The Role of the Graphene Oxide (GO) and PEO Treated-Zinc Oxide (ZnO/PEO) Intermediate Electrode Buffer Layer in Vacuum-Free Quantum Dots Solar Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Graphene Oxide
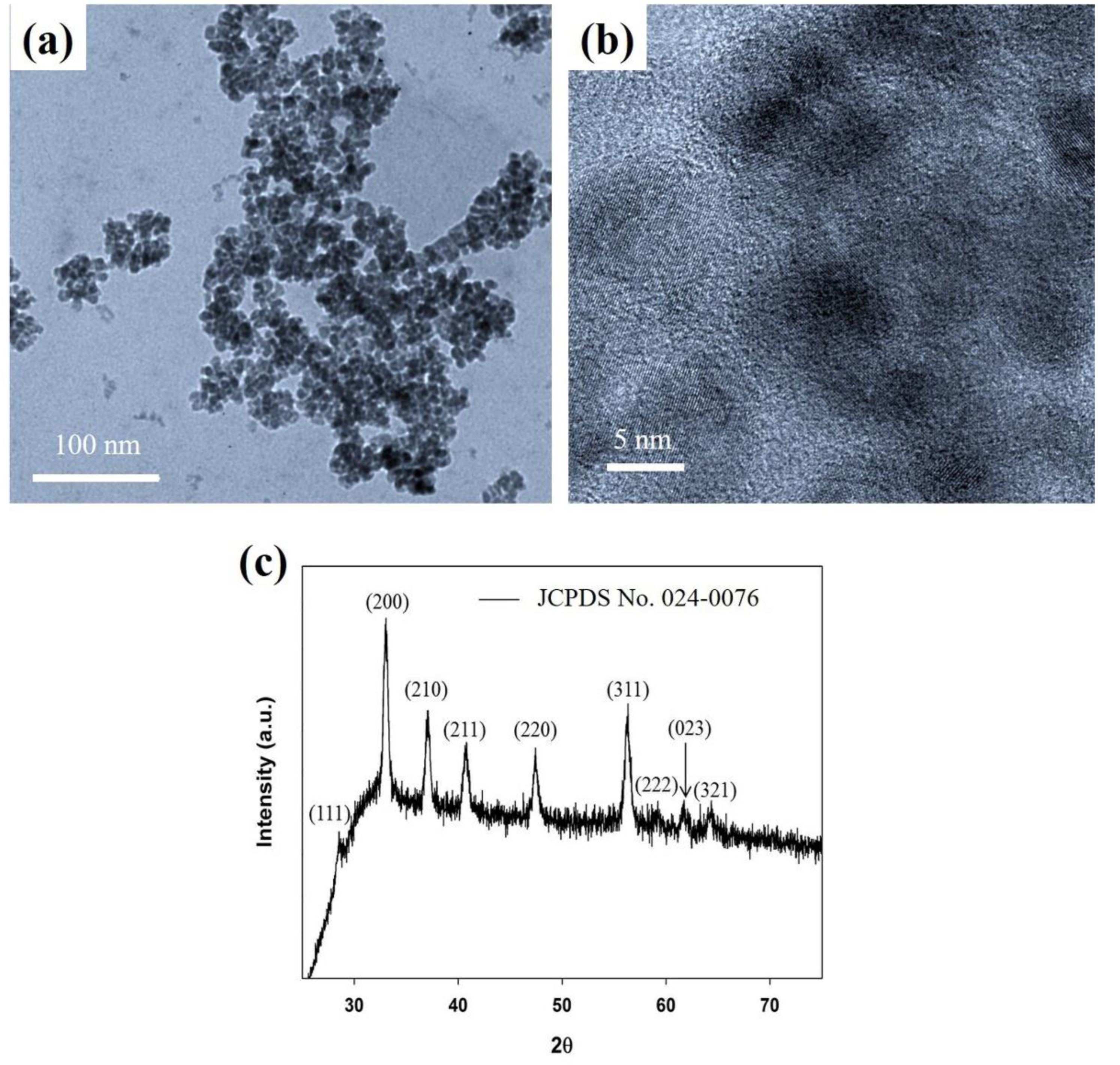
2.2. Synthesis of Iron Pyrite (FeS2) Spherical Nanoparticles (SNPs)
2.3. Synthesis of Zinc Oxide (ZnO) Spherical Nanoparticles
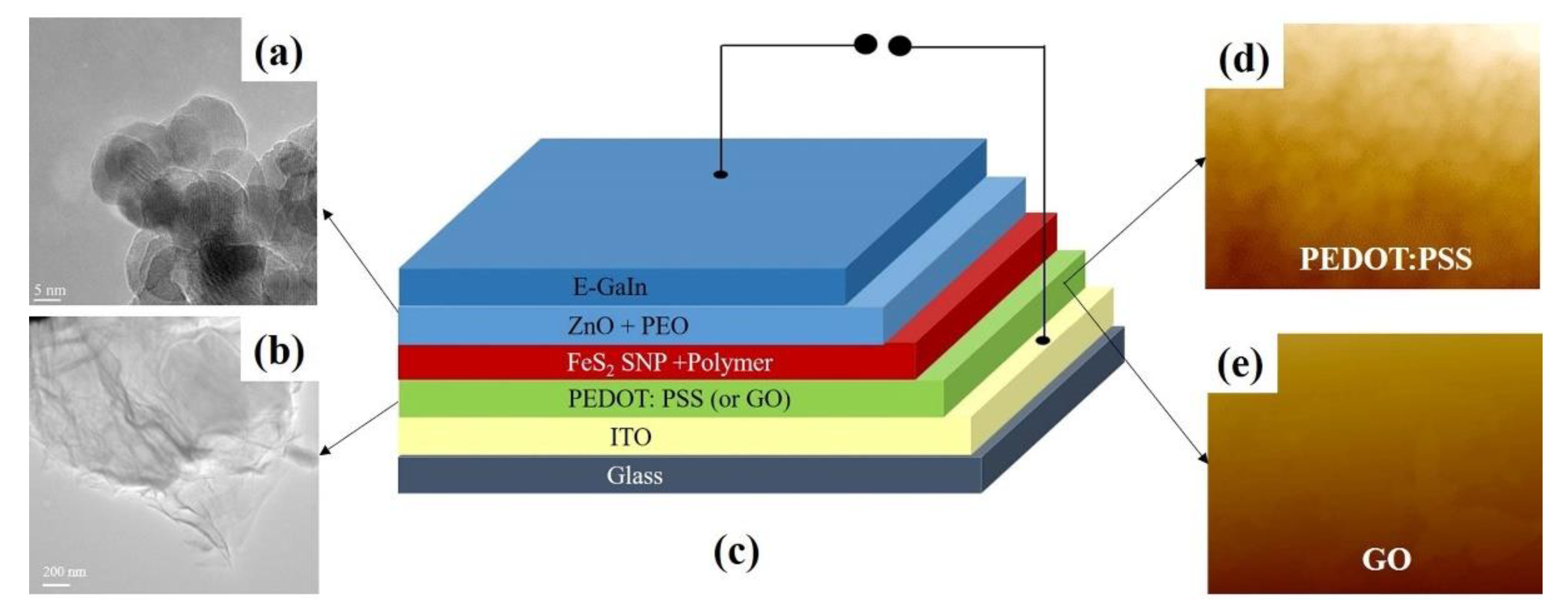
2.4. Device Fabrication
2.5. Characterization
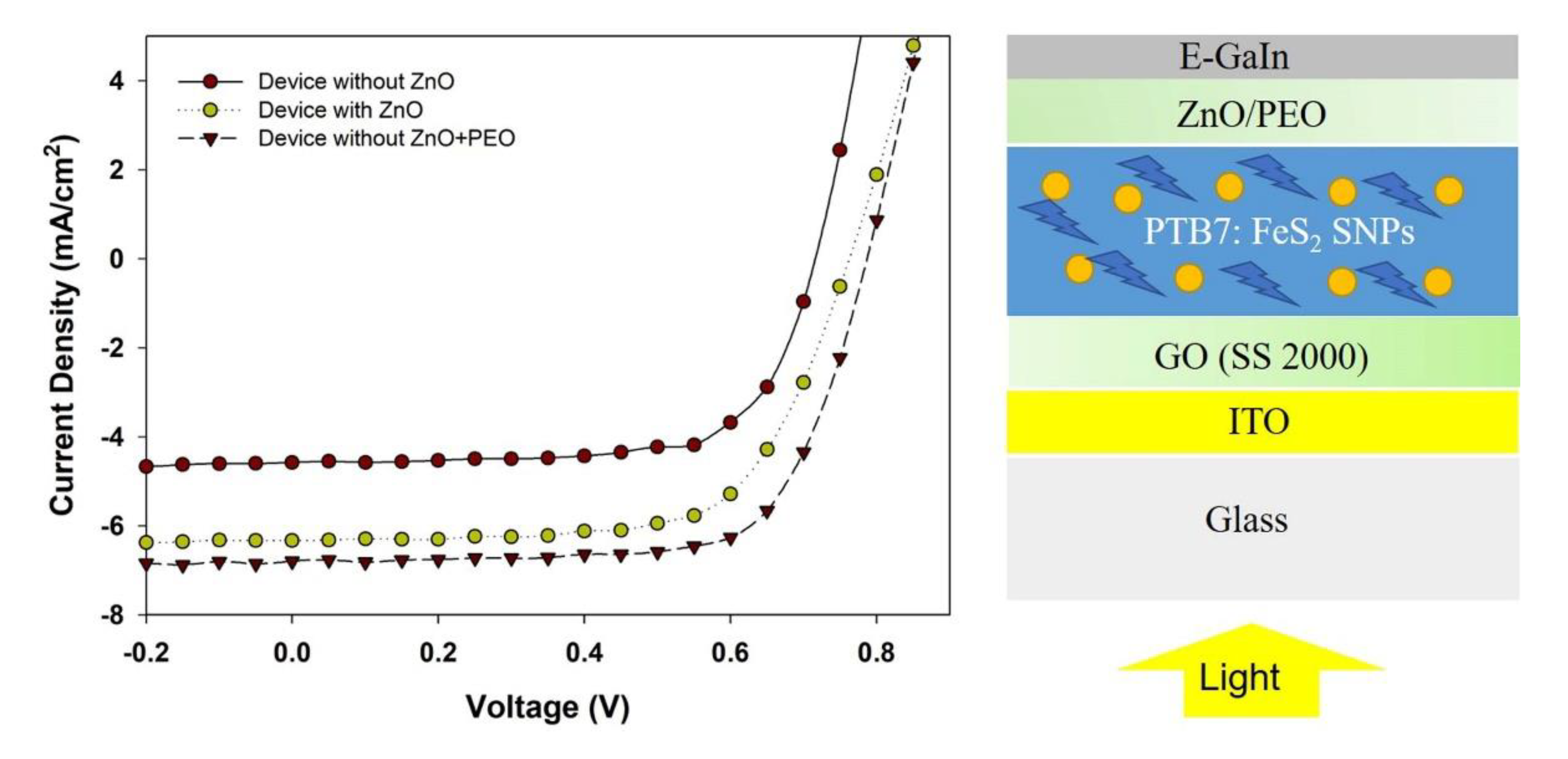
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaikh, S.M.F.; Rahman, G.; Mane, R.S.; Joo, O.S. Bismuth oxide nanoplates-based efficient DSSCs: Influence of ZnO surface passivation layer. Electrochim. Acta 2013, 111, 593–600. [Google Scholar] [CrossRef]
- Blakers, A.; Zin, N.; McIntosh, K.R.; Fong, K. High-efficiency silicon solar cells. Energy Procedia 2013, 33, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Fradi, K.; Bouich, A.; Slimi, B.; Chtourou, R. Towards improving the optoelectronics properties of MAPbI3(1−x)B3x/ZnO heterojunction by bromine doping. Optik 2022, 249, 168283. [Google Scholar] [CrossRef]
- Khalate, S.A.; Kate, R.S.; Deokate, R.J. A review on energy economics and the recent research and development in energy and the Cu2ZnSnS4 (CZTS) solar cells: A focus towards efficiency. Sol. Energy 2018, 169, 616–633. [Google Scholar]
- Steinmann, V.; Kronenberg, N.M.; Lenze, M.R.; Graf, S.M.; Hertel, D.; Meerholz, K.; Burckstummer, H.; Tulyakova, E.V.; Würthner, F. Simple, Highly Efficient Vacuum-Processed Bulk Heterojunction Solar Cells Based on Merocyanine Dyes. Adv. Energy Mater. 2011, 1, 888–893. [Google Scholar] [CrossRef]
- She, X.J.; Liu, J.; Zhang, J.Y.; Gao, X.; Wang, S.D. Spatial profile of charge storage in organic field-effect transistor nonvolatile memory using polymer electret. Appl. Phys. Lett. 2013, 103, 184. [Google Scholar] [CrossRef]
- Yang, B.; Xiao, Z.; Huang, J. Polymer aggregation correlated transition from Schottky-junction to bulk heterojunction organic solar cells. Appl. Phys. Lett. 2014, 104, 54. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Yang, C.; Gong, X.; Lee, K.; Heeger, A.J. Thermally stable, efficient polymer solar cells with nanoscale control of the interpenetrating network morphology. Adv. Funct. Mater. 2005, 14, 1617–1622. [Google Scholar] [CrossRef]
- Ma, B.; Woo, C.H.; Miyamoto, Y.; Fréchet, J.M. Rapid room-temperature synthesis and characterization of nanocrystals of a prototypical zeolitic imidazolate framework. Chem. Mater. 2009, 21, 1410–1412. [Google Scholar]
- Chiang, C.H.; Wu, C.G. Bulk heterojunction perovskite–PCBM solar cells with high fill factor. Nat. Photonics 2016, 10, 196–200. [Google Scholar] [CrossRef]
- You, J.B.; Meng, L.; Song, T.B.; Guo, T.F.; Yang, Y.M.; Chang, W.H.; Hong, Z.R.; Chen, H.J.; Zhou, H.P.; Chen, Q.; et al. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nanotechnol. 2016, 11, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zeng, M.; Li, Y.; Liu, T.; Luo, Z.; Xu, Y.; Ping, L.; Nan, Z.; Jianfeng, L.; Yuan, L.; et al. Rational anode engineering enables progresses for different types of organic solar cells. Adv. Energy Mater. 2021, 11, 2100492. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Q.; Huang, C.; Yang, T.; Shi, S.; Yao, S.; Donglou, R.; Tao, L.; Guangye, Z.; Zou, B. Controlling the Treatment Time for Ideal Morphology towards Efficient Organic Solar Cells. Molecules 2022, 27, 5713. [Google Scholar] [CrossRef]
- Wu, Z.W.; Bai, S.; Xiang, J.; Yuan, Z.C.; Yang, Y.G.; Cui, W.; Gao, X.Y.; Liu, Z.; Jin, Y.Z.; Sun, B.Q. Efficient planar heterojunction perovskite solar cells employing graphene oxide as hole conductor. Nanoscale 2014, 6, 10505–10510. [Google Scholar] [CrossRef] [PubMed]
- Agresti, A.; Pescetelli, S.; Cina, L.; Konios, D.; Kakavelakis, G.; Kymakis, E.; Carlo, A.D. Efficiency and stability enhancement in perovskite solar cells by inserting lithium-neutralized graphene oxide as electron transporting layer. Adv. Funct. Mater. 2016, 26, 2686–2694. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Murali, S.; Cai, W.W.; Li, X.S.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef]
- Jeong, W.L.; Park, S.H.; Jho, Y.D.; Joo, S.K.; Lee, D.S. The Role of the Graphene Oxide (GO) and Reduced Graphene Oxide (RGO) Intermediate Layer in CZTSSe Thin-Film Solar Cells. Materials 2022, 15, 3419. [Google Scholar] [CrossRef]
- Lee, D.Y.; Na, S.I.; Kim, S.S. Graphene oxide/PEDOT: PSS composite hole transport layer for efficient and stable planar heterojunction perovskite solar cells. Nanoscale 2016, 8, 1513–1522. [Google Scholar] [CrossRef]
- Oosterhout, S.D.; Wienk, M.M.; van Bavel, S.S.; Thiedmann, R.; Koster, L.J.A.; Gilot, J.; Loos, J.; Schmidt, V.; Janssen, R.A.J. The effect of three-dimensional morphology on the efficiency of hybrid polymer solar cells. Nat. Mater. 2009, 8, 818–824. [Google Scholar] [CrossRef]
- Shao, S.; Zheng, K.; Zidek, K.; Chabera, P.; Pullerits, T.; Zhang, F. Optimizing ZnO nanoparticle surface for bulk heterojunction hybrid solar cells. Sol. Energy Mater. Sol. Cells 2013, 118, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.R.; Voigt, J.A.; Liu, J.; Mckenzie, B.; Mcdermott, M.J.; Rodriguez, M.A.; Konishi, H.; Xu, H. Complex and oriented ZnO nanostructures. Nat. Mater. 2003, 2, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Qu, S.; Jia, C.; Liu, K.; Tan, F. Modified synthesis of FeS2 quantum dots for hybrid bulk-heterojunction solar cells. Mat. Lett. 2015, 157, 235–238. [Google Scholar] [CrossRef]
- Luo, L.; Luan, W.; Yuan, B.; Zhang, C.; Jin, L. High Efficient and Stable Solid Solar Cell: Based on FeS2 nanocrystals and P3HT:PCBM. Energy Procedia 2015, 75, 2181–2186. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Puthussery, J.; Seefeld, S.; Berry, N.; Gibbs, M.; Law, M. Colloidal iron pyrite (FeS2) nanocrystal inks for thin-film photovoltaics. J. Am. Chem. Soc. 2011, 133, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S. Magnetic properties of 3d transition-metal dichalcogenides with the pyrite structure. J. Appl. Phys. 1979, 50, 2308. [Google Scholar] [CrossRef]
- Ennaoui, A.; Fiechter, S.; Pettenkofer, C.; Alonsovante, N.; Buker, K.; Bronold, M.; Hopfner, C.; Tributsch, H. Iron disulfide for solar energy conversion. Sol. Energy Mater. Sol. Cells 1993, 29, 289. [Google Scholar] [CrossRef]
- Yuan, B.X.; Luan, W.L.; Tu, S.T. One-step synthesis of cubic FeS2 and flower-like FeSe2 particles by a solvothermal reduction process. Dalton Trans. 2012, 41, 772. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.T.N.; Nguyen, T.P.N.; Pham, V.T.H.; Trinh, K.T.; Lee, S.H.; Park, C. Structural, morphological, and optoelectronic properties of rod-like iron pyrite nanocrystals for solar cell applications. Jpn. J. Appl. Phys. 2015, 54, 045001. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, S.H.; Noh, J.; Han, S.H. Performance and Stability of Electroluminescent Device with Self-Assembled Layers of Poly(3,4-ethylenedioxythiophene) Poly(styrenesulfonate) and Polyelectrolytes. Thin Solid Films 2006, 510, 305–310. [Google Scholar] [CrossRef]
- Lagemaat, J.; Barnes, T.M.; Rumbles, G.; Shaheen, S.E.; Coutts, T.J.; Weeks, C.; Levitsky, I.; Peltola, J.; Glatkowski, P. Organic Solar Cells with Carbon Nanotubes Replacing In2O3:Sn as the Transparent Electrode. Appl. Phys. Lett. 2006, 88, 233503. [Google Scholar] [CrossRef]
- Li, S.S.; Tu, K.H.; Lin, C.C.; Chen, C.W.; Chhowalla, M. Solution-processable graphene oxide as an efficient hole transport layer in polymer solar cells. ACS Nano 2010, 4, 3169–3174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, I.; Dikin, D.A.; Piner, R.D.; Ruoff, R.S. Tunable electrical conductivity of individual graphene oxide sheets reduced at “low” temperatures. Nano Lett. 2008, 8, 4283–4287. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.Y.; Lee, C.S.; Lai, S.L.; Fung, M.K.; Wong, F.L.; Sun, H.Y.; Lau, K.M.; Lee, S.T. Efficient organic photovoltaic devices using a combination of exciton blocking layer and anodic buffer layer. J. Appl. Phys. 2006, 100, 094506. [Google Scholar] [CrossRef]
- Lin, C.; Tseng, S.; Liu, Y.; Tai, Y.; Chattopadhyay, S.; Lin, C.; Lee, J.; Hwang, J.; Hsu, Y.; Chen, L.; et al. Suppressing series resistance in organic solar cells by oxygen plasma treatment. Appl. Phys. Lett. 2008, 92, 233302. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, Y.; Huang, J.; Xu, X.; Cui, S.; Lu, Z. Hole transport layer selection toward efficient colloidal PbS quantum dot solar cells. Opt. Express 2019, 27, A1340. [Google Scholar] [CrossRef]




| Device Structure | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| Pristine | 2.09 | 0.675 | 65.70 | 0.93 |
| With PEDOT | 3.40 | 0.725 | 60.01 | 1.48 |
| With GO (SS 1000) | 4.32 | 0.725 | 65.01 | 2.00 |
| With GO (SS 2000) | 4.58 | 0.670 | 70.43 | 2.30 |
| With GO (SS 3000) | 2.87 | 0.725 | 61.42 | 1.26 |
| Device Structure | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| Without ZnO | 4.58 | 0.67 | 70.43 | 2.30 |
| With ZnO | 6.33 | 0.77 | 65.74 | 3.14 |
| With ZnO + PEO | 6.70 | 0.77 | 70.04 | 3.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.B.; Jo, Y.; Lam, N.H.; Jung, J.H.; Kim, C.-D.; Truong, N.T.N. The Role of the Graphene Oxide (GO) and PEO Treated-Zinc Oxide (ZnO/PEO) Intermediate Electrode Buffer Layer in Vacuum-Free Quantum Dots Solar Cell. Metals 2022, 12, 2096. https://doi.org/10.3390/met12122096
Kang SB, Jo Y, Lam NH, Jung JH, Kim C-D, Truong NTN. The Role of the Graphene Oxide (GO) and PEO Treated-Zinc Oxide (ZnO/PEO) Intermediate Electrode Buffer Layer in Vacuum-Free Quantum Dots Solar Cell. Metals. 2022; 12(12):2096. https://doi.org/10.3390/met12122096
Chicago/Turabian StyleKang, Seung Beom, Younjung Jo, Nguyen Hoang Lam, Jae Hak Jung, Chang-Duk Kim, and Nguyen Tam Nguyen Truong. 2022. "The Role of the Graphene Oxide (GO) and PEO Treated-Zinc Oxide (ZnO/PEO) Intermediate Electrode Buffer Layer in Vacuum-Free Quantum Dots Solar Cell" Metals 12, no. 12: 2096. https://doi.org/10.3390/met12122096





