Effect of Particle Size of MgO on the Sinter Strength before and after Reduction and Its Mechanism Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization
2.2. Procedures
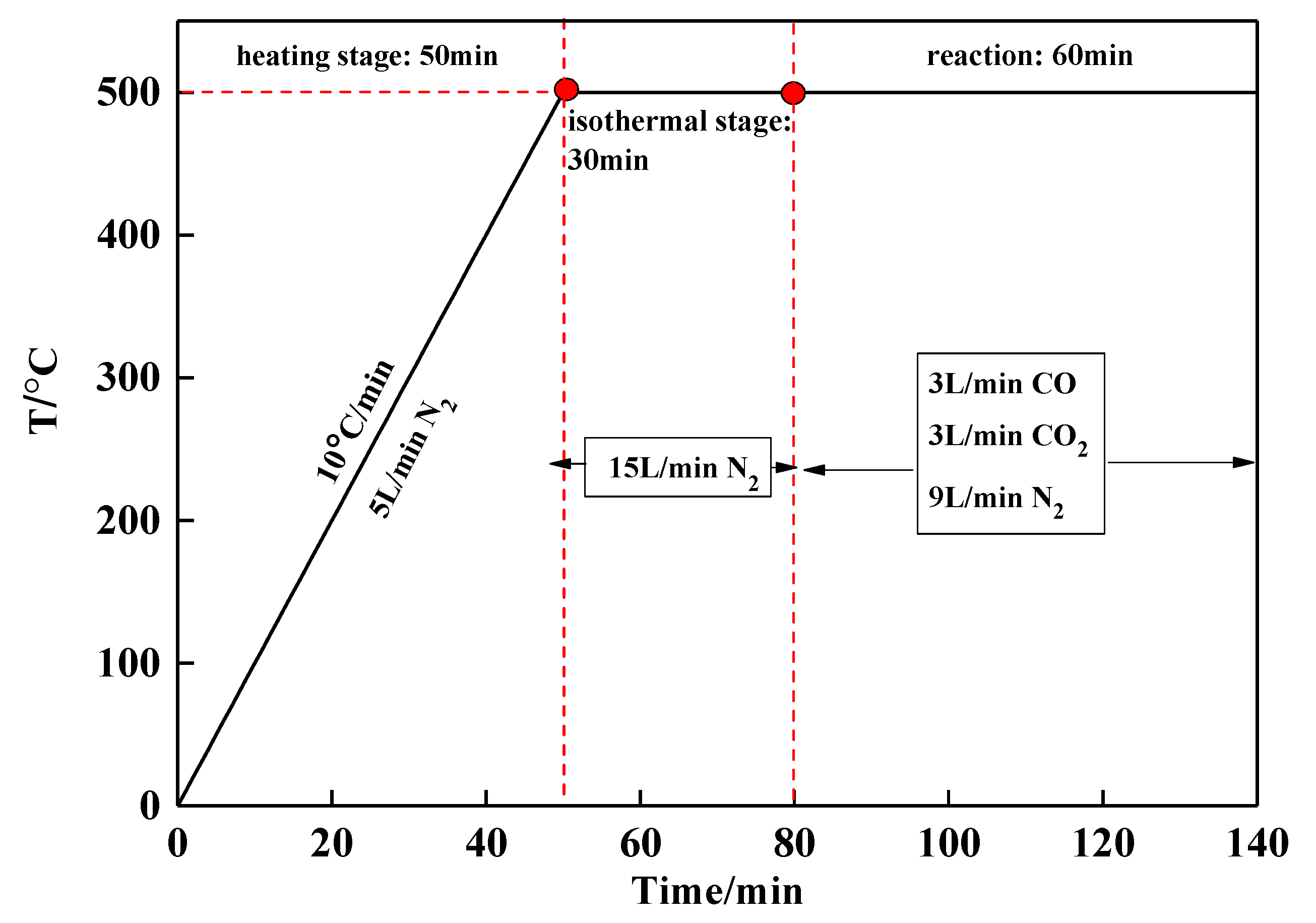
2.3. Analysis Methods
3. Results
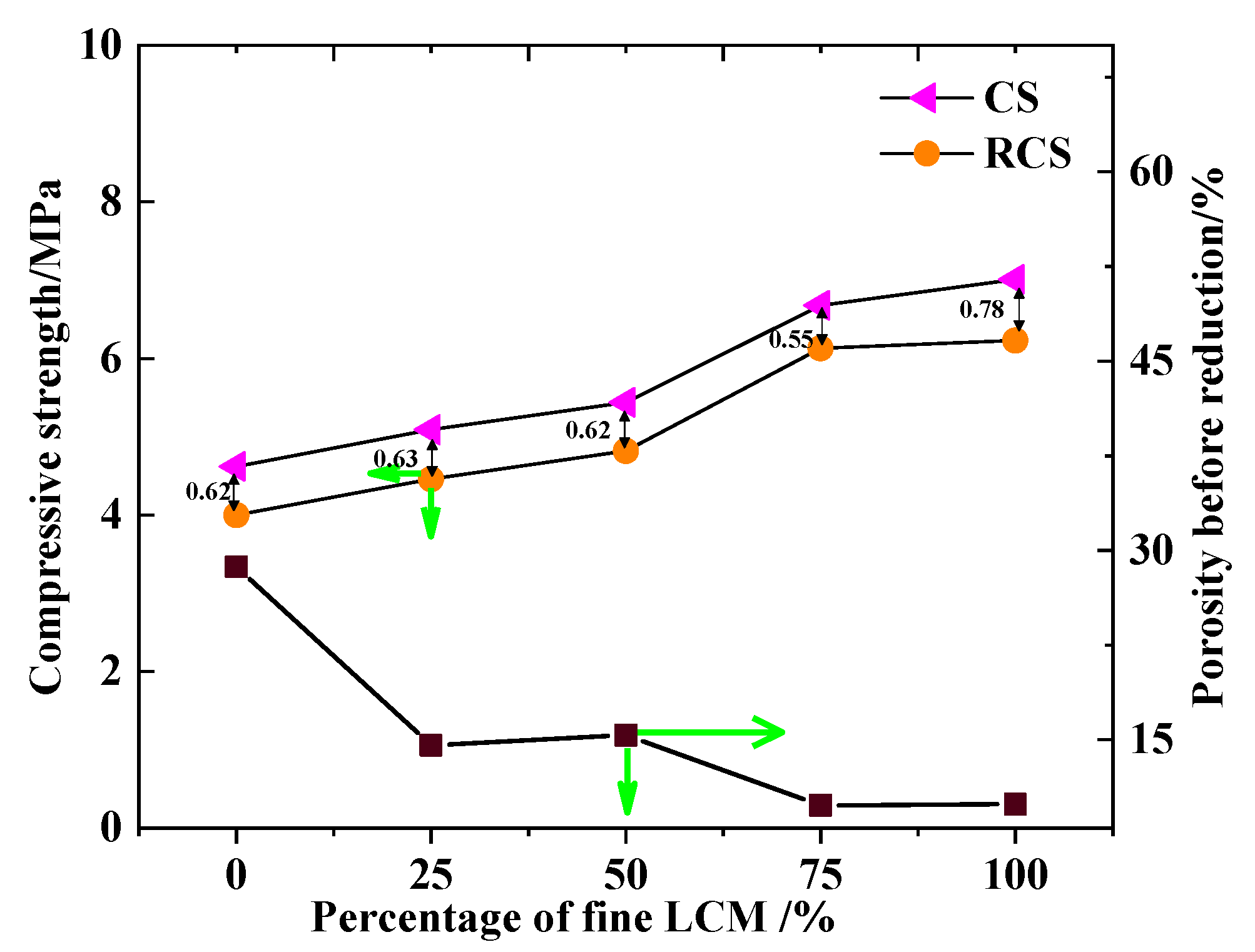
3.1. Experimental Results of Fe2O3-MgO Series and Fe2O3-MgO-CaO Series
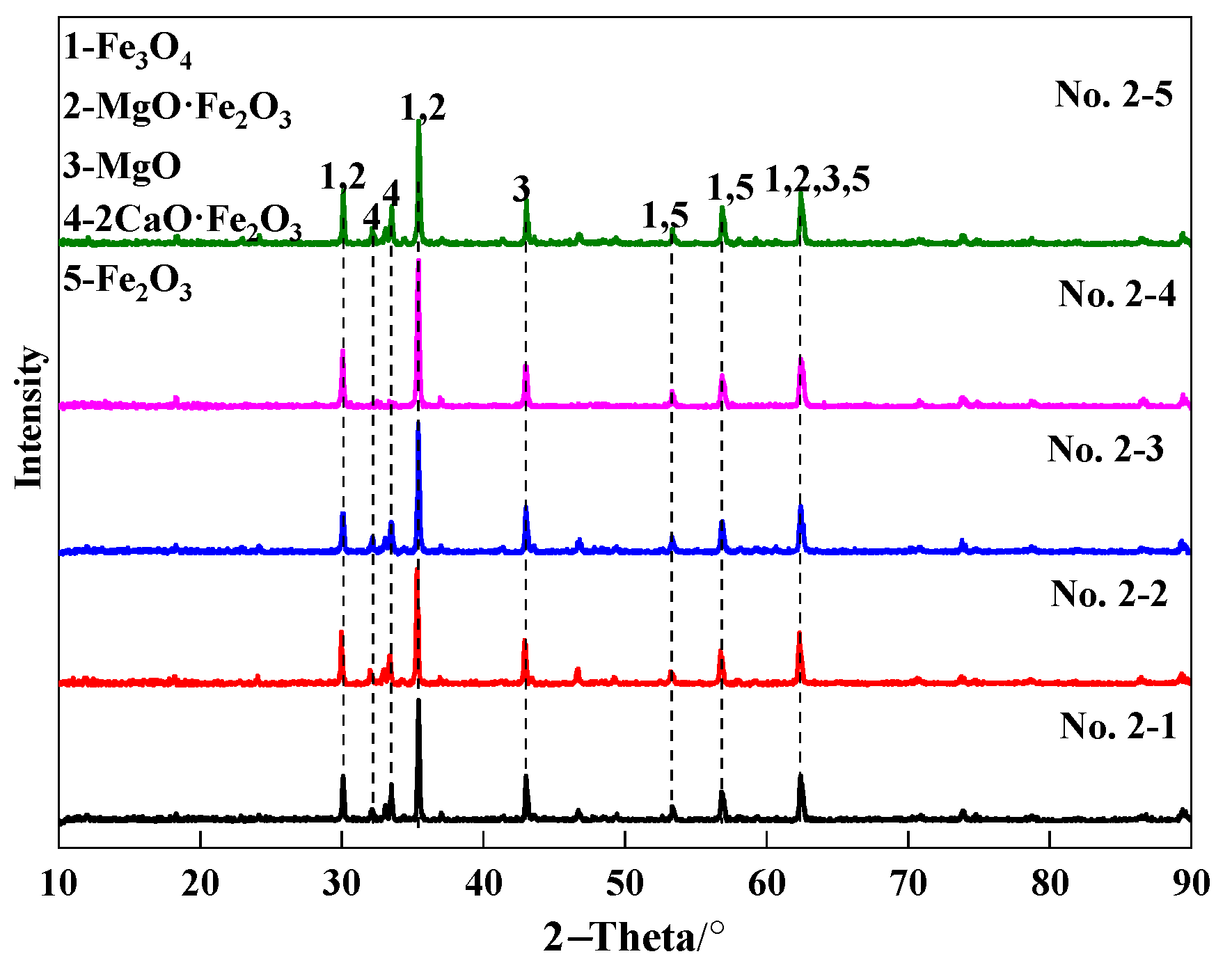
3.2. XRD and SEM Analysis on the Sample of Fe2O3-MgO
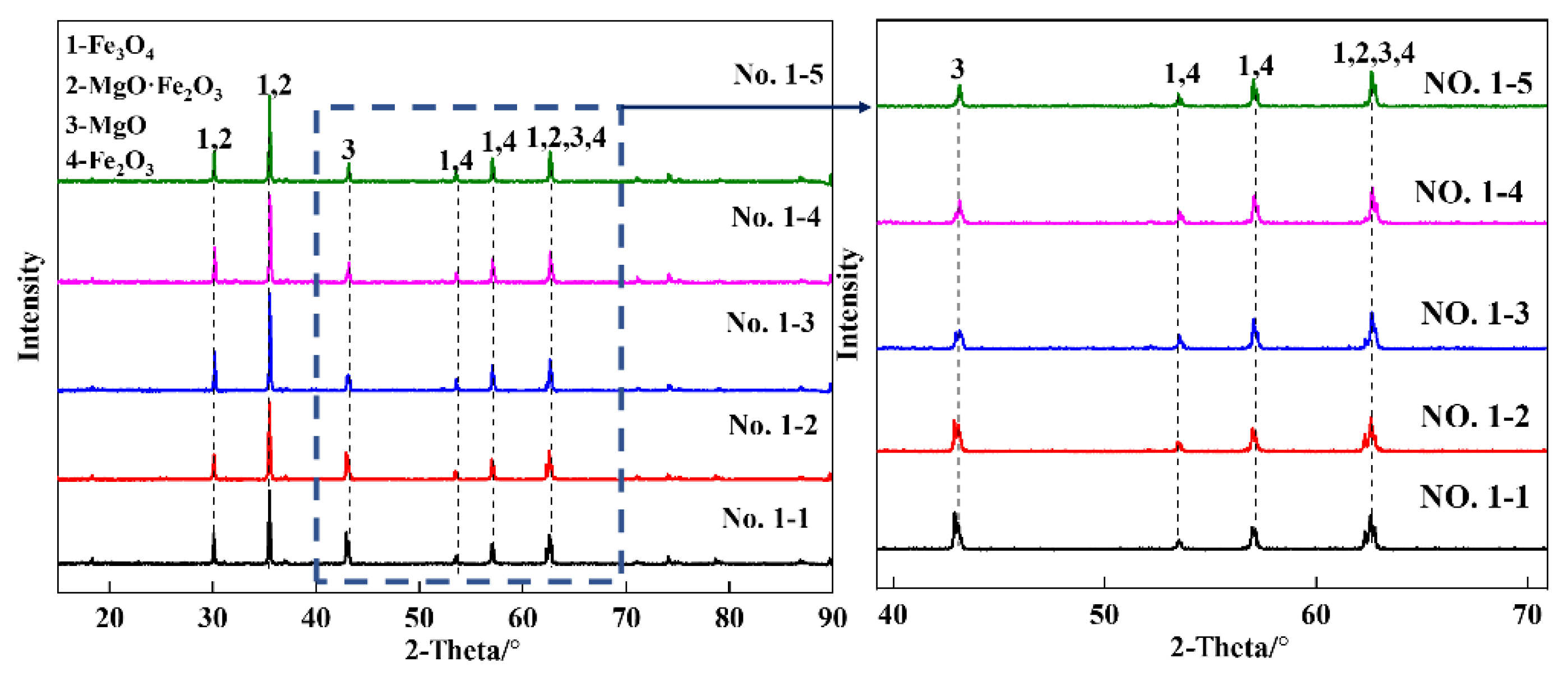
3.2.1. Fe2O3-MgO System Sample before Reduction
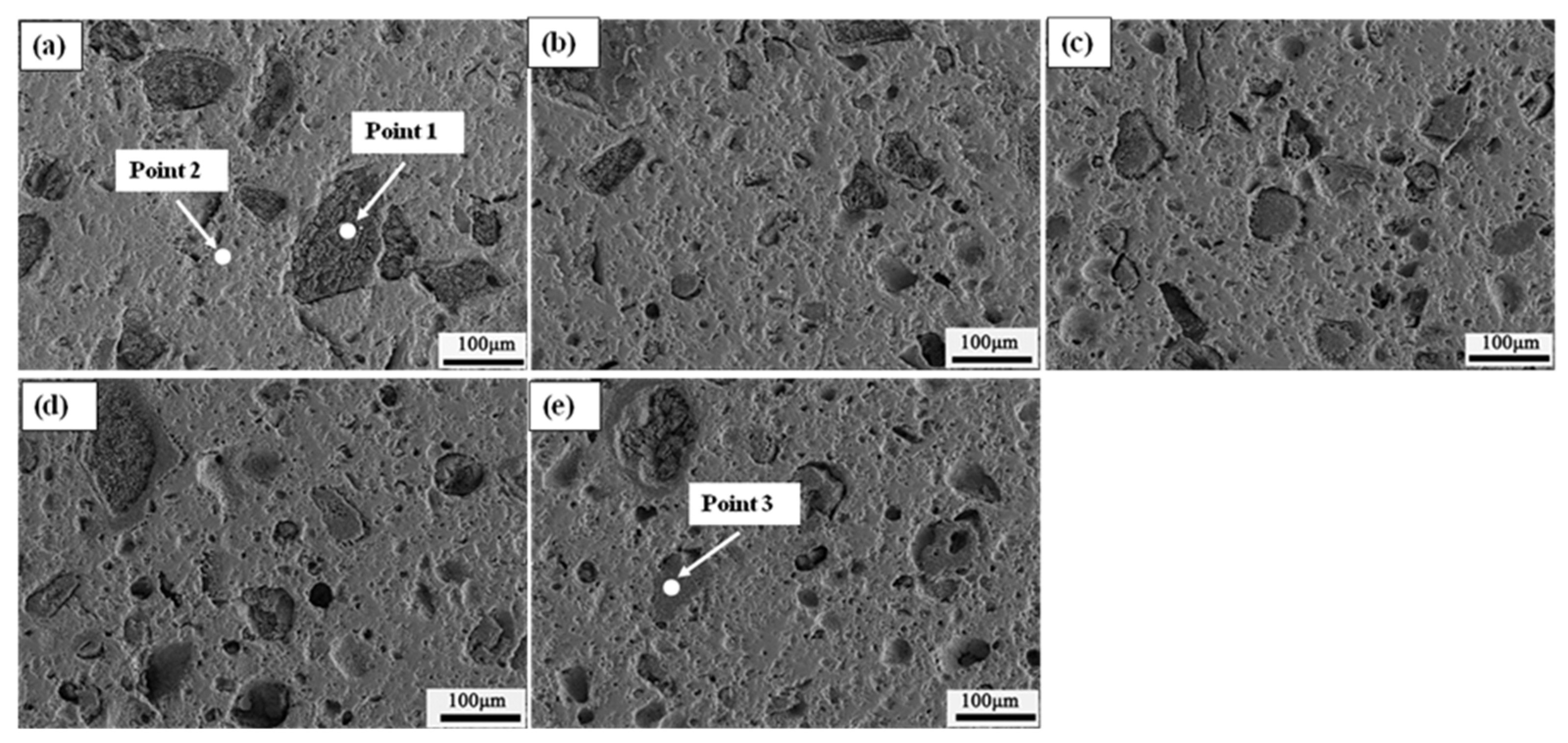
3.2.2. Fe2O3-MgO System Sample after Reduction
3.3. XRD and SEM Analysis on the Sample of Fe2O3-MgO-CaO
3.3.1. Fe2O3-MgO-CaO System Sample before Reduction
3.3.2. Fe2O3-MgO-CaO System Sample after Reduction
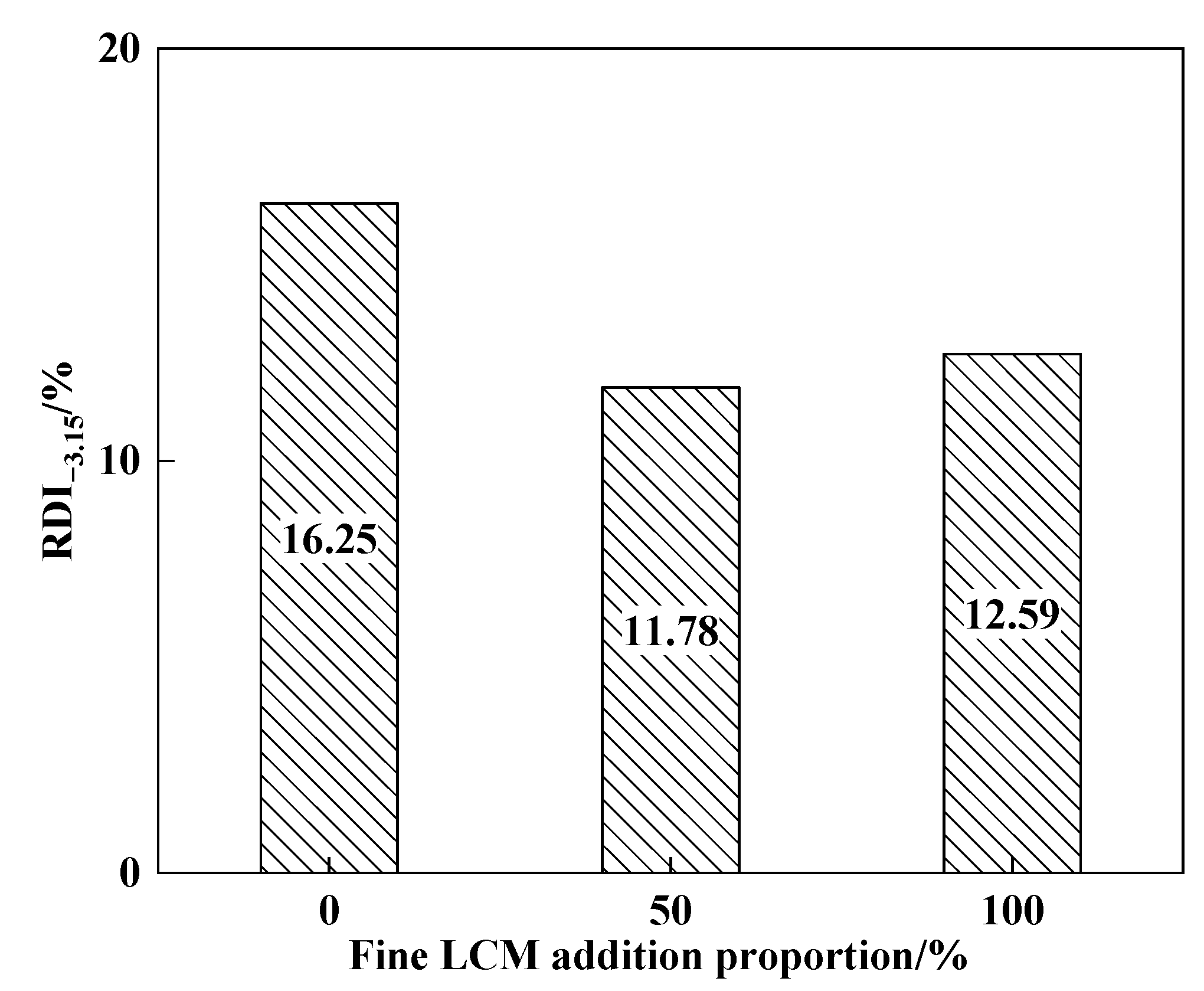
3.4. Results of Sintering Pot
4. Discussion
4.1. Effect of Particle Size with LCM on Strength before Reduction
4.2. Effect of Particle Size with LCM on Strength after Reduction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, X.D.; Mao, C.; Zhu, J.M.; Xu, H.F.; Wang, G.F.; Zhao, B.J. Properties of Low-MgO ironmaking blast furnace slags. ISIJ Int. 2018, 58, 1402–1405. [Google Scholar] [CrossRef] [Green Version]
- Ueda, S.; Kon, T.; Miki, T.; Kim, S.J.; Nogami, H. Effects of Al2O3 and MgO on softening, melting, and permeation properties of CaO-FeO-SiO2 on a coke bed. Metall. Mater. Trans. B. 2016, 47, 2371–2377. [Google Scholar] [CrossRef]
- Iljana, M.; Kemppainen, A.; Paananen, T.; Mattila, O.; Heikkinen, E.P.; Fabriitius, T. Evaluating the reduction-softening behaviour of blast furnace burden with an advance test. ISIJ Int. 2016, 56, 1705–1714. [Google Scholar] [CrossRef] [Green Version]
- George, H.L.; Monaghan, B.J.; Longbottom, R.J.; Chew, S.J.; Austin, P.R. Flow of molten slag through coke channels. ISIJ Int. 2013, 53, 1172–1179. [Google Scholar] [CrossRef] [Green Version]
- Shen, F.M.; Gao, Q.J.; Jiang, X.; Wei, G.; Zhen, H.Y. Effect of magnesia on the compressive strength of pellets. Int. J. Miner. Metall. Mater. 2014, 21, 431–437. [Google Scholar] [CrossRef]
- Umadevi, T.; Roy, A.K.; Mahapatra, P.C.; Prabhu, M.; Ranjan, M. Influence of magnesia on iron ore sinter properties and productivity—Use of dolomite and dunite. Steel Res. Int. 2009, 80, 800–807. [Google Scholar]
- Shen, F.M.; Jiang, X.; Wu, G.S.; Wei, G.; Li, X.G.; Shen, Y.S. Proper MgO addition in blast furnace operation. ISIJ Int. 2006, 46, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.M.; Liu, Q.; Shao, H.J. Effect of MgO on highly basic sinters with high Al2O3. Mining Metall Explor. 2021, 38, 2175–2183. [Google Scholar] [CrossRef]
- Higuchi, K.; Nishimura, T.; Shioda, T.; Pettersson, M.; Sikstrom, P. Reaction behaviors of mixed burdens consisting of pellets and sintered ores in an experimental blast furnace. Tetsu-to-Hagane 2021, 107, 336–344. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, S.T.; Jiang, T.; Xue, X.X. Influence of MgO in form of magnesite on properties and mineralogy of high chromium, vanadium, titanium magnetite sinters. Ironmak. Steelmak. 2014, 42, 217–225. [Google Scholar] [CrossRef]
- Zhang, L.H.; Yang, S.T.; Tang, W.; Xue, X.X. Investigations of MgO on sintering performance and metallurgical property of high-chromium vanadium-titanium magnetite. Minerals 2019, 9, 324. [Google Scholar] [CrossRef]
- Guo, H.; Shen, F.M.; Jiang, X.; Gao, Q.J.; Ding, G.G. Effects of MgO additive on metallurgical properties of fluxed-pellet. J. Cent. South. Univ. 2019, 26, 3238–3251. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Chou, J.L.; Shi, B.J.; Pan, J. Influence of MgO on low temperature reduction and mineralogical changes of sinter in simulated COREX shaft furnace reducing conditions. Minerals 2019, 9, 272. [Google Scholar] [CrossRef] [Green Version]
- Pan, F.; Zhu, Q.S.; Du, Z.; Sun, H.Y. Migration behavior of the MgO and its influence on the reduction of Fe3O4-MgO sinter. ISIJ Int. 2018, 58, 652–659. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Chu, M.S.; Xue, X.X. Optimized use of MgO flux in the agglomeration of high-chromium vanadium-titanium magnetite. Int. J. Miner. Metall. Mater. 2015, 22, 371–380. [Google Scholar] [CrossRef]
- Zhang, Y.M. Pellet Production Technology; Metallurgical Industry Press: Beijing, China, 2005. [Google Scholar]
- Suresh, S. Fatigue of Material; Cambridge University Press: New York, UY, USA, 1998. [Google Scholar]
- Zhu, D.Q.; Yang, C.C.; Pan, J.; Li, X.B. Comparison of the oxidation behavior of high FeO chromite and magnetite concentrates relevant to the induration of ferrous pellets. Metall. Mater. Trans. B 2016, 47, 2919–2930. [Google Scholar] [CrossRef]
- Yan, J.; Zou, G.; Liu, L.; Zhang, D.; Bai, H.; Wu, A.P.; Zhou, Y.N. Sintering Mechanisms and mechanical properties of joints bonded using silver nanoparticles for electronic packaging applications. Weld World 2015, 59, 427–432. [Google Scholar] [CrossRef]
- Jing, M.F.; Li, G.S.; Jiang, X.; Shen, F.M. Effect of penetration behavior of calcium ferrite melts into hematite on the strength of sinter. J. NORTHEAST. Univ. 2008, 8, 1135–1138. [Google Scholar]
- Eisenhüttenleute, V.D.; Allibert, M. Slag Atlas, 2nd ed.; Verlag Stahleisen GmbH: Düsseldorf, Germany, 1995. [Google Scholar]
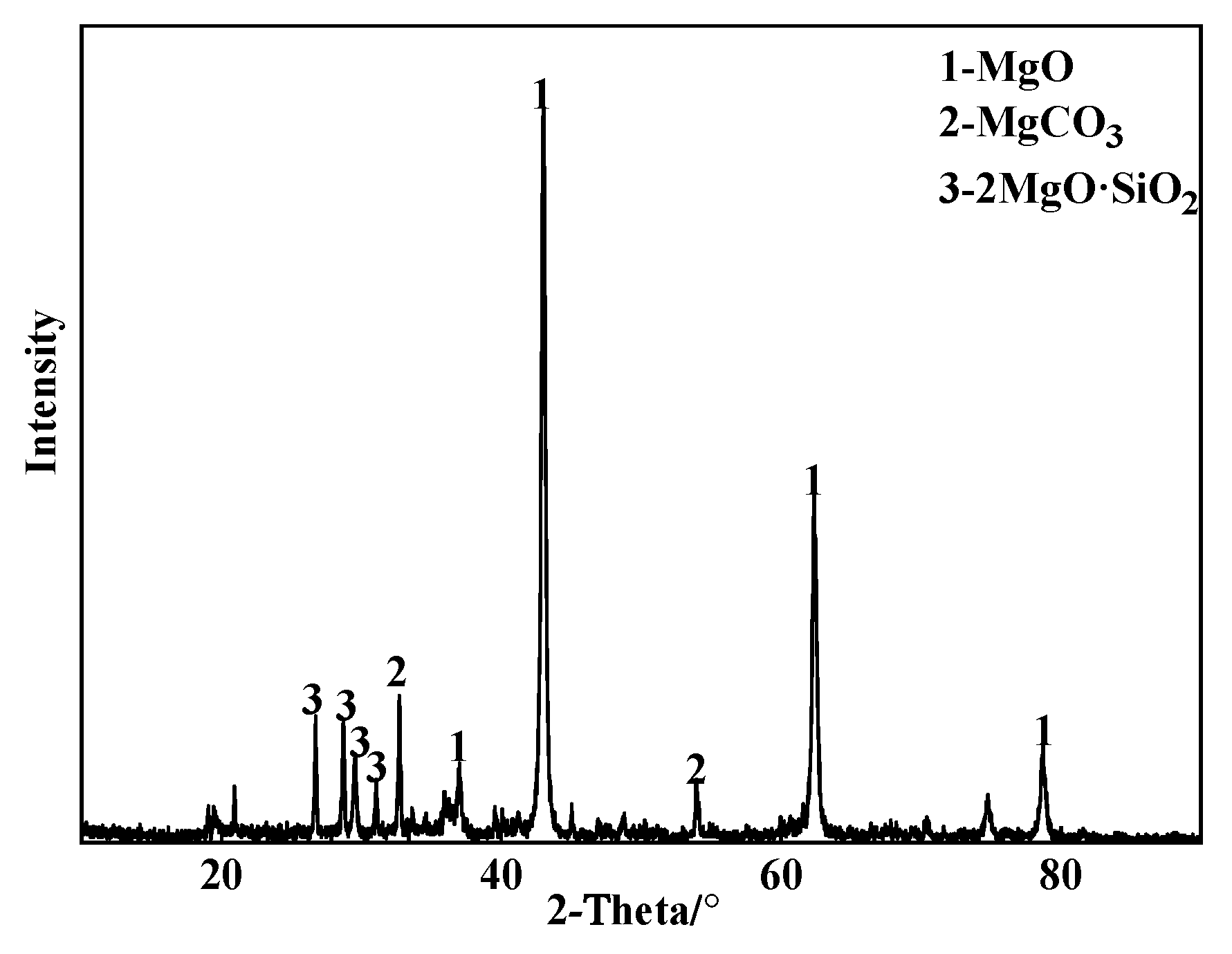
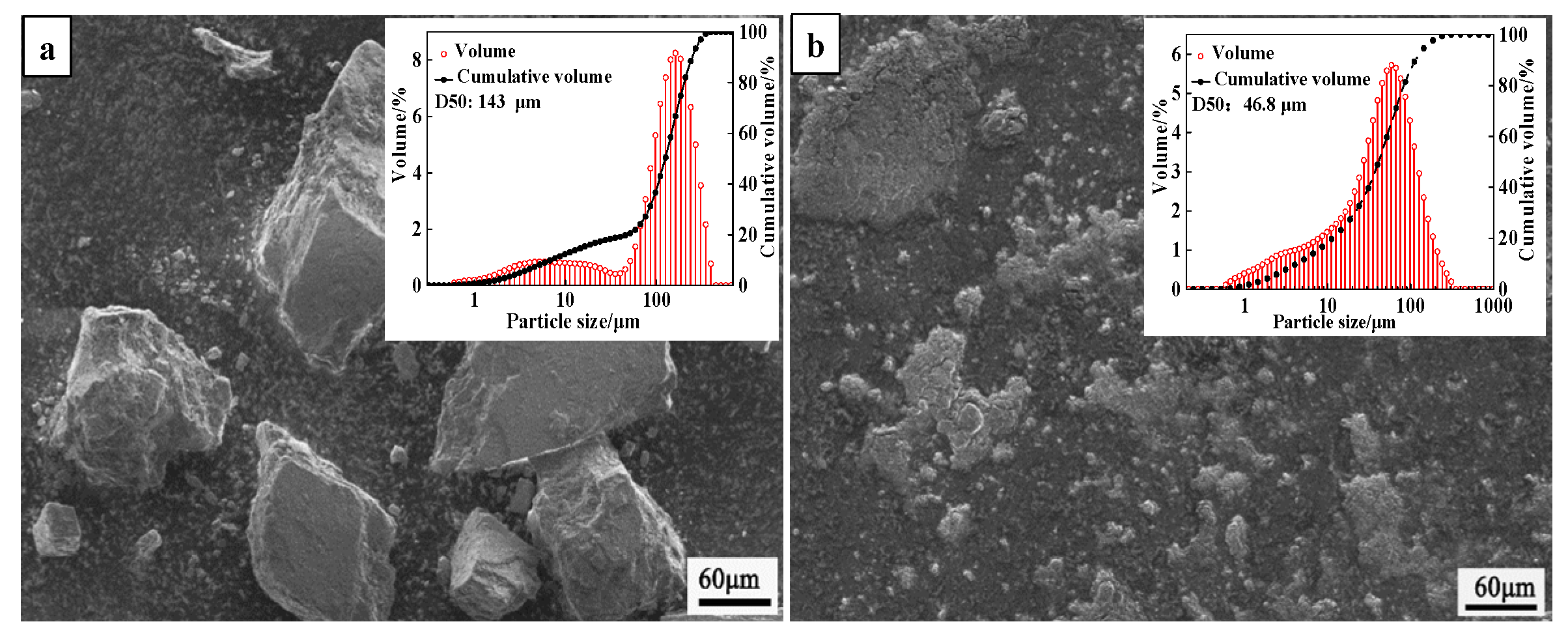
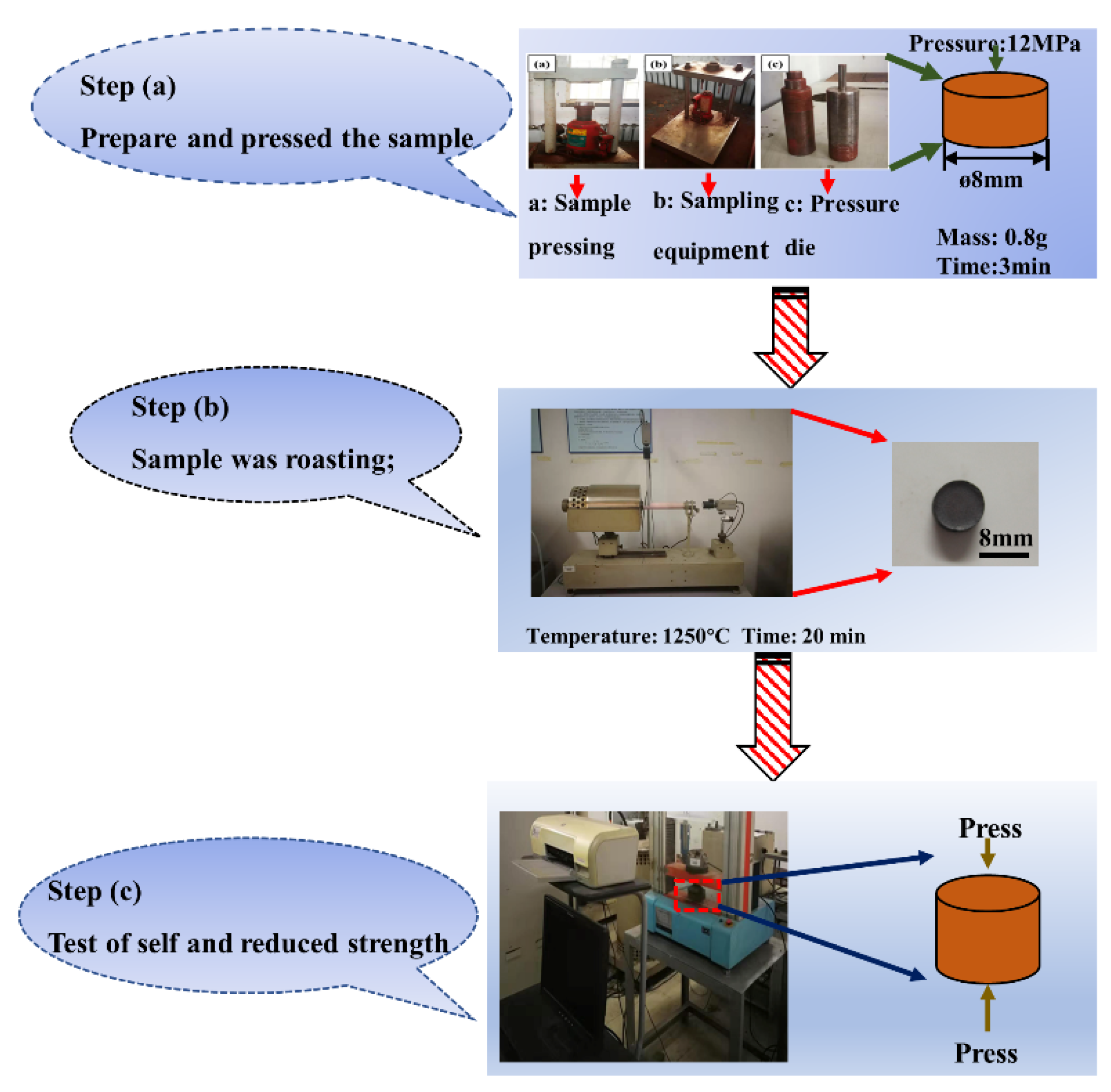
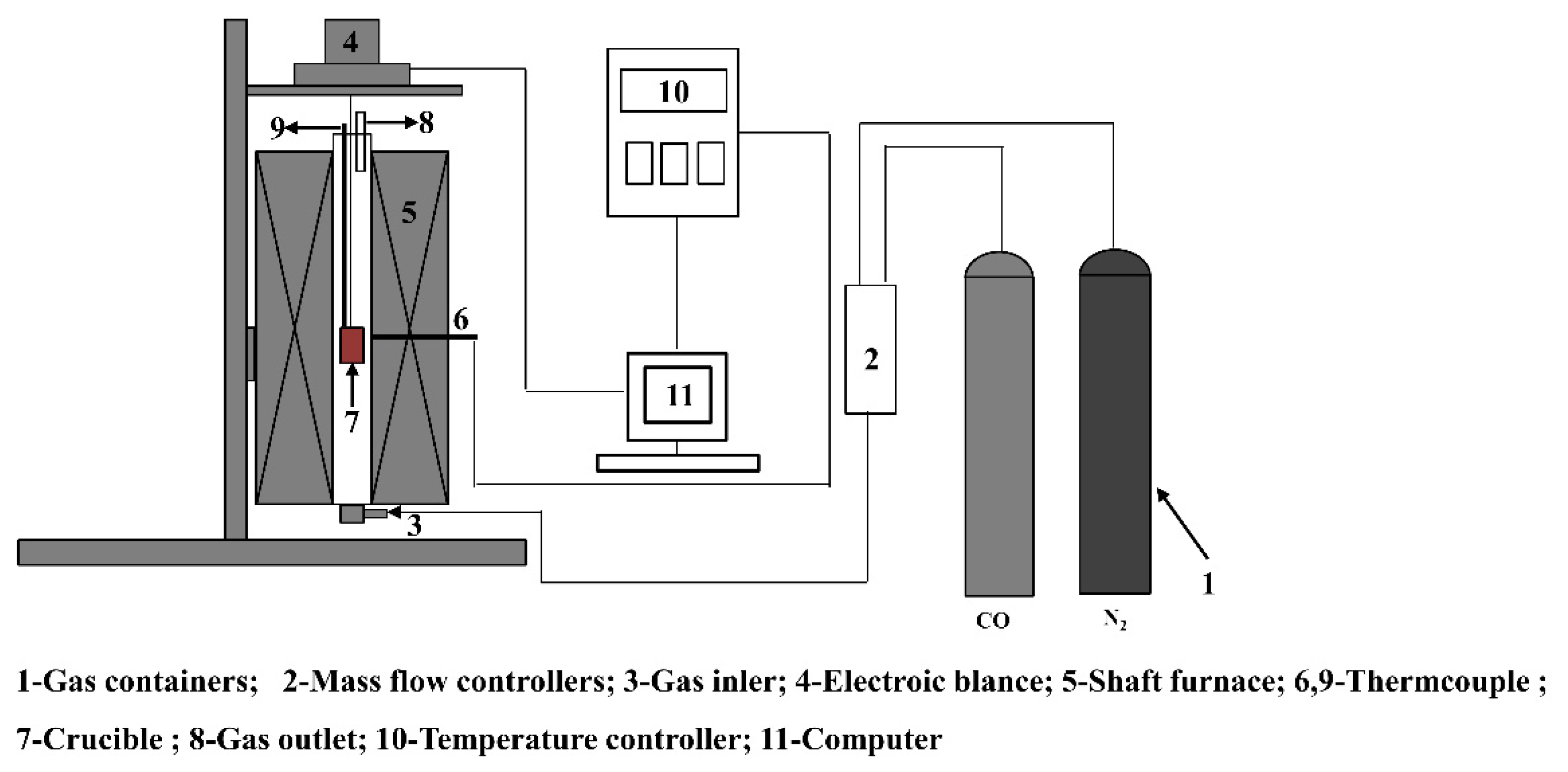







| MgO | CaO | SiO2 | Al2O3 | LOI |
|---|---|---|---|---|
| 71.35 | 2.76 | 5.78 | 0.74 | 17.84 |
| CaO | MgO | SiO2 | H2O | LOI |
|---|---|---|---|---|
| 31.3 | 19.01 | 1.36 | 0.07 | 44.56 |
| Group | No. | Fe2O3 (mol)/Weight (g) | MgO (mol)/ Weight (g) | CaO (mol)/Weight (g) | Coarse LCM (%)/Weight (g) | Fine LCM (%)/Weight (g) |
|---|---|---|---|---|---|---|
| 1 | 1–1 | 1/0.640 | 1/0.160 | 0 | 100/0.220 | 0 |
| 1–2 | 1/0.640 | 1/0.160 | 0 | 75/0.165 | 25/0.055 | |
| 1–3 | 1/0.640 | 1/0.160 | 0 | 50/0.110 | 50/0.110 | |
| 1–4 | 1/0.640 | 1/0.160 | 0 | 25/0.055 | 75/0.165 | |
| 1–5 | 1/0.640 | 1/0.160 | 0 | 0 | 100/0.220 | |
| 2 | 2–1 | 1/0.500 | 1/0.125 | 1/0.175 | 100/0.175 | 0 |
| 2–2 | 1/0.500 | 1/0.125 | 1/0.175 | 75/0.131 | 25/0.044 | |
| 2–3 | 1/0.500 | 1/0.125 | 1/0.175 | 50/0.088 | 50/0.088 | |
| 2–4 | 1/0.500 | 1/0.125 | 1/0.175 | 25/0.044 | 75/0.131 | |
| 2–5 | 1/0.500 | 1/0.125 | 1/0.175 | 0 | 100/0.175 |
| No. | Blended Ore | Return Mine | LCM | QuickLime | Coke | Outsourced Coke | Fly Ash | Fine LCM Proportion |
|---|---|---|---|---|---|---|---|---|
| No.1 | 66.63 | 15.57 | 3.53 | 8.69 | 2.70 | 1.23 | 1.64 | 0 |
| No.2 | 66.63 | 15.57 | 3.53 | 8.69 | 2.70 | 1.23 | 1.64 | 50 |
| No.3 | 66.63 | 15.57 | 3.53 | 8.69 | 2.70 | 1.23 | 1.64 | 100 |
| Zone | Mass Percent/% | Components | ||||
|---|---|---|---|---|---|---|
| Mg | Fe | O | Si | Ca | ||
| Point 1 | 52.35 | 5.79 | 40.89 | 0.46 | 0.51 | MgO |
| Point 2 | 6.99 | 60.34 | 32.19 | - | 0.49 | Fe2O3 |
| Point 3 | 27.25 | 9.78 | 43.36 | 16.81 | 2.79 | MgO·Fe2O3 |
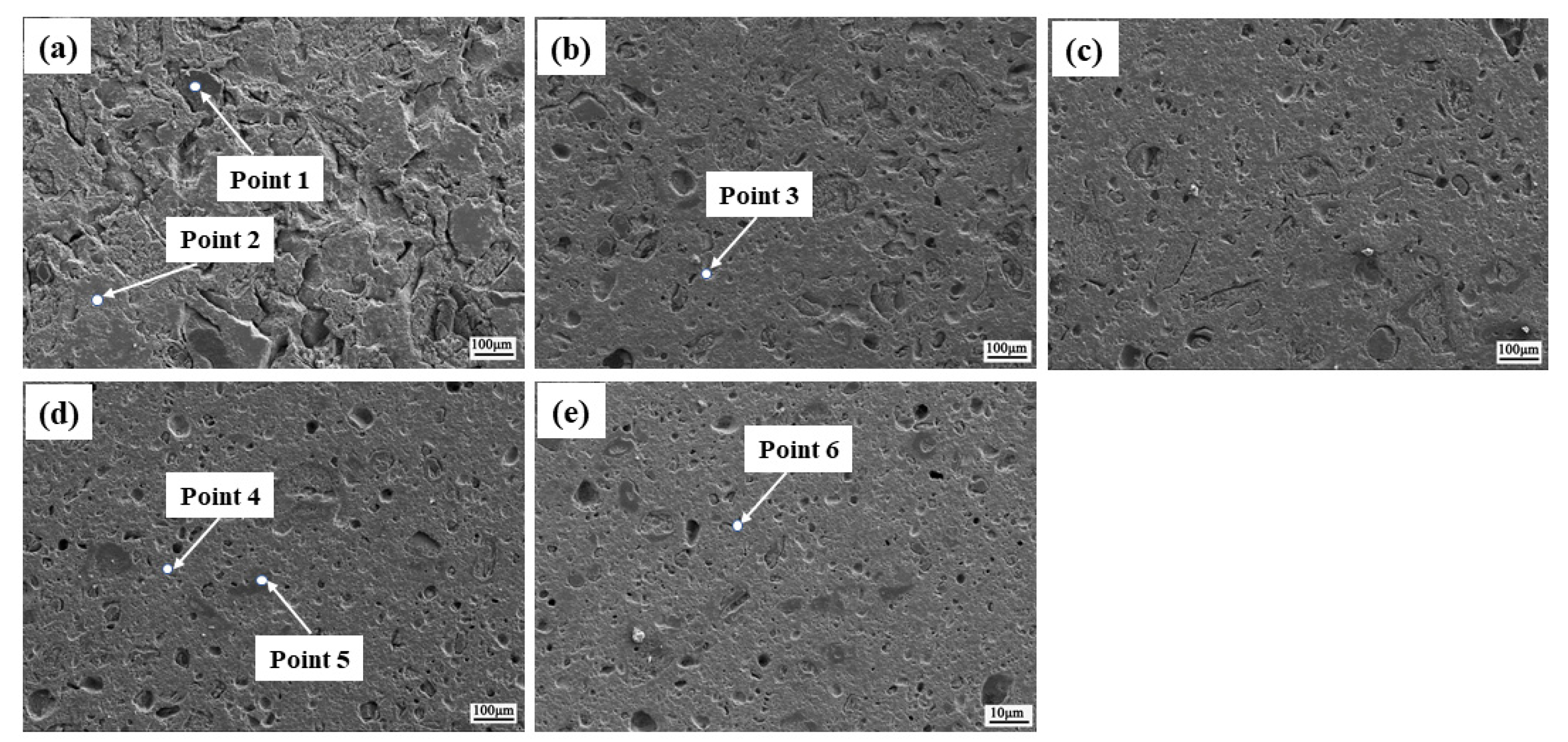
| Zone | Mass Percent/% | Components | ||||
|---|---|---|---|---|---|---|
| Mg | Fe | O | Si | Ca | ||
| Point 1 | 57.23 | 1.99 | 40.77 | - | - | MgO |
| Point 2 | 1.18 | 68.94 | 29.87 | - | - | Fe2O3 |
| Point 3 | 7.01 | 62.35 | 30.64 | - | - | Fe2O3, Fe3O4, MgO·Fe2O3 |
| Point 4 | 9.27 | 58.07 | 32.66 | - | - | Fe2O3, Fe3O4, |
| Point 5 | 27.62 | 10.17 | 44.43 | 15.45 | 2.33 | MgO·Fe2O3,slag |
| Point 6 | 8.79 | 58.86 | 32.35 | - | - | Fe3O4 |
| Zone | Mass Percent/% | Component | ||||
|---|---|---|---|---|---|---|
| Mg | Fe | O | Si | Ca | ||
| Point 1 | 51.75 | 6.51 | 40.97 | 0.77 | - | MgO |
| Point 2 | 1.79 | 38.60 | 35.38 | 0.97 | 23.26 | Fe2O3 |
| Point 3 | 12.62 | 0.98 | 46.29 | 27.09 | 13.02 | MgO·Fe2O3 |
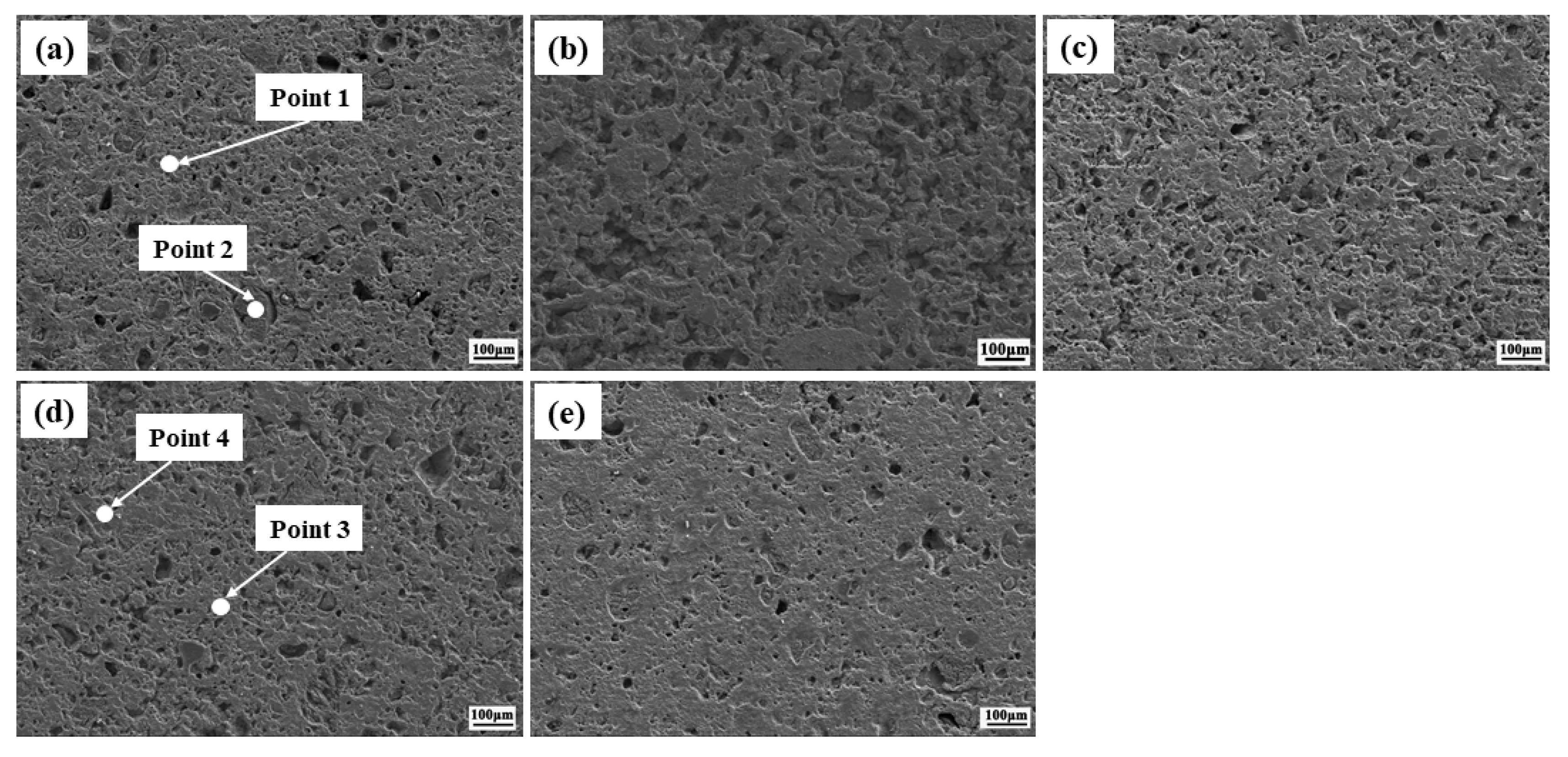
| Zone | Mass Percent/% | Components | ||||
|---|---|---|---|---|---|---|
| Mg | Fe | O | Si | Ca | ||
| Point 1 | 8.19 | 56.97 | 33.01 | - | 1.84 | MgO·Fe2O3 |
| Point 2 | 60.21 | 1.01 | 38.75 | - | - | MgO |
| Point 3 | 4.46 | 40.26 | 37.62 | 1.16 | 16.51 | Fe3O4, Fe2O3 |
| Point 4 | 1.03 | 35.17 | 36.10 | 1.68 | 26.02 | CaO·Fe2O3 |
| LCM | Particle Radius (μm) | Surface Area (m2) | Density (g/m3) | Mass (g) | Specific Surface Area (m2/g) | R |
|---|---|---|---|---|---|---|
| coarse | 71.5 | 6.42 × 10−8 | 2700 | 4.131 × 10−9 | 15.541 | 3.19 × 10−4 |
| fine | 23.4 | 6.88 × 10−8 | 2700 | 1.450 × 10−9 | 47.447 | 3.42 × 10−5 |
| Sample | System | Components | EDS Points | Change a |
|---|---|---|---|---|
| Sample after reduction | Fe2O3-MgO | Fe2O3 | Figure 11-Point 2 | D |
| MgO | Figure 11-Point 1 | D | ||
| Fe3O4 | Figure 11-Point 6 | D | ||
| MgO·Fe2O3 | - | I | ||
| Slag | Figure 11-Point 5 | I | ||
| Fe2O3-MgO-CaO | Fe2O3 | - | D | |
| MgO | Figure 15-Point 2 | D | ||
| Fe3O4 | - | D | ||
| MgO·Fe2O3 | Figure 15-Point 1 | I | ||
| CaO·Fe2O3 | Figure 15-Point 4 | I |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, J.; Zhang, C.; Chang, S.; Yang, J. Effect of Particle Size of MgO on the Sinter Strength before and after Reduction and Its Mechanism Analysis. Metals 2022, 12, 2088. https://doi.org/10.3390/met12122088
Cong J, Zhang C, Chang S, Yang J. Effect of Particle Size of MgO on the Sinter Strength before and after Reduction and Its Mechanism Analysis. Metals. 2022; 12(12):2088. https://doi.org/10.3390/met12122088
Chicago/Turabian StyleCong, Junqiang, Chunyin Zhang, Shengzhao Chang, and Jialong Yang. 2022. "Effect of Particle Size of MgO on the Sinter Strength before and after Reduction and Its Mechanism Analysis" Metals 12, no. 12: 2088. https://doi.org/10.3390/met12122088
APA StyleCong, J., Zhang, C., Chang, S., & Yang, J. (2022). Effect of Particle Size of MgO on the Sinter Strength before and after Reduction and Its Mechanism Analysis. Metals, 12(12), 2088. https://doi.org/10.3390/met12122088





