Thermodynamics of Vacuum Chloride Volatilization of Ni, Co, Mn, Li, Al, and Cu in Spent Lithium−Ion Battery
Abstract
:1. Introduction
2. Thermodynamic Calculation
3. Results and discussion
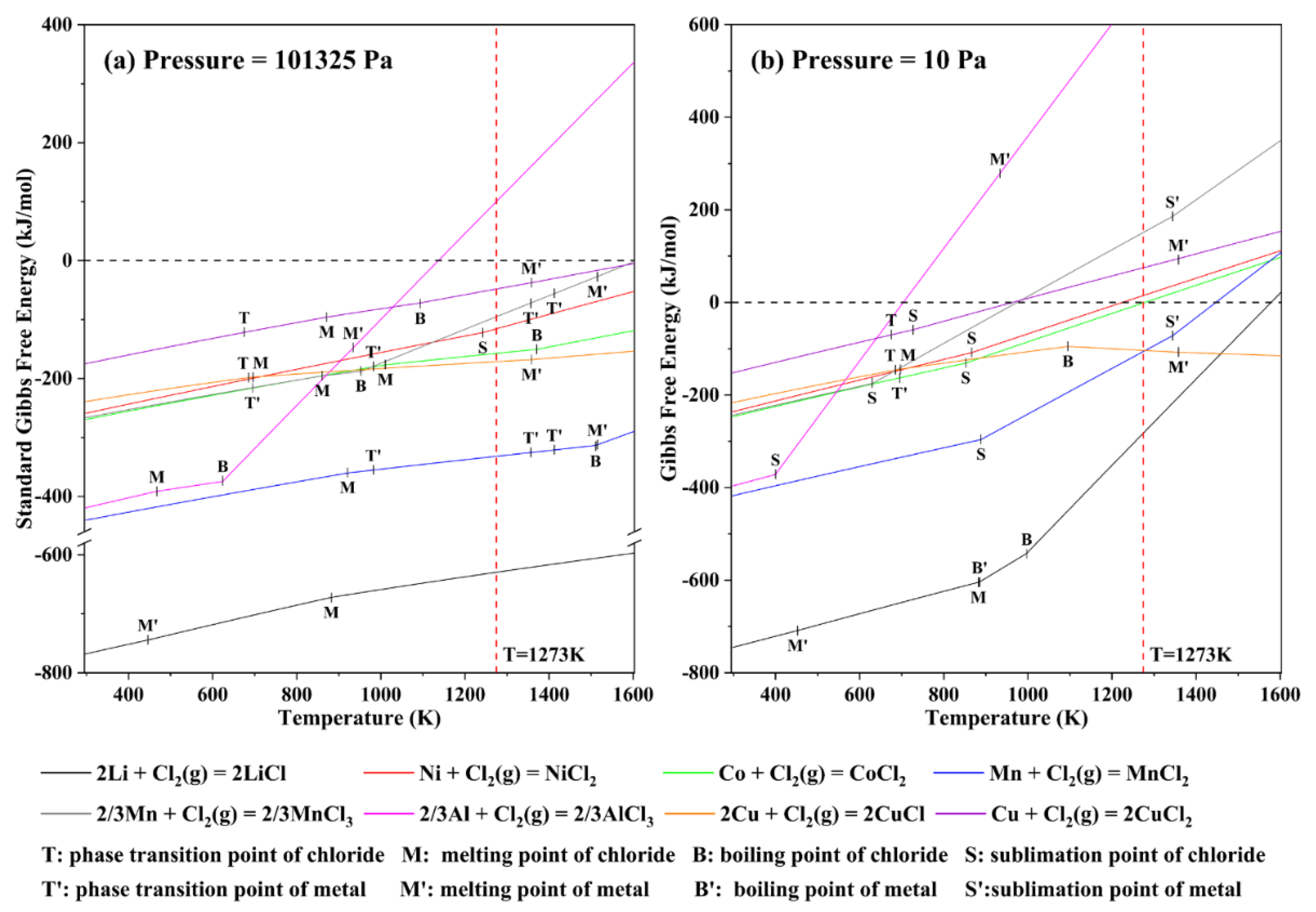
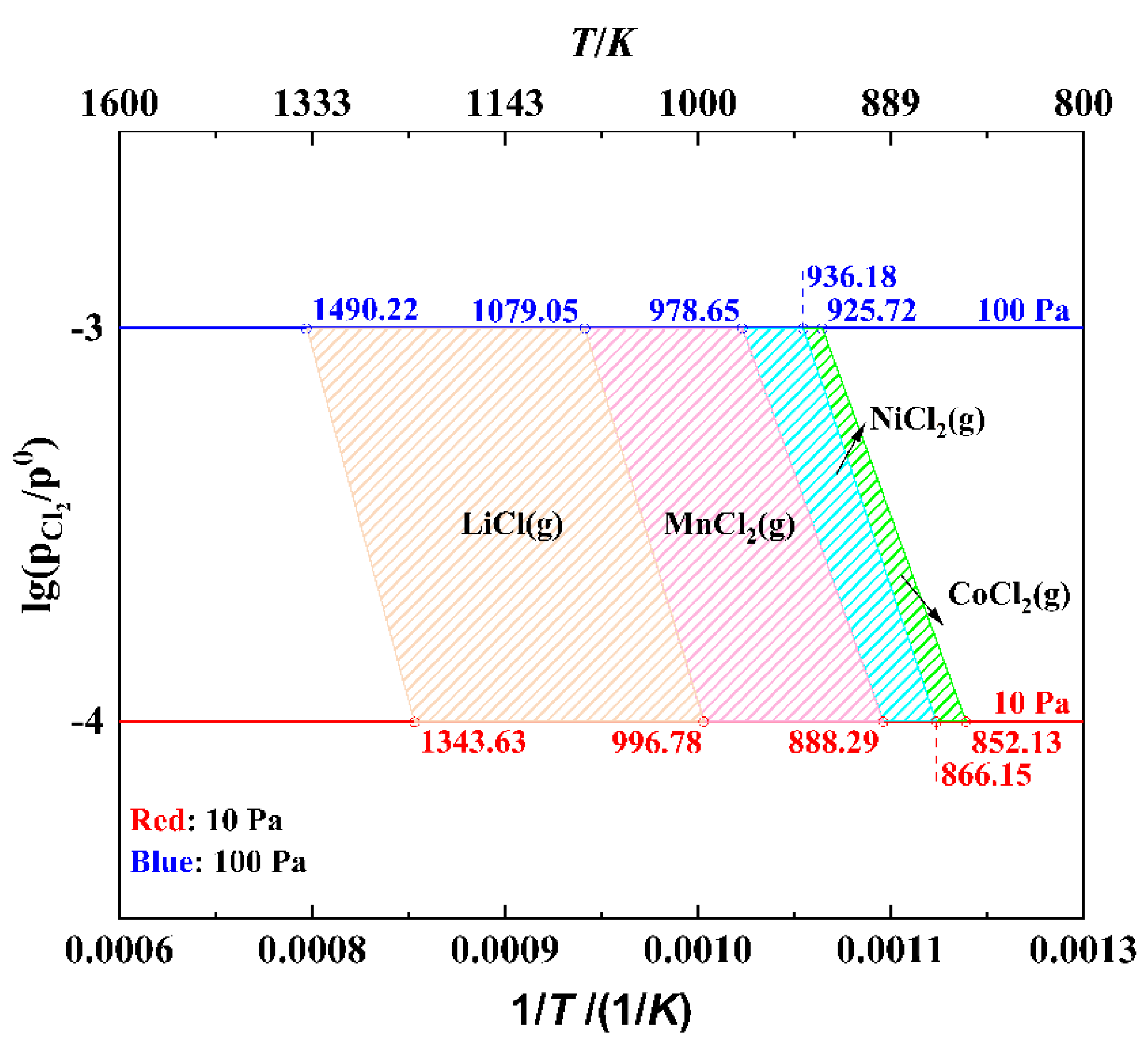
3.1. Analysis of Dominant Area of Ni−Co−Mn−Li−Cl System
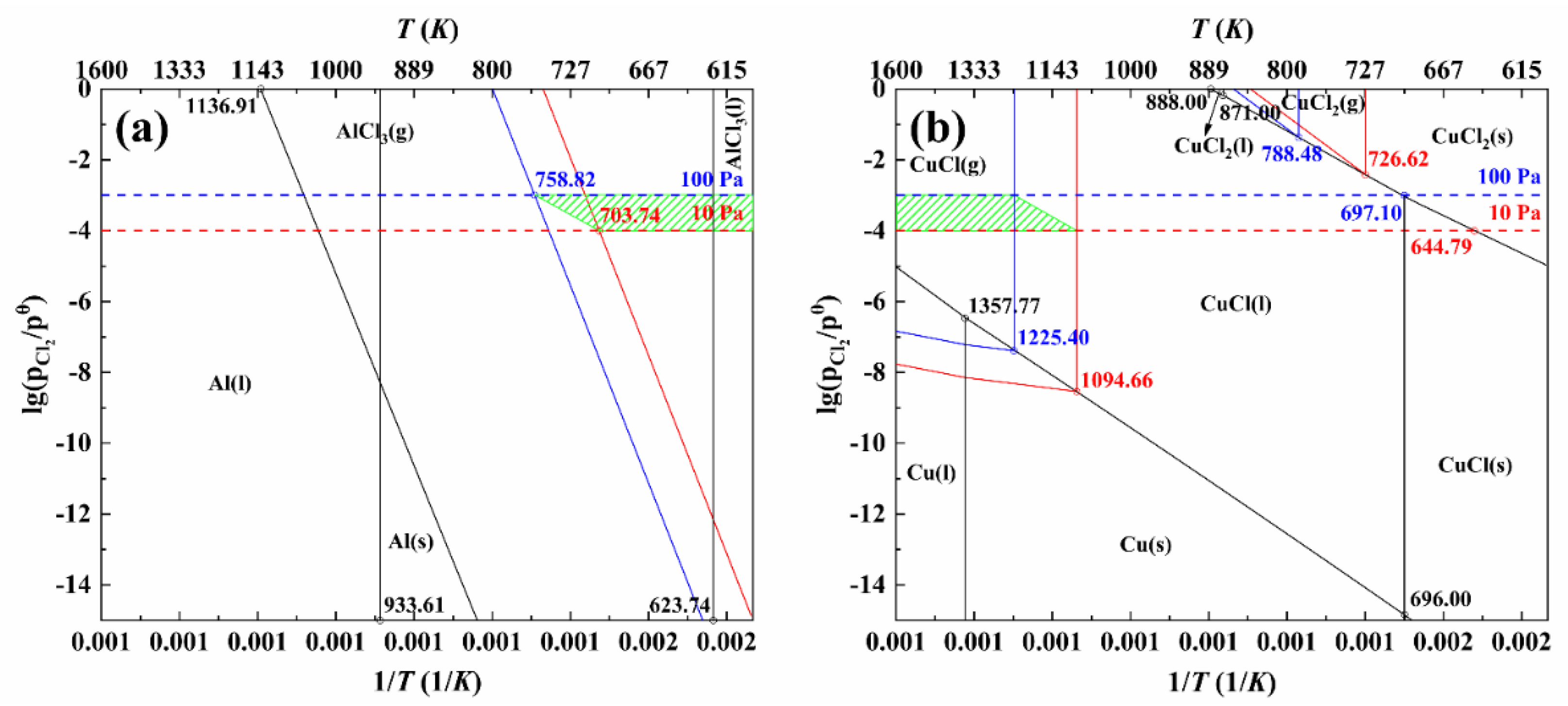
3.2. Analysis of Dominant Area of Al−Cu−Cl System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, H.; Lie, J.; Liu, J. Rapid Leaching of Valuable Metals from Spent Lithium−Ion Batteries with Microwave Irradiation Using Organic and Inorganic Acid. J. Sustain. Metall. 2021, 7, 630–641. [Google Scholar] [CrossRef]
- Jung, J.C.; Sui, P.; Zhang, J. A review of recycling spent lithium−ion battery cathode materials using hydrometallurgical treatments. J. Energy Storage 2021, 35, 102217. [Google Scholar] [CrossRef]
- Fu, Y.P.; He, Y.Q.; Chen, H.C.; Ye, C.L.; Lu, Q.C.; Li, R.N.; Xie, W.N.; Wang, J. Effective leaching and extraction of valuable metals from electrode material of spent lithium−ion batteries using mixed organic acids leachant. J. Ind. Eng. Chem. 2019, 79, 154–162. [Google Scholar] [CrossRef]
- Lu, Q.Q.; Chen, L.L.; Li, X.W.; Chao, Y.H.; Sun, J.; Ji, H.Y.; Zhu, W.S. Sustainable and Convenient Recovery of Valuable Metals from Spent Li−Ion Batteries by a One−Pot Extraction Process. ACS Sustain. Chem. Eng. 2021, 9, 13851–13861. [Google Scholar] [CrossRef]
- Gu, S.; Zhang, L.; Fu, B.T.; Wang, X.P.; Ahn, J.W. Feasible route for the recovery of strategic metals from mixed lithium−ion batteries cathode materials by precipitation and carbonation. Chem. Eng. J. 2021, 420, 127561. [Google Scholar] [CrossRef]
- Qiu, R.J.; Huang, Z.; Zheng, J.Y.; Song, Q.B.; Ruan, J.J.; Tang, Y.T.; Qiu, R.L. Energy models and the process of fluid−magnetic separation for recovering cobalt micro−particles from vacuum reduction products of spent lithium ion batteries. J. Clean. Prod. 2021, 279, 123230. [Google Scholar] [CrossRef]
- Kurashima, K.; Kumagai, S.; Kameda, T.; Saito, Y.; Yoshioka, T. Heavy metal removal from municipal solid waste fly ash through chloride volatilization using poly(vinyl chloride) as chlorinating agent. J. Mater. Cycles Waste 2020, 22, 1270–1283. [Google Scholar] [CrossRef]
- Kurashima, K.; Matsuda, K.; Kumagai, S.; Kameda, T.; Saito, Y.; Yoshioka, T. A combined kinetic and thermodynamic approach for interpreting the complex interactions during chloride volatilization of heavy metals in municipal solid waste fly ash. Waste Manag. 2019, 87, 204–217. [Google Scholar] [CrossRef]
- Hong, Y.X.; Ning, X.A.; Lu, X.W.; Li, Y.; Lai, X.J.; Zeng, J.; Chen, C.H. Effect of chlorine on the volatilization of heavy metals by roasting iron tailings. China Environ. Sci. 2020, 40, 2276–2286. [Google Scholar] [CrossRef]
- Shen, W.Q.; Zhu, N.W.; Xi, Y.H.; Huang, J.L.; Li, F.; Wu, P.X.; Dang, Z. Effects of medical waste incineration fly ash on the promotion of heavy metal chlorination volatilization from incineration residues. J. Haz. Mat. 2021, 425, 128037. [Google Scholar] [CrossRef]
- Okabe, P.; Rappleye, D.; Newton, M.; Simpson, M.F. Development of metallic nuclear material purification process via simultaneous chlorination and volatilization. J. Nucl. Mater. 2021, 543, 152626. [Google Scholar] [CrossRef]
- Grause, G.; Yamamoto, N.; Kameda, T.; Yoshioka, T. Removal of lead from cathode ray tube funnel glass by chloride volatilization. Int. J. Environ. Sci. Technol. 2014, 11, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.Y.; Sun, S.Y. Chlorination Transformation and Volatilization of Heavy Metals in Fly Ash from the Incineration during the Disposal Process with Higher Temperature. Environ. Sci. 2012, 33, 3279–3287. [Google Scholar] [CrossRef]
- Xue, K.; Jiao, F.; Li, W.; Liu, W.; Qin, W.Q.; Yang, C.R. Using Magnesium Chloride to Volatilize Impurity Metals from Waste Magnesia−Chromium Refractories. JOM 2022, 74, 1350–1359. [Google Scholar] [CrossRef]
- Hu, G.C.; Hu, N.X.; Wu, J.J.; Ma, W.H. Research progress in recovery of valuable metals in cathode materials for lithium−ion batteries. Chin. J. Nonferrous Met. 2021, 31, 3320–3343. [Google Scholar]
- Huang, Y.; Shao, P.H.; Yang, L.M.; Zheng, Y.F.; Sun, Z.; Fang, L.L.; Lv, W.G.; Yao, Z.W.; Wang, L.H.; Luo, X.B. Thermochemically driven crystal phase transfer via chlorination roasting toward the selective extraction of lithium from spent LiNi1/3Co1/3Mn1/3O2. Resour. Conserv. Recycl. 2021, 174, 105757. [Google Scholar] [CrossRef]
- Yu, S.R.; Zhang, B.; Zhang, W.S. Effects of chlorine on the volatilization of heavy metals during the co−combustion of sewage sludge. Waste Manag. 2017, 62, 204–210. [Google Scholar] [CrossRef]
- Van Winkel, S.; Scheunis, L.; Verhaeghe, F.; Blanpain, B.; Malfliet, A. Chlorine Addition to Existing Zinc Fuming Processes: A Thermodynamic Study. J. Sustain. Metall. 2020, 5, 538–550. [Google Scholar] [CrossRef]
- Kameda, T.; Fukushima, S.; Grause, G.; Yoshioka, T. Metal recovery from wire scrap via a chloride volatilization process: Poly(vinyl chloride) derived chlorine as volatilization agent. Thermochim. Acta 2013, 562, 65–69. [Google Scholar] [CrossRef]
- Khan, M.N.; Tlili, I. Innovative thermodynamic parametric investigation of gas and steam bottoming cycles with heat exchanger and heat recovery steam generator: Energy and exergy analysis. Energy Rep. 2018, 4, 497–506. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, F.; Wei, D.X.; Han, J.H.; Xia, Y.J.; Jiang, J.; Zhong, M.W.; Hirata, A.; Watanabe, K. Vapor phase dealloying kinetics of MnZn alloys. ACTA Mater. 2021, 212, 116916. [Google Scholar] [CrossRef]
- Xing, Z.X.; Cheng, G.J.; Yang, H.; Xue, X.X.; Jiang, P.G. Mechanism and application of the ore with chlorination treatment: A review. Miner. Eng. 2020, 154, 106404. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.A.; Lv, G.Z.; Zhang, J.Z.; Dou, Z.H.; Zhang, W.G.; Pan, X.J.; Wang, Y.X. Thermodynamics Analysis on Process of Pelletizing Chlorination of Fly Ash. In Light Metals 2018; Minerals Metals & Materials, Series, Martin, O., Eds.; Springer: Phoenix, AZ, USA, 2018; pp. 89–95. [Google Scholar] [CrossRef]
- Yu, Q.C.; Wang, P.Y.; Yang, B.; Cui, C.L.; Zhang, H.; Wang, C. Carbothermic Reduction and Chlorination of Alumina with Coal in Vacuum. Adv. Mater. Res. 2012, 443–444, 862–865. [Google Scholar] [CrossRef]
- Yu, W.Z.; Yang, B.; Zhu, F.L.; Jiang, W.L.; Yu, Q.C.; Xu, B.Q. Investigation of chlorination process in aluminum production by carbothermic−chlorination reduction of Al2O3 under vacuum. Vacuum 2012, 86, 1113–1117. [Google Scholar] [CrossRef]
- Moodley, S.; Eric, R.H.; Kucukkaragoz, C.; Kale, A. Chlorination of Titania Feedstocks; Jiang, T., Hwang, J., Masset, P., Yucel, o., Padilla, R., Zhou, G., Eds.; Wiley: Orlando, FL, USA, 2012; pp. 93–104. [Google Scholar]



| Chloride | Melting Point (K) | Boiling Point (K) | |
|---|---|---|---|
| 101,325 Pa | 10 Pa | ||
| LiCl | 883.00 | 1701.55 | 996.78 |
| NiCl2 | 1304.00 | 1242.79 * | 866.15 * |
| CoCl2 | 1010.00 | 1371.13 | 852.13 * |
| MnCl2 | 923.00 | 1510.24 | 888.29 * |
| MnCl3 | 860.00 | 953.96 | 630.15 * |
| AlCl3 | 465.70 | 623.74 | 399.46 * |
| CuCl | 696.00 | 1950.80 | 1094.66 |
| CuCl2 | 871.00 | 1094.04 | 726.62 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, W.; Hu, G.; Ding, J.; Wu, J.; Ma, W. Thermodynamics of Vacuum Chloride Volatilization of Ni, Co, Mn, Li, Al, and Cu in Spent Lithium−Ion Battery. Metals 2022, 12, 2183. https://doi.org/10.3390/met12122183
Luo W, Hu G, Ding J, Wu J, Ma W. Thermodynamics of Vacuum Chloride Volatilization of Ni, Co, Mn, Li, Al, and Cu in Spent Lithium−Ion Battery. Metals. 2022; 12(12):2183. https://doi.org/10.3390/met12122183
Chicago/Turabian StyleLuo, Wen, Guochen Hu, Junshuai Ding, Jijun Wu, and Wenhui Ma. 2022. "Thermodynamics of Vacuum Chloride Volatilization of Ni, Co, Mn, Li, Al, and Cu in Spent Lithium−Ion Battery" Metals 12, no. 12: 2183. https://doi.org/10.3390/met12122183
APA StyleLuo, W., Hu, G., Ding, J., Wu, J., & Ma, W. (2022). Thermodynamics of Vacuum Chloride Volatilization of Ni, Co, Mn, Li, Al, and Cu in Spent Lithium−Ion Battery. Metals, 12(12), 2183. https://doi.org/10.3390/met12122183






