Evolution and Formation of Non-Metallic Inclusions during Electroslag Remelting of Ce-Bearing 15Cr-22Ni-1Nb Austenitic Heat-Resistant Steel
Abstract
:1. Introduction
2. Experimental
2.1. ESR Experimental Procedure
2.2. Compositional Analysis and Inclusions Characterization
3. Results and Discussion
3.1. Steel Composition
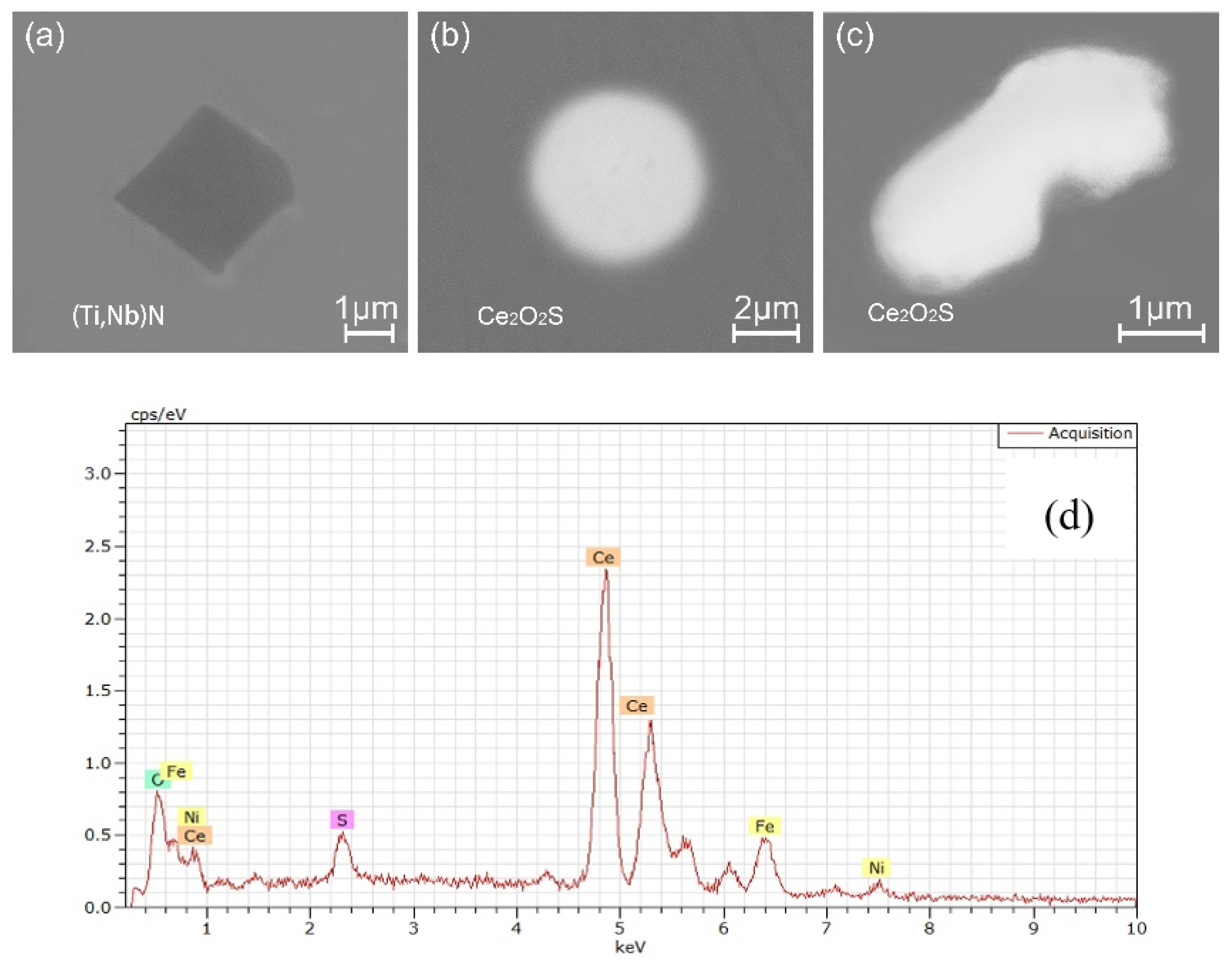
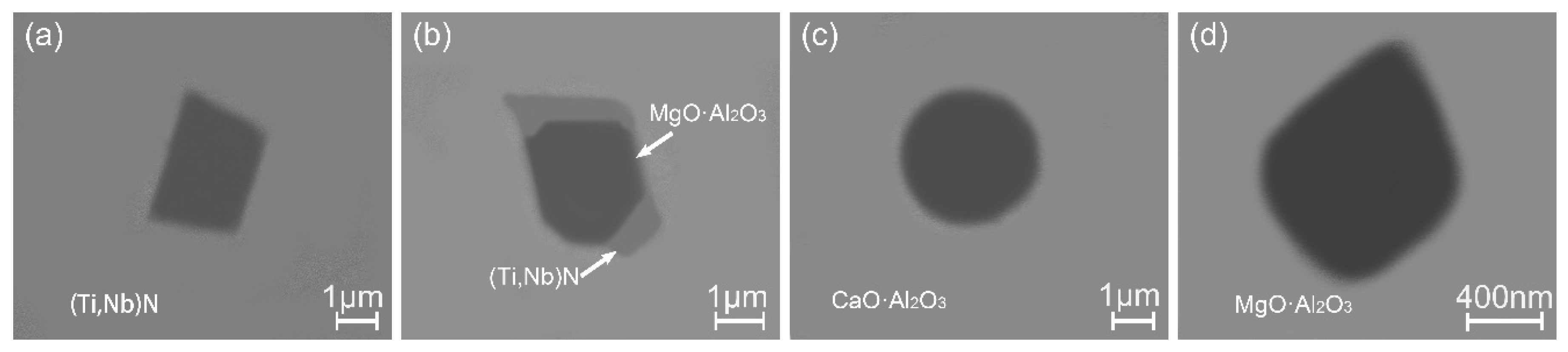
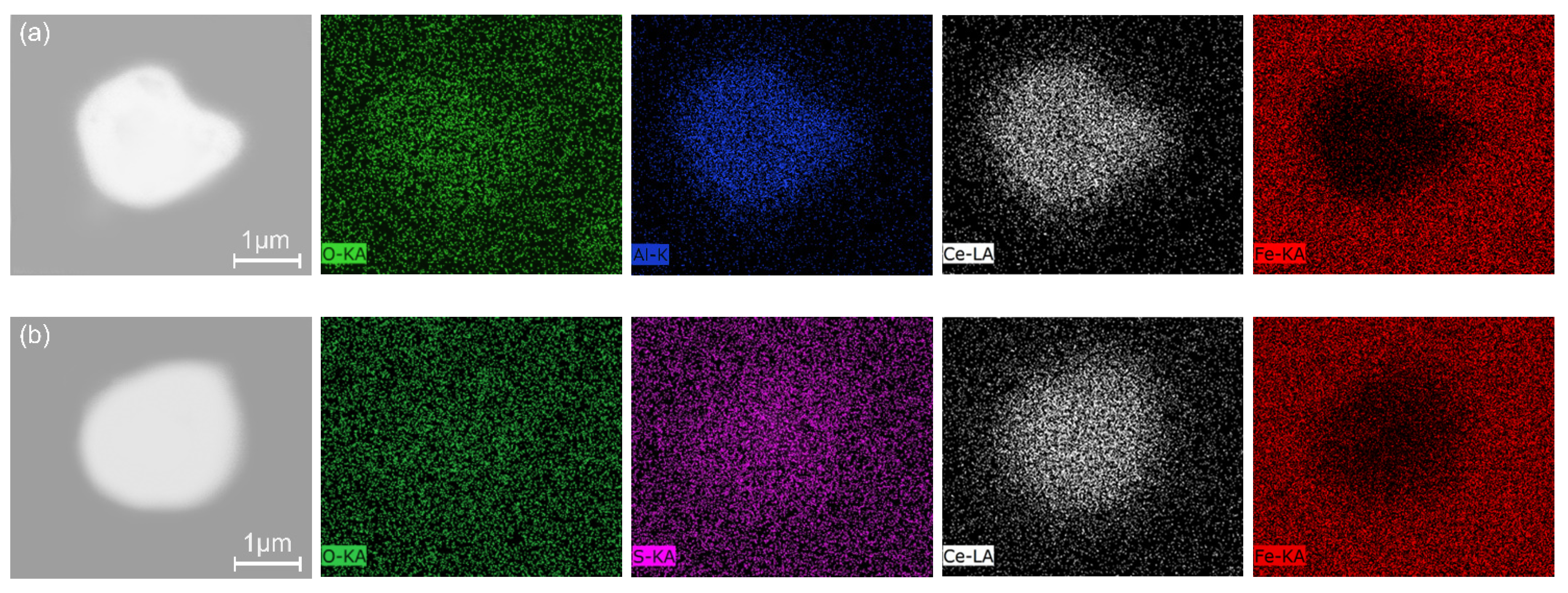
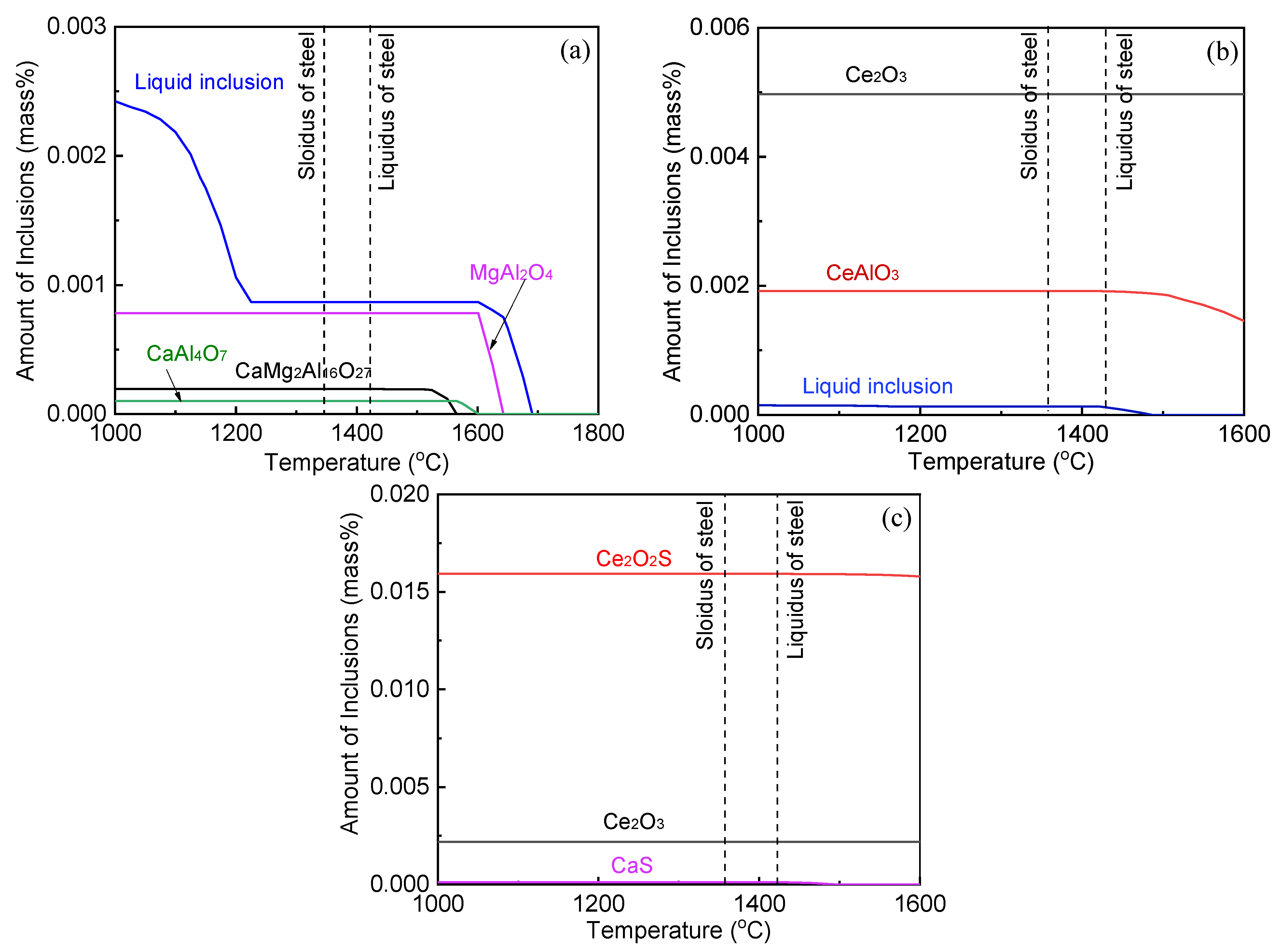
3.2. Inclusions in Consumable Steel Electrode
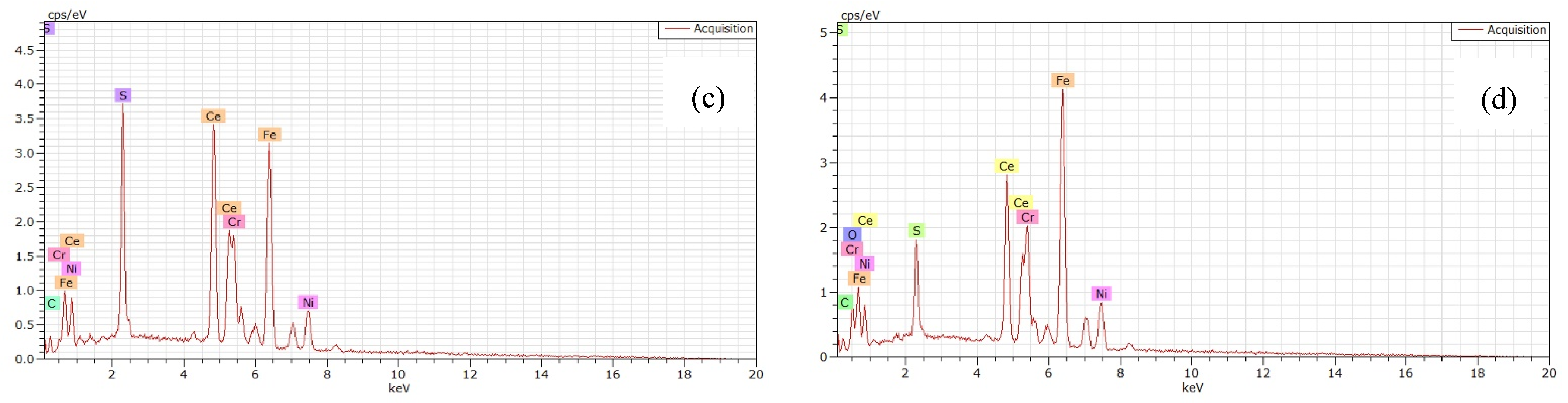
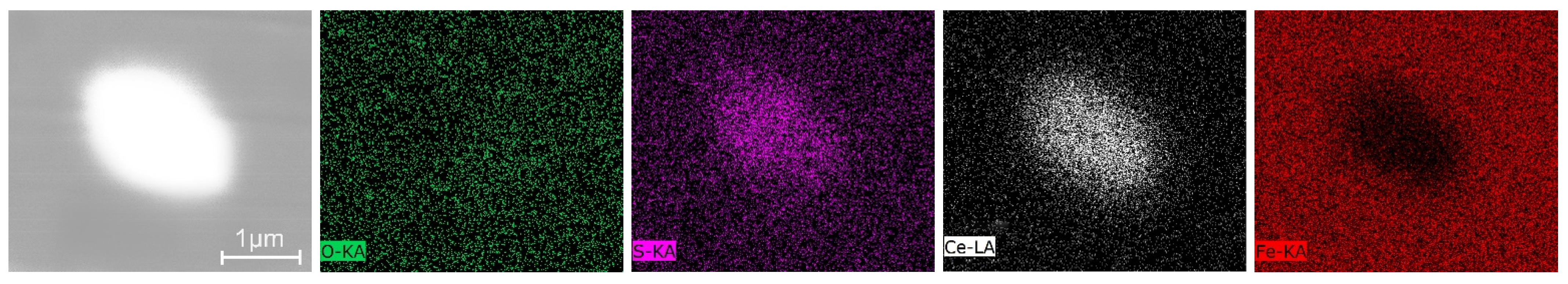
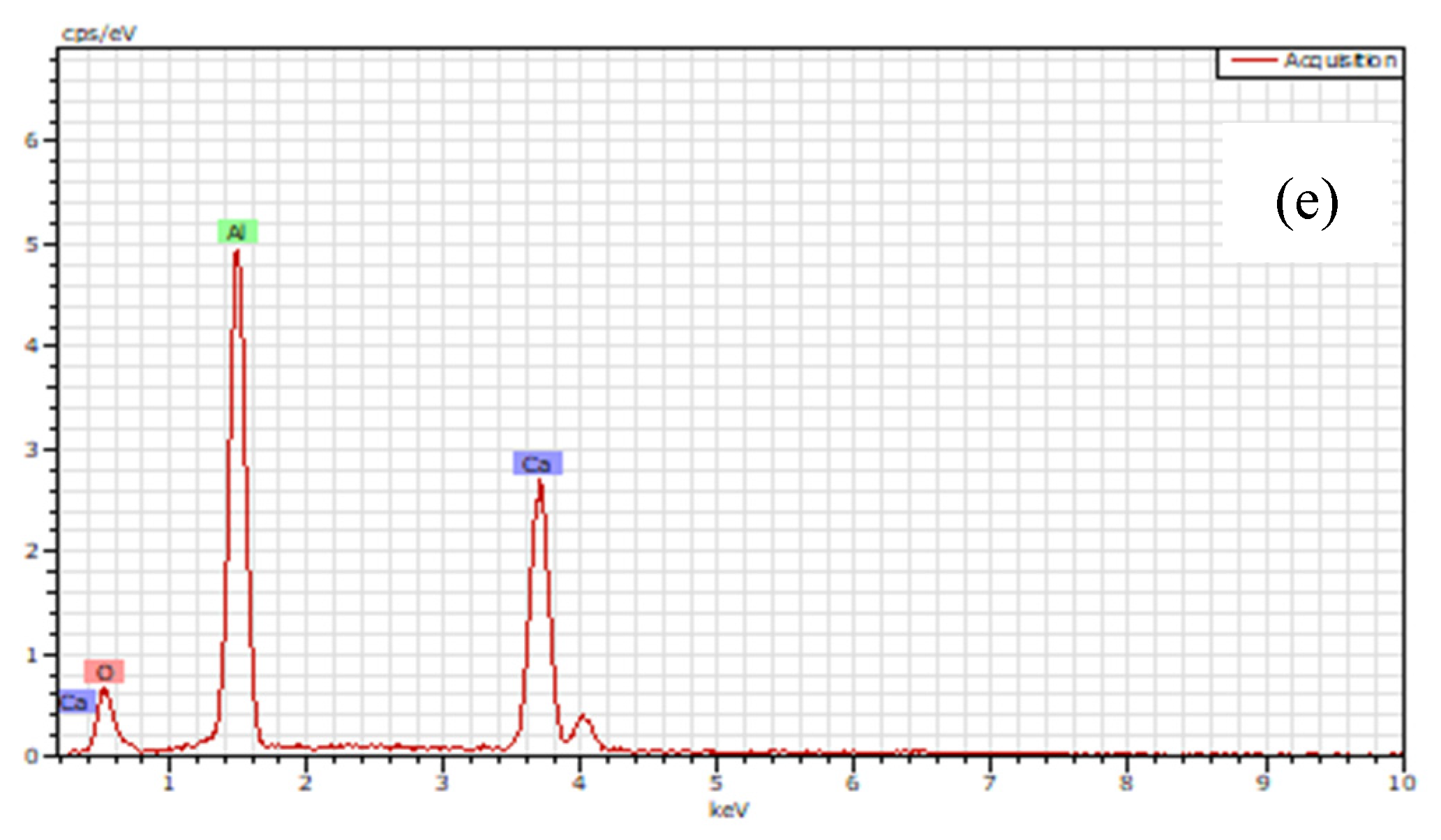
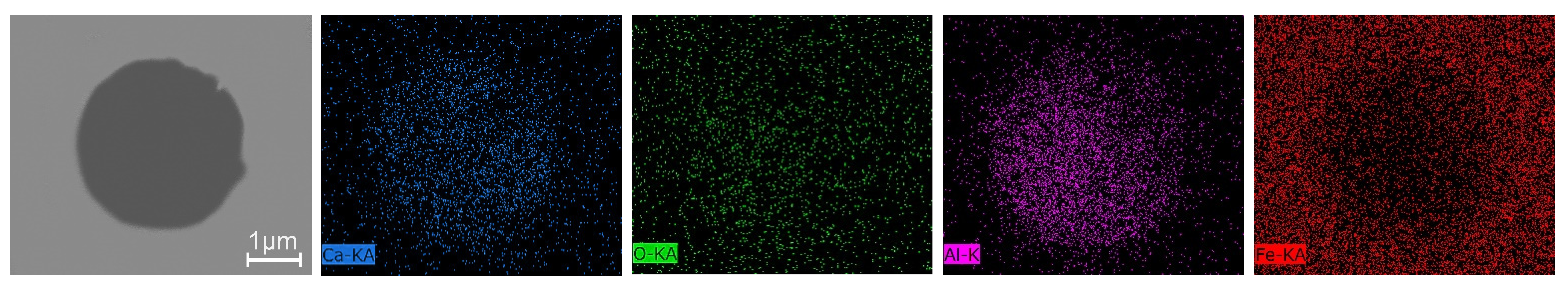
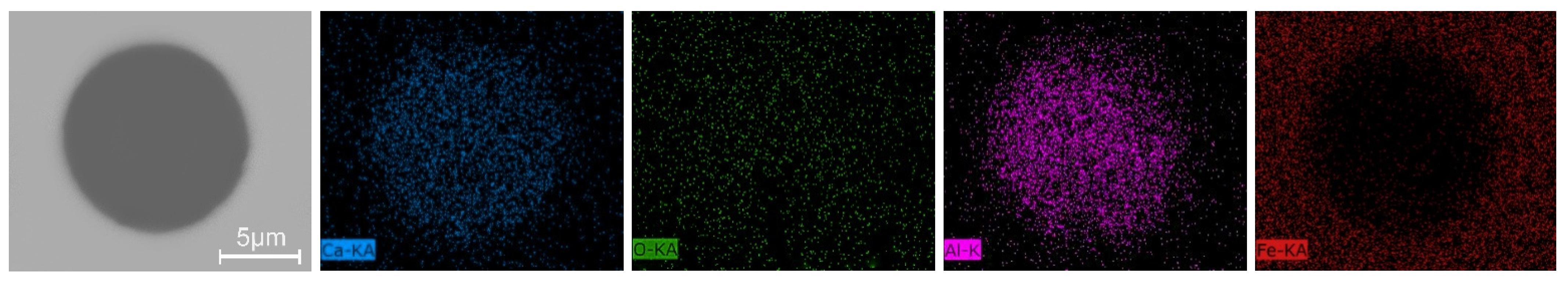
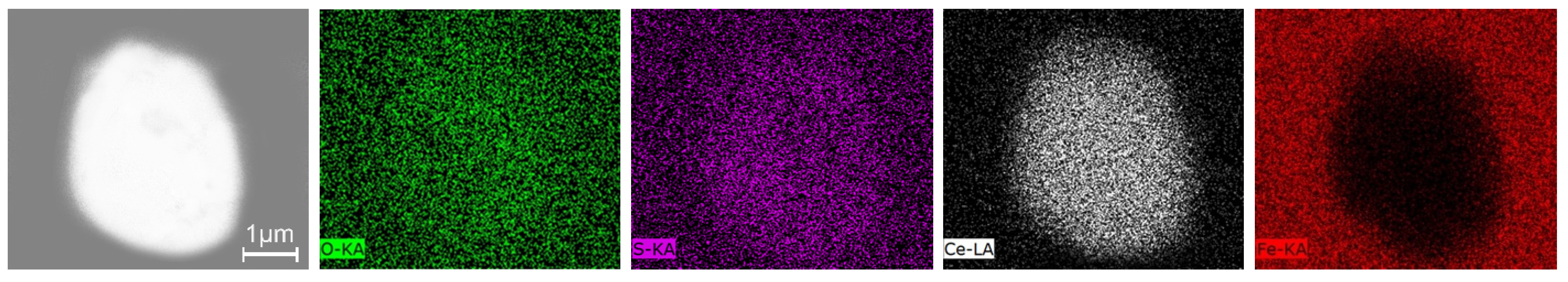
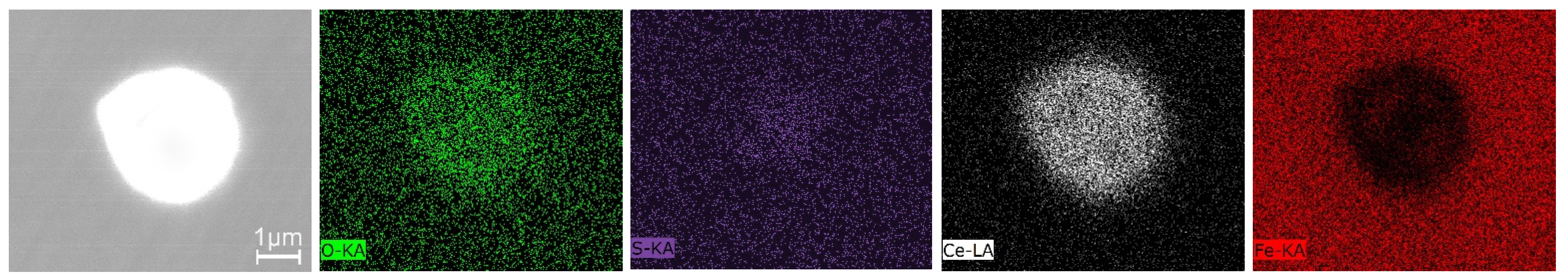
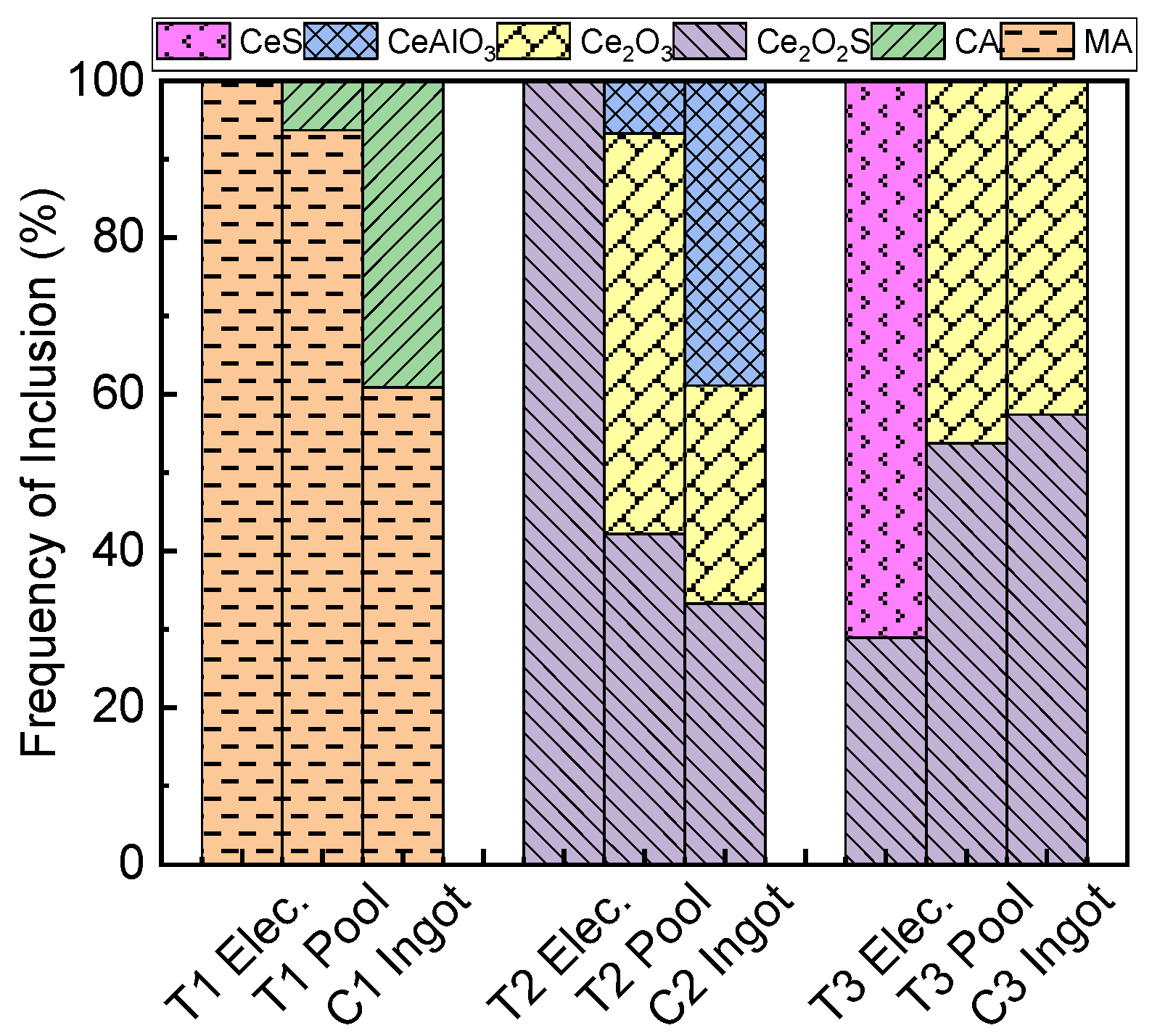
3.3. Inclusions in the Liquid Metal Pool and Remelted Ingots
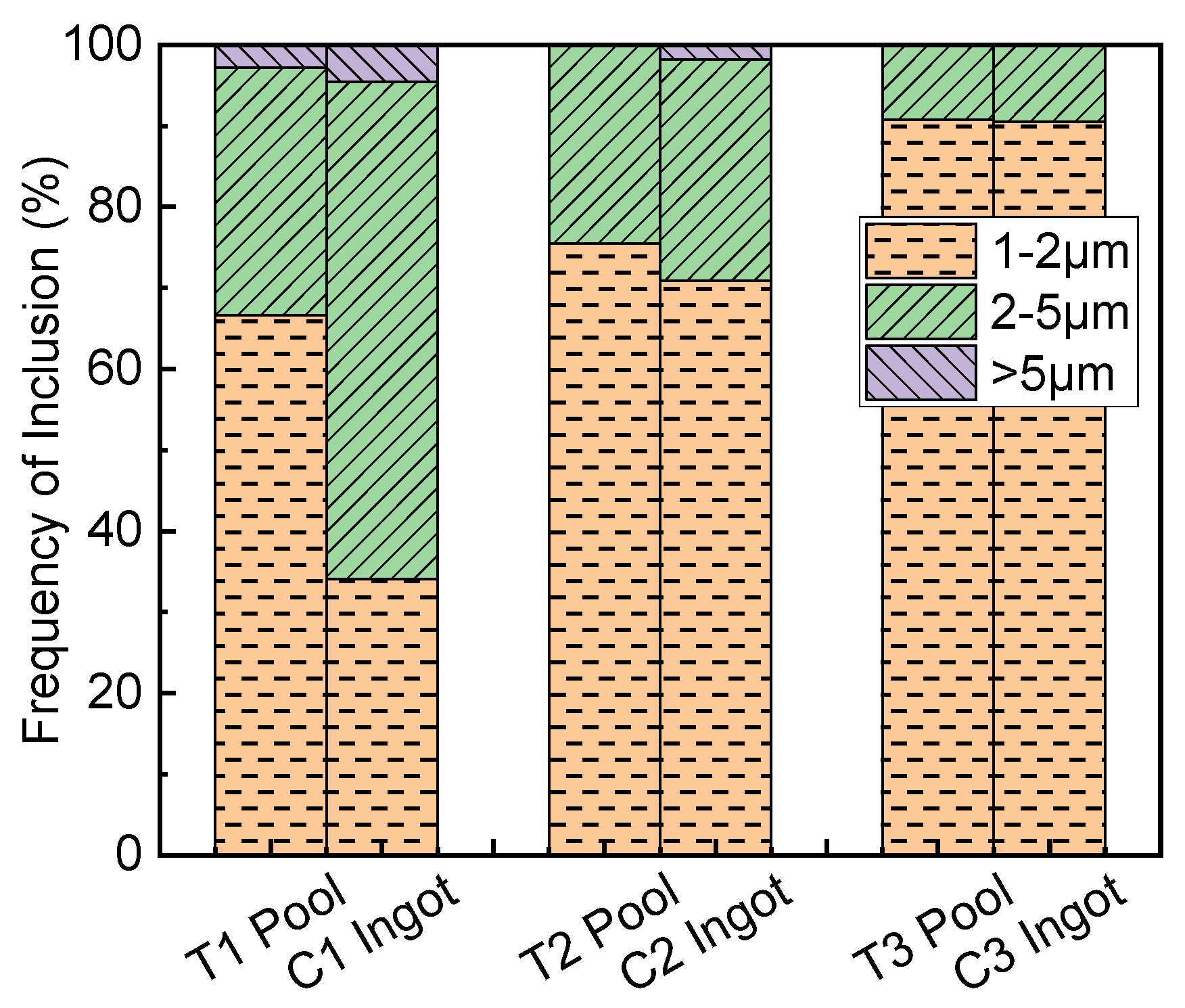
3.4. Size Distribution and Types of Inclusions
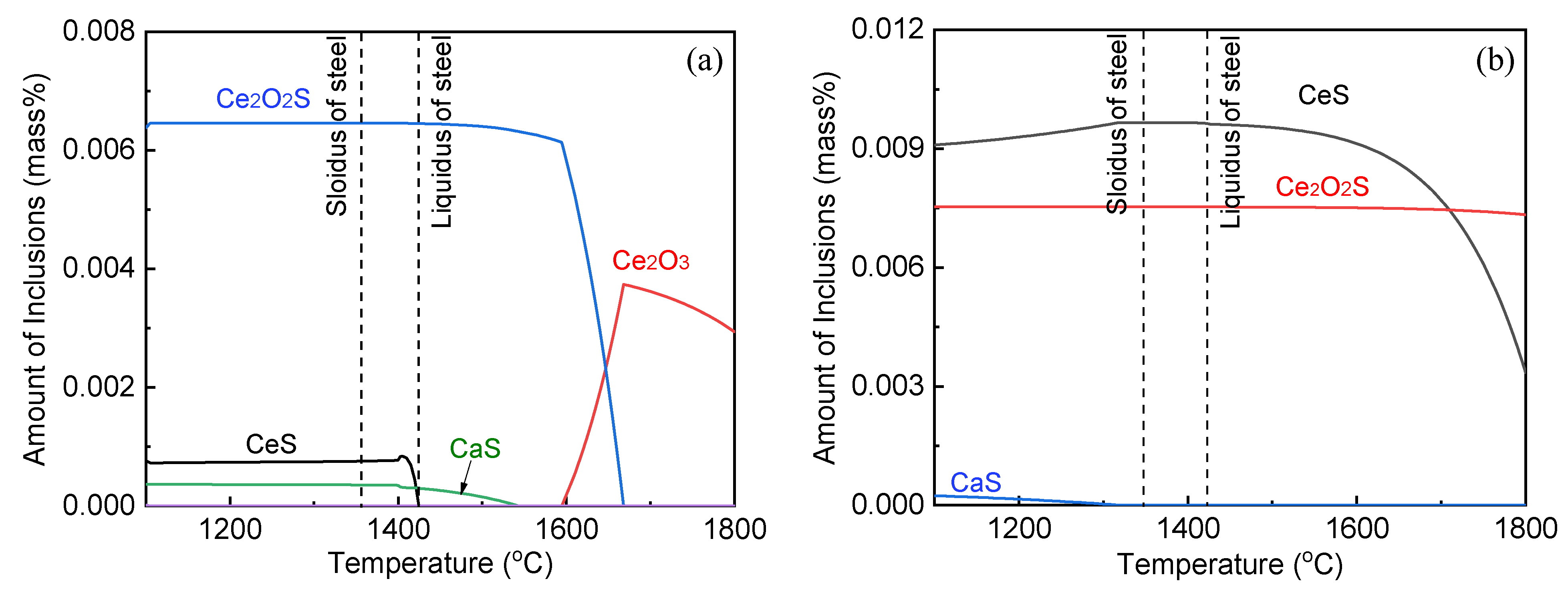
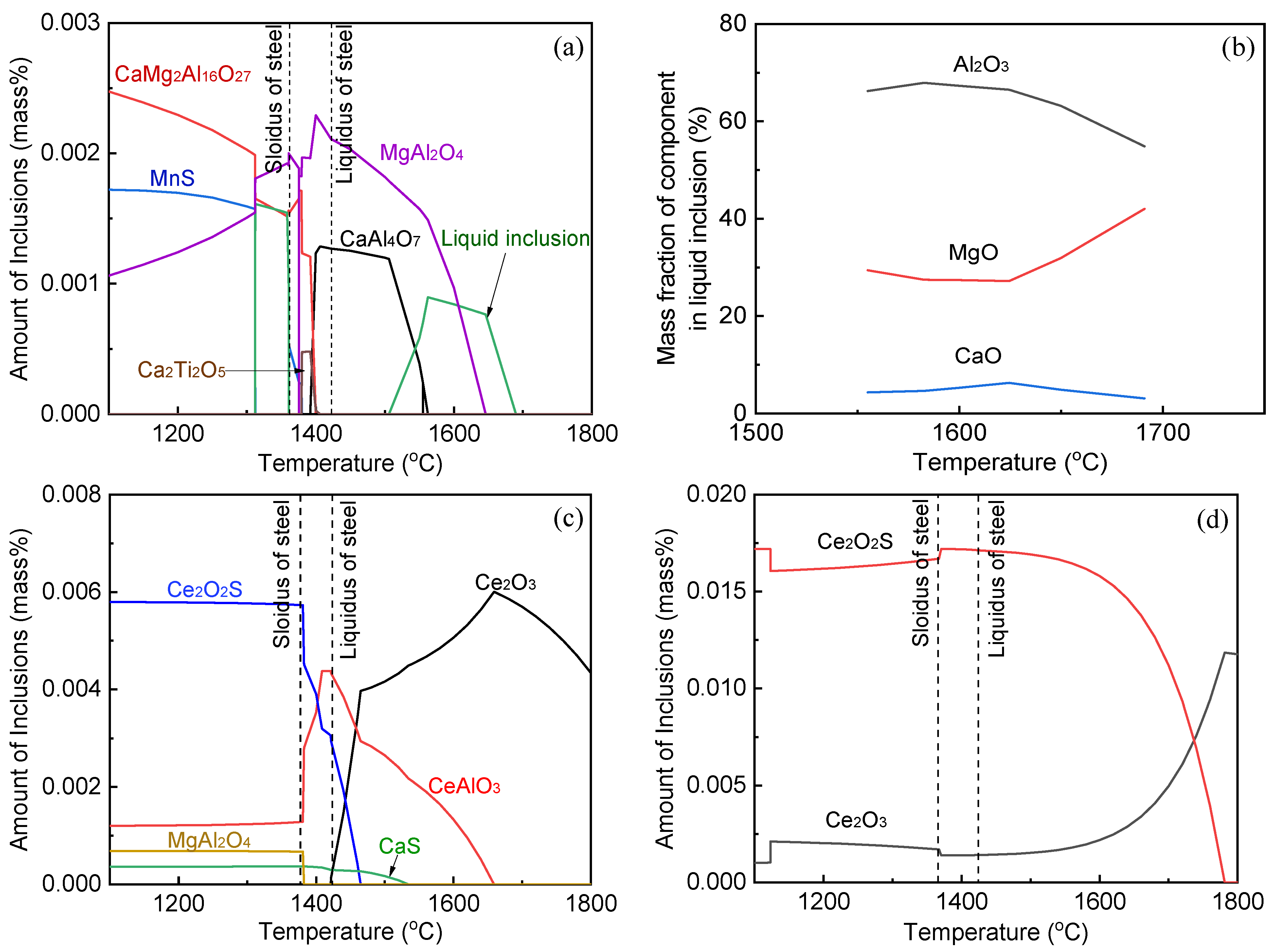
3.5. Evolution Mechanism of Inclusions during Remelted Remelting
4. Conclusions
- The Al pickup in the steel is caused by the Al addition for deoxidation during the ESR process, rather than the reduction of Al2O3 in the slag by Ce. The soluble oxygen pickup is generated in liquid steel due to the decomposition of FeO in the slag and desulfurization during the protective argon gas atmosphere ESR.
- The oxide inclusions in Ce-free electrode are MgO·Al2O3, part of which are removed by molten slag absorption during the ESR. The oxide inclusions in liquid metal pool are mainly MgO·Al2O3 and CaO–Al2O3 (6% in number fraction). The soluble oxygen that arising from reoxidation of liquid steel during the ESR react with soluble calcium and aluminum to form CaO–Al2O3 inclusions. MgO·Al2O3 inclusions are originated from reoxidation products and the relics from the electrode.
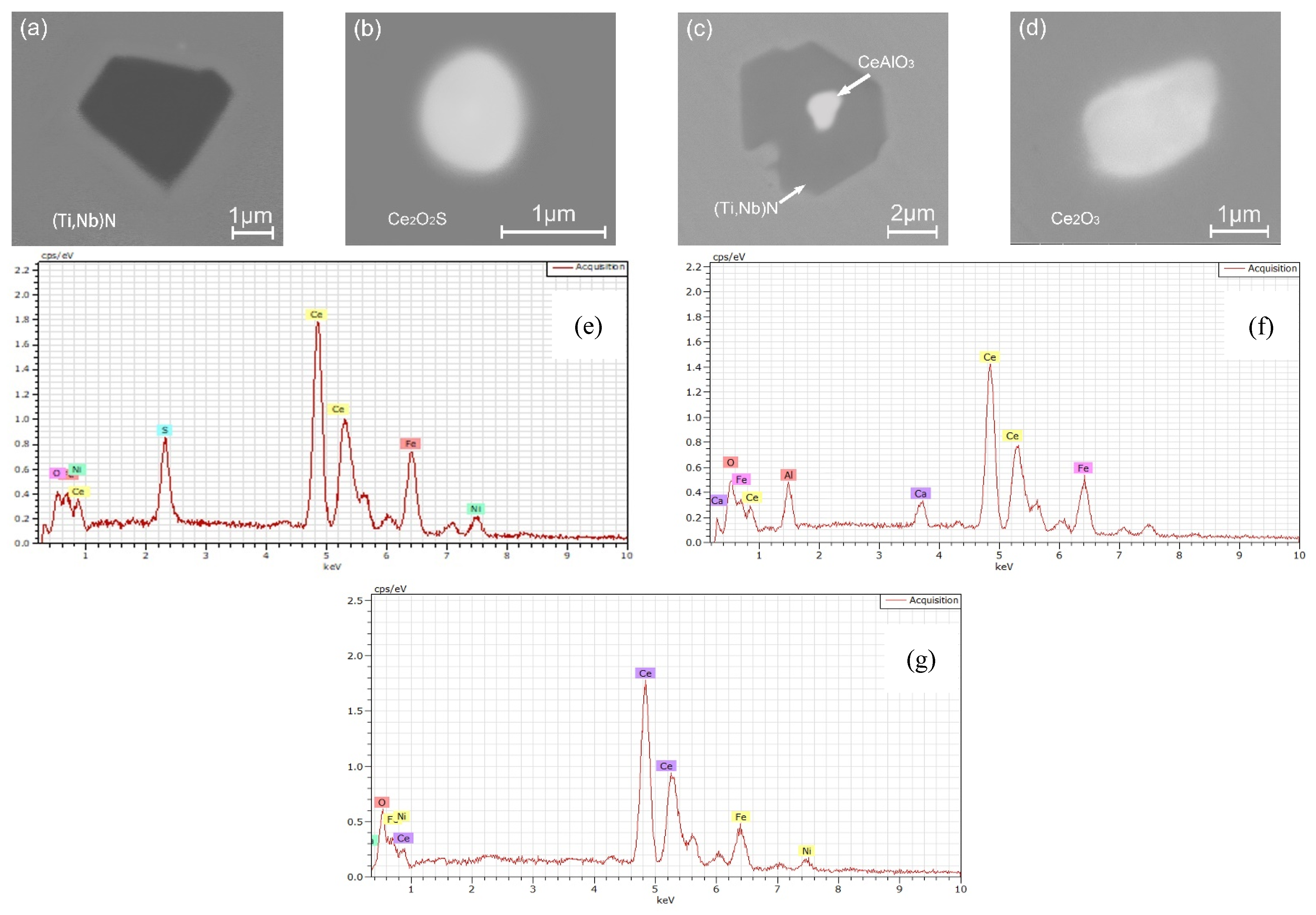
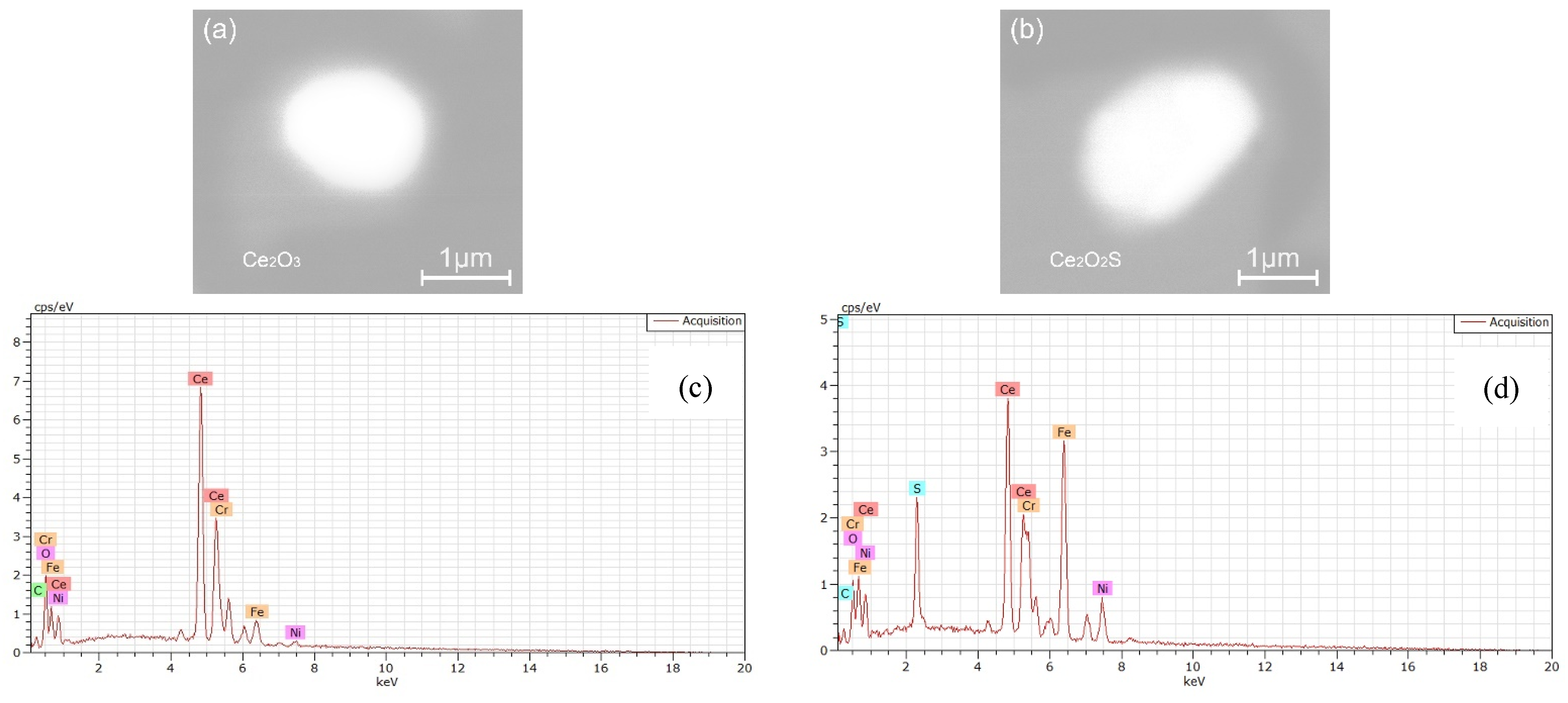
- The oxide inclusions in the electrode with 0.016 mass% Ce are Ce2O2S. Part of Ce2O2S inclusions are removed during ESR in two ways: (I) dissociated into soluble oxygen and soluble elements in liquid steel, (II) absorbed by molten slag. The oxide inclusions in the liquid metal pool are Ce2O3, CeAlO3, and Ce2O2S. CeAlO3 inclusions are reoxidation product, and Ce2O2S inclusions are the relics from the electrode. The proportions of Ce2O3, CeAlO3 and Ce2O2S inclusions in ingot are 28%, 39%, and 33%, respectively.
- The rare-earth inclusions in the electrode with 0.300 mass% Ce are Ce2O2S and CeS. The CeS inclusions are fully removed during ESR. Part of Ce2O2S inclusions are removed by slag adsorption. The oxide inclusions in liquid metal pool are Ce2O3 (reoxidation products, 46% in number proportion) and Ce2O2S (54% in number fraction). Ce2O2S inclusions in liquid metal pool are originated from the relics of electrode and reoxidation products. The proportion of Ce2O3 and Ce2O2S inclusions in remelted ingots are 43% and 57%, respectively.
- No fresh oxide inclusions are generated during the solidification of liquid steel. The differences in the number proportions of different types of inclusions between liquid metal pool and remelted ingot are attributed to the removal through floatation before full solidification of liquid steel during the ESR.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kondrat’Ev, S.Y.; Kraposhin, V.S.; Anastasiadi, G.P.; Talis, A.L. Experimental observation and crystallographic description of M7C3 carbide transformation in Fe–Cr–Ni–C HP type alloy. Acta Mater. 2015, 100, 275–281. [Google Scholar] [CrossRef]
- Hedaiat, F.; Dehmolaei, R.; Khorasanian, M.; Lotfi, B. Long-term oxidation behaviour and thermal stability of heat-resistant stainless steel claddings deposited on AISI 316 stainless steel by the GTAW process. Surf. Coat. Technol. 2021, 424, 127605. [Google Scholar] [CrossRef]
- Mazánová, V.; Heczko, M.; Polák, J. Fatigue crack initiation and growth in 43Fe-25Ni-22.5Cr austenitic steel at a temperature of 700 °C. Int. J. Fatigue 2018, 114, 11–21. [Google Scholar] [CrossRef]
- Guo, H.; Yang, S.F.; Wang, T.T.; Yuan, H.; Zhang, Y.L.; Li, J.S. Microstructure evolution and acicular ferrite nucleation in inclusion-engineered steel with modified MgO@C nanoparticle addition. J. Mater. Sci. Technol. 2022, 99, 277–287. [Google Scholar] [CrossRef]
- Xu, X.; Benaarbia, A.; Allen, D.J.; Jepson, M.A.E.; Sun, W. Investigation of microstructural evolution and creep rupture behaviour of 9% Cr MarBN steel welds. Mater. Sci. Eng. A 2020, 791, 139546. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Li, S.; Li, G.; Wang, Q.; Hui, W.; Weng, Y. On the critical inclusion size of high strength steels under ultra-high cycle fatigue. Mater. Sci. Eng. A 2006, 427, 167–174. [Google Scholar] [CrossRef]
- Meurling, F.; Melander, A.; Tidesten, M.; Westin, L. Influence of carbide and inclusion contents on the fatigue properties of high speed steels and tool steels. Int. J. Fatigue 2001, 23, 215–224. [Google Scholar] [CrossRef]
- Shibaeva, T.V.; Laurinavichyute, V.K.; Tsirlina, G.A.; Arsenkin, A.M.; Grigorovich, K.V. The effect of microstructure and non-metallic inclusions on corrosion behavior of low carbon steel in chloride containing solutions. Corros. Sci. 2014, 80, 299–308. [Google Scholar] [CrossRef]
- Tomita, Y. Effect of morphology of nonmetallic inclusions on tensile properties of quenched and tempered 0.4C-Cr-Mo-Ni steel. Mater. Charact. 1995, 34, 121–128. [Google Scholar] [CrossRef]
- Garrison, W.M.; Wojcieszynski, A.L. A discussion of the effect of inclusion volume fraction on the toughness of steel. Mater. Sci. Eng. A. 2007, 464, 321–329. [Google Scholar] [CrossRef]
- Shi, C.B.; Wang, S.J.; Li, J.; Cho, J.W. Non-metallic inclusions in electroslag remelting: A review. J. Iron Steel. Res. Int. 2021, 28, 1483–1503. [Google Scholar] [CrossRef]
- Wang, M.J.; Mu, S.M.; Sun, F.F.; Wang, Y. Influence of rare earth elements on microstructure and mechanical properties of cast high-speed steel rolls. J. Rare Earths 2007, 25, 490–494. [Google Scholar] [CrossRef]
- Chen, R.C.; Wang, Z.G.; He, J.G.; Zhu, F.S.; Li, C.H. Effects of rare earth elements on microstructure and mechanical properties of H13 die steel. Metals 2020, 10, 918. [Google Scholar] [CrossRef]
- Wang, L.M.; Du, X.J.; Gan, Y.; Liu, L.; Ye, X.N.; Jiang, L.Z. Effect of rare earths on oxidation resistance of heat resistant steel. J. Rare Earths 2010, 28, 489–491. [Google Scholar] [CrossRef]
- Gao, J.Z.; Fu, P.X.; Liu, H.W.; Li, D.Z. Effects of rare earth on the microstructure and impact toughness of H13 Steel. Metals 2015, 5, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.C.; Yu, J.T.; Li, H.B.; Jiang, Z.H.; Geng, Y.F.; Feng, H.; Zhang, B.B.; Zhu, H.C. Refinement mechanism of cerium addition on solidification structure and sigma phase of super austenitic stainless steel S32654. J. Mater. Sci. Technol. 2022, 102, 105–114. [Google Scholar] [CrossRef]
- Xu, Y.W.; Song, S.; Wang, J.W. Effect of rare earth cerium on the creep properties of modified 9Cr–1Mo heat-resistant steel. Mater. Lett. 2015, 161, 616–619. [Google Scholar] [CrossRef]
- Wang, H.P.; Xiong, L.; Zhang, L.; Wang, Y.; Shu, Y.Y.; Zhou, Y. Investigation of RE-O-S-As inclusions in high carbon steels. Metall. Mater. Trans. B 2017, 48, 2849–2858. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Bao, X.R.; Chen, L.; Jin, Z.L.; Ren, H.P. Rare Earth microalloied elements′ influence on the organization and capability of X65 pipeline steel. J. Rare Earths 2010, 28, 497–500. [Google Scholar] [CrossRef]
- Chen, L.; Ma, X.C.; Wang, L.M.; Ye, X.N. Effect of rare earth element yttrium addition on microstructures and properties of a 21Cr–11Ni austenitic heat-resistant stainless steel. Mater. Des. 2011, 32, 2206–2212. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Wang, J.F.; Zhou, S.; Wang, X.D. Effects of rare earth addition on microstructure and mechanical properties of a Fe–15Mn–1.5Al–0.6C TWIP steel. Mater. Sci. Eng. A 2014, 608, 106–113. [Google Scholar] [CrossRef]
- Wang, Y.G.; Liu, C.J. Agglomeration characteristics of various inclusions in Al-killed molten steel containing rare earth element. Metall. Mater. Trans. B 2020, 51, 2585–2595. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, G.G.; Li, S.J.; Dai, W.X. Effect of cerium on the behavior of inclusions in H13 steel. Steel Res. Int. 2018, 89, 1800371. [Google Scholar] [CrossRef]
- Liu, H.H.; Fu, P.X.; Liu, H.W.; Cao, Y.F.; Sun, C.; Du, N.Y.; Li, D.Z. Effects of rare earth elements on microstructure evolution and mechanical properties of 718H pre-hardened mold steel. J. Mater. Sci. Technol. 2020, 50, 245–256. [Google Scholar] [CrossRef]
- Feng, H.; Lu, P.C.; Li, H.B.; Jiang, Z.H. Effect of Mg pretreatment and Ce addition on cleanliness and inclusion evolution in high-nitrogen stainless bearing steels. Metall. Mater. Trans. B 2022, 53, 864–876. [Google Scholar] [CrossRef]
- Geng, R.M.; Li, J.; Shi, C.B. Effect of Ce on inclusion evolution and HAZ mechanical properties of Al-killed high-strength steel. Ironmak. Steelmak. 2021, 48, 796–802. [Google Scholar] [CrossRef]
- Geng, R.M.; Li, J.; Shi, C.B. Evolution of inclusions with Ce addition and Ca treatment in Al-killed steel during RH refining process. ISIJ Int. 2021, 61, 1506–1513. [Google Scholar] [CrossRef]
- Shi, C.B.; Yu, W.T.; Wang, H.; Li, J.; Jiang, M. Simultaneous modification of alumina and MgO·Al2O3 inclusions by calcium treatment during electroslag remelting of stainless tool steel. Metall. Mater. Trans. B 2016, 48, 146–161. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Z.H.; Geng, X.; Chen, M.J.; Cui, S. Effect of rare earth–magnesium alloy on inclusion evolution in industrial production of die steel. Steel Res. Int. 2019, 90, 1900103. [Google Scholar] [CrossRef]
- Shi, C.B.; Zheng, D.L.; Guo, B.S.; LI, J.; Jiang, F. Evolution of oxide–sulfide complex inclusions and its correlation with steel cleanliness during electroslag rapid remelting (ESRR) of tool steel. Metall. Mater. Trans. B 2018, 49, 3390–3402. [Google Scholar] [CrossRef]
- Wang, S.J.; Shi, C.B.; Liang, Y.J.; Wan, X.X.; Zhu, X. Evolution and formation of non-metallic inclusions during electroslag remelting of a heat-resistant steel for ultra-supercritical power plants. Metall. Mater. Trans. B 2022, 53, 3095–3114. [Google Scholar] [CrossRef]
- Gao, S.; Wang, M.; Guo, J.L.; Wang, H.; Zhi, J.G.; Bao, Y.P. Characterization transformation of inclusions using rare earth Ce treatment on Al-Killed titanium alloyed interstitial free steel. Steel Res. Int. 2019, 90, 1900194. [Google Scholar] [CrossRef]
- Geng, R.M.; Li, J.; Shi, C.B.; Zhi, J.G.; Lu, B. Effect of Ce-La on inclusion evolution in Al-killed high strength steel. Metall. Res. Technol. 2020, 117, 616. [Google Scholar] [CrossRef]
- Shi, C.B.; Wang, H.; Li, J. Effects of reoxidation of liquid steel and slag composition on the chemistry evolution of inclusions during electroslag remelting. Metall. Mater. Trans. B 2018, 49, 1675–1689. [Google Scholar] [CrossRef]
- Shi, C.B. Deoxidation of electroslag remelting (ESR)—A review. ISIJ Int. 2020, 60, 1083–1096. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.L.; Liu, C.J.; Jiang, M.F. Effect of rare earths on impact toughness of a low-carbon steel. Mater. Des. 2012, 33, 306–312. [Google Scholar] [CrossRef]
- Kruger, D.; Garbers, C.A. Characteristics and modification of non-metallic inclusions in titanium-stabilized aisi 409 ferritic stainless steel. Metall. Mater. Trans. B 2017, 48, 1514–1532. [Google Scholar] [CrossRef]
- Wen, T.J.; Ren, Q.; Zhang, L.F.; Wang, J.J.; Ren, Y.; Zhang, J.; Yang, W.; Xu, A.J. Evolution of nonmetallic inclusions during the electroslag remelting process. Steel Res. Int. 2021, 92, 2000629. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, L.F. Effect of cerium content on inclusions in an ultra-low-carbon aluminum-killed steel. Metall. Mater. Trans. B 2020, 51, 589–600. [Google Scholar] [CrossRef]
- Adabavazeh, Z.; Hwang, W.S.; Su, Y.H. Effect of adding cerium on microstructure and morphology of Ce-based inclusions formed in low-carbon steel. Sci. Rep. 2017, 7, 46503. [Google Scholar] [CrossRef]
- Shi, C.B.; Zheng, X.; Yang, Z.B.; Lan, P.; Li, J.; Jiang, F. Effect of melting rate of electroslag rapid remelting (ESRR) on the microstructure and carbides in a hot work tool steel. Metals Mater. Int. 2021, 27, 3603–3616. [Google Scholar] [CrossRef]
- Klancnik, U.; Kosec, B.; Mrvar, P.; Medved, J. Non-equilibrium solidification and microsegregation in centrifugally cast high speed steel for rolls. J. Min. Metall. B 2018, 54, 59–66. [Google Scholar] [CrossRef]







| Trials No. | C | Si | Mn | Mo | Ti | Cr | Ni | Al | Nb | S | Ca | Ce | Mg | N | O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | 0.02 | 0.5 | 0.83 | 2.49 | 0.18 | 15.1 | 22 | 0.15 | 0.98 | 0.0032 | 0.0002 | 0 | 0.0004 | 0.0071 | 0.0010 |
| T2 | 0.02 | 0.5 | 0.89 | 2.53 | 0.14 | 14.9 | 22 | 0.10 | 0.99 | 0.0009 | 0.0002 | 0.016 | 0.0003 | 0.0120 | 0.0006 |
| T3 | 0.02 | 0.5 | 0.78 | 2.50 | 0.21 | 14.6 | 22 | 0.15 | 1.03 | 0.0025 | 0.0002 | 0.300 | 0.0003 | 0.0031 | 0.0007 |
| Ingot No. | C | Si | Mn | Mo | Ti | Cr | Ni | Al | Nb | S | Ca | Ce | Mg | N | O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 0.02 | 0.5 | 0.82 | 2.60 | 0.13 | 15.5 | 22 | 0.19 | 1.00 | 0.0007 | 0.0002 | 0 | 0.0004 | 0.0076 | 0.0016 |
| C2 | 0.02 | 0.5 | 0.89 | 2.60 | 0.12 | 15.4 | 22 | 0.23 | 1.00 | 0.0007 | 0.0002 | 0.0055 | 0.0003 | 0.0140 | 0.0013 |
| C3 | 0.02 | 0.5 | 0.83 | 2.50 | 0.15 | 14.6 | 22 | 0.15 | 1.02 | 0.0016 | 0.0002 | 0.0630 | 0.0003 | 0.0054 | 0.0018 |
| C | Si | Mn | Mo | Ti | Cr | Ni | Al | Mg | Ca | S | Ce | N | O | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | −0.421 | −0.066 | −0.021 | 0.005 | −1.8 | −0.033 | 0.006 | −1.17 | −300 | - | −0.133 | −0.57 | 0.057 | −0.17 |
| S | 0.111 | 0.075 | −0.026 | - | −0.27 | −0.0105 | - | 0.041 | −1.82 | −110 | −0.046 | −1.91 | 0.01 | −0.27 |
| Al | 0.091 | 0.056 | 0.035 | - | 0.004 | - | −0.029 | 0.045 | −0.3 | −0.047 | 0.035 | −0.52 | −0.057 | −1.98 |
| Ce | −0.077 | - | - | - | −3.62 | - | - | −2.25 | - | - | −8.36 | −0.003 | −6.612 | −5.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Shi, C.; Wang, S.; Li, J.; Zhu, X. Evolution and Formation of Non-Metallic Inclusions during Electroslag Remelting of Ce-Bearing 15Cr-22Ni-1Nb Austenitic Heat-Resistant Steel. Metals 2022, 12, 2094. https://doi.org/10.3390/met12122094
Wang Z, Shi C, Wang S, Li J, Zhu X. Evolution and Formation of Non-Metallic Inclusions during Electroslag Remelting of Ce-Bearing 15Cr-22Ni-1Nb Austenitic Heat-Resistant Steel. Metals. 2022; 12(12):2094. https://doi.org/10.3390/met12122094
Chicago/Turabian StyleWang, Zhongwei, Chengbin Shi, Shijun Wang, Jing Li, and Xin Zhu. 2022. "Evolution and Formation of Non-Metallic Inclusions during Electroslag Remelting of Ce-Bearing 15Cr-22Ni-1Nb Austenitic Heat-Resistant Steel" Metals 12, no. 12: 2094. https://doi.org/10.3390/met12122094






