The Evolution of Surface Oxides during TiFe0.9M0.1 (M = Ni, Mn) Activation: An In Situ XPS Investigation
Abstract
1. Introduction
2. Experimental Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abe, J.O.; Popoola, A.; Ajenifuja, E.; Popoola, O. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Rusman, N.A.A.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Moroz, S.; Tan, X.F.; Pierce, J.; Greaves, M.; Duguid, A.; Dumur, K.; Ng, J. Systems based on hypo-eutectic Mg–Mg2Ni alloys for medium to large scale hydrogen storage and delivery. J. Alloys Compd. 2013, 580, S329–S332. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. Formation and Properties of Iron Titanium Hydride. Inorg. Chem. 1974, 13, 218–222. [Google Scholar] [CrossRef]
- Lys, A.; Fadonougbo, J.O.; Faisal, M.; Suh, J.-Y.; Lee, Y.-S.; Shim, J.-H.; Park, J.; Cho, Y.W. Enhancing the Hydrogen Storage Properties of AxBy Intermetallic Compounds by Partial Substitution: A Short Review. Hydrogen 2020, 1, 38–63. [Google Scholar] [CrossRef]
- Fadonougbo, J.O.; Park, K.B.; Na, T.-W.; Park, C.-S.; Park, H.-K.; Ko, W.-S. An integrated computational and experimental method for predicting hydrogen plateau pressures of TiFe1-xMx-based room temperature hydrides. Int. J. Hydrogen Energy 2022, 47, 17673–17682. [Google Scholar] [CrossRef]
- Sandrock, G.D.; Reilly, J.J.; Johnson, J.R. Metallurgical considerations in the production and use of FeTi alloys for hydrogen storage. In Proceedings of the 11th Intersociety Energy Conversion Engineering Conference, State Line, NV, USA, 12 September 1976. [Google Scholar]
- Zhang, Y.-H.; Li, C.; Yuan, Z.-M.; Qi, Y.; Guo, S.-H.; Zhao, D.-L. Research progress of TiFe-based hydrogen storage alloys. J. Iron Steel Res. Int. 2022, 29, 537–551. [Google Scholar] [CrossRef]
- Mintz, M.; Vaknin, S.; Biderman, S.; Hadari, Z. Hydrides of ternary TiFexM1−x (M = Cr, Mn, Co, Ni) intermetallics. J. Appl. Phys. 1981, 52, 463–467. [Google Scholar] [CrossRef]
- Jain, P.; Gosselin, C.; Huot, J. Effect of Zr, Ni and Zr7Ni10 alloy on hydrogen storage characteristics of TiFe alloy. Int. J. Hydrogen Energy 2015, 40, 16921–16927. [Google Scholar] [CrossRef]
- Park, K.B.; Fadonougbo, J.O.; Park, C.-S.; Lee, J.-H.; Na, T.-W.; Kang, H.-S.; Ko, W.-S.; Park, H.-K. Effect of Fe substitution on first hydrogenation kinetics of TiFe-based hydrogen storage alloys after air exposure. Int. J. Hydrogen Energy 2021, 46, 30780–30789. [Google Scholar] [CrossRef]
- Lee, S.-M.; Perng, T.-P. Correlation of substitutional solid solution with hydrogenation properties of TiFe1−xMx (M = Ni, Co, Al) alloys. J. Alloys Compd. 1999, 291, 254–261. [Google Scholar] [CrossRef]
- Zadorozhnyy, V.Y.; Klyamkin, S.; Zadorozhnyy, M.Y.; Bermesheva, O.; Kaloshkin, S. Mechanical alloying of nanocrystalline intermetallic compound TiFe doped by aluminum and chromium. J. Alloys Compd. 2014, 586, S56–S60. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Cuevas, F.; Latroche, M. Hydrogen storage properties of Mn and Cu for Fe substitution in TiFe0.9 intermetallic compound. J. Alloys Compd. 2021, 851, 156075. [Google Scholar] [CrossRef]
- Ali, W.; Hao, Z.; Li, Z.; Chen, G.; Wu, Z.; Lu, X.; Li, C. Effects of Cu and Y substitution on hydrogen storage performance of TiFe0.86Mn0.1Y0.1−xCux. Int. J. Hydrogen Energy 2017, 42, 16620–16631. [Google Scholar] [CrossRef]
- Patel, A.K.; Duguay, A.; Tougas, B.; Schade, C.; Sharma, P.; Huot, J. Microstructure and first hydrogenation properties of TiFe alloy with Zr and Mn as additives. Int. J. Hydrogen Energy 2020, 45, 787–797. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Dreistadt, D.M.; Capurso, G.; Jepsen, J.; Cuevas, F.; Latroche, M. Fundamental hydrogen storage properties of TiFe-alloy with partial substitution of Fe by Ti and Mn. J. Alloys Compd. 2021, 874, 159925. [Google Scholar] [CrossRef]
- Yang, T.; Wang, P.; Xia, C.; Liu, N.; Liang, C.; Yin, F.; Li, Q. Effect of chromium, manganese and yttrium on microstructure and hydrogen storage properties of TiFe-based alloy. Int. J. Hydrogen Energy 2020, 45, 12071–12081. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, S.-I.; Faisal, M.; Kim, H.; Lee, Y.-S.; Suh, J.-Y.; Shim, J.-H.; Huh, J.-Y.; Cho, Y.W. Effect of Cr addition on room temperature hydrogenation of TiFe alloys. Int. J. Hydrogen Energy 2021, 46, 19478–19485. [Google Scholar] [CrossRef]
- Lee, S.-M.; Perng, T.-P. Effect of the second phase on the initiation of hydrogenation of TiFe1−xMx (M = Cr, Mn) alloys. Int. J. Hydrogen Energy 1994, 19, 259–263. [Google Scholar] [CrossRef]
- Kim, H.; Faisal, M.; Lee, S.-I.; Jung, J.Y.; Kim, H.-J.; Hong, J.; Lee, Y.-S.; Shim, J.-H.; Cho, Y.W.; Kim, D.H. Activation of Ti–Fe–Cr alloys containing identical AB2 fractions. J. Alloys Compd. 2021, 864, 158876. [Google Scholar] [CrossRef]
- Emami, H.; Edalati, K.; Matsuda, J.; Akiba, E.; Horita, Z. Hydrogen storage performance of TiFe after processing by ball milling. Acta Mater. 2015, 88, 190–195. [Google Scholar] [CrossRef]
- Chiang, C.H.; Chin, Z.H.; Perng, T.P. Hydrogenation of TiFe by high-energy ball milling. J. Alloys Compd. 2000, 307, 259–265. [Google Scholar] [CrossRef]
- Edalati, K.; Matsuda, J.; Yanagida, A.; Akiba, E.; Horita, Z. Activation of TiFe for hydrogen storage by plastic deformation using groove rolling and high-pressure torsion: Similarities and differences. Int. J. Hydrogen Energy 2014, 39, 15589–15594. [Google Scholar] [CrossRef]
- Edalati, K.; Matsuda, J.; Iwaoka, H.; Toh, S.; Akiba, E.; Horita, Z. High-pressure torsion of TiFe intermetallics for activation of hydrogen storage at room temperature with heterogeneous nanostructure. Int. J. Hydrogen Energy 2013, 38, 4622–4627. [Google Scholar] [CrossRef]
- Vega, L.; Leiva, D.; Neto, R.L.; Silva, W.; Silva, R.; Ishikawa, T.; Kiminami, C.; Botta, W. Mechanical activation of TiFe for hydrogen storage by cold rolling under inert atmosphere. Int. J. Hydrogen Energy 2018, 43, 2913–2918. [Google Scholar] [CrossRef]
- Zadorozhnyy, V.Y.; Milovzorov, G.; Klyamkin, S.; Zadorozhnyy, M.Y.; Strugova, D.; Gorshenkov, M.; Kaloshkin, S. Preparation and hydrogen storage properties of nanocrystalline TiFe synthesized by mechanical alloying. Prog. Nat. Sci. Mater. Int. 2017, 27, 149–155. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, J.; Wang, Q. Reactivation behaviour of TiFe hydride. J. Alloys Compd. 1994, 215, 91–95. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Berti, N.; Cuevas, F.; Latroche, M.; Baricco, M. Substitutional effects in TiFe for hydrogen storage: A comprehensive review. Mater. Adv. 2021, 2, 2524–2560. [Google Scholar] [CrossRef]
- Gosselin, C.; Santos, D.; Huot, J. First hydrogenation enhancement in TiFe alloys for hydrogen storage. J. Phys. D Appl. Phys. 2017, 50, 375303. [Google Scholar] [CrossRef]
- Ulate-Kolitsky, E.; Tougas, B.; Neumann, B.; Schade, C.; Huot, J. First hydrogenation of mechanically processed TiFe-based alloy synthesized by gas atomization. Int. J. Hydrogen Energy 2021, 46, 7381–7389. [Google Scholar] [CrossRef]
- Patel, A.K.; Duguay, A.; Tougas, B.; Neumann, B.; Schade, C.; Sharma, P.; Huot, J. Study of the Microstructural and First Hydrogenation Properties of TiFe Alloy with Zr, Mn and V as Additives. Processes 2021, 9, 1217. [Google Scholar] [CrossRef]
- Schober, T.; Westlake, D. The activation of FeTi for hydrogen storage-A different view. Scr. Metall. 1981, 15, 913–918. [Google Scholar] [CrossRef]
- Schlapbach, L.; Riesterer, T. The activation of FeTi for hydrogen absorption. Appl. Phys. A 1983, 32, 169–182. [Google Scholar] [CrossRef]
- Modi, P.; Aguey-Zinsou, K.-F. Titanium-iron-manganese (TiFe0.85Mn0.15) alloy for hydrogen storage: Reactivation upon oxidation. Int. J. Hydrog. Energy 2019, 44, 16757–16764. [Google Scholar] [CrossRef]
- Lin, H.-J.; Li, H.-W.; Shao, H.; Lu, Y.; Asano, K. In situ measurement technologies on solid-state hydrogen storage materials: A review. Mater. Today Energy 2020, 17, 100463. [Google Scholar] [CrossRef]
- Faisal, M.; Suh, J.-Y.; Lee, Y.-S. Understanding first cycle hydrogenation properties of Ti–Fe–Zr ternary alloys. Int. J. Hydrogen Energy 2021, 46, 4241–4251. [Google Scholar] [CrossRef]
- Madian, M.; Wang, Z.; Gonzalez-Martinez, I.; Oswald, S.; Giebeler, L.; Mikhailova, D. Ordered Ti-Fe-O nanotubes as additive-free anodes for lithium ion batteries. Appl. Mater. Today 2020, 20, 100676. [Google Scholar] [CrossRef]
- Chávez-Díaz, M.P.; Luna-Sánchez, R.M.; Vazquez-Arenas, J.; Lartundo-Rojas, L.; Hallen, J.M.; Cabrera-Sierra, R. XPS and EIS studies to account for the passive behavior of the alloy Ti-6Al-4V in Hank’s solution. J. Solid State Electrochem. 2019, 23, 3187–3196. [Google Scholar] [CrossRef]
- Modi, P.; Liu, W.; Aguey-Zinsou, K.-F. Effect of chromium addition on the reactivation of the titanium-iron-manganese (TiFe0.85Mn0.15) alloy. J. Alloys Compd. 2022, 891, 161943. [Google Scholar] [CrossRef]
- Wu, X.; Chen, C.; Hao, J.; Zhao, T.; Ma, H.; Lu, Y.; Ren, Z. Effect of Phosphating and Heat Treatment on Magnetic Properties of Fe-3.3 Si-6.5 Cr Soft Magnetic Composites. J. Supercond. Nov. Magn. 2020, 33, 1889–1897. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Kesler, V.G.; Pervukhina, N.V.; Zhang, Z. Ti 2p and O 1s core levels and chemical bonding in titanium-bearing oxides. J. Electron Spectrosc. Relat. Phenom. 2006, 152, 18–24. [Google Scholar] [CrossRef]
- Biesinger, M. X-ray Photoelectron Spectroscopy (XPS) Reference Pages; Surface Science Western, University of Western Ontario: London, ON, Canada, 2015. [Google Scholar]
- Pan, R.; Li, Y.; Fang, F.; Cao, W.; Heb, Y. Surface valence states of Mn ions and magnetic properties of La0.67Sr0.33MnO3 films. Int. J. Mater. Sci. Appl. 2016, 5, 222–227. [Google Scholar]
- Hengne, A.M.; Samal, A.K.; Enakonda, L.R.; Harb, M.; Gevers, L.E.; Anjum, D.H.; Hedhili, M.N.; Saih, Y.; Huang, K.-W.; Basset, J.-M. Ni–Sn-supported ZrO2 catalysts modified by indium for selective CO2 hydrogenation to methanol. ACS Omega 2018, 3, 3688–3701. [Google Scholar] [CrossRef] [PubMed]
- Park, K.B.; Ko, W.-S.; Fadonougbo, J.O.; Na, T.-W.; Im, H.-T.; Park, J.-Y.; Kang, J.-W.; Kang, H.-S.; Park, C.-S.; Park, H.-K. Effect of Fe substitution by Mn and Cr on first hydrogenation kinetics of air-exposed TiFe-based hydrogen storage alloy. Mater. Charact. 2021, 178, 111246. [Google Scholar] [CrossRef]
- Park, K.B.; Fadonougbo, J.O.; Na, T.-W.; Lee, T.W.; Kim, M.; Lee, D.H.; Kwon, H.G.; Park, C.-S.; Kim, Y.D.; Park, H.-K. On the first hydrogenation kinetics and mechanisms of a TiFe0.85Cr0.15 alloy produced by gas atomization. Mater. Charact. 2022, 192, 112188. [Google Scholar] [CrossRef]
- Clarke, R.; Giddey, S.; Badwal, S. Stand-alone PEM water electrolysis system for fail safe operation with a renewable energy source. Int. J. Hydrogen Energy 2010, 35, 928–935. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ryu, J.-H.; Lee, I.-B. Feasibility Analysis of Integrated Power-to-Ammonia Processes Employing Alkaline Electrolysis and Air Separation. J. Chem. Eng. Jpn. 2022, 55, 51–60. [Google Scholar] [CrossRef]
- Toghyani, S.; Afshari, E.; Baniasadi, E. A parametric comparison of three fuel recirculation system in the closed loop fuel supply system of PEM fuel cell. Int. J. Hydrogen Energy 2019, 44, 7518–7530. [Google Scholar] [CrossRef]

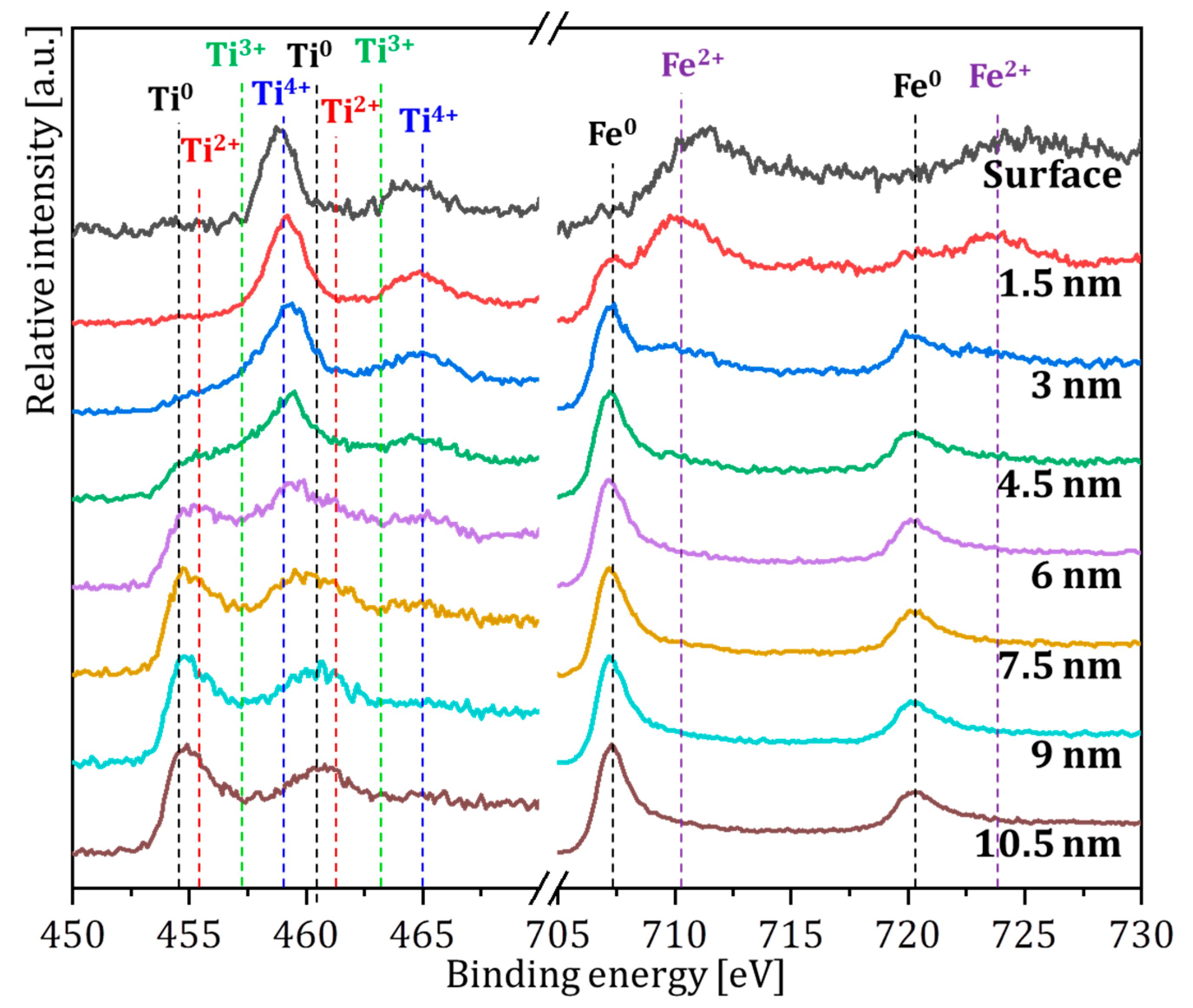
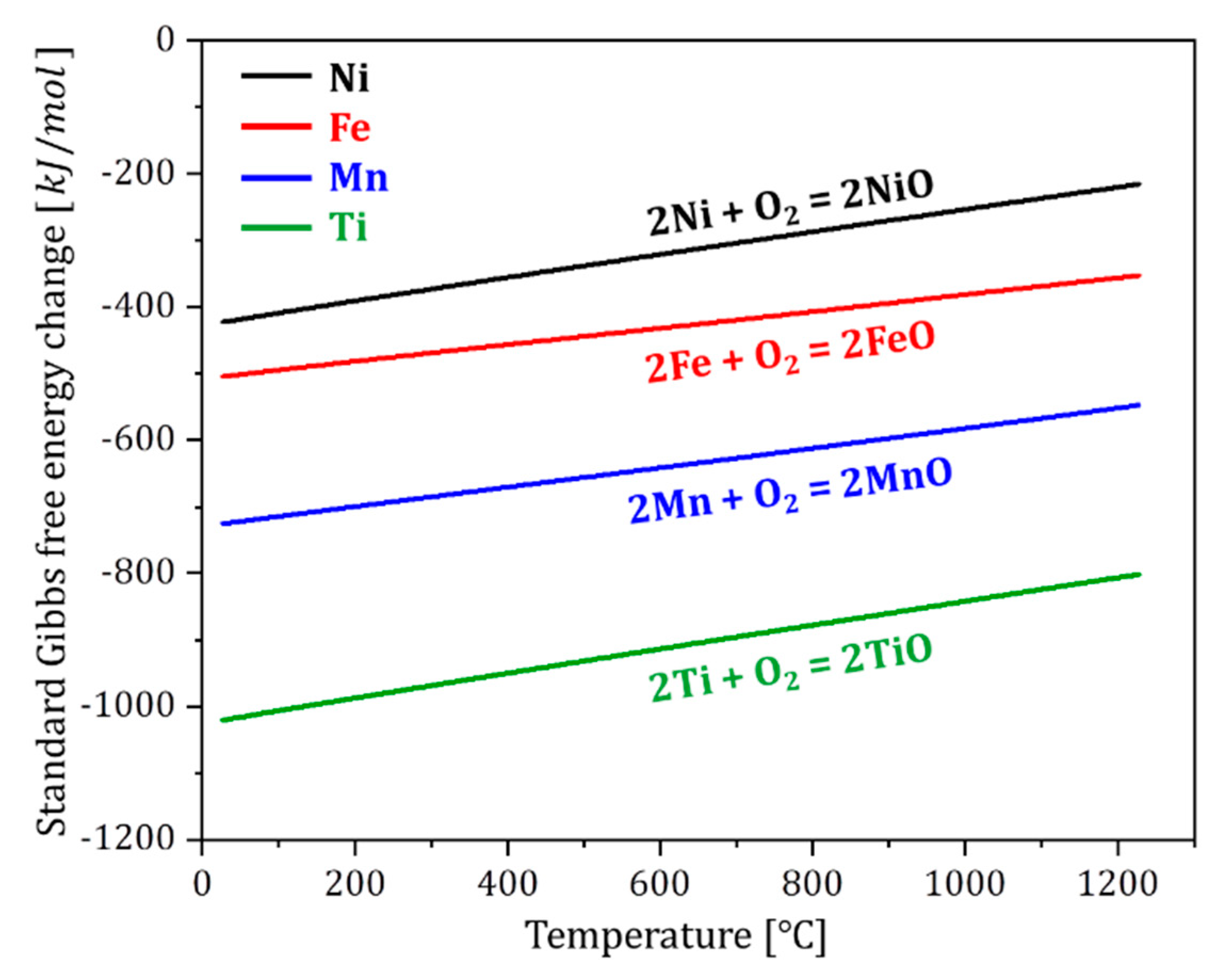
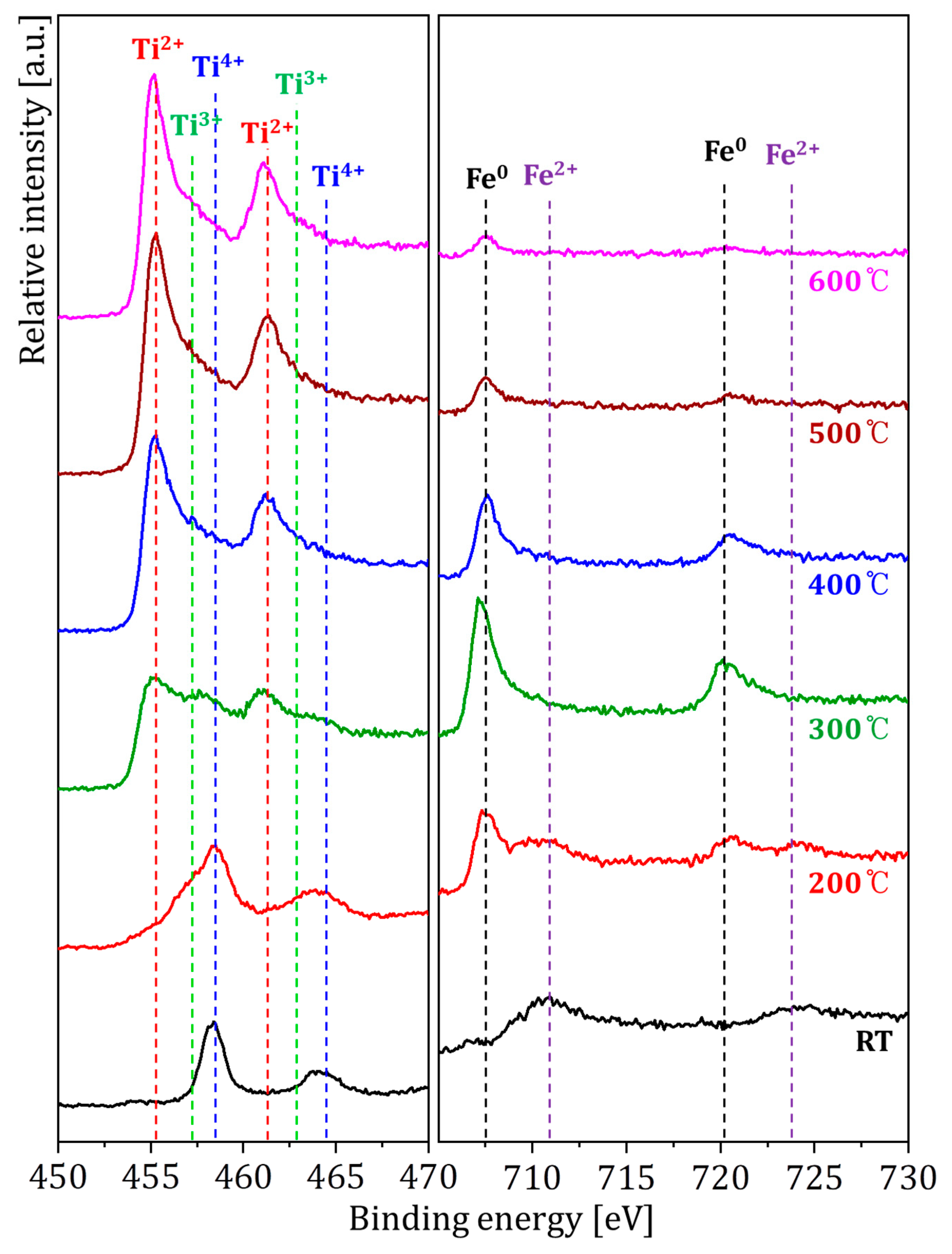
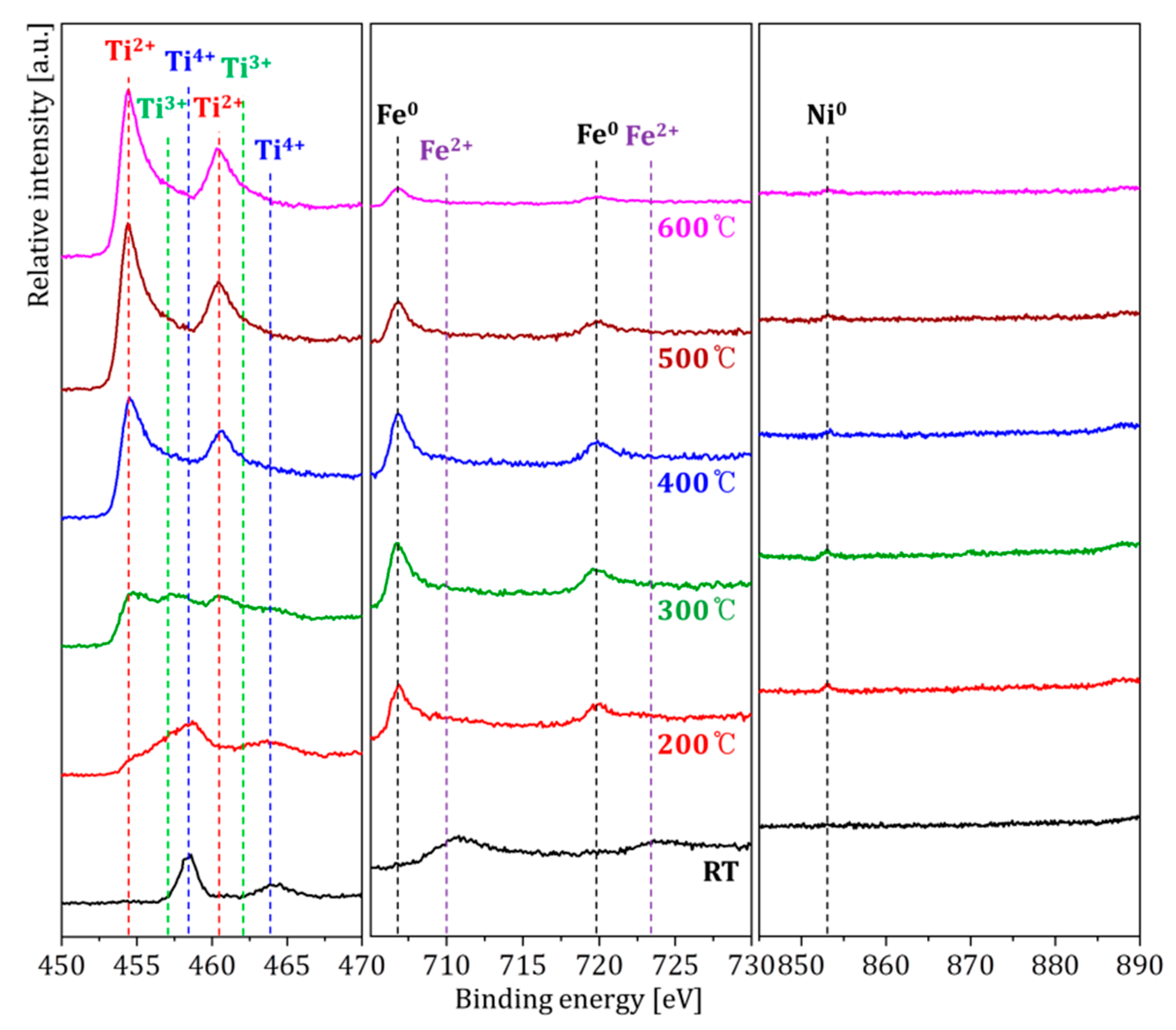
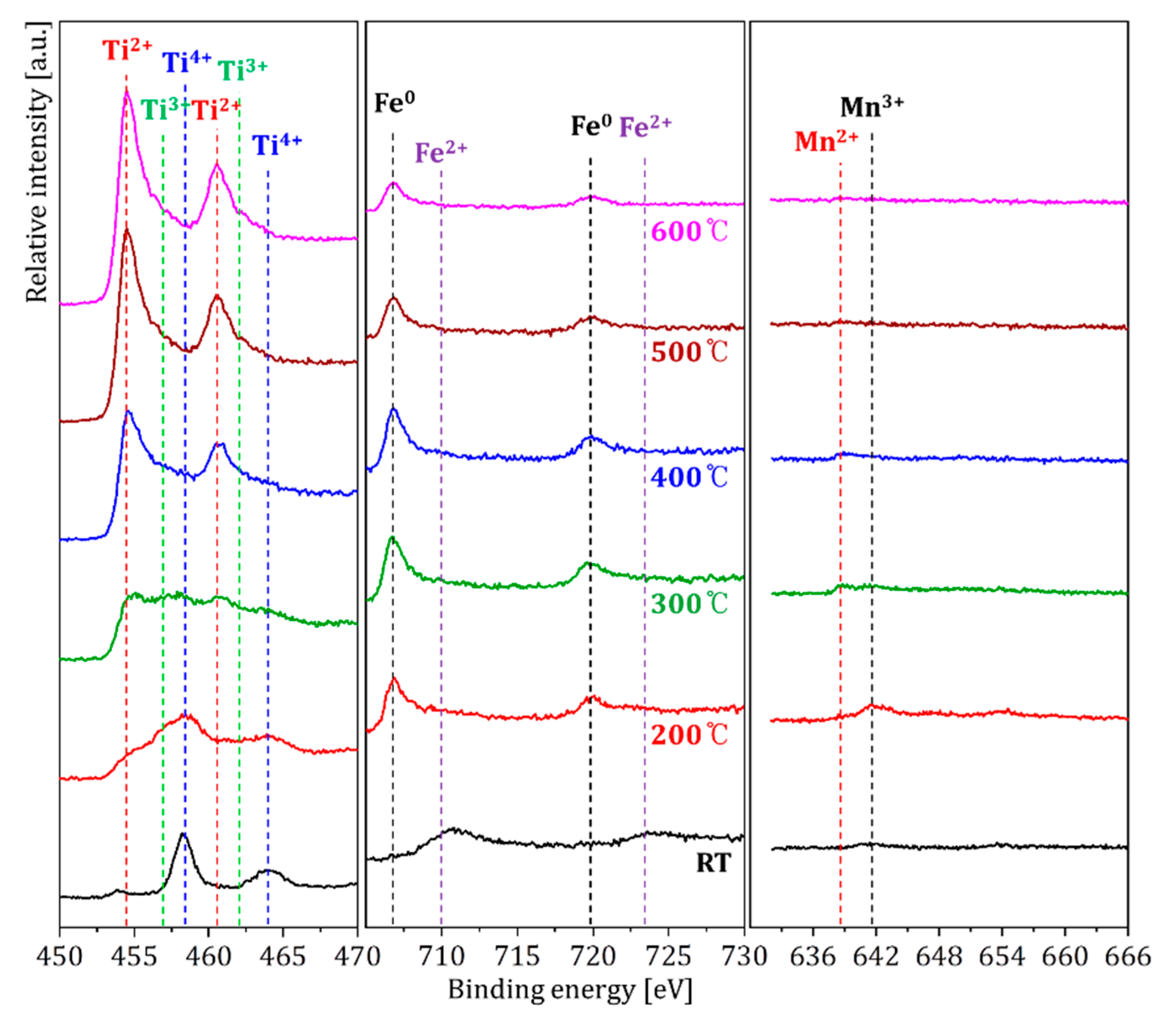
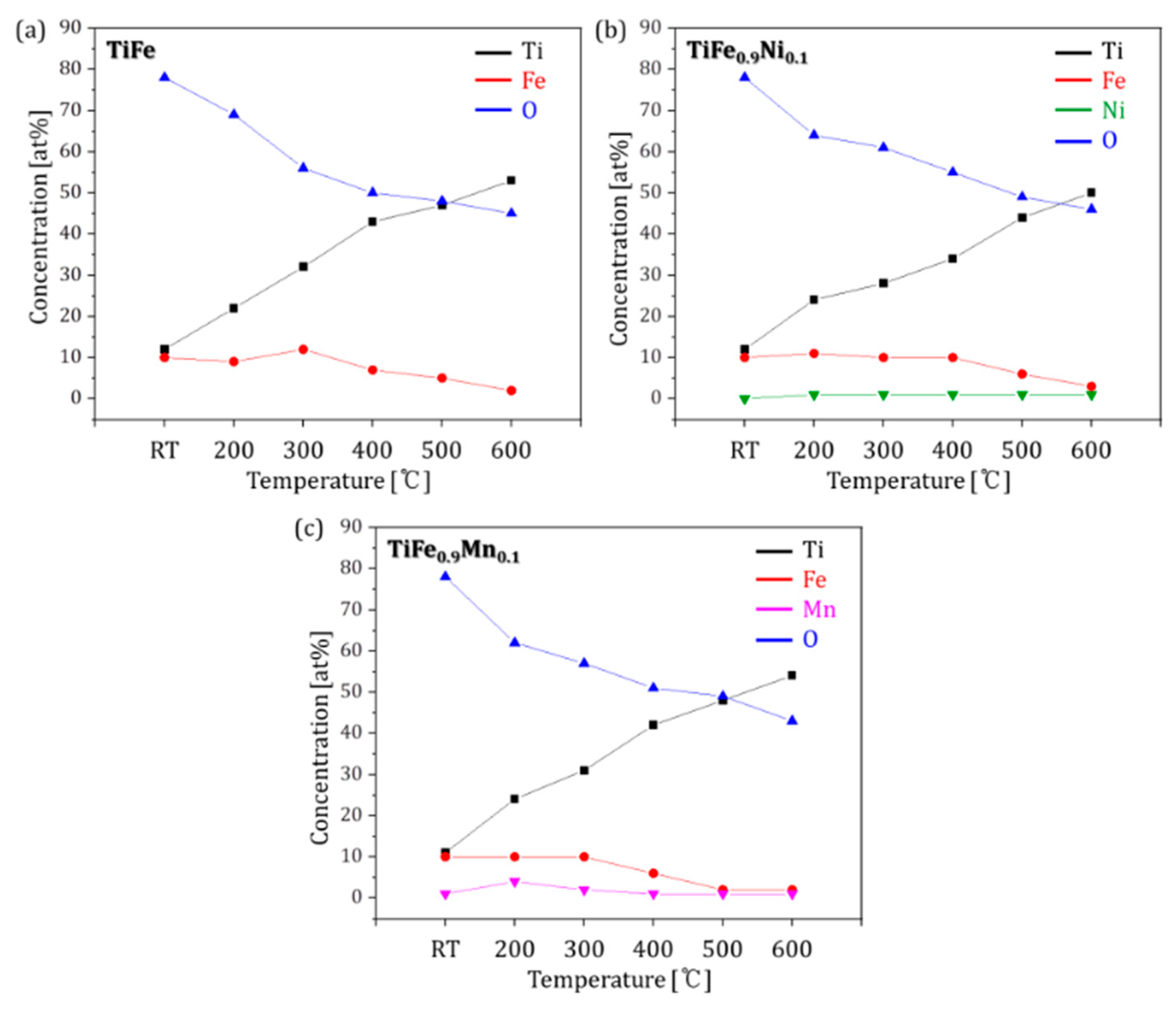
| Temperatures | Composition (at. %) | ||
|---|---|---|---|
| TiFe | TiFe0.9Ni0.1 | TiFe0.9Mn0.1 | |
| Room Temperature (RT) | Ti12Fe10O78 | Ti12Fe10O78 | Ti11Fe10Mn1O78 |
| 200 °C | Ti22Fe9O69 | Ti24Fe11Ni1O64 | Ti24Fe10Mn4O62 |
| 300 °C | Ti32Fe12O56 | Ti28Fe10Ni1O61 | Ti31Fe10Mn2O57 |
| 400 °C | Ti43Fe7O50 | Ti34Fe10Ni1O55 | Ti42Fe6Mn1O51 |
| 500 °C | Ti47Fe5O48 | Ti44Fe6Ni1O49 | Ti48Fe2Mn1O49 |
| 600 °C | Ti53Fe2O45 | Ti50Fe3Ni1O46 | Ti54Fe2Mn1O43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.B.; Fadonougbo, J.O.; Bae, J.-S.; Kang, G.B.; Choi, J.I.; Kim, Y.D.; Na, T.-W.; Park, H.-K. The Evolution of Surface Oxides during TiFe0.9M0.1 (M = Ni, Mn) Activation: An In Situ XPS Investigation. Metals 2022, 12, 2093. https://doi.org/10.3390/met12122093
Park KB, Fadonougbo JO, Bae J-S, Kang GB, Choi JI, Kim YD, Na T-W, Park H-K. The Evolution of Surface Oxides during TiFe0.9M0.1 (M = Ni, Mn) Activation: An In Situ XPS Investigation. Metals. 2022; 12(12):2093. https://doi.org/10.3390/met12122093
Chicago/Turabian StylePark, Ki Beom, Julien O. Fadonougbo, Jong-Seong Bae, Gyu Byeong Kang, Jong In Choi, Young Do Kim, Tae-Wook Na, and Hyung-Ki Park. 2022. "The Evolution of Surface Oxides during TiFe0.9M0.1 (M = Ni, Mn) Activation: An In Situ XPS Investigation" Metals 12, no. 12: 2093. https://doi.org/10.3390/met12122093
APA StylePark, K. B., Fadonougbo, J. O., Bae, J.-S., Kang, G. B., Choi, J. I., Kim, Y. D., Na, T.-W., & Park, H.-K. (2022). The Evolution of Surface Oxides during TiFe0.9M0.1 (M = Ni, Mn) Activation: An In Situ XPS Investigation. Metals, 12(12), 2093. https://doi.org/10.3390/met12122093







