, S.L.; Mukasyan, A.S. Nickel Oxide Reduction by Hydrogen: Kinetic and Structural Transformations, American Chemical Society (ACS). J. Phys. Chem. 2015, 119, 16131–16138. [Google Scholar]
- Lassner, E.; Schubert, W. Tungsten, Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds; Springer Science+ Business Media: New York, NY, USA, 1999. [Google Scholar] [CrossRef]
- Haubner, R.; Schubert, W.D.; Lassner, E.; Lux, B. Mechanism of technical reduction of tungsten: Part 2 hydrogen reduction of tungsten blue oxide to tungsten powder. J. Refract. Met. Hard Mater. 1983, 2, 108–115. [Google Scholar]
- Schubert, W.D.; Lassner, E. Production and characterization of hydrogen-reduced submicron tungsten powders. Part II: Controlled decomposition of APT and hydrogen reduction of the oxides. J. Refract. Met. Hard Mater. 1991, 10, 171–183. [Google Scholar] [CrossRef]
- Parson, D. The reduction of tungsten oxides by hydrogen. Electrochem. Technol. 1965, 3, 280–283. [Google Scholar]
- Pierre, G.R.S.; Ebihara, W.T.; Pool, M.J.; Speiser, R. Tungsten-oxygen system. Trans. TMS-AIME 1962, 224, 259–264. [Google Scholar]
- Ackennann, R.J.; Rauh, E.G. A Thermodynamic Study of the Tungsten-Oxygen System at High Temperatures1. J. Phys. Chem. 1963, 67, 2596–2601. [Google Scholar] [CrossRef]
- Chang, L.L.Y.; Phillips, B.J. Phase Relations in Refractory Metal-Oxygen Systems. J. Am. Ceram. Soc. 1969, 52, 527–533. [Google Scholar] [CrossRef]
- Sahle, W.; Berglund, S. The morphology of slightly reduced tungsten trioxide equilibrated with CO-CO2 gaseous buffers with and without water addition. J. Less-Common Metals. 1981, 79, 271–280. [Google Scholar] [CrossRef]
- Spreitzer, D.; Schenk, J. Reduction of Iron Oxides with Hydrogen—A Review. Steel Res. Int. 2019, 90, 1900108. [Google Scholar] [CrossRef]
- Abdel Halim, K.S.; Ramadan, M.; Shawabkeh, A.; Abufara, A. Synthesis and characterization of metallic materials for membrane technology. Beni-Suef Univ. J. Basic Appl. Sci. 2013, 2, 72–79. [Google Scholar] [CrossRef]
- Abdel Halim, K.S.; Nasr, M.I.; El-Geassy, A.A. Developed model for reduction mechanism of iron ore pellets under load. Ironmak. Steelmak. 2011, 38, 189–196. [Google Scholar] [CrossRef]


| Ea Values (kJ/mol) and the Corresponding Rate-Controlling Step | Oxide | Ea Initial (kJ/mol) | Ea Initial (kJ/mol) | |
|---|---|---|---|---|
| Ea Values | Rate-Controlling Step | |||
| 8–16 | Gas diffusion | NiO | 9.6 | 13.24 |
| 29–42 | Combined gas diffusion and interfacial chemical reaction | Fe2O3 | 12.43 | 21.65 |
| >90 | Solid-state diffusion | WO3 | 23.68 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halim, K.S.A.; El-Geassy, A.A.; Ramadan, M.; Nasr, M.I.; Hussein, A.; Fathy, N.; Alghamdi, A.S. Reduction Behavior and Characteristics of Metal Oxides in the Nanoscale. Metals 2022, 12, 2182. https://doi.org/10.3390/met12122182
Halim KSA, El-Geassy AA, Ramadan M, Nasr MI, Hussein A, Fathy N, Alghamdi AS. Reduction Behavior and Characteristics of Metal Oxides in the Nanoscale. Metals. 2022; 12(12):2182. https://doi.org/10.3390/met12122182
Chicago/Turabian StyleHalim, K. S. Abdel, A. A. El-Geassy, Mohamed Ramadan, M. I. Nasr, A. Hussein, Naglaa Fathy, and Abdulaziz S. Alghamdi. 2022. "Reduction Behavior and Characteristics of Metal Oxides in the Nanoscale" Metals 12, no. 12: 2182. https://doi.org/10.3390/met12122182
APA StyleHalim, K. S. A., El-Geassy, A. A., Ramadan, M., Nasr, M. I., Hussein, A., Fathy, N., & Alghamdi, A. S. (2022). Reduction Behavior and Characteristics of Metal Oxides in the Nanoscale. Metals, 12(12), 2182. https://doi.org/10.3390/met12122182






