Bio-Assisted Leaching of Non-Ferrous Metals from Waste Printed Circuit Boards—Importance of Process Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. PCBs
2.2. Microorganisms and Culturing Conditions
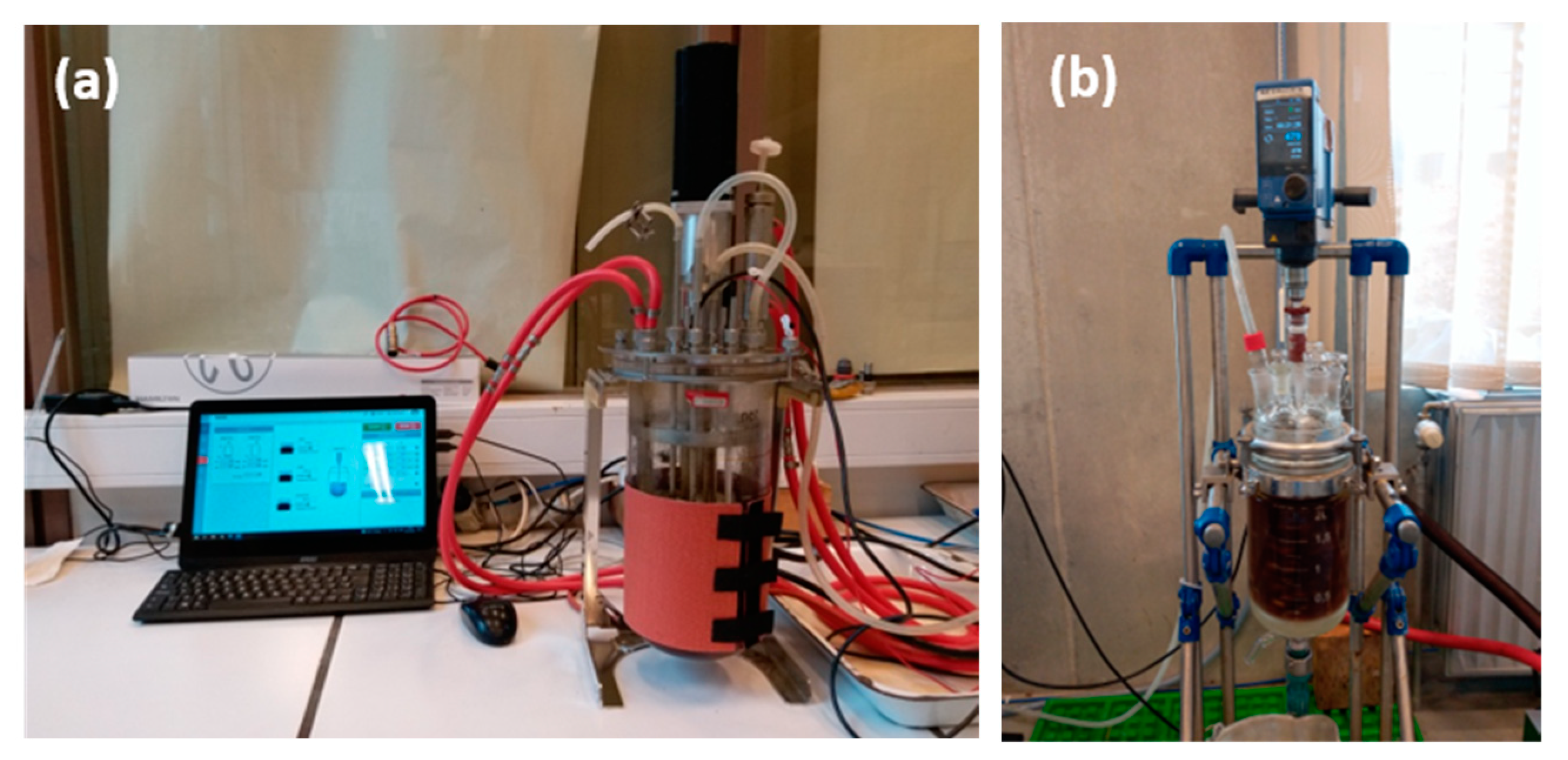
2.3. Bioleaching of PCBs
2.4. Analysis
3. Results and Disscussion
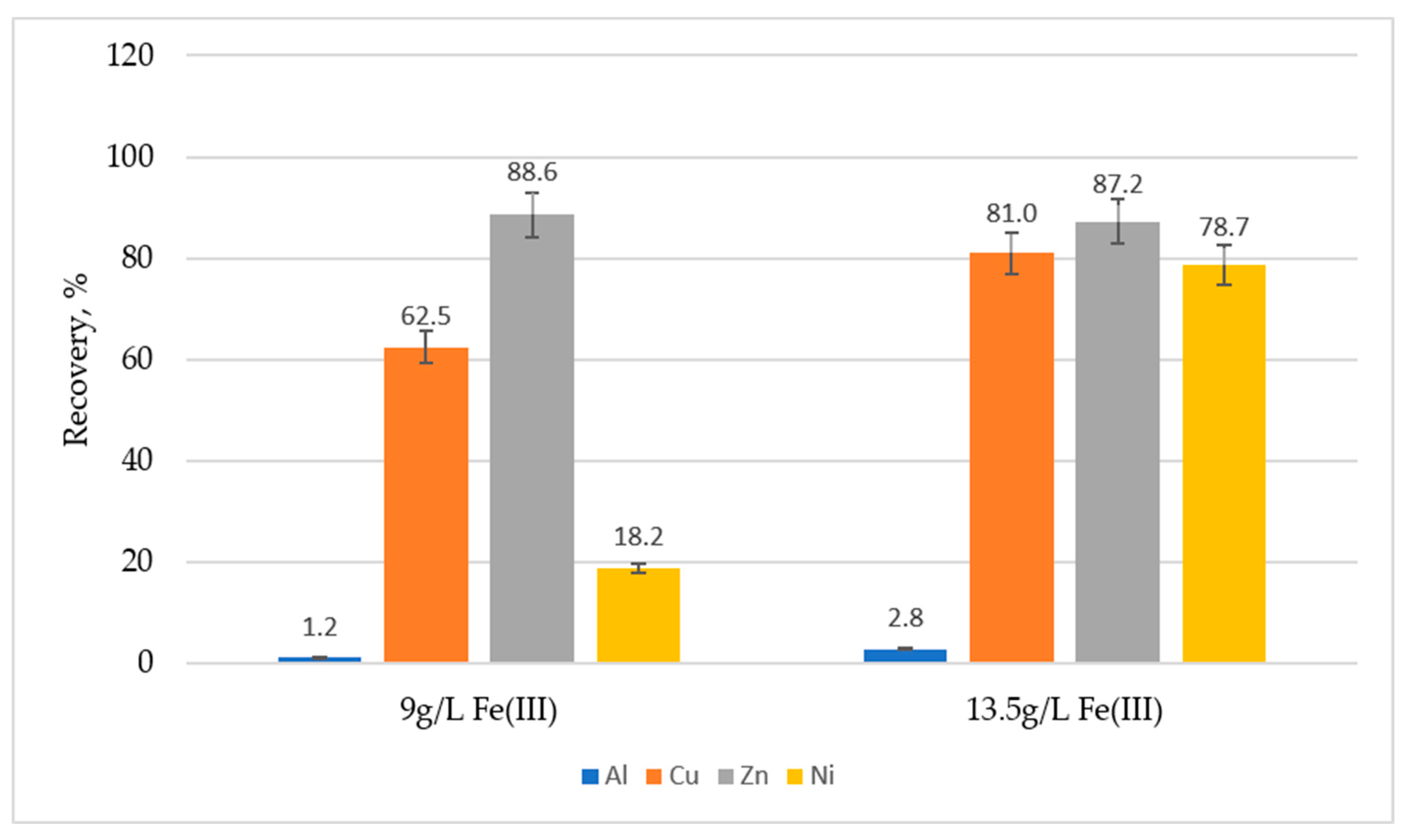
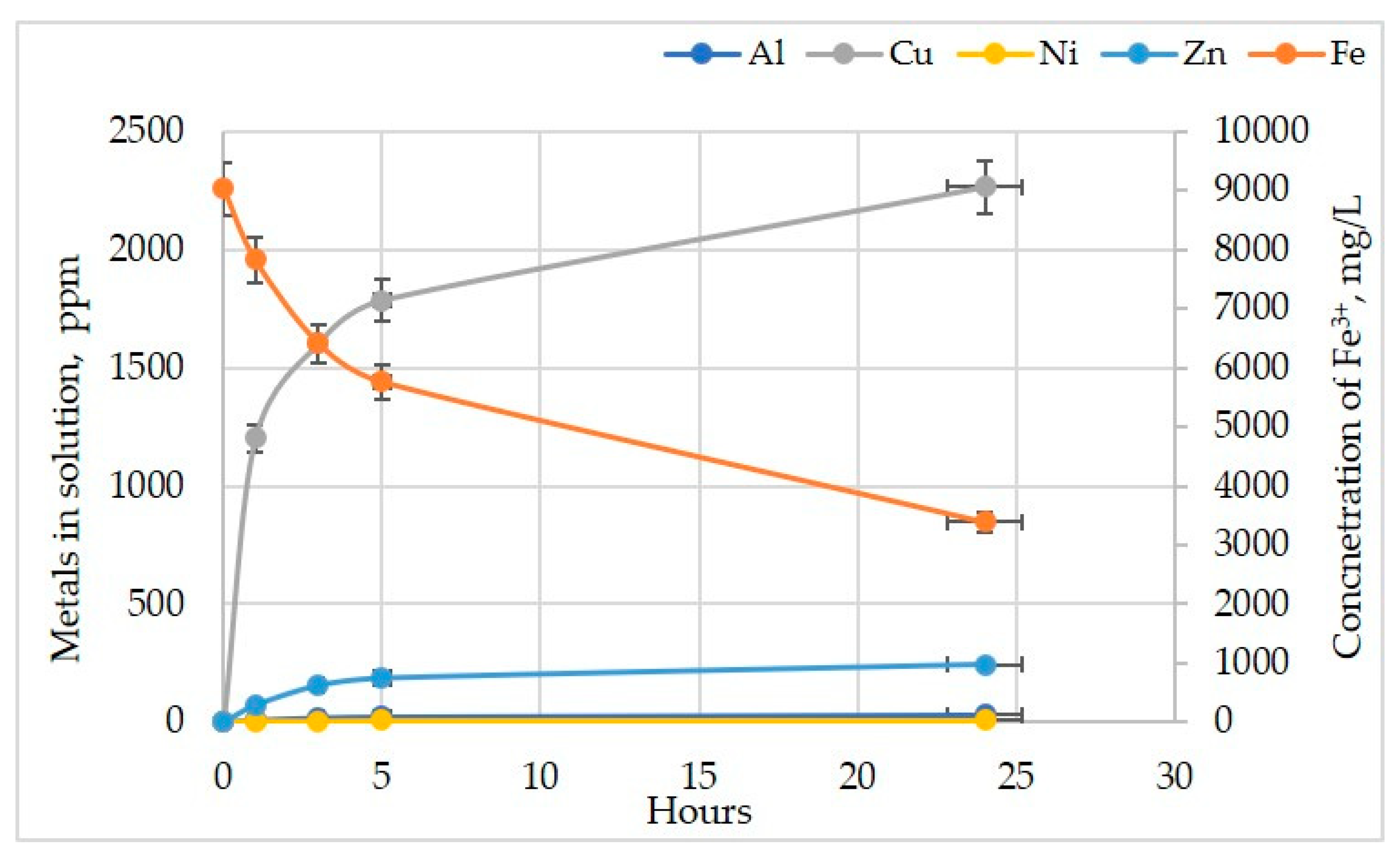
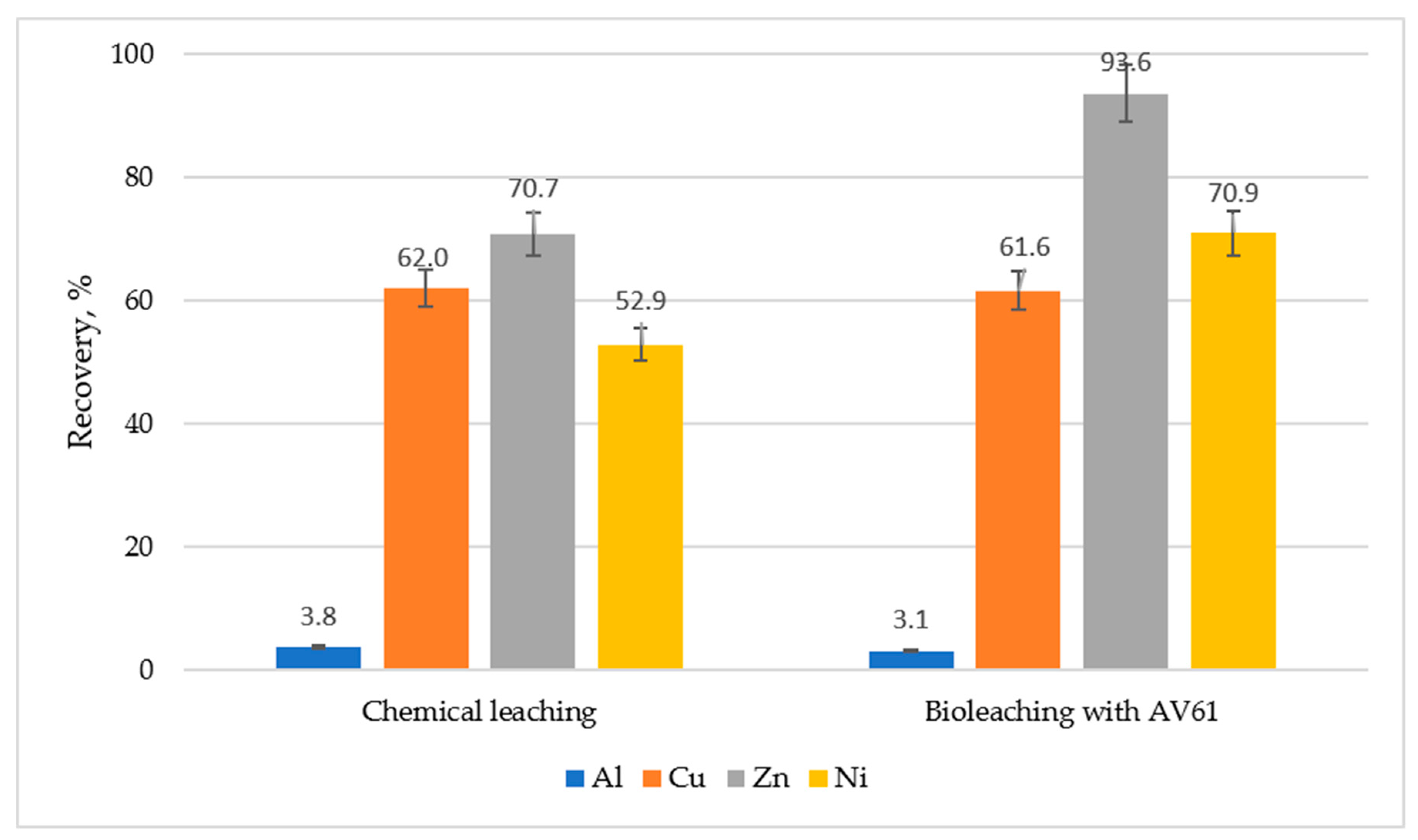
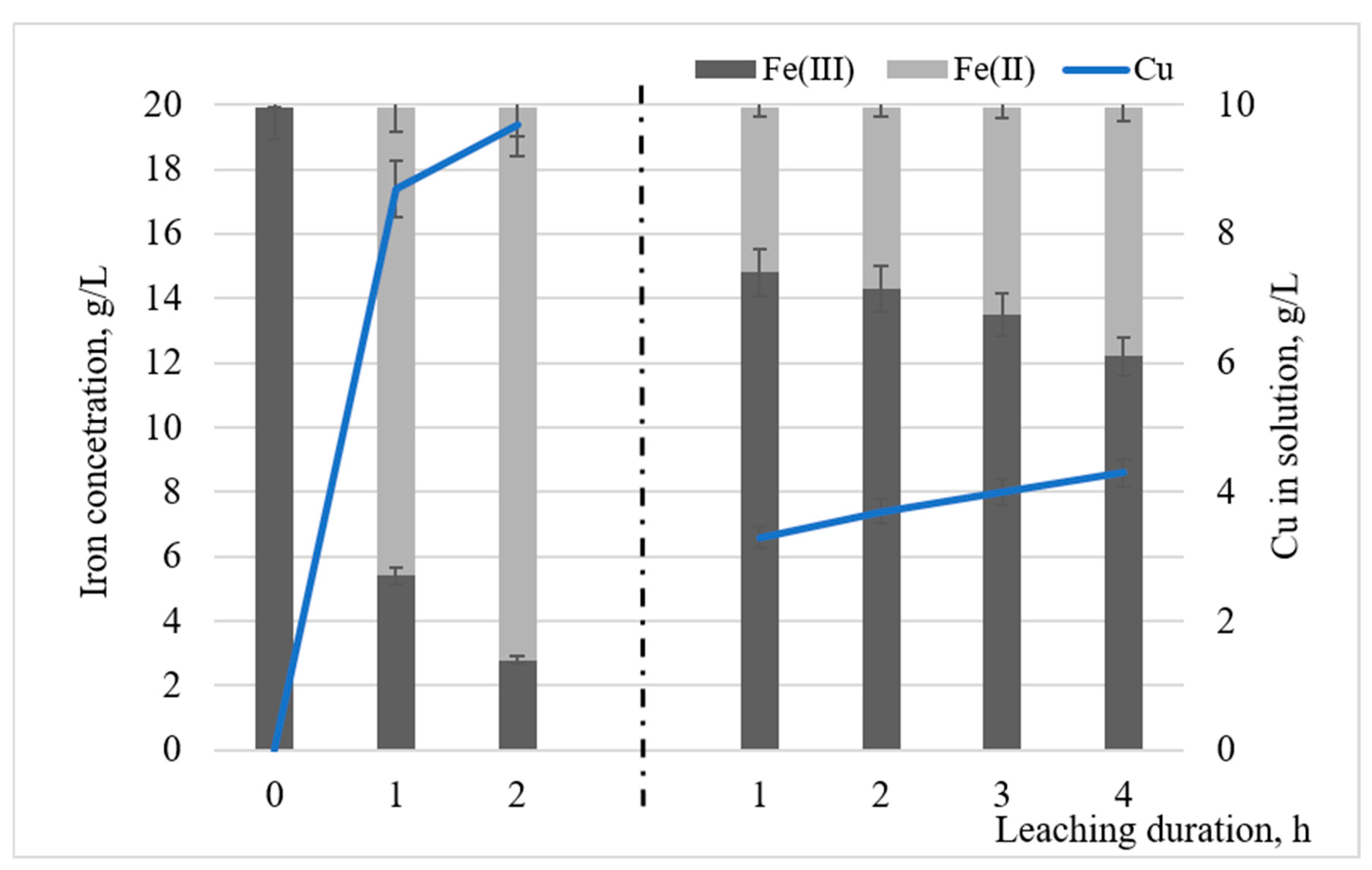
3.1. Effect of Initial Concentration of Fe(III), Pulp Density and pH on Metals Recovery
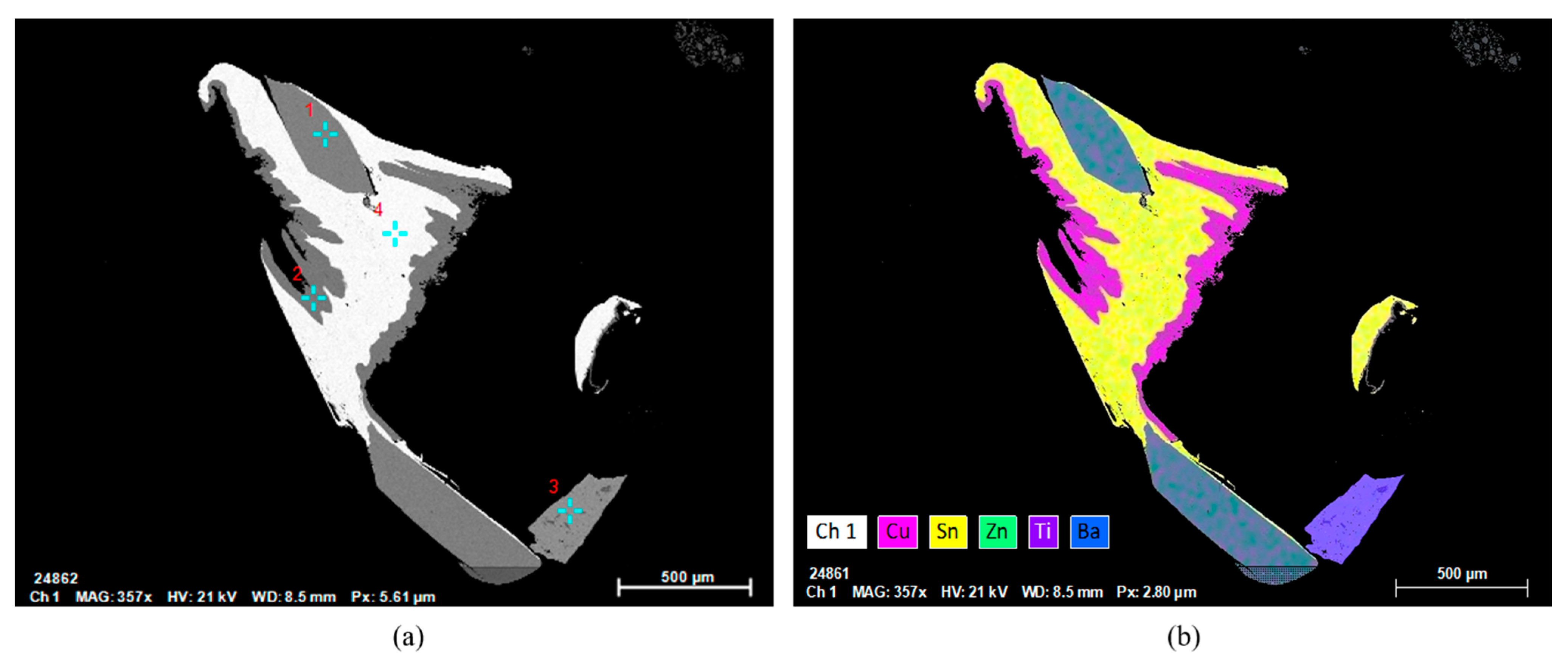
3.2. Mineralogy of the Leached PCBs
3.3. Visual Appearance of PCBs before and after Leaching
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumari, A.; Jha, M.; Singh, R.P. Recovery of metals from pyrolyzed PCBs by hydrometallurgical techniques. Hydrometallurgy 2016, 165, 97–105. [Google Scholar] [CrossRef]
- Hadi, P.; Xu, M.; Lin, C.S.K.; Hui, C.-W.; Mckay, G. Review: Waste printed circuit board recycling techniques and product utilization. J. Hazard. Mater. 2015, 283, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Sodha, A.B.; Shah, M.B.; Qureshi, S.A.; Tipre, D.R.; Dave, S.R. Decouple and compare the role of abiotic factors and developed iron and sulfur oxidizers for enhanced extraction of metals from television printed circuit boards. Sep. Sci. Technol. 2019, 54, 4. [Google Scholar] [CrossRef]
- Sadia, I.; Srivastava, R.R.; Kima, H.; NimraI, I. Biotechnological recycling of hazardous waste PCBs using Sulfobacillus thermosulfidooxidans through pretreatment of toxicant metals: Process optimization and kinetic studies. Chemosphere 2022, 286, 131978. [Google Scholar]
- Islam, A.; Ahmed, T.; Awual, M.R.; Rahman, A.; Sultana, M.; Aziz, A.A.; Monir, M.U.; Teo, S.H.; Hasan, M. Advances in sustainable approaches to recover metals from e-waste—A review. J. Clean. Prod. 2020, 244, 118815. [Google Scholar] [CrossRef]
- Lehner, T. Integrated recycling of non-ferrousmetal at Boliden Ltd. In Proceedings of the 1998 IEEE International Symposium on Electronics and the Environment, Oak Brook, IL, USA, 6 May 1998; pp. 42–47. [Google Scholar]
- Bleiwas, D.; Kelly, T. Obsolete Computers, “Gold Mines”, or High-Tech Trach? Resource Recovery from Recycling; United States Geological Survey 7; USDS: Beaverton, OR, USA, 2001. [CrossRef]
- He, W.; Li, G.; Ma, X.; Wang, H.; Huang, J.; Xu, M.; Huang, C. WEEE recovery strategies and the WEEE treatment status in China. J. Hazard. Mater. 2006, 136, 502–512. [Google Scholar] [CrossRef]
- Deveci, H.; Yazıcı, E.Y.; Aydın, U.; Yazıcı, R.; Akcil, A. Extraction of copper from scrap TV boards by sulphuric acid leaching under oxidising conditions. In Proceedings of the Going Green-CARE INNOVATION 2010 Conference (2010), Vienna, Austria, 8–11 November 2010; p. 45. [Google Scholar]
- Kaya, M. Recovery of metals from electronic waste by physical and chemical recycling processes. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2016, 10, 232–243. [Google Scholar] [CrossRef]
- Moyo, T.; Chirume, B.H.; Petersen, J. Assessing alternative pre-treatment methods to promote metal recovery in the leaching of printed circuit boards. Resour. Conserv. Recycl. 2020, 152, 104545. [Google Scholar] [CrossRef]
- Dave, S.; Sodha, A.; Tipre, D. Microbial technology for metal recovery from e-waste printed circuit boards. J. Bacteriol. Mycol. 2018, 6, 241–247. [Google Scholar] [CrossRef]
- Vasile, C.; Brebu, M.A.; Totolin, M.; Yanik, J.A.L.E.; Karayildirim, T.A.M.E.R.; Darie, H. Feedstock recycling from the printed circuit boards of used computers. Energy Fuels 2008, 22, 1658–1665. [Google Scholar] [CrossRef]
- Birloaga, I.; De Michelis, I.; Ferella, F.; Buzatu, M.; Vegliò, F. Study on the influence of various factors in the hydrometallurgical processing of waste printed circuit boards for copper and gold recovery. Waste Manag. 2013, 33, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Oishi, T.; Koyama, K.; Alam, S.; Tanaka, M.; Lee, J.C. Recovery of high purity copper cathode from printed circuit boards using ammoniacal sulfate or chloride solutions. Hydrometallurgy 2007, 89, 82–88. [Google Scholar] [CrossRef]
- Fogarasi, S.; Imre-Lucaci, F.; Imre-Lucaci, Á.; Ilea, P. Copper recovery and gold enrichment from waste printed circuit boards by mediated electrochemical oxidation. J. Hazard. Mater. 2014, 273, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Hubau, A.; Minter, M.; Changes, A.; Joulian, C.; Silvente, C.; Guezennec, A.G. Recovery of metals in a double-stage continuous bioreactor for acid bioleaching of printed circuit boards (PCBs). Sep. Purif. Technol. 2020, 238, 116481. [Google Scholar] [CrossRef]
- Sodha, A.; Qureshi, S.; Khatri, B.; Tipre, D.; Dave, S. Enhancement in iron oxidation and multi-metal extraction from waste television printed circuit boards by iron oxidizing Leptospirillum feriphillum isolated from coal sample. Waste Biomass Valor. 2017, 10, 671–680. [Google Scholar] [CrossRef]
- Jadhav, U.; Hocheng, H. Enzymatic bioleaching of metals from printed circuit board. Clean Technol. Environ. Policy 2015, 17, 947–956. [Google Scholar] [CrossRef]
- Kiddee, P.; Naidu, R.; Wong, M.H. Metals and polybrominated diphenyl ethers leaching from electronic waste in simulated landfills. J. Hazard. Mater. 2013, 252–253, 243–249. [Google Scholar] [CrossRef]
- Mankhand, T.; Singh, K.; Kumar, S.; Gupta, S.; Das, S. Pyrolysis of printed circuit boards. Int. J. Metall. Mater. Sci. Eng. 2012, 6, 102–107. [Google Scholar] [CrossRef]
- Akcil, A.; Erust, C.; Sekhar, G.C.; Ozgun, M.; Sahin, M.; Tuncuk, A. Precious metal recovery from waste printed circuit boards using cyanide and non cyanide lixiviants—A review. Waste Manag. 2015, 45, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Jadhao, P.; Chauhan, G.; Pant, K.K.; Nigam, K.D.P. Greener approach for the extraction of copper metal from electronic waste. Waste Manag. 2015, 57, 102–112. [Google Scholar] [CrossRef]
- Krebs, W.; Brombacher, C.; Bosshard, P.P.; Bachofen, R.; Brandl, H. Microbial recovery of metals from solids. FEMS Microbiol. Rev. 1997, 20, 605–617. [Google Scholar] [CrossRef]
- Vermeulen, F.; Nicolay, X. Sequential bioleaching of copper from brake pads residues using encapsulated bacteria. Miner. Eng. 2017, 106, 39–45. [Google Scholar] [CrossRef]
- Tunchuk, A.; Stazi, V.; Akcil, A.; Yazici, E.Y.; Deveci, H. Aqueous metal recovery techniques ftrom e-scrap: Hydrometallurgy in recycling. Miner. Eng. 2012, 25, 28–37. [Google Scholar] [CrossRef]
- Mrazikova, A.; Kadukova, J.; Marcincakova, R.; Velgosova, O.; Willner, J.; Fornalczyk, A.; Saturnus, M. The effect of specific conditions on Cu, Ni, Zn and Al recovery from PCBs waste using acidophilic bacterial strains. Arch. Metall. Mater. 2016, 61, 261–264. [Google Scholar] [CrossRef][Green Version]
- Tapia, J.; Duenas, A.; Cheje, N.; Soclle, G.; Patino, N.; Ancalla, W.; Tenorio, S.; Denos, J.; Taco, H.; Cao, W.; et al. Bioleaching of heavy metals from printed circuit boards with an Acidophilic iron-oxidizing microbial consortium in stirred tank reactor. Bioengineering 2022, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Modin, O.; Fuad, N.; Rauch, S. Microbial electrochemical recovery of zinc. Electrochim. Acta. 2017, 248, 58–63. [Google Scholar] [CrossRef]
- Hong, Y.; Valix, M. Bioleaching of electronic waste using acidophilic sulfur oxidizing bacteria. J. Clean. Prod. 2014, 65, 465–472. [Google Scholar] [CrossRef]
- Brandl, H.; Bosshard, R.; Wegmann, M. Computer-munching microbes: Metal leaching from electronic scrap by bacteria and fungi. Hydrometallurgy 2001, 59, 319–326. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, N.; Shen, W.; Zhang, T.; Wu, P. Bioleaching of copper from metal concentrates of waste printed circuit boards by a newly isolated Acidithiobacillus ferrooxidans strain Z1. J. Mater. Cycles Waste Manag. 2017, 19, 247–255. [Google Scholar] [CrossRef]
- Zhu, N.; Xiang, Y.; Zhang, T.; Wu, P.; Danga, Z.; Li, P.; Wu, J. Bioleaching of metal concentrates of waste printed circuit boards by mixed culture of acidophilic bacteria. J. Hazard. Mater. 2011, 192, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.C.; Wang, Y.P.; Peng, T.J.; Shen, L.; Yu, R.L.; Liu, Y.D.; Chen, M.; Li, J.K.; Wu, X.L.; Zeng, W.M. Recycling of metals from pretreated waste printed circuit boards effectively. J. Biosci. Bioeng. 2017, 123, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Cezac, P.; Hoadley, A.F.; Contamine, F.; D’hugues, P. A review of sulfide minerals microbially assisted leaching in stirred tank reactors. Int. Biodeterior. Biodegrad. 2017, 119, 118–146. [Google Scholar]
- Murugesan, M.P.; Kannan, K.; Selvaganapathy, T. Bioleaching recovery of copper from printed circuit boards and optimization of various parameters using response surface methodology (RSM). Mater. Today Proc. 2020, 26 Pt 2, 2720–2728. [Google Scholar] [CrossRef]
- Ilyas, S.; Anwar, M.A.; Niazi, S.; Ghauri, M.A. Bioleaching of metals from electronic scrap by moderately thermophilic acidophilic bacteria. Hydrometallurgy 2007, 88, 180–188. [Google Scholar] [CrossRef]
- Shah, M.; Tipre, D.; Purohit, M.; Dave, S. Development of two-step process for enhanced biorecovery of Cu-Zn-Ni from computer printed circuit boards. J. Biosci. Bioeng. 2015, 120, 167–173. [Google Scholar] [CrossRef]
- Marques, A.C.; Cabrera, J.M.; Malfatti, C.F. Printed circuit boards: A review on the perspective of sustainability. J. Environ. Manag. 2013, 131, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Tang, J.; Liu, W.; Zhou, Q. Optimizing mixed culture of two acidophiles to improve copper recovery from printed circuit boards (PCBs). J. Hazard. Mater. 2013, 250–251, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, A.A.; Shetty, V.K.; Saidutta, M.B. Bioleaching of copper from electronic waste using Acinetobacter sp. Cr B2 in a pulsed plate column operated in batch and sequential batch mode. J. Environ. Chem. Eng. 2017, 5, 1599–1607. [Google Scholar] [CrossRef]
- Anshu, P.; Subrata, H. Feasibility of bioleaching of selected metals from electronic waste by Acidiphilium acidophilum. Waste Biomass Valorization 2017, 9, 871–877. [Google Scholar] [CrossRef]
- Amiri, F.; Mousavi, S.M.; Yaghmaei, S.; Barati, M. Bioleaching kinetics of a spent refinery catalyst using Aspergillus niger at optimal conditions. Biochem. Eng. J. 2012, 67, 208–217. [Google Scholar] [CrossRef]
- Santhiya, D.; Ting, Y.P. Use of adapted Aspergillus niger in the bioleaching of spent refinery processing catalyst. J. Biotechnol. 2006, 121, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Falguni, P.; Lakshmi, B. Bioleaching of copper and nickel from mobile phone printed circuit board using Aspergillus fumigatus A2DS. Environ. Chem. Lett. 2019, 17, 1873–1879. [Google Scholar]
- Pradhan, J.; Kumar, S. Metals bioleaching from electronic waste by Chromobacterium violaceum and Pseudomonads sp. Waste Manag. Res. 2012, 30, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.B.; Natarajan, G.; Abdul Rahim, M.N.; Tan, H.T.; Chung, M.C.; Ting, Y.P.; Yew, W.S. Enhancing gold recovery from electronic waste via lixiviant metabolic engineering in Chromobacterium violaceum. Sci. Rep. 2016, 3, 2236. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Jeong, J.; Lee, S.; Pandey, B.; Lee, J. Evaluation of bioleaching factors on gold recovery from ore by cyanide-producing bacteria. Miner. Eng. 2013, 48, 20–24. [Google Scholar] [CrossRef]
- Hubau, A.; Minter, M.; Changes, A.; Joulian, C.; Perez, C.; Guezennec, A.G. Continuous production of a biogenic ferric iron lixiviant for the bioleaching of printed circuit boards (PCBs). Hydrometallurgy 2018, 180, 180–191. [Google Scholar] [CrossRef]
- Bas, A.D.; Deveci, H.; Yazici, E.Y. Bioleaching of copper from low grade scrap TV circuit boards using mesophilic bacteria. Hydrometallurgy 2013, 138, 65–70. [Google Scholar] [CrossRef]
- Guezennec, A.G.; Joulian, C.; Delort, C.; Bodenan, F.; Hedrich, S.; d’Hugues, P. CO2 mass gtransfer in bioleaching reactors: CO2 enrichment applied to a complex copper concetrate. Hydrometallurgy 2018, 180, 277–286. [Google Scholar] [CrossRef]
- Ilyas, S.; Ruan, C.; Bhatti, H.N.; Ghauri, M.A.; Anwar, M.A. Column bioleaching of metals from electronic scrap. Hydrometallurgy 2010, 101, 135–140. [Google Scholar] [CrossRef]
- Sodha, A.B.; Tipre, D.R.; Dave, S.R. Optimisation of biohydrometallurgical batch reactor process for copper extraction and recovery from non-pulverized waste printed circuit boards. Hydrometallurgy 2020, 191, 105170. [Google Scholar] [CrossRef]
- Boxall, N.J.; Cheng, K.Y.; Bruckard, W.; Kaksonen, A.H. Application of indirect non-contact bioleaching for extracting metals from waste lithium-ion batteries. J. Hazard. Mater. 2018, 360, 504–511. [Google Scholar] [CrossRef]
- Yken, J.V.; Cheng, K.Y.; Boxall, N.J.; Nikoloski, A.N.; Moheimani, N.; Valix, M.; Sahajwalla, V.; Kaksonen, A.H. Potential of metals leaching from printed circuit boards with biological and chemical lixiviants. Hydrometallurgy 2020, 196, 105433. [Google Scholar] [CrossRef]
- Erkmen, O. Practice 4—Most probable number technique. In Microbiological Analysis of Foods and Food Processing Environments; Elsevier: Amsterdam, The Netherlands, 2022; pp. 31–37. [Google Scholar]
- Lucchesi, C.A.; Hirn, C.F. EDTA Titration of total Iron in Iron(II) and Iron(III) mixtures. Application to Iron driers. Anal. Chem. 1960, 32, 1191–1193. [Google Scholar] [CrossRef]
- Bouzahzah, H.; Benzaazoua, M.; Mermillod-Blondin, R.; Pirard, E. A novel procedure for polished section preparation for automated mineralogy avoiding internal particle settlement. In Proceedings of the 12th International Congress for Applied Mineralogy (ICAM), Istanbul, Turkey, 10–12 August 2015. [Google Scholar]
- Vardanyan, A.; Vardanyan, N.; Abrahamyan, N.; Aatach, M.; Gaydardzhiev, S. Sequential biologically assisted extraction of Cu and Zn from printed circuit boards (PCB). Int. J. Environ. Stud. 2022. [Google Scholar] [CrossRef]
- Guezennec, A.G.; Bru, K.; Jacob, J.; d’Hugues, P. Co-processing of sulfidic mining wastes and metal-rich post-consumer wastes by biohydrometallurgy. Miner. Eng. 2015, 75, 45–53. [Google Scholar] [CrossRef]








| Metal, % | Concentration |
|---|---|
| Cu | 21.53 |
| Al | 6.95 |
| Pb | 3.2 |
| Zn | 0.78 |
| Ni | 5.12 |
| Fe | 3.86 |
| Sn | 1.98 |
| Ag | 0.097 |
| Duration | Number of Cells, Cells/mL |
|---|---|
| Initial (0 min) | 0.6 × 109 |
| 1 h | 0.25 × 107 |
| 3 h | 0.6 × 105 |
| 5 h | 0.25 × 105 |
| 24 h | 0.6 × 10 |
| Spectrum | Oxygen | Aluminum | Silicon | Phosphorus | Sulfur | Calcium | Iron | Copper | Bromine | Tin |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40.22 | 20.65 | 13.11 | 4.17 | 21.85 | |||||
| 2 | 45.45 | 8.76 | 21.5 | 17.39 | 1.45 | 5.45 | ||||
| 3 | 100 | |||||||||
| 4 | 36.09 | 1.84 | 4.76 | 0.87 | 1.25 | 10.74 | 2.69 | 17.3 | 24.46 | |
| 5 | 29.21 | 0.95 | 5.91 | 1.36 | 17.42 | 4.01 | 4.3 | 36.85 |
| Spectrum | Al | Si | P | S | Ca | Fe | Cu | Sn |
|---|---|---|---|---|---|---|---|---|
| 1 | 100 | |||||||
| 2 | 8.87 | 24.86 | 17.82 | 5.68 | ||||
| 3 | 0.57 | 3.03 | 0.78 | 13.07 | 6.51 | 53.33 | ||
| 4 | 2.46 | 1.2 | 15.22 | 8.12 | 50.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vardanyan, A.; Vardanyan, N.; Aâtach, M.; Malavasi, P.; Gaydardzhiev, S. Bio-Assisted Leaching of Non-Ferrous Metals from Waste Printed Circuit Boards—Importance of Process Parameters. Metals 2022, 12, 2092. https://doi.org/10.3390/met12122092
Vardanyan A, Vardanyan N, Aâtach M, Malavasi P, Gaydardzhiev S. Bio-Assisted Leaching of Non-Ferrous Metals from Waste Printed Circuit Boards—Importance of Process Parameters. Metals. 2022; 12(12):2092. https://doi.org/10.3390/met12122092
Chicago/Turabian StyleVardanyan, Arevik, Narine Vardanyan, Mohamed Aâtach, Pierre Malavasi, and Stoyan Gaydardzhiev. 2022. "Bio-Assisted Leaching of Non-Ferrous Metals from Waste Printed Circuit Boards—Importance of Process Parameters" Metals 12, no. 12: 2092. https://doi.org/10.3390/met12122092
APA StyleVardanyan, A., Vardanyan, N., Aâtach, M., Malavasi, P., & Gaydardzhiev, S. (2022). Bio-Assisted Leaching of Non-Ferrous Metals from Waste Printed Circuit Boards—Importance of Process Parameters. Metals, 12(12), 2092. https://doi.org/10.3390/met12122092








