Vacuum Carbon Reducing Iron Oxide Scale to Prepare Porous 316 Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Raw Materials
2.2. Methods
3. Results and Discussion
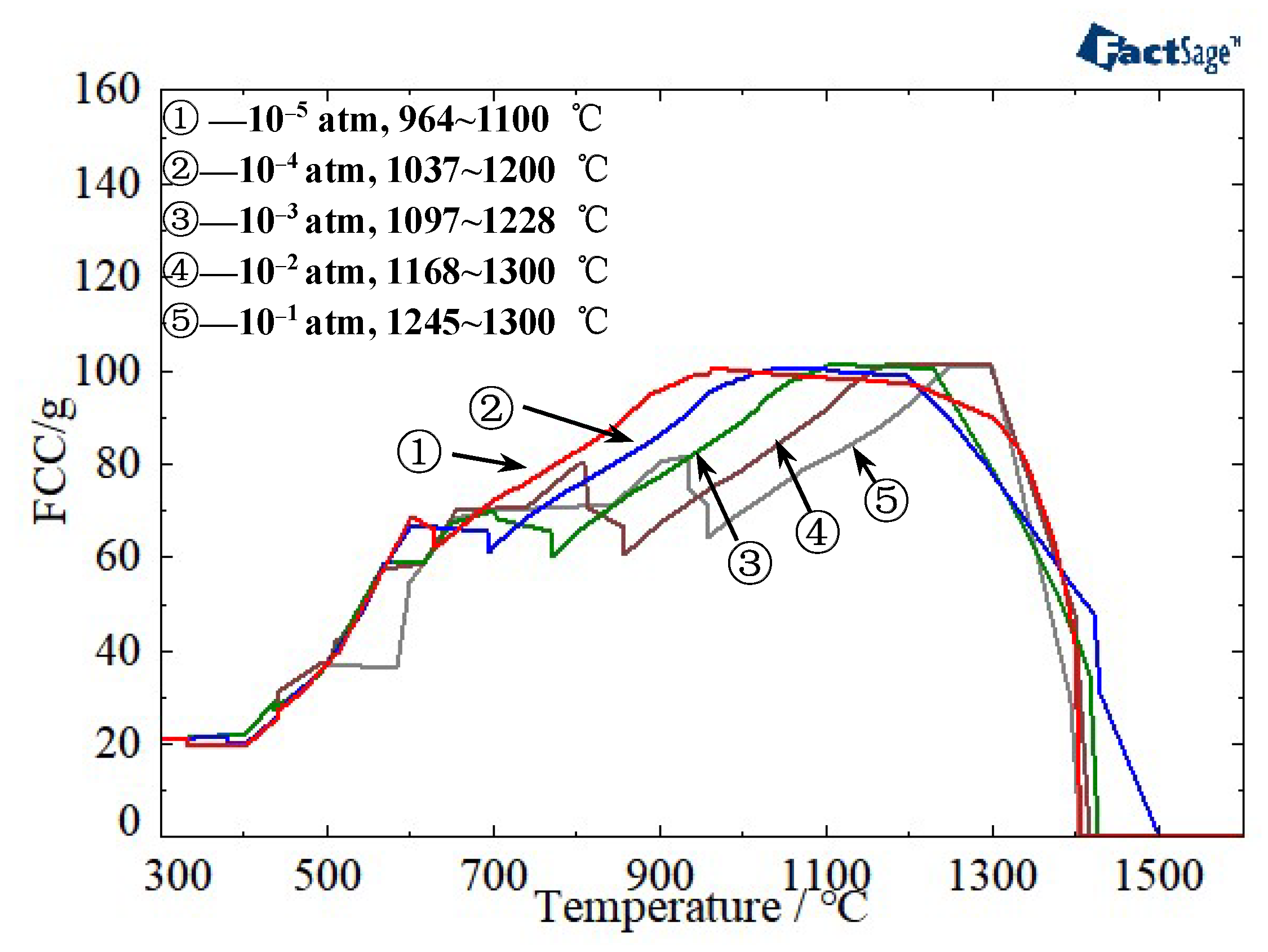
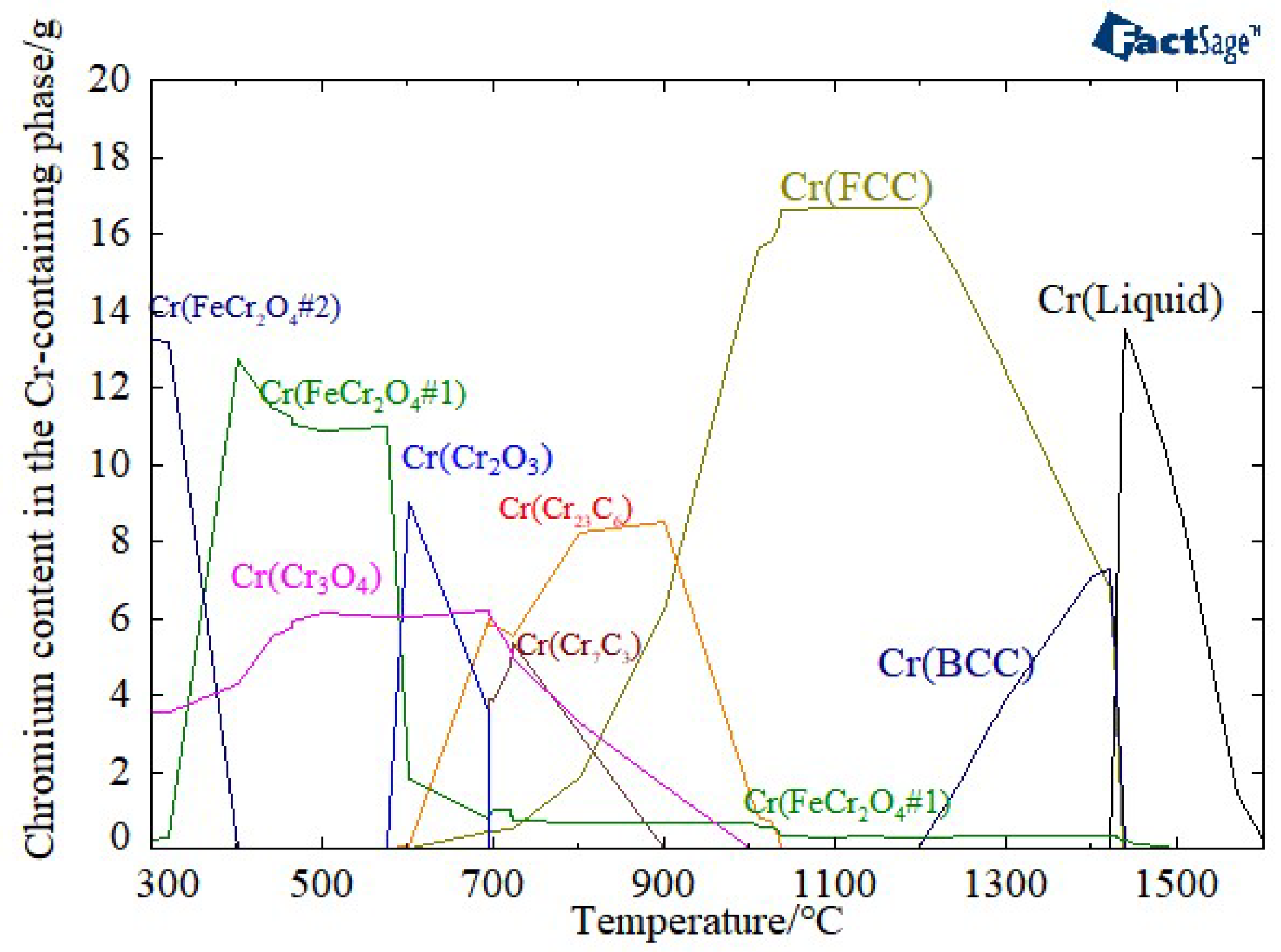
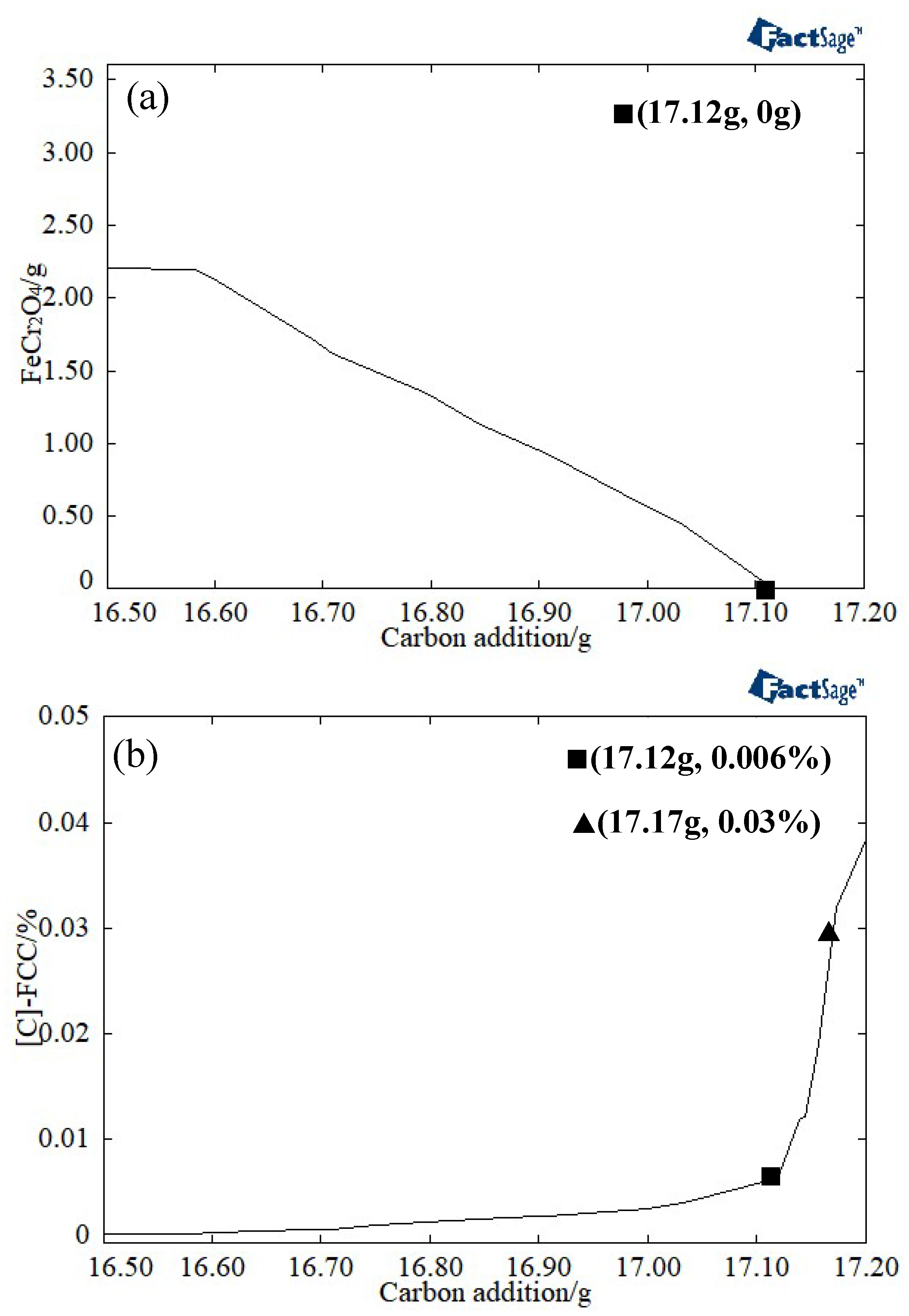
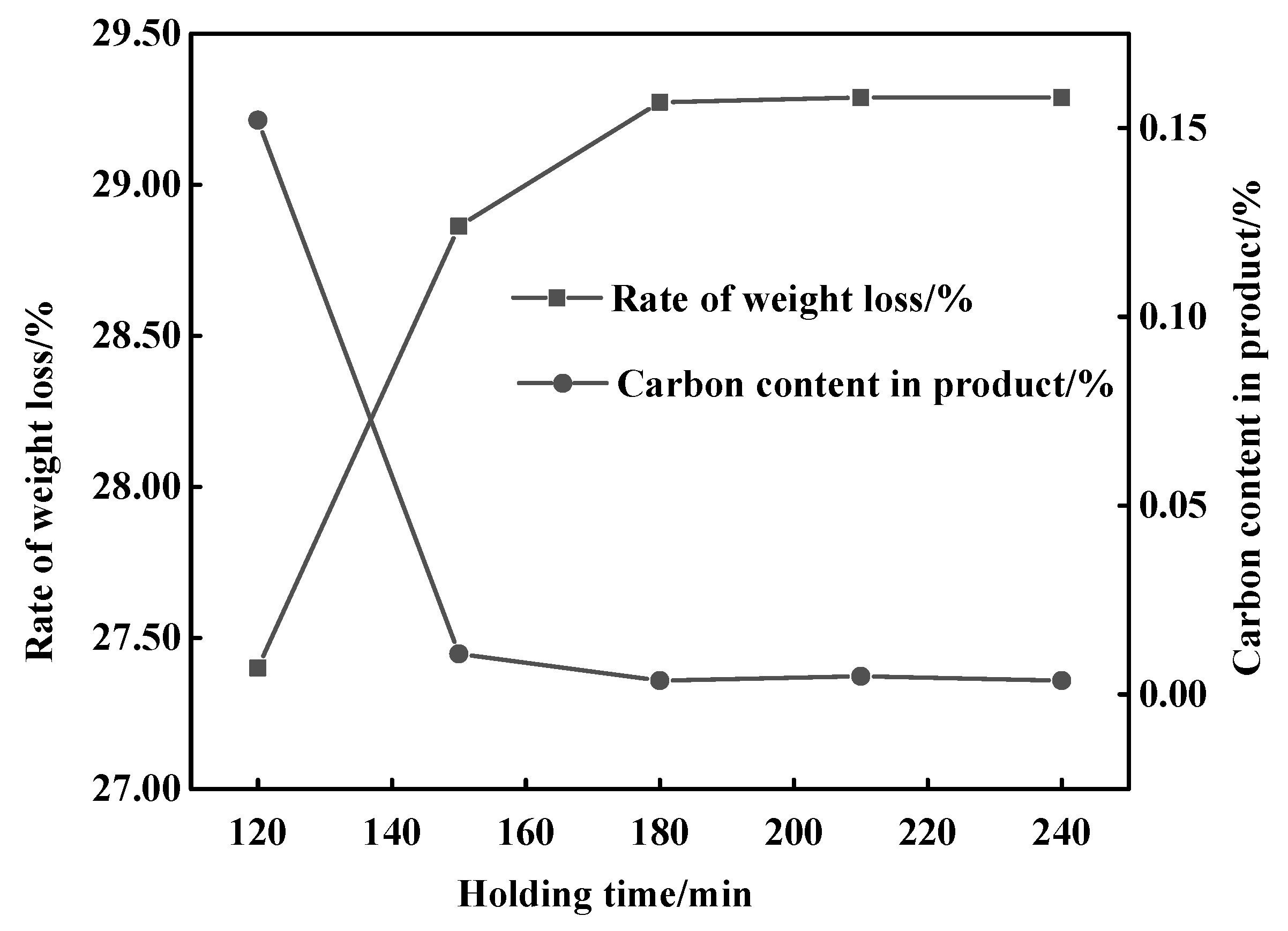
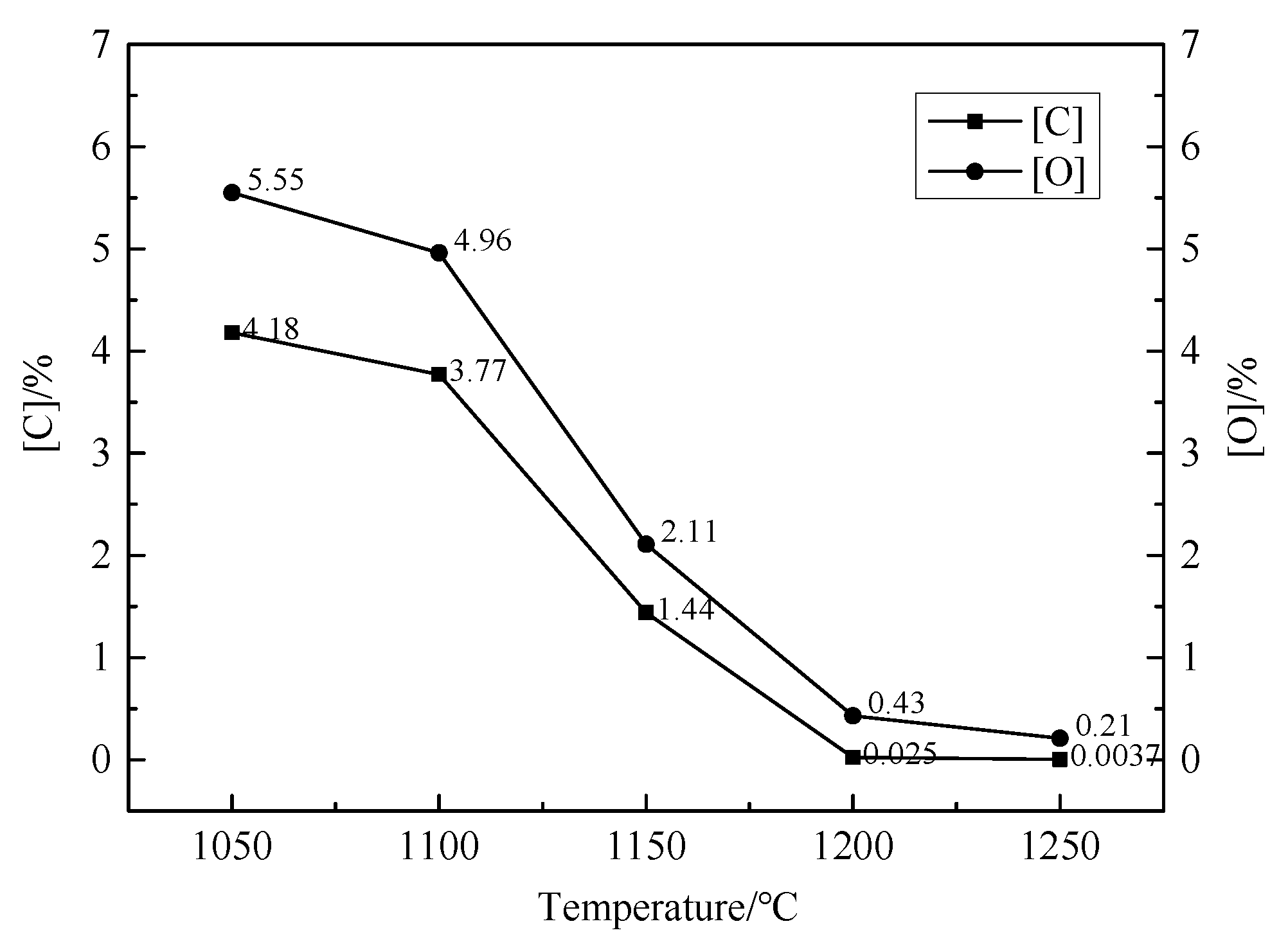
3.1. Determination of the Reduction Sintering System
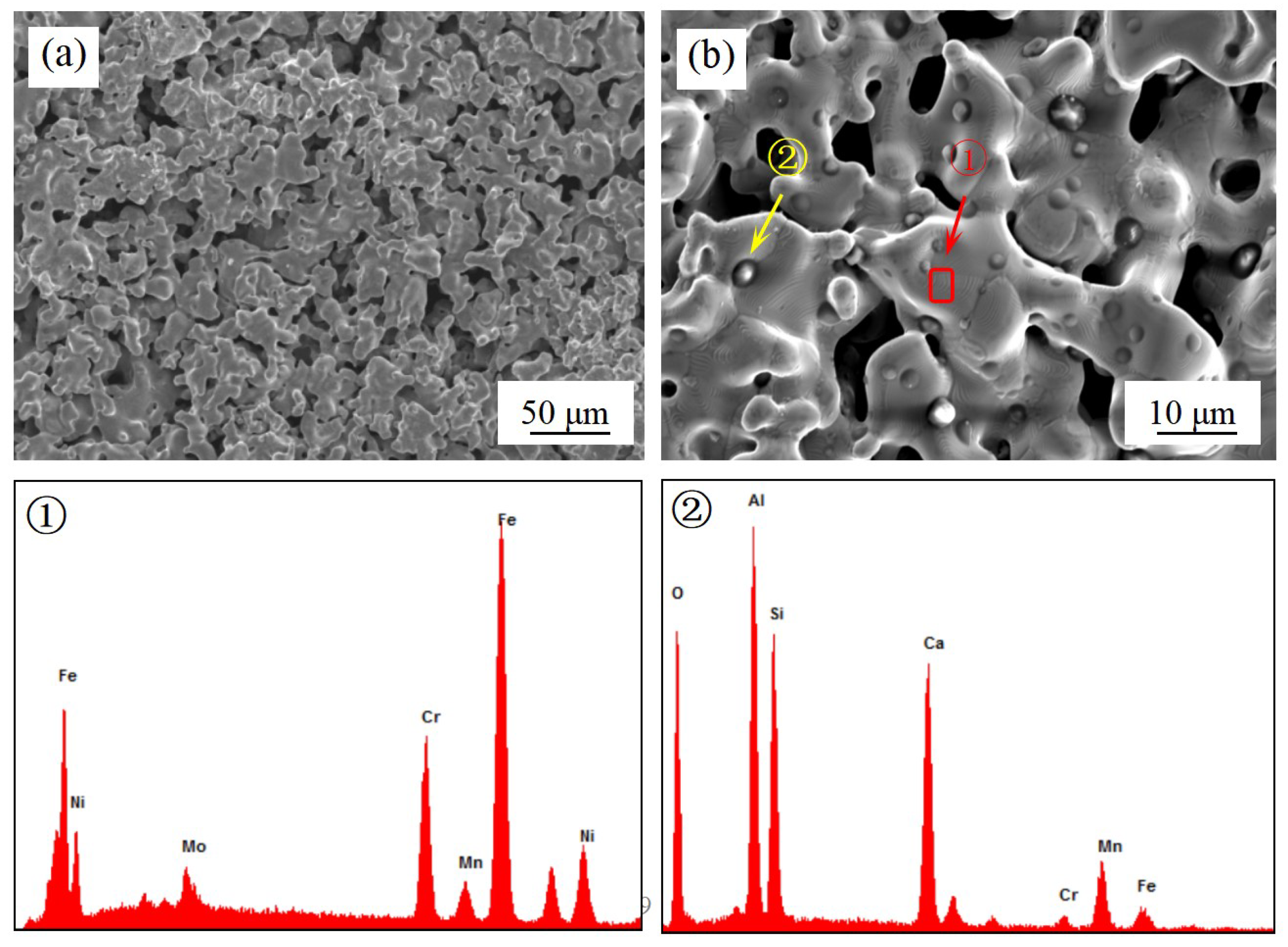
3.2. Microstructure of Porous 316 Stainless Steel
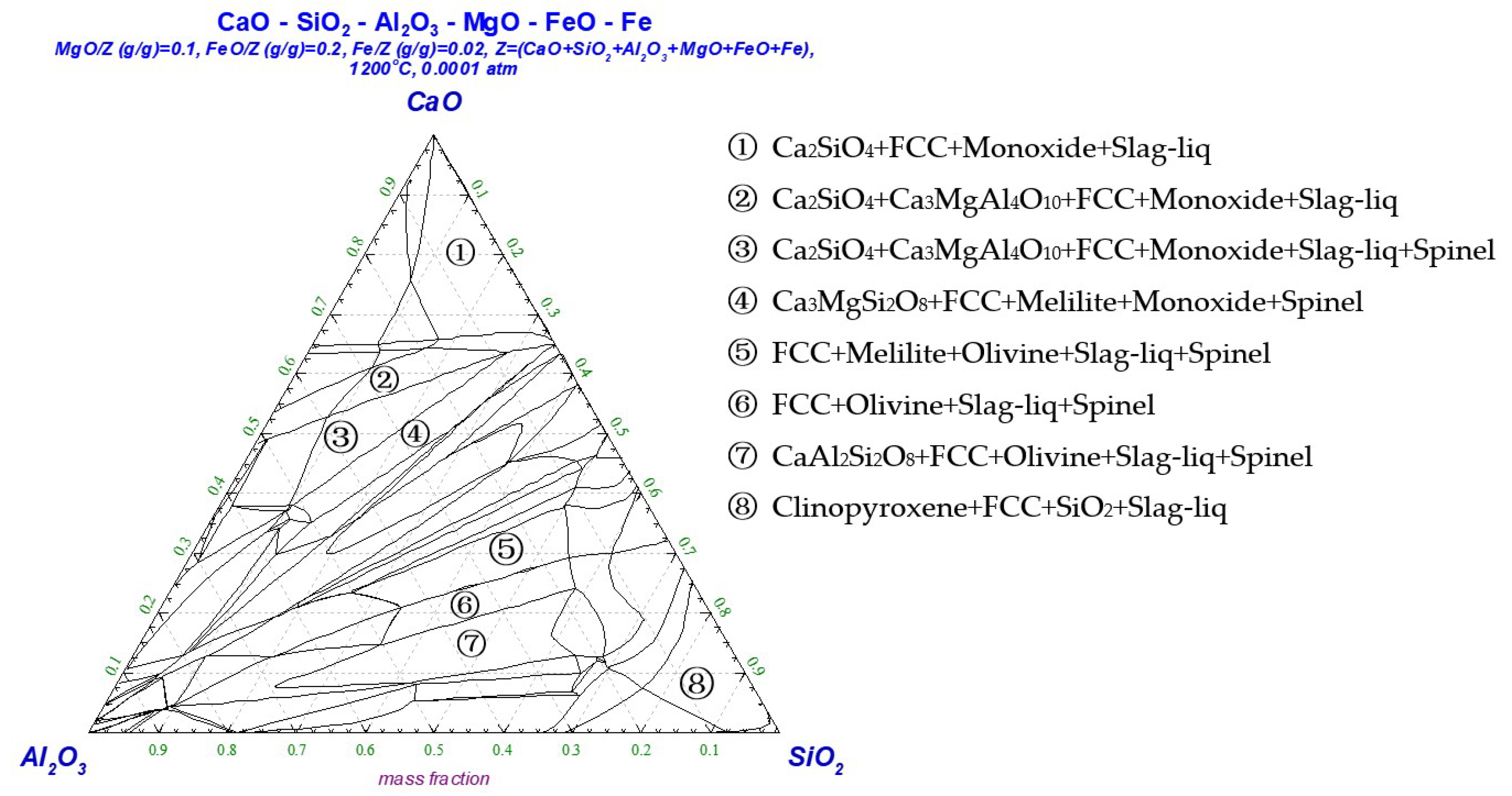
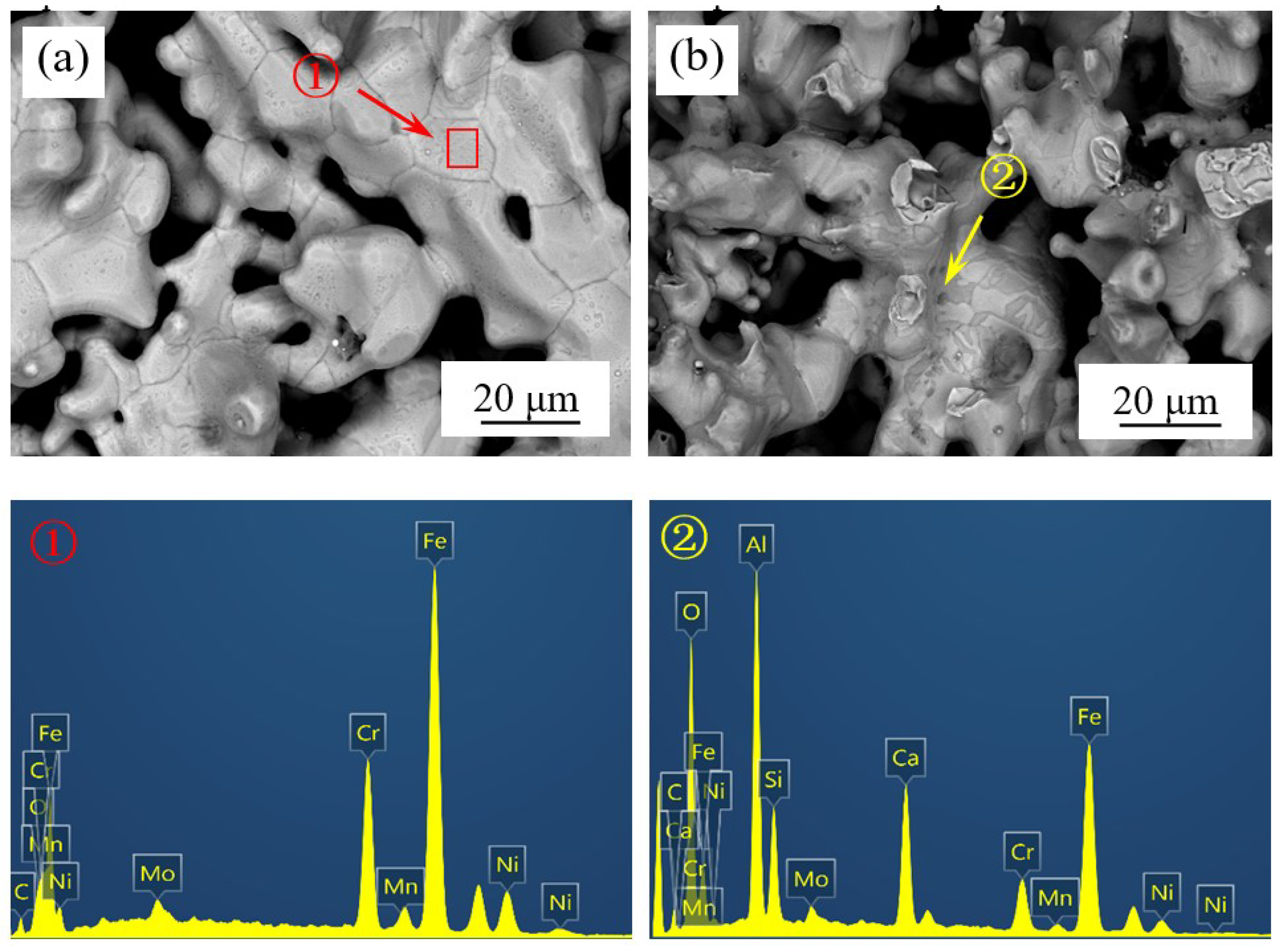
3.3. Growing up of Impurity Particles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hao, L.; Li, T.J.; Xie, Z.L.; Duan, Q.J.; Zhang, G.Y. The Oxidation Behaviors of Indefinite Chill Roll and High Speed Steel Materials. Metals 2020, 10, 1095. [Google Scholar] [CrossRef]
- 2021 Crude Steel Production Data Analytics. China Business Intelligence Network. Available online: https://baijiahao.baidu.com/s?id=1725663902517027102&wfr=spider&for=pc (accessed on 22 October 2022).
- Liu, J.X.; He, Y.; Xue, X.X. A new and simple route to prepare γ-Fe2O3 with iron oxide scale. Mater. Lett. 2018, 6, 112. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.; Gan, W.L.; Feng, H.; He, H.Y.; Ni, H.W. Synthesis of Ni-Zn ferrite from zinc in Zn-contain dust and iron in scale. J. Iron Steel Res. 2021, 33, 911–919. [Google Scholar]
- Yang, C. Basic charactaristics and comprehensive utilization of iron-bearing solid wastes from metallurgical enterprises. Environ. Eng. 2012, 30, 287–289. [Google Scholar]
- Zhao, C.H.; Wada, T.; Andrade, V.D.; Williams, G.J.; Gelb, J.; Li, L.; Thieme, J.; Kato, H.; Chen-Wiegart, Y.K. 3D Morphological and Chemical Evolution of Nanoporous Stainless Steel by Liquid Metal Dealloying. ACS Appl. Mater. Interfaces 2017, 10, 1021. [Google Scholar]
- Bencina, M.; Junkar, I.; Vesel, A.; AlešIgli, M.M. Nanoporous Stainless Steel Materials for Body Implants of Synthesizing Procedure. Nanomaterials 2022, 12, 2924. [Google Scholar] [CrossRef]
- Allioux, F.M.; Benavides, D.O.; Miren, E.; Kong, L.X.; Tanaka, D.A.P.; Dumée, L.F. Preparation of Porous Stainless Steel Hollow-Fibers through Multi-Modal Particle Size Sintering towards Pore Engineering. Membranes 2017, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Mercadelli, E.; Gondolini, A.; Pinasco, P.; Sanson, A. Stainless Steel Porous Substrates Produced by Tape Casting. Met. Mater. Int. 2017, 23, 184–192. [Google Scholar] [CrossRef]
- Abdullah, Z.; Ismail, A.; Ahmad, S. The Influence of Porosity on Corrosion Attack of Austenitic Stainless Steel. J. Phys. 2017, 10, 1088. [Google Scholar] [CrossRef]
- Xu, X.B.; Liu, P.S.; Chen, G.F.; Li, C.P. Sound Absorption Performance of Highly Porous Stainless Steel Foam with Reticular Structure. Met. Mater. Int. 2021, 27, 3316–3324. [Google Scholar] [CrossRef]
- Fousová, M.; Kubásek, J.; Vojtěch, D.; Fojt, J.; Čapek, J. 3D printed porous stainless steel for potential use in medicine. Mater. Sci. Eng. 2017, 10, 1088. [Google Scholar] [CrossRef]
- Dudek, A.; Włodarczyk, R. Effect of sintering atmosphere on properties of porous stainless steel for biomedical applications. Mater. Sci. Eng. C 2013, 33, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.; Chalivendra, V.; Rahbar, N.; Wakil, S.E. Mechanical characterization and modeling of graded porous stainless steel specimens for possible bone implant applications. Int. J. Eng. Sci. 2012, 53, 67–73. [Google Scholar] [CrossRef]
- Kato, K.; Yamamoto, A.; Ochiai, S.; Wada, M.; Daigo, Y.; Kita, K.; Omori, K. Cytocompatibility and mechanical properties of novel porous 316 L stainless steel. Mater. Sci. Eng. C 2013, 33, 2736–2743. [Google Scholar] [CrossRef] [PubMed]
- Groarke, R.; Danilenkoff, C.; Karam, S.; McCarthy, E.; Michel, B.; Mussatto, A.; Sloane, J.; O’Neill, A.; Raghavendra, R. 316L Stainless Steel Powders for Additive Manufacturing: Relationships of Powder Rheology, Size, Size Distribution to Part Properties. Materials 2020, 13, 5537. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.; Lim, K.S.; Park, J.K.; Song, J.H.; Oh, S.H.; Kim, J.W.; Zhang, Z.J.; Park, C.; Koh, J.T. Evaluation of cellular response and drug delivery efficacy of nanoporous stainless steel material. Biomater. Res. 2021, 10, 1186. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.B.; Li, J.; Yang, K. Preliminary Study on Porous High-Manganese 316L Stainless Steel Through Physical Vacuum Dealloying. Acta Metall. Sin. 2017, 30, 731–734. [Google Scholar] [CrossRef]
- Kirichenko, O.V.; Klimenko, V.N.; Shapoval, I.V.; Valeeva, I.K. Filtering properties of porous materials made of thin stainless steel fibers. Powder Metall. Met. Cer. 2015, 54, 151–155. [Google Scholar] [CrossRef]
- Amel-Farzad, H.; Peivandi, M.T.; Yusof-Sani, S.M.R. In-body corrosion fatigue failure of a stainless steel orthopedic implant with a rare collection of different damage mechanisms. Eng. Fail. Anal. 2007, 14, 1205–1217. [Google Scholar] [CrossRef]
- Li, W.Q.; Qu, Z.G.; Zhang, B.L.; Zhao, K.; Tao, W.Q. Thermal behavior of porous stainless-steel fiber felt saturated with phase change material. Energy 2013, 5, 846–852. [Google Scholar] [CrossRef]
- Kazantseva, N.; Krakhmalev, P.; Åsberg, M.; Koemets, Y.; Karabanalov, M.; Davydov, D.; Ezhov, I.; Koemets, O. Micromechanisms of Deformation and Fracture in Porous L-PBF 316L Stainless Steel at Different Strain Rates. Metals 2021, 11, 1870. [Google Scholar] [CrossRef]
- Krakhmalev, P.; Fredriksson, G.; Svensson, K.; Yadroitsev, I. Microstructure, solidification texture, and thermal stability of 316 L stainless steel manufactured by laser powder bed fusion. Metals 2018, 8, 643. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Lin, G.L.; Khonsari, M.M.; Zhang, J.H.; Yang, H.Y. Material characterization and lubricating behaviors of porous stainless steel fabricated by selective laser melting. J. Mater. Process. Technol. 2018, 262, 41–52. [Google Scholar] [CrossRef]
- Feng, P.; Liu, Y.; Wang, Y.; Li, K.; Zhao, X.U.; Tang, H.P. Sintering behaviors of porous 316L stainless steel fiber felt. J. Cent. South Univ. 2015, 22, 793–799. [Google Scholar] [CrossRef]
- Ao, Q.B.; Wang, J.Z.; Ma, J.; Ge, Y. Sound Absorption Properties of Stainless Steel Fiber Porous Materials before/after Corrosion. Mater. Sci. 2018, 933, 367–372. [Google Scholar] [CrossRef]
- Zhang, W.P.; Li, L.J.; Gao, J.X.; Huang, J.M.; Zhang, X.K. The Effect of Porosity on Mechanical Properties of Porous FeCrN Stainless Steel. J. Phys. 2021, 10, 1088. [Google Scholar] [CrossRef]
- Öztürk, B.; Topcu, A.; Cora, Ö.N. Influence of processing parameters on the porosity, thermal expansion, and oxidation behavior of consolidated Fe22Cr stainless steel powder. Powder Technol. 2021, 382, 199–207. [Google Scholar] [CrossRef]
- Ma, D.; Guo, P.M.; Pang, J.M.; Zhao, P. Theoretical Analysis and Industrial Experiment of Direct Alloying of Molybdenum Oxide for 316L Steel. Iron Steel. 2014, 49, 27–30. [Google Scholar]
- Wang, G.P.; Liu, C.J. Theoretical and experimental research of molybdenum oxide alloying for 316L stainless steel smelting process. J. Iron Steel Rea. Int. 2018, 30, 354–358. [Google Scholar]
- Stavropoulos, P.; Panagiotopoulou, V.C.; Papacharalampopoulos, A.; Aivaliotis, P.; Georgopoulos, D.; Konstantinos, S. A Framework for CO2 Emission Reduction in Manufacturing Industries: A Steel Industry Case. Designs 2022, 6, 22. [Google Scholar] [CrossRef]
- Mysteel.com. Available online: https://feigang.mysteel.com/m/22/1008/10/AF407D5E375A3AA4.html (accessed on 22 October 2022).
- Mukasheva, N.Z.; Kosdauletovb, N.Y.; Suleimen, B.T. Comparison of Iron and Chromium Reduction from Chrome Ore Concentrates by Solid Carbon and Carbon Monoxide. Solid State Phenom. 2020, 299, 1152–1157. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, M.F.; Wang, D.Y. Material Balance Calculation of Chromium Ore Smelting Reduction and Direct Alloying Process in a Converter. Adv. Mater. Res. 2012, 485, 574–577. [Google Scholar] [CrossRef]
- You, Y.; Wei, B.K.; You, W.; Wang, Z.L.; Jiang, B.L. Compound Control Method of Carbon Content in Argon—Oxygen Refining Ferrochromium Alloy. Trans. Indian Inst. Met. 2021, 10, 1007. [Google Scholar] [CrossRef]
- Teng, J.W.; Gong, X.J.; Yang, B.B.; Yu, S.; Lai, R.L.; Liu, J.T.; Li, Y.P. High temperature oxidation behavior of a novel Ni-Cr-W-Al-Ti superalloy. Corros. Sci. 2022, 10, 1016. [Google Scholar] [CrossRef]
- Wang, Y.X.; Tang, Y.J.; Wan, W.; Zhang, X. High-Temperature Oxidation Resistance of CrMoN Films. J. Mater. Eng. Perform. 2020, 10, 1007. [Google Scholar] [CrossRef]
- Kryukova, R.E.; Goryushkina, V.F.; Bendrea, Y.V.; Bashchenkoa, L.P.; Kozyreva, N.A. Thermodynamic Aspects of Cr2O3 Reduction by Carbon. Steel Trans. 2019, 49, 843–847. [Google Scholar] [CrossRef]
- Li, J.; Ren, X.; Zhang, Y.; Hou, H.; Gao, X. Effect of superplastic deformation on precipitation behavior of sigma phase in 3207 duplex stainless steel. Mater. Int. 2021, 31, 334–340. [Google Scholar] [CrossRef]
- Liu, R.; Dang, X.T.; Peng, Y.T.; Wu, T. Microstructure and Wear Behavior of Laser Cladded CoCrNiMox Coatings on the Low Carbon Steel. Crystals 2022, 12, 1229. [Google Scholar] [CrossRef]
- Tu, Y.; Zhang, Y.; Su, Z.; Jiang, T. Mineralization mechanism of limonitic laterite sinter under different fuel dosage: Effect of FeO. Powder Technol. 2022, 10, 1016. [Google Scholar] [CrossRef]
- Liao, J.; Zhao, B. Experimental Studies in Phase Equilibrium of the System “FeO-SiO2-MgO-Al2O3-Cr2O3” at Iron Saturation. Metal. Mater. Trans. B 2021, 52, 2364–2374. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.S.; Xue, Q.G. Kinetics of the Volume Shrinkage of a Magnetite/Carbon Composite Pellet during Solid-State Carbothermic Reduction. Metals 2018, 8, 1050. [Google Scholar] [CrossRef]
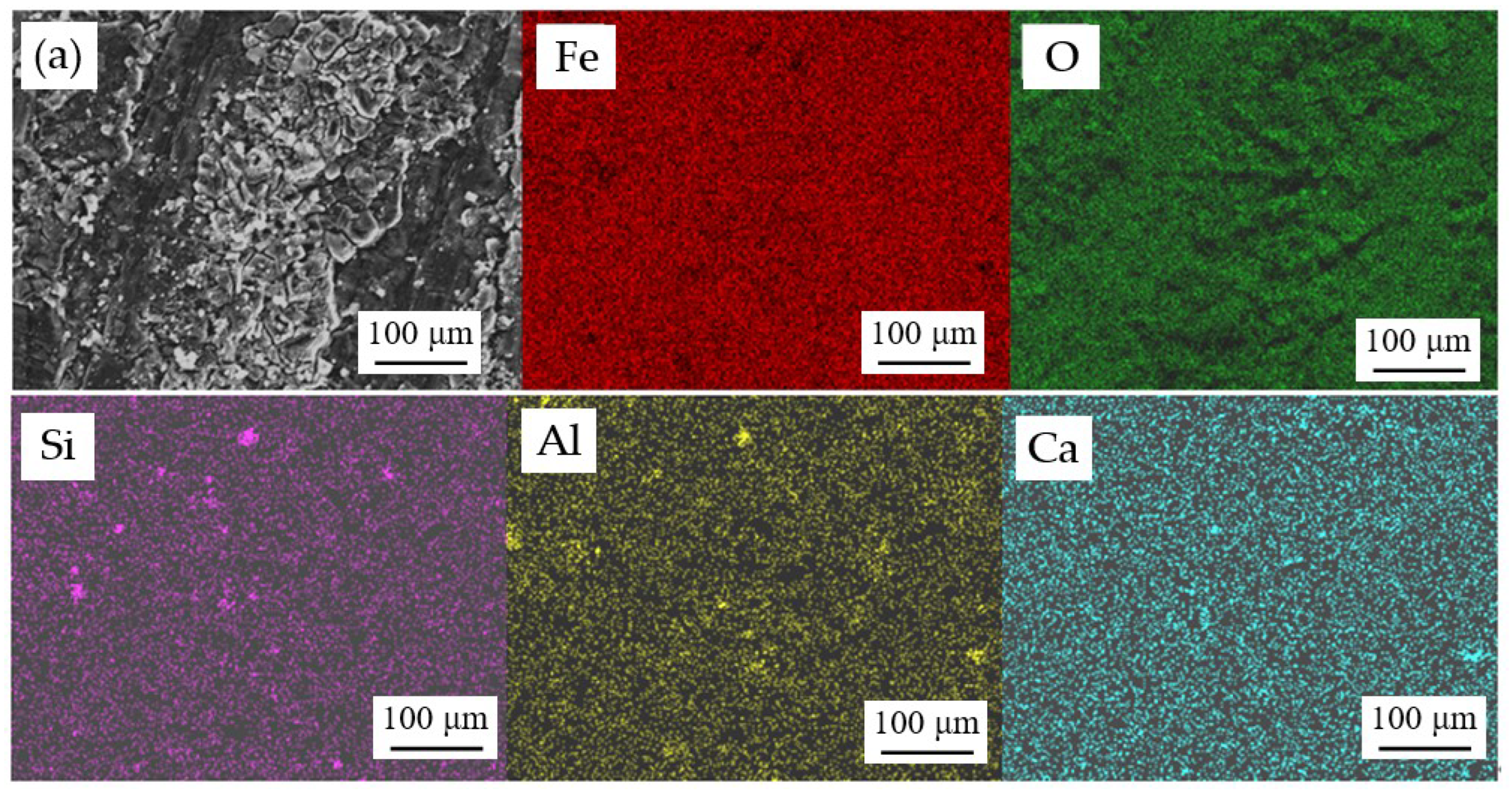
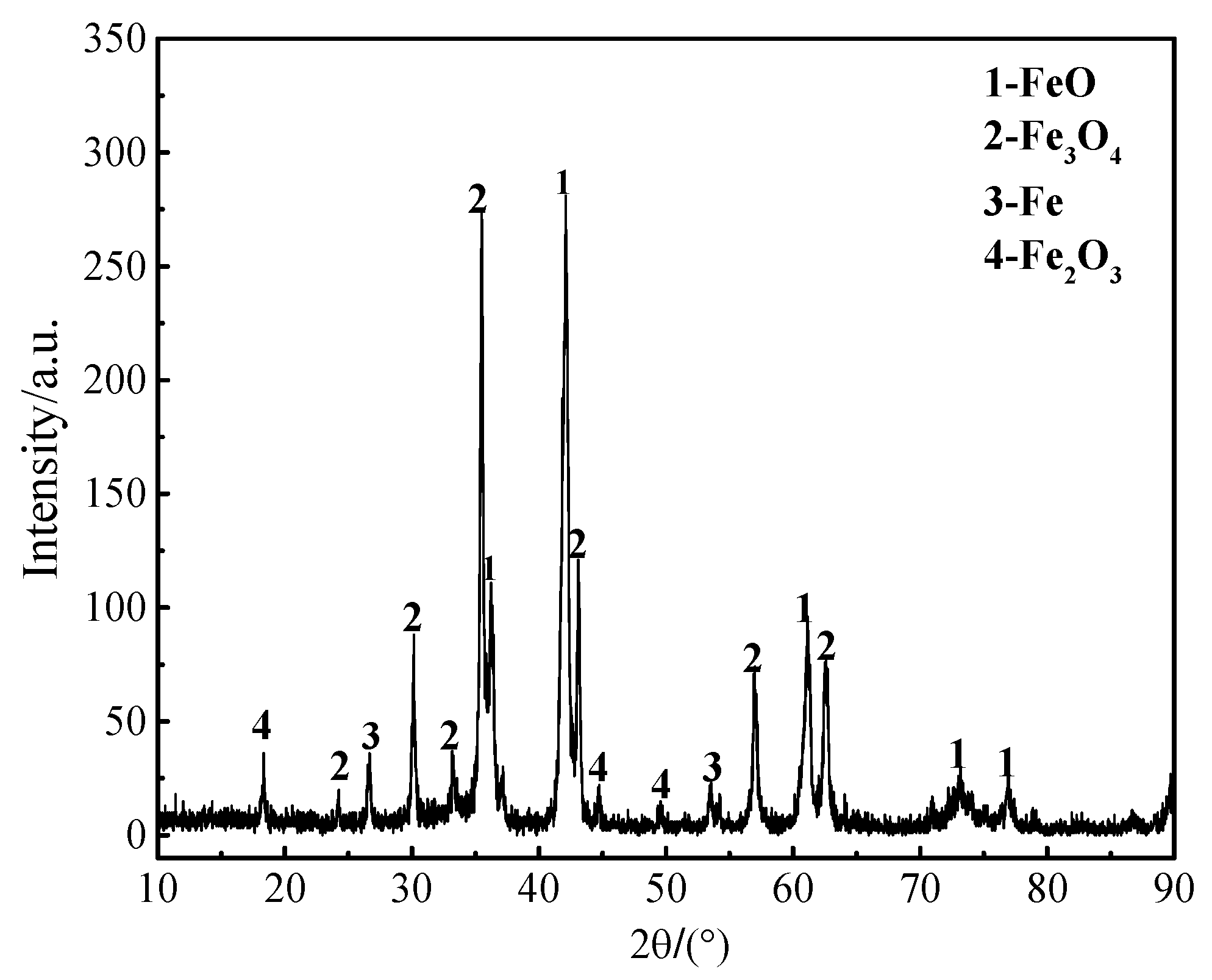
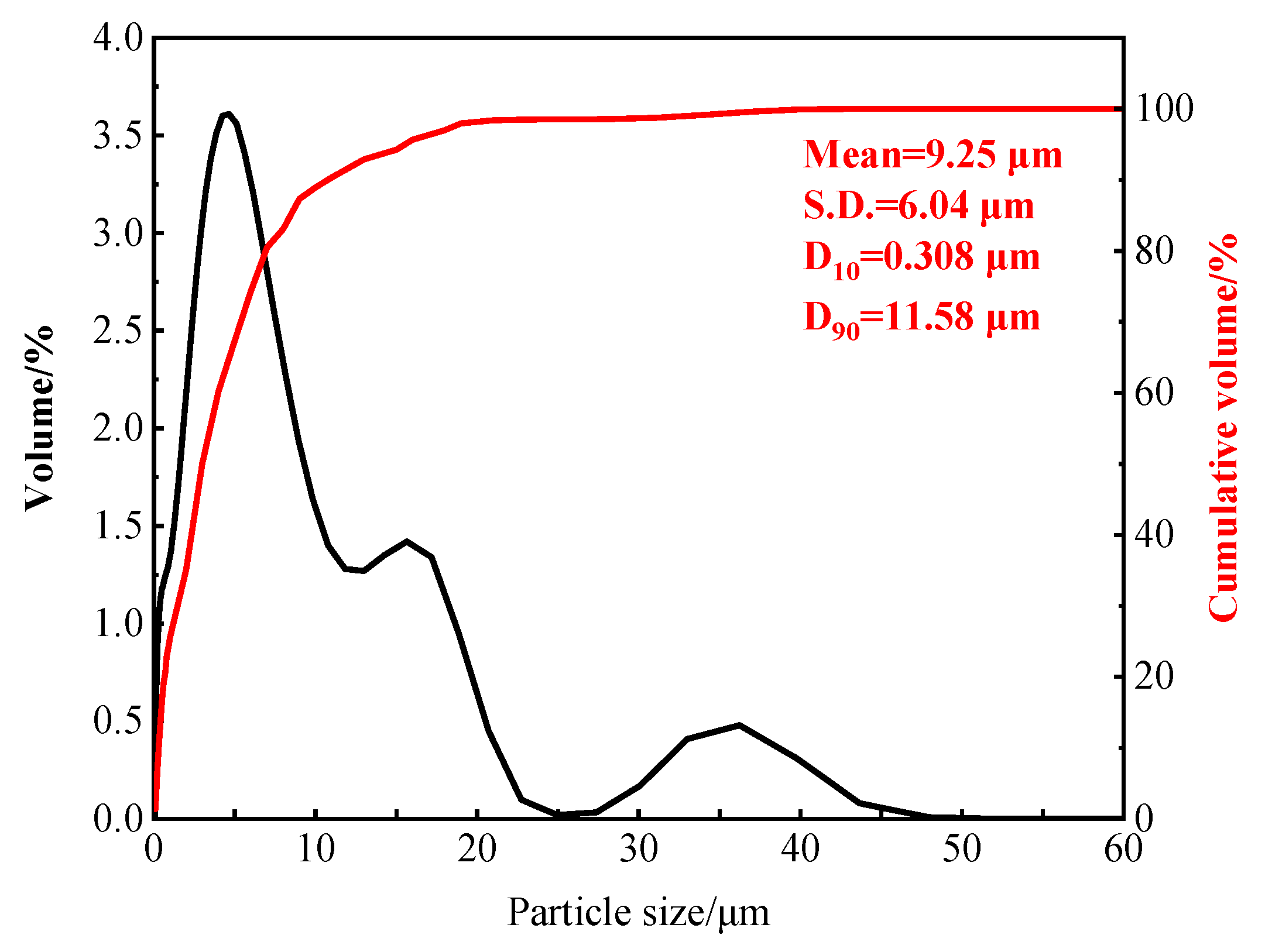


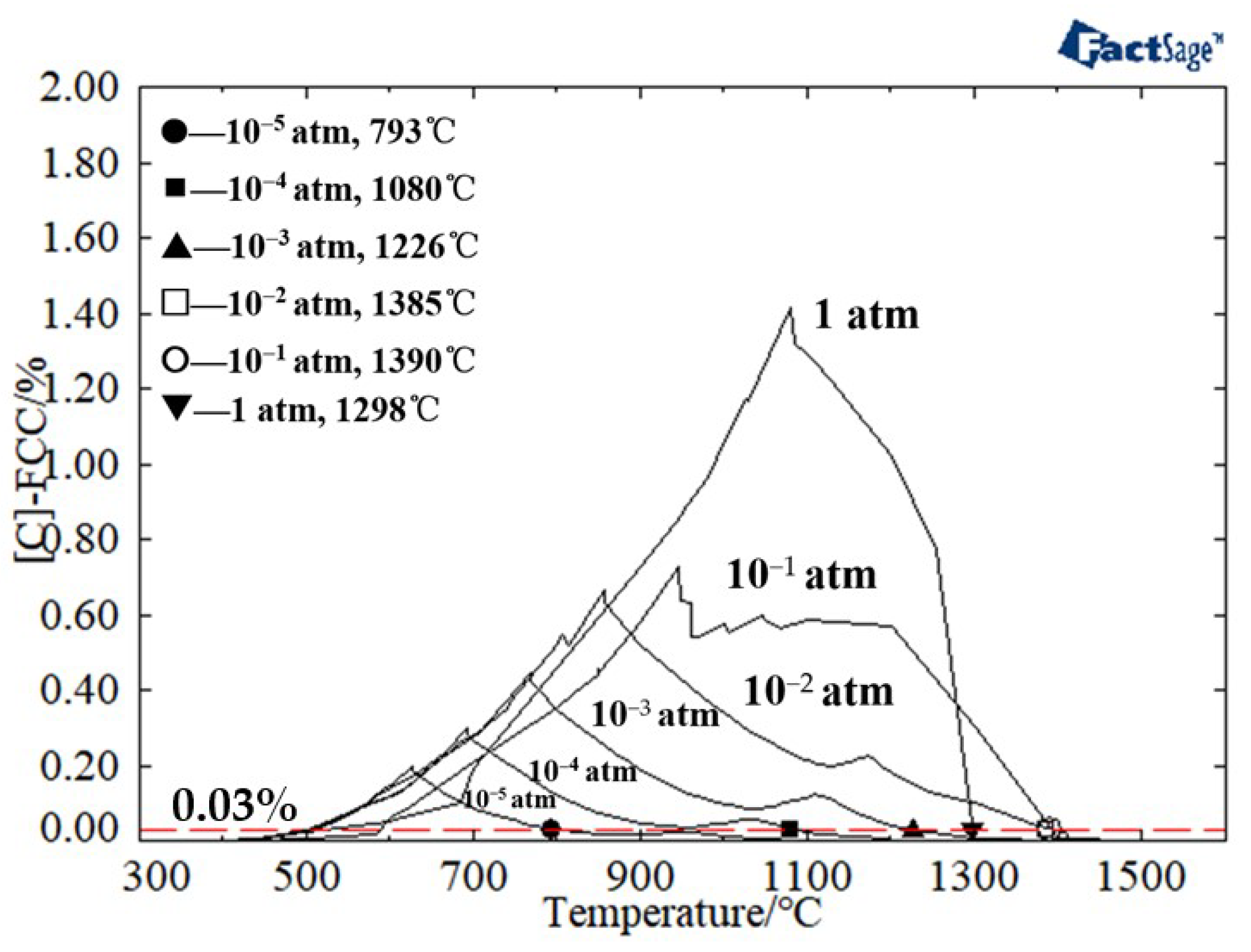




| Component | C | Cr | Ni | Mn | Mo | S | P | Fe |
|---|---|---|---|---|---|---|---|---|
| Steel specification | ≤0.03 | 16~18 | 10~14 | ≤2.0 | 2~3 | ≤0.030 | ≤0.035 | Bal |
| Median | ≤0.03 | 17 | 12 | ≤2.0 | 2.5 | ≤0.030 | ≤0.035 | Bal |
| TFe | FeO | Fe2O3 | SiO2 | CaO | Al2O3 | MgO | S | P | LOI |
|---|---|---|---|---|---|---|---|---|---|
| 73.17 | 58.63 | 39.38 | 0.30 | 0.36 | 0.23 | 0.39 | 0.02 | 0.01 | 0.68 |
| Auxiliary Materials | Purity | Manufacturer |
|---|---|---|
| Chromium metal powder | ≥99.9% | Zhongmai Metal Materials Co., Ltd., Nangong, China |
| Nickel metal powder | ≥99.9% | Zhongmai Metal Materials Co., Ltd., Nangong, China |
| Molybdenum metal powder | ≥99.9% | Zhongmai Metal Materials Co., Ltd., Nangong, China |
| Graphite powder | ≥99.9% | Kermel Chemical Reagent Co., Ltd., Tianjin, China |
| Iron Oxide Scale | C | Cr | Ni | Mo |
|---|---|---|---|---|
| 90.88 | 16.93 | 17.00 | 12.00 | 2.50 |
| Raw Material | Metal Chromium | Metal Nickle | Metal Molybdenum |
|---|---|---|---|
| Yield | 98.71 | 100.00 | 97.20 |
| Fe | Ni | Mn | Mo | Cr | C | O | S | P |
|---|---|---|---|---|---|---|---|---|
| 66.89 | 12.00 | 1.98 | 2.49 | 16.60 | 0.025 | 0.20 | 0.010 | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Peng, J.; Chang, H.; Wang, Y. Vacuum Carbon Reducing Iron Oxide Scale to Prepare Porous 316 Stainless Steel. Metals 2022, 12, 2118. https://doi.org/10.3390/met12122118
Zhang F, Peng J, Chang H, Wang Y. Vacuum Carbon Reducing Iron Oxide Scale to Prepare Porous 316 Stainless Steel. Metals. 2022; 12(12):2118. https://doi.org/10.3390/met12122118
Chicago/Turabian StyleZhang, Fang, Jun Peng, Hongtao Chang, and Yongbin Wang. 2022. "Vacuum Carbon Reducing Iron Oxide Scale to Prepare Porous 316 Stainless Steel" Metals 12, no. 12: 2118. https://doi.org/10.3390/met12122118
APA StyleZhang, F., Peng, J., Chang, H., & Wang, Y. (2022). Vacuum Carbon Reducing Iron Oxide Scale to Prepare Porous 316 Stainless Steel. Metals, 12(12), 2118. https://doi.org/10.3390/met12122118






