Effects of Electrolytic Copper Foil Roughness on Lithium-Ion Battery Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
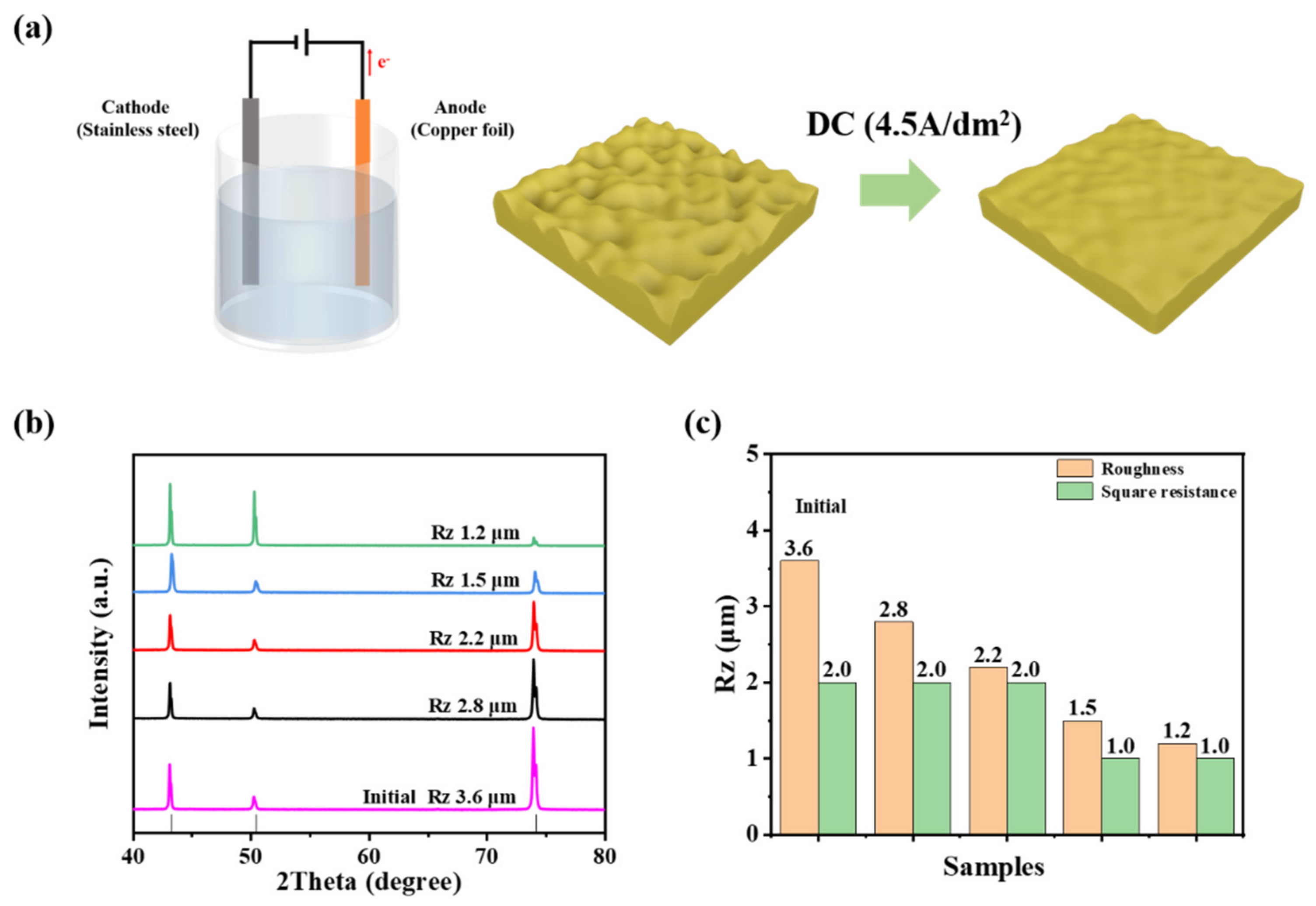
2.2. Modification of the Electrolytic Copper Foil
2.3. Material Characterization
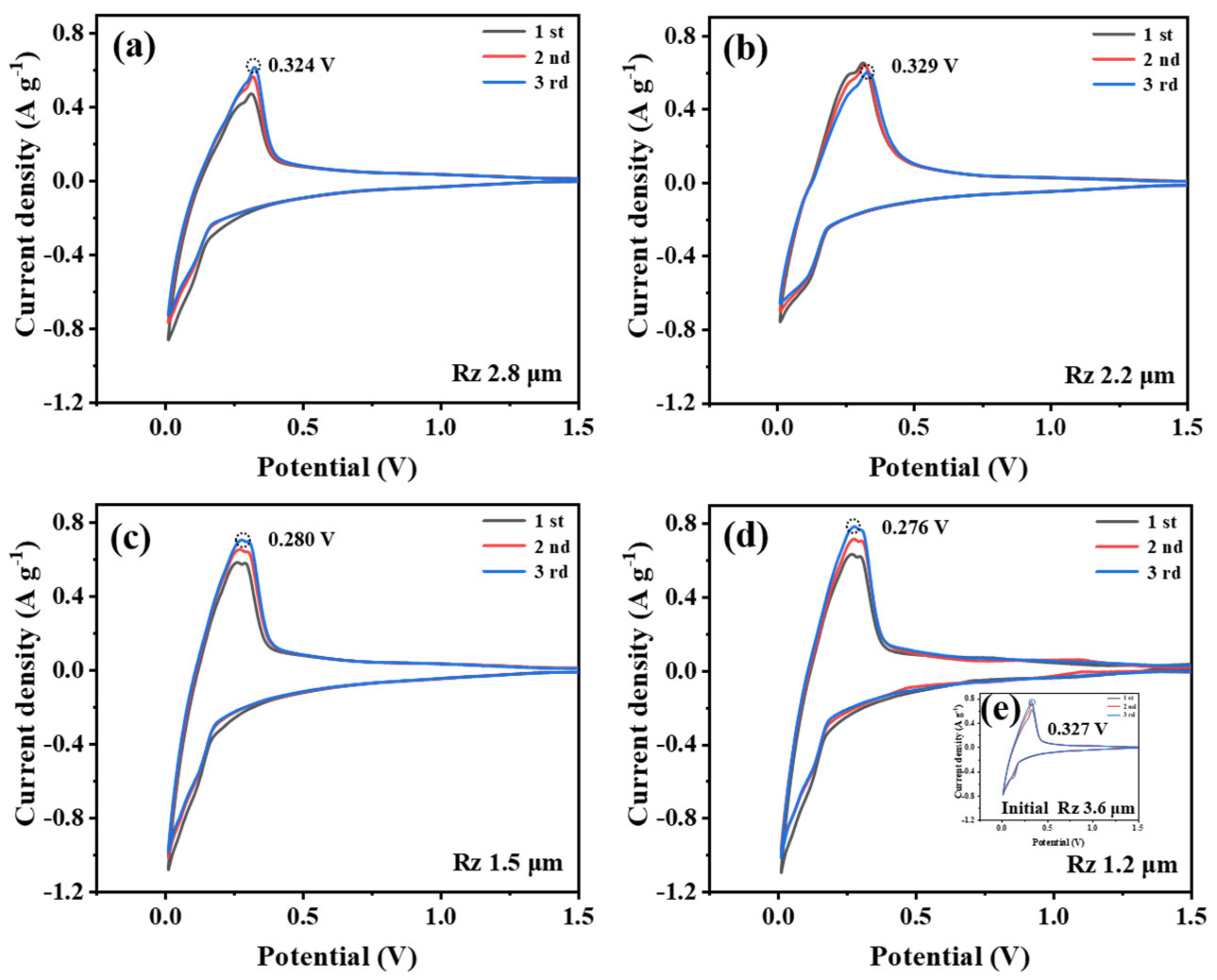
2.4. Electrode Sheet Preparation and Electrochemical Testing
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Wang, X.; Gao, H.; Yan, S.; Chen, S.; Liu, X.; Hu, X. Rolled electrodeposited copper foil with modified surface morphology as anode current collector for high performance lithium-ion batteries. Surf. Coatings Technol. 2021, 410, 126881. [Google Scholar] [CrossRef]
- Fan, X.-Y.; Ke, F.-S.; Wei, G.-Z.; Huang, L.; Sun, S.-G. Sn–Co alloy anode using porous Cu as current collector for lithium ion battery. J. Alloys Compd. 2009, 476, 70–73. [Google Scholar] [CrossRef]
- Arai, S.; Kitamura, T. Simple Method for Fabrication of Three-Dimensional (3D) Copper Nanostructured Architecture by Electrodeposition. ECS Electrochem. Lett. 2014, 3, D7–D9. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Chen, H.; Wen, M.; Shen, K.; Chen, Q.; Hou, G.; Tang, Y. Lithiophilic 3D Copper-Based Magnetic Current Collector for Lithium-Free Anode to Realize Deep Lithium Deposition. Adv. Funct. Mater. 2021, 32, 2110110. [Google Scholar] [CrossRef]
- Reyter, D.; Rousselot, S.; Mazouzi, D.; Gauthier, M.; Moreau, P.; Lestriez, B.; Guyomard, D.; Roué, L. An electrochemically roughened Cu current collector for Si-based electrode in Li-ion batteries. J. Power Sources 2013, 239, 308–314. [Google Scholar] [CrossRef]
- Pan, C.; Chen, S.-J.; Huang, Y.-H.; Wang, L.; Luo, J.-L.; Fu, X.-Z. A facile method to fabricate lightweight copper coated polyimide film current collectors for lithium-ion batteries. J. Power Sources 2022, 528, 231207. [Google Scholar] [CrossRef]
- Long, J.; Liu, H.; Xie, Y.; Tang, W.; Fu, T.; Tang, Y.; Lu, L.; Ding, X.; Tang, X. Three-Dimensional Copper Foil-Powder Sintering Current Collector for a Silicon-Based Anode Lithium-Ion Battery. Materials 2018, 11, 1338. [Google Scholar] [CrossRef]
- Liu, H.; Wang, E.; Zhang, Q.; Ren, Y.; Guo, X.; Wang, L.; Li, G.; Yu, H. Unique 3D nanoporous/macroporous structure Cu current collector for dendrite-free lithium deposition. Energy Storage Mater. 2019, 17, 253–259. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, S.; Jiang, X.; Yao, X.; Xu, X.; Peng, A.; Wang, L.; Xue, Q. High-performances of Li4Ti5O12 anodes for lithium-ion batteries via modifying the Cu current collector through magnetron sputtering amorphous carbon. J. Alloys Compd. 2020, 830, 154682. [Google Scholar] [CrossRef]
- Xu, X.; Shen, K.; Wen, M.; Wu, Q.; Hou, G.; Cao, H.; Wu, L.; Zheng, G.; Tang, Y. Facile synthesis of three-dimensional Cu/Fe3O4 nanowires as binder-free anode for lithium-ion batteries. Appl. Surf. Sci. 2018, 450, 356–363. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2020, 36, 147–170. [Google Scholar] [CrossRef]
- Wei, C.; Tang, J.; Xu, G.; Xia, S.; Ye, N.; Zhao, Y.; Yan, J. Electroless Deposition of Automatically Shedded Thin Copper Foils. ACS Appl. Mater. Interfaces 2020, 12, 28831–28839. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Yu, M.; Fan, G.; Liu, J.; Wang, Y.; Zhao, K.; Ding, J.; Cheng, F. Growing Nanostructured CuO on Copper Foil via Chemical Etching to Upgrade Metallic Lithium Anode. ACS Appl. Mater. Interfaces 2021, 13, 6367–6374. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Qu, R.; Zhou, L.; Zhang, D.; Chen, J.; He, X.; Wang, L.; Wang, H.; Dou, H. In situ preparation of CuCl cubic particles on the commercial copper foil: Its significant facilitation to the electrochemical performance of the commercial graphite and its unexpected photochromic behavior. J. Alloys Compd. 2020, 835, 155302. [Google Scholar] [CrossRef]
- Shen, C.; Hu, G.; Cheong, L.; Huang, S.; Zhang, J.; Wang, D. Direct Observation of the Growth of Lithium Dendrites on Graphite Anodes by Operando EC-AFM. Small Methods 2017, 2, 1700298. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Fan, B.; Shan, H.; Chen, Q.; Jiang, C.; Hou, G.; Tang, Y. Study on the relationship between crystal plane orientation and strength of electrolytic copper foil. J. Alloys Compd. 2021, 884, 161044. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, C.; Sun, Y.; Yi, R.; Cai, Y.; Li, Y.; Mitrovic, I.; Taylor, S.; Chalker, P.; Yang, L.; et al. 3D-structured multi-walled carbon nanotubes/copper nanowires composite as a porous current collector for the enhanced silicon-based anode. J. Alloys Compd. 2019, 803, 505–513. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, W.; Zhang, X.; Ke, Y.; Qiu, Z.; Luo, J.; Tang, Y.; Wang, C.; Yuan, Y.; Huang, Y. A review on structuralized current collectors for high-performance lithium-ion battery anodes. Appl. Energy 2020, 276, 115464. [Google Scholar] [CrossRef]
- Xia, T.; Liang, T.; Xiao, Z.; Chen, J.; Liu, J.; Zhong, S. Nanograined copper foil as a high-performance collector for lithium-ion batteries. J. Alloys Compd. 2020, 831, 154801. [Google Scholar] [CrossRef]
- Wen, S.; Li, Z.; Zou, C.; Zhong, W.; Wang, C.; Chen, J.; Zhong, S. Improved performances of lithium-ion batteries by conductive polymer modified copper current collector. New J. Chem. 2021, 45, 10541–10548. [Google Scholar] [CrossRef]
- Toigo, C.; Frankenberger, M.; Billot, N.; Pscherer, C.; Stumper, B.; Distelrath, F.; Schubert, J.; Pettinger, K.; Arbizzani, C. Improved Li4 Ti5 O12 electrodes by modified current collector surface. Electrochim. Acta 2021, 392, 138978. [Google Scholar] [CrossRef]
- Luo, J.; Yuan, W.; Huang, S.; Zhao, B.; Chen, Y.; Liu, M.; Tang, Y. From Checkerboard-Like Sand Barriers to 3D Cu@CNF Composite Current Collectors for High-Performance Batteries. Adv. Sci. 2018, 5, 1800031. [Google Scholar] [CrossRef]
- Xiao, J.Z.; Chen, J.; Liu, J.; Liang, T.; Zhong, S. Microcrystalline copper foil as a high performance collector for lithium-ion batteries. J. Power Sources 2019, 438, 226973. [Google Scholar] [CrossRef]
- Jia, S.; Wang, Y.; Liu, X.; Zhao, S.; Zhao, W.; Huang, Y.; Li, Z.; Lin, Z. Hierarchically porous CuO nano-labyrinths as binder-free anodes for long-life and high-rate lithium ion batteries. Nano Energy 2019, 59, 229–236. [Google Scholar] [CrossRef]
- Jeong, C.U.; Lee, S.-Y.; Kim, J.; Cho, K.Y.; Yoon, S. Embossed aluminum as a current collector for high-rate lithium cathode performance. J. Power Sources 2018, 398, 193–200. [Google Scholar] [CrossRef]
- Jeon, H.; Cho, I.; Jo, H.; Kim, K.; Ryou, M.-H.; Lee, Y.M. Highly rough copper current collector: Improving adhesion property between a silicon electrode and current collector for flexible lithium-ion batteries. RSC Adv. 2017, 7, 35681–35686. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, Z.; Li, X.; Wang, X.; Liu, D.; Hu, D.; Qiao, L.; He, D. Template-free synthesized Ni nanofoams as nanostructured current collectors for high-performance electrodes in lithium ion batteries. J. Mater. Chem. A 2013, 1, 10002–10007. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, F.; Wang, Y.; Jia, L.; Hao, J. Manufacturing profile-free copper foil using laser shock flattening. Int. J. Mach. Tool. Manuf. 2020, 152, 103542. [Google Scholar]
- Chen, G.; Yang, G.; Zhou, S.; Tawfiq, K.; Wei, H.; Wang, B. Electropolishing of surfaces: Theory and applications. Surf. Eng. 2017, 33, 149–166. [Google Scholar]
- Till, G.; David, S.; Ajinkya, M.; Chris, M.; Arno, K.; Gunther, R. Classification of calendering-induced electrode defects and their influence on subsequent processes of lithium-ion battery production. Enegry Technol.-Ger. 2020, 8, 1900026. [Google Scholar]
- Yoon, S.; Jang, H.-S.; Kim, S.; Kim, J.; Cho, K.Y. Crater-like architectural aluminum current collectors with superior electrochemical performance for Li-ion batteries. J. Electroanal. Chem. 2017, 797, 37–41. [Google Scholar] [CrossRef]
- Fei, X.; Dong, Z.; Gong, B.; Zhao, X. Lightweight Through-Hole Copper Foil as a Current Collector for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 42266–42275. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zuo, D.; Pei, X.; Mu, C.; Chen, K.; Chen, Q.; Hou, G.; Tang, Y. Effects of Electrolytic Copper Foil Roughness on Lithium-Ion Battery Performance. Metals 2022, 12, 2110. https://doi.org/10.3390/met12122110
Zhang J, Zuo D, Pei X, Mu C, Chen K, Chen Q, Hou G, Tang Y. Effects of Electrolytic Copper Foil Roughness on Lithium-Ion Battery Performance. Metals. 2022; 12(12):2110. https://doi.org/10.3390/met12122110
Chicago/Turabian StyleZhang, Jianli, Dengyu Zuo, Xiaozhe Pei, Chengfa Mu, Keyu Chen, Qiang Chen, Guangya Hou, and Yiping Tang. 2022. "Effects of Electrolytic Copper Foil Roughness on Lithium-Ion Battery Performance" Metals 12, no. 12: 2110. https://doi.org/10.3390/met12122110
APA StyleZhang, J., Zuo, D., Pei, X., Mu, C., Chen, K., Chen, Q., Hou, G., & Tang, Y. (2022). Effects of Electrolytic Copper Foil Roughness on Lithium-Ion Battery Performance. Metals, 12(12), 2110. https://doi.org/10.3390/met12122110





