Tin Removal from Tin-Bearing Iron Concentrate with a Roasting in an Atmosphere of SO2 and CO
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterization
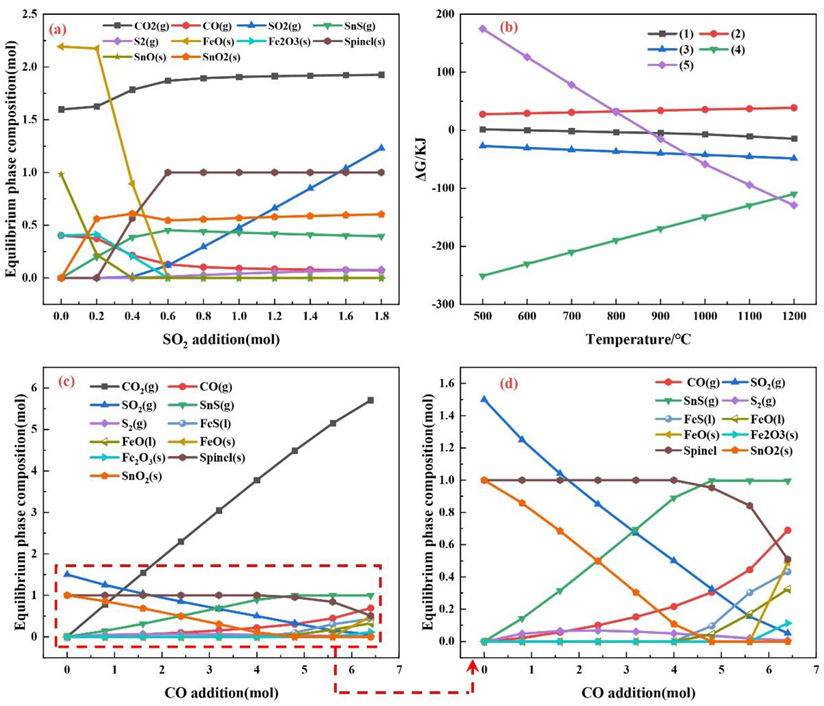
3. Thermodynamic Analysis
4. Results and Discussion
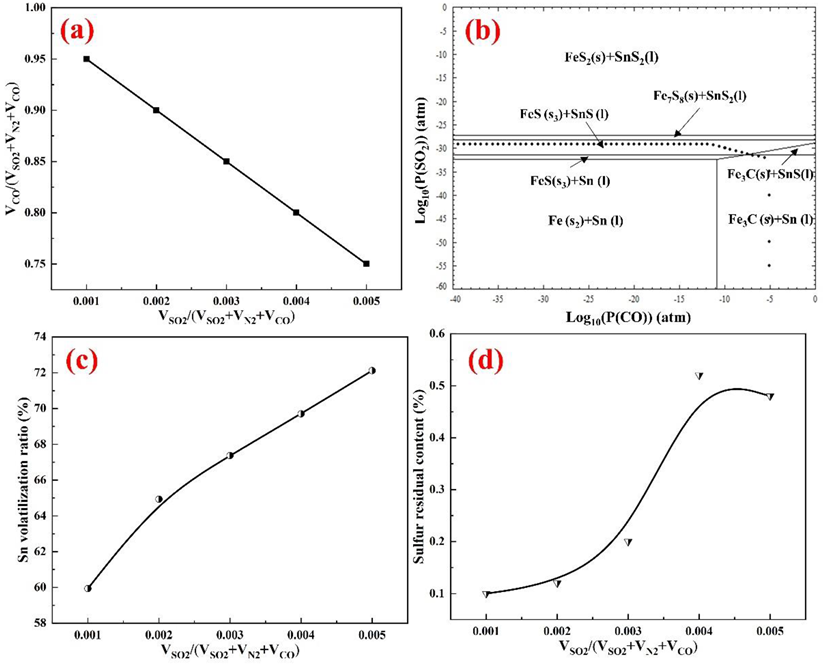
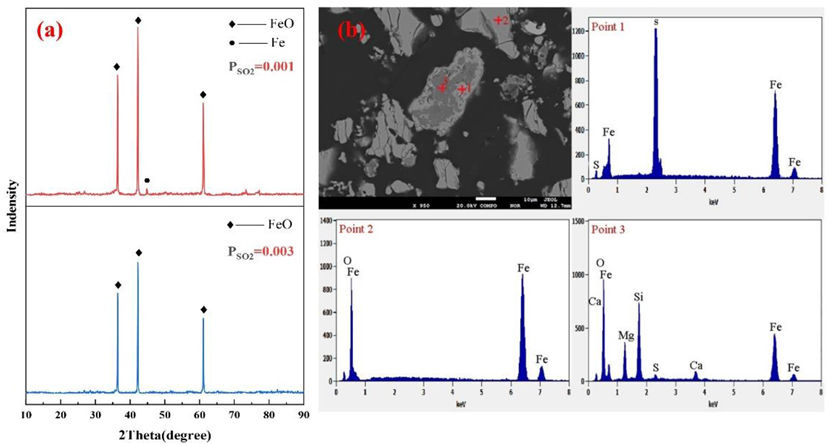
4.1. Effects of the SO2 Partial Pressure
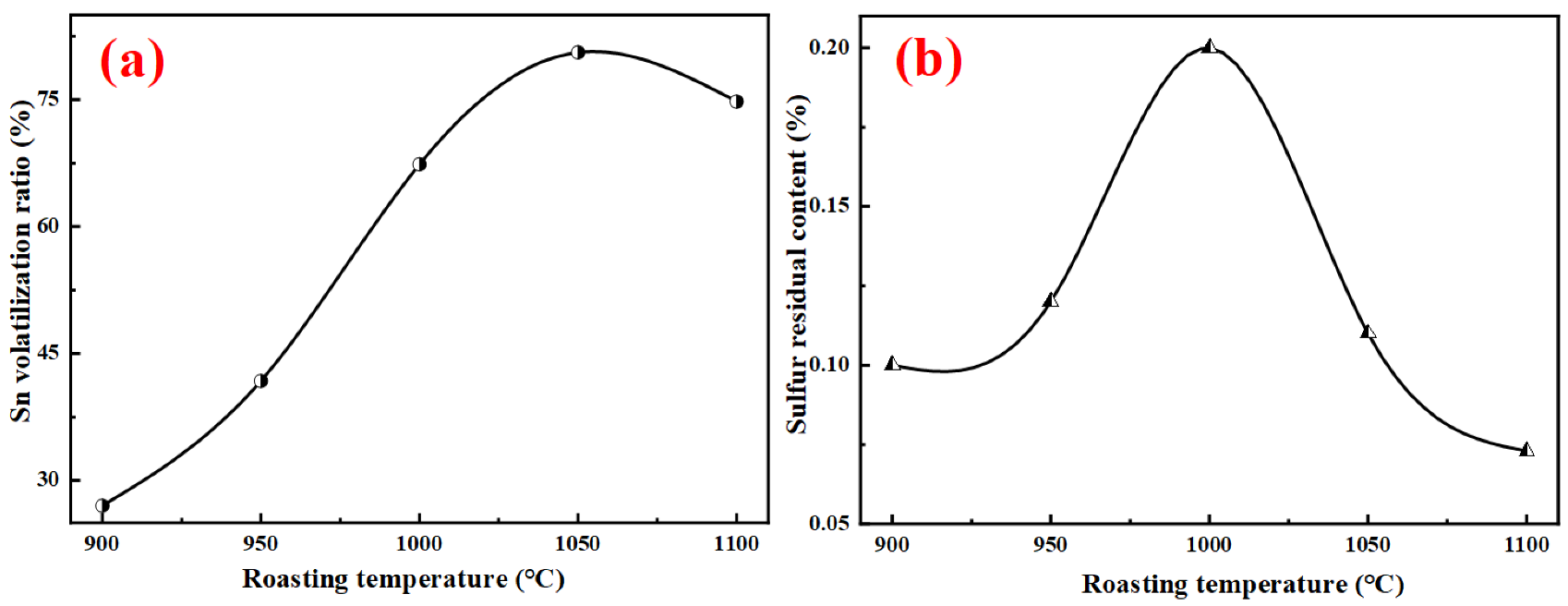
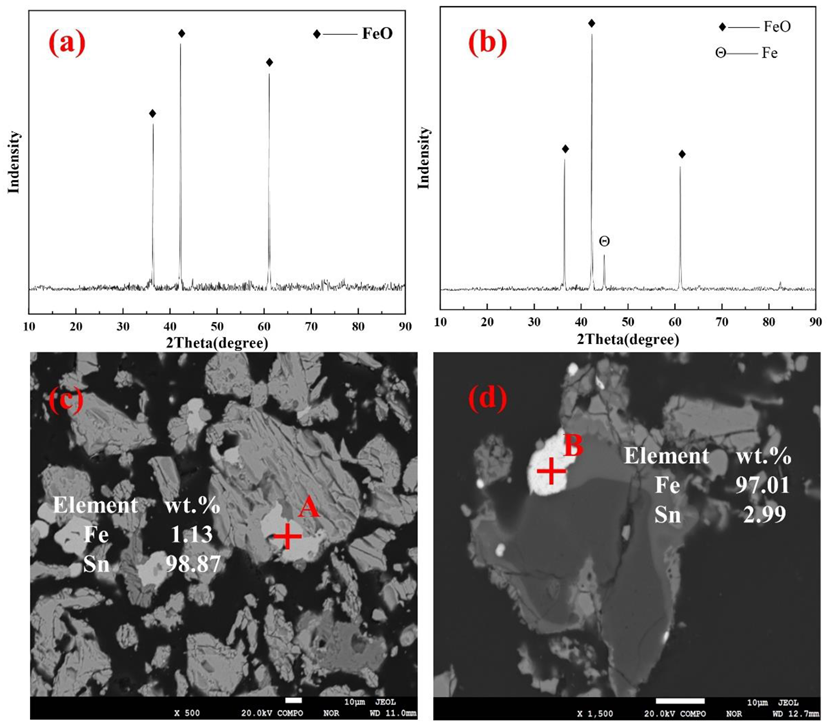
4.2. Effects of Roasting Temperature
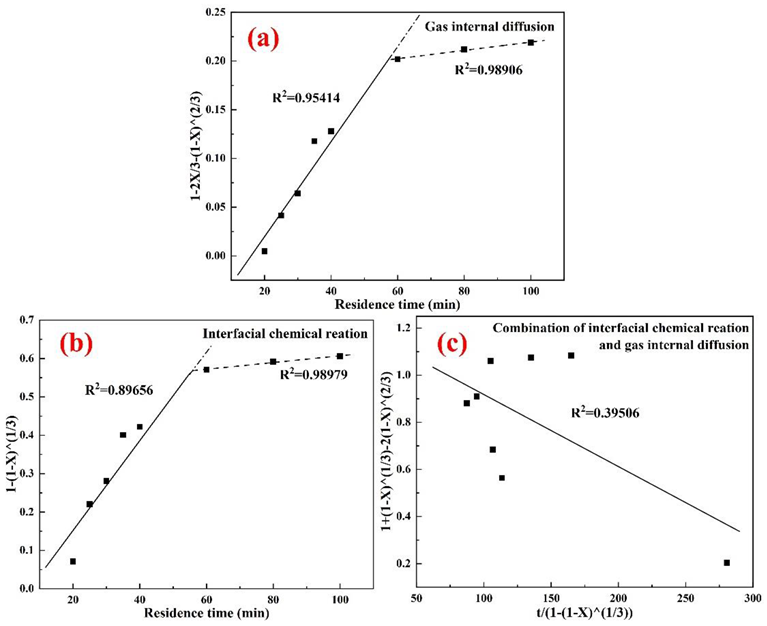
4.3. Effects of the Residence Time
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Sun, H.H.; Bai, J.; Li, L.T. Innovative methodology for comprehensive utilization of iron ore tailings: Part 1. The recovery of iron from iron ore tailings using magnetic separation after magnetizing roasting. J. Hazard. Mater. 2010, 174, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Cao, Z.; Wang, S.; Zhong, H. Phase transformation of iron in limonite ore by microwave roasting with addition of alkali lignin and its effects on magnetic separation. J. Alloys Compd. 2017, 722, 651–661. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T.; Elmandy, A.M.; Abdel-Khalek, N.A.; Gornostayev, S. Improvement of phosphorus removal from iron ore using combined microwave pretreatment and ultrasonic treatment. Sep. Purif. Technol. 2015, 156, 724–737. [Google Scholar] [CrossRef]
- Yu, Y.; Li, L.; Wang, J.Y.; Li, K.Z.; Wang, H. Phase transformation of Sn in tin-bearing iron concentrates by roasting with FeS2 in CO-CO2 mixed gases and its effects on Sn separation. J. Alloys Compd. 2018, 750, 8–16. [Google Scholar] [CrossRef]
- Yu, Y.; Li, L.; Wang, J.Y.; Wang, J.C.; Li, K.Z. Sn separation from Sn-bearing iron concentrates by roasting with waste tire rubber in N2 + CO + CO2 mixed gases. J. Hazard. Mater. 2019, 371, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, L.; Sang, X.L. Removing tin from tin-bearing iron concentrates with sulfidation roasting using high sulfur coal. ISIJ Int. 2016, 56, 57–62. [Google Scholar] [CrossRef]
- Zhang, R.J.; Li, L.; Li, K.Z.; Yu, Y.; Wang, H. Reduction and sulfurization behavior of tin phases in tin-bearing iron concentrates with sulfates in sulfur-bearing stone coal. ISIJ Int. 2018, 58, 453–459. [Google Scholar] [CrossRef]
- Zhang, R.J.; Li, L.; Li, K.Z.; Yu, Y. Reduction roasting of tin-Bearing iron concentrates using pyrite. ISIJ Int. 2016, 56, 953–959. [Google Scholar] [CrossRef]
- Su, Z.J.; Zhang, Y.B.; Liu, B.B.; Zhou, Y.L.; Jiang, T.; Li, G.H. Reduction behavior of SnO2 in the tin-bearing iron concentrates under CO-CO2 atmosphere. Part I: Effect of magnetite. Powder Technol. 2016, 292, 251–259. [Google Scholar] [CrossRef]
- Wang, Z.W.; Wang, C.Y.; Lu, H.M. Investigation on removal of tin from Sn-Bearing iron concentrates by reduction roasting. Mining Metall. 2005, 14, 63–66. [Google Scholar] [CrossRef]
- Yu, Y.; Li, L. Transformation behaviour of sulfur from gypsum waste (CaSO4·2H2O) while roasting with tin-bearing iron concentrate for tin removal and iron recovery. ISIJ Int. 2020, 60, 2291–2300. [Google Scholar] [CrossRef]
- Pandey, R.; Biswas, R.; Chakrabarti, T.; Devotta, S. Flue gas desulfurization: Physicochemical and biotechnological approaches. Crit. Rev. Environ. Sci. Technol. 2005, 35, 571–622. [Google Scholar] [CrossRef]
- Bates, T.; Lamb, B.; Guenther, A.; Dignon, J.; Stoiber, R. Sulfur emissions to the atmosphere from natural sources. J. Atmos. Chem. 1992, 14, 315–337. [Google Scholar] [CrossRef]
- Ma, K.; Deng, J.; Ma, P.; Sun, C.; Zhou, Q.; Xu, J. A novel plant-internal route of recycling sulfur from the flue gas desulfurization (FGD) ash through sintering process: From lab-scale principles to industrial practices. J. Environ. Chem. Eng. 2021, 10, 106957. [Google Scholar] [CrossRef]
- Lim, J.; Cho, H.; Kim, J. Optimization of wet flue gas desulfurization system using recycled waste oyster shell as high-grade limestone substitutes. J. Cleaner Prod. 2021, 318, 128492. [Google Scholar] [CrossRef]
- Lim, J.; Choi, Y.; Kim, G.; Kim, J. Modeling of the wet flue gas desulfurization system to utilize low-grade limestone. Korean J. Chem. Eng. 2020, 37, 2085–2093. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Li, L.; Ren, Q.; Yang, X.; Jiang, Z.; Zhang, Z. Utilization of low-quality desulfurized ash from semi-dry flue gas desulfurization by mixing with hemihydrate gypsum. Fuel 2019, 255, 115783. [Google Scholar] [CrossRef]
- Fang, D.; Liao, X.; Zhang, X.; Teng, A.; Xue, X. A novel resource utilization of the calcium-based semi-dry flue gas desulfurization ash: As a reductant to remove chromium and vanadium from vanadium industrial wastewater. J. Hazard. Mater. 2018, 342, 436–445. [Google Scholar] [CrossRef]
- Matsushima, N.; Li, Y.; Nishioka, M.; Sadakata, M.; Qi, H.; Xu, X. Novel dry-desulfurization process using Ca(OH)2/fly ash sorbent in a circulating fluidized bed. Environ. Sci. Technol. 2004, 38, 6867–6874. [Google Scholar] [CrossRef]
- Li, Y.; You, C.; Song, C. Adhesive carrier particles for rapidly hydrated sorbent for moderate-temperature dry flue gas desulfurization. Environ. Sci. Technol. 2010, 44, 4692–4696. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Zhang, Z.; Nie, M.; Wang, L.; Zhang, H. Adsorption of condensable particulate matter from coal-fired flue gas by activated carbon. Sci. Total Environ. 2021, 778, 146245. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dou, J.; Duan, X.; Chai, H.; Oliveira, J.; Yu, J. Adverse effects of inherent CaO in coconut shell-derived activated carbon on its performance during flue gas desulfurization. Environ. Sci. Technol. 2009, 54, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Cheng, S.; Li, J.; Zhang, H.; Jia, J.; Sun, X. Effect of wet flue gas desulfurization (WFGD) on fine particle (PM2.5) emission from coal-fired boilers. J. Environ. Sci. 2019, 77, 32–42. [Google Scholar] [CrossRef]
- Zhang, Y.Y. Behaviour Pattern of Stannous Sulfide under Vacuum and High Temperature and Experimental Study of Vacuum Distillation of Sulfur Slag. Master’s. Thesis, Kunming University of Science and Technology, Kunming, China, 2014. [Google Scholar]
- Guo, Y.F. Study on Strengthening of Solid-State Reduction and Comprehensive Utilization of Vanadiferous Titanomagnetite. Ph.D. Thesis, Central South University of Technology, Changsha, China, 2007. [Google Scholar]
- Zhang, G.H.; Chou, K.C.; Zhao, H.L. Reduction kinetics of FeTiO3 powder by hydrogen. ISIJ Int. 2012, 52, 1986–1989. [Google Scholar] [CrossRef]
- Mo, D.C. Kinetics for Metallurgy, 1st ed.; Central South University Press: Changsha, China, 1987; pp. 154–196. [Google Scholar]
- Padilla, R.; Sohn, H.Y. The reduction of stannic oxide with carbon. Metall. Mater. Trans. B 1979, 10, 109–115. [Google Scholar] [CrossRef]
- Zhang, C.F.; Peng, B.; Peng, J. Electric arc furnace dust non-isothermal reduction kinetics. Trans. Nonferrous Met. Soc. China 2000, 10, 524–530. [Google Scholar]
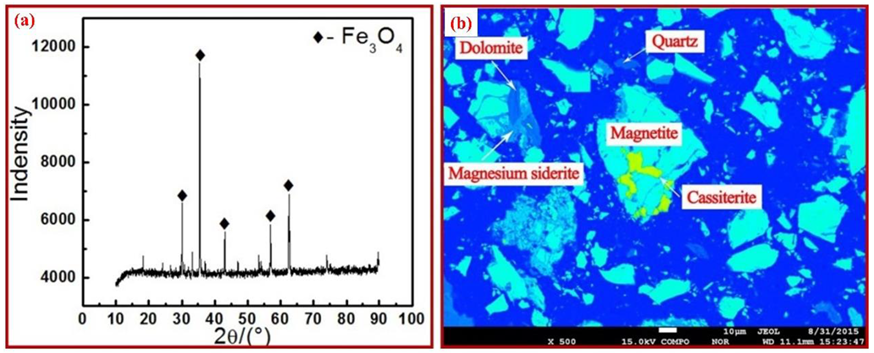
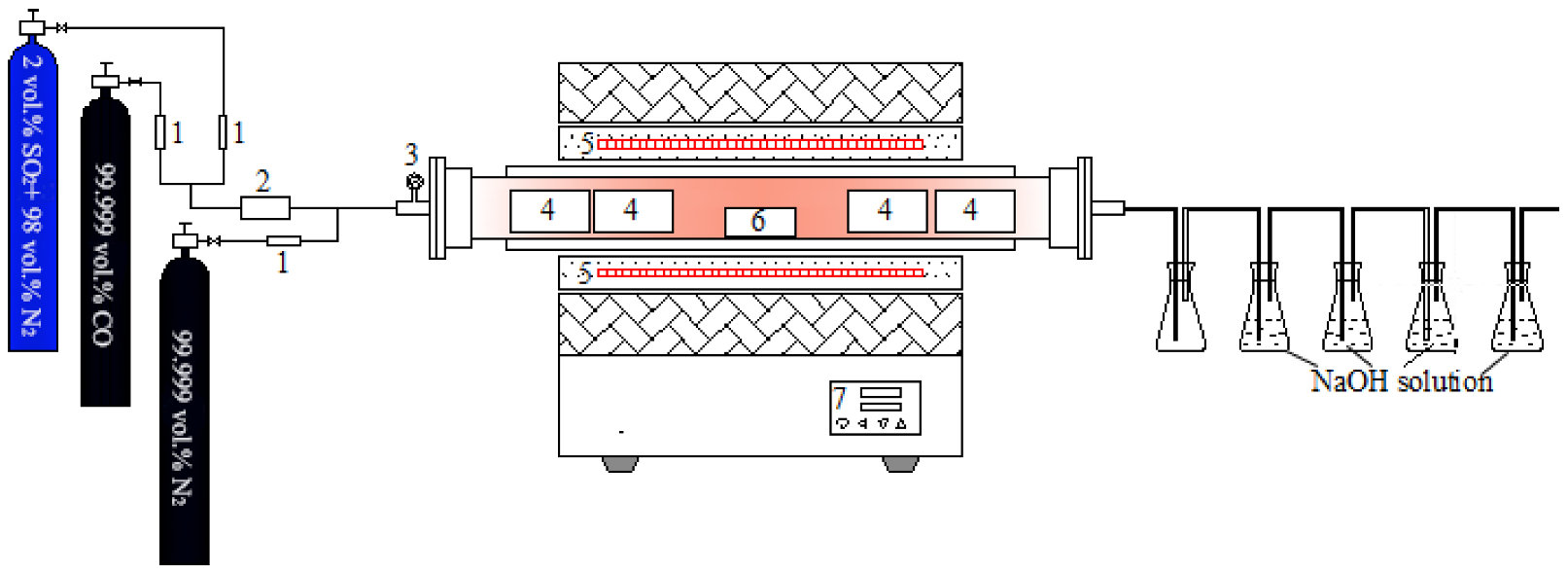

| Element | Fe | SiO2 | Sn | Zn | CaO | Al2O3 | Pb | Others |
|---|---|---|---|---|---|---|---|---|
| Content | 65.52 | 0.91 | 0.39 | 0.04 | 2.30 | 0.87 | 0.1 | 29.87 |
| Controlling Step | Kinetic Equation [26] |
|---|---|
| Interfacial chemical reaction | t = a [1 − (1 − X)1/3] |
| Gas internal diffusion | t = b [1 − 2X/3 − (1 − X)2/3] |
| Combination of interfacial chemical reaction and gas internal diffusion | t = a1 [1 − (1 − X)1/3] + b1 [1 − 2X/3 − (1 − X)2/3] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Xu, Z.; Wang, S. Tin Removal from Tin-Bearing Iron Concentrate with a Roasting in an Atmosphere of SO2 and CO. Metals 2022, 12, 1974. https://doi.org/10.3390/met12111974
Li L, Xu Z, Wang S. Tin Removal from Tin-Bearing Iron Concentrate with a Roasting in an Atmosphere of SO2 and CO. Metals. 2022; 12(11):1974. https://doi.org/10.3390/met12111974
Chicago/Turabian StyleLi, Lei, Zhipeng Xu, and Shiding Wang. 2022. "Tin Removal from Tin-Bearing Iron Concentrate with a Roasting in an Atmosphere of SO2 and CO" Metals 12, no. 11: 1974. https://doi.org/10.3390/met12111974
APA StyleLi, L., Xu, Z., & Wang, S. (2022). Tin Removal from Tin-Bearing Iron Concentrate with a Roasting in an Atmosphere of SO2 and CO. Metals, 12(11), 1974. https://doi.org/10.3390/met12111974







