Microstructure and Mechanical Properties of Novel Heat Resistant Cast Al-Cu-Yb(Gd)-Mg-Mn-Zr Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
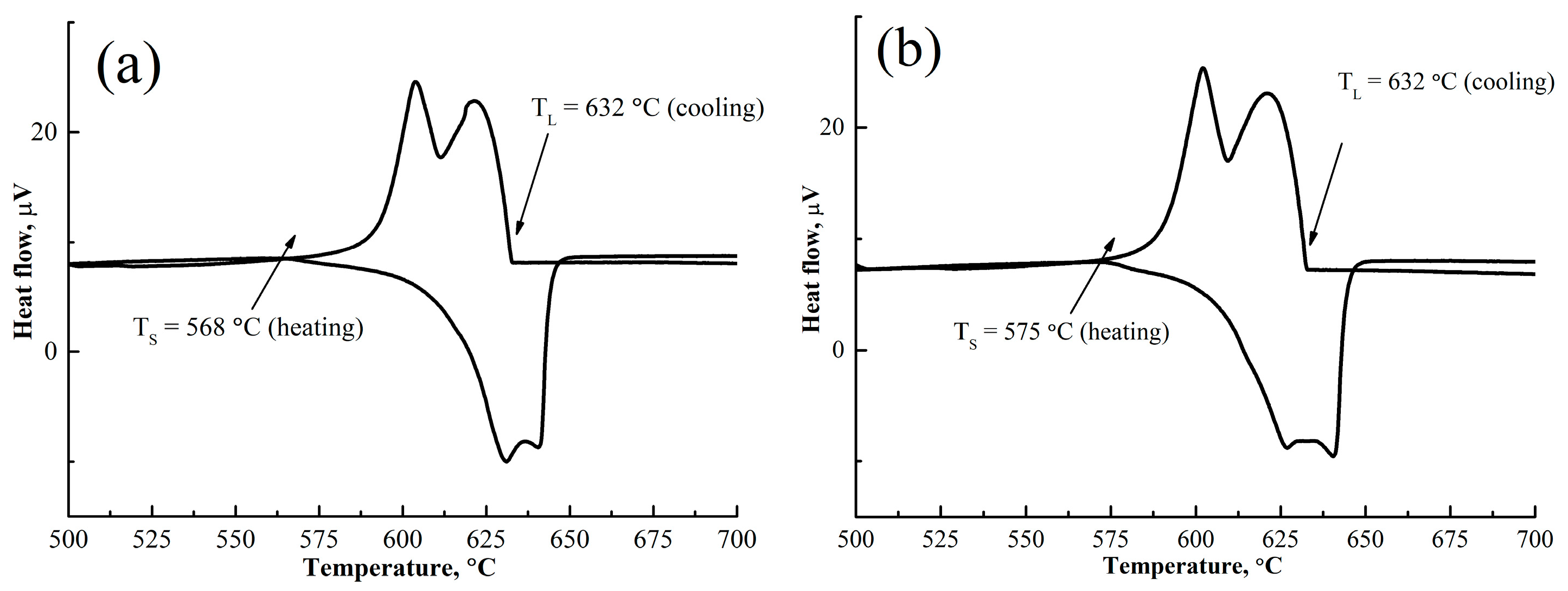
- Ytterbium in combination with Zr and Ti provide greater refining than gadolinium. The average grains of the AlCuYbMg and AlCuGdMg alloys are 60 ± 12 and 100 ± 15 µm, respectively.
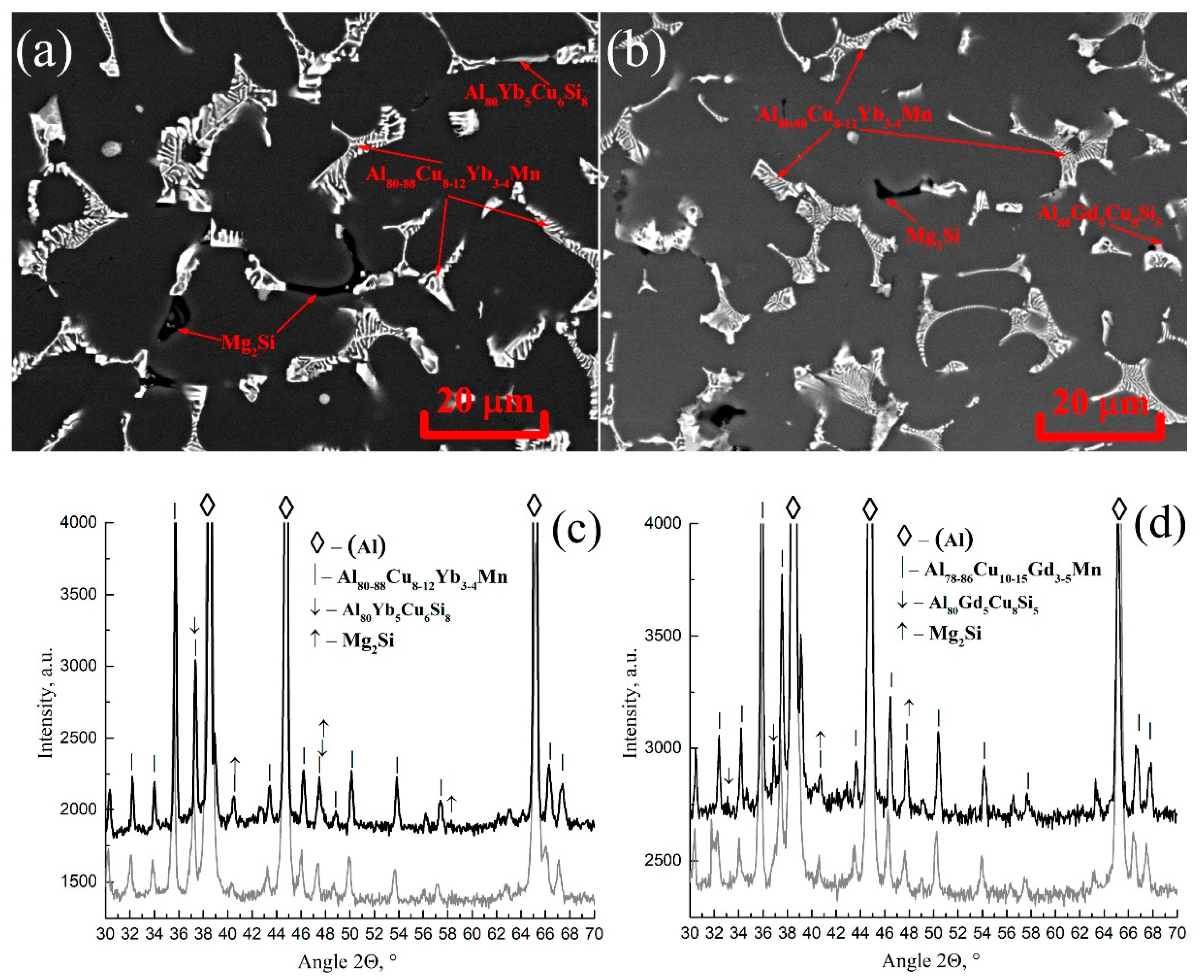
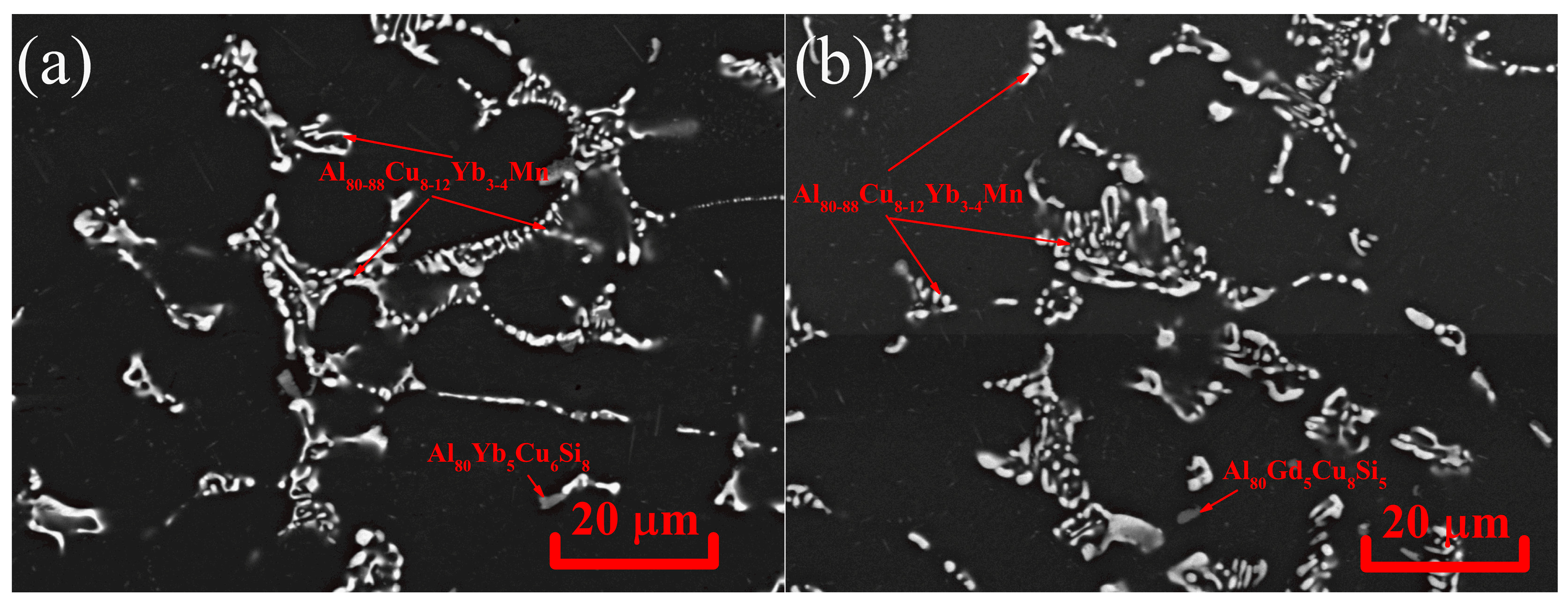
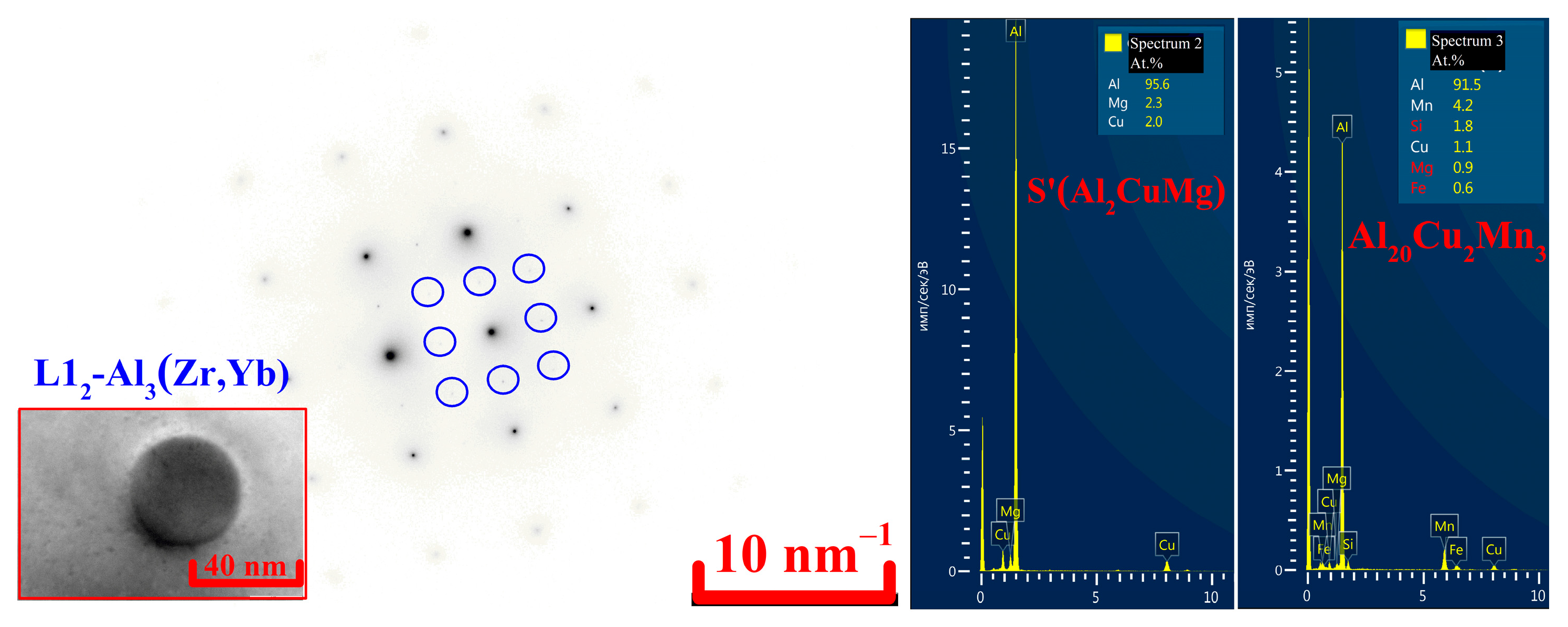
- Al80-88Cu8-12Yb3-4Mn and Al78-86Cu10-15Gd3-5Mn phases of the solidification origin form in the investigated AlCuYbMg and AlCuGdMg alloys. In addition, Mn-rich phase particles with about 10%Mn were identified in the AlCuYbMg alloy. The formula of this phase can be written as Al22Cu3Mn2Yb. Magnesium led to the Mg2Si phase solidification.
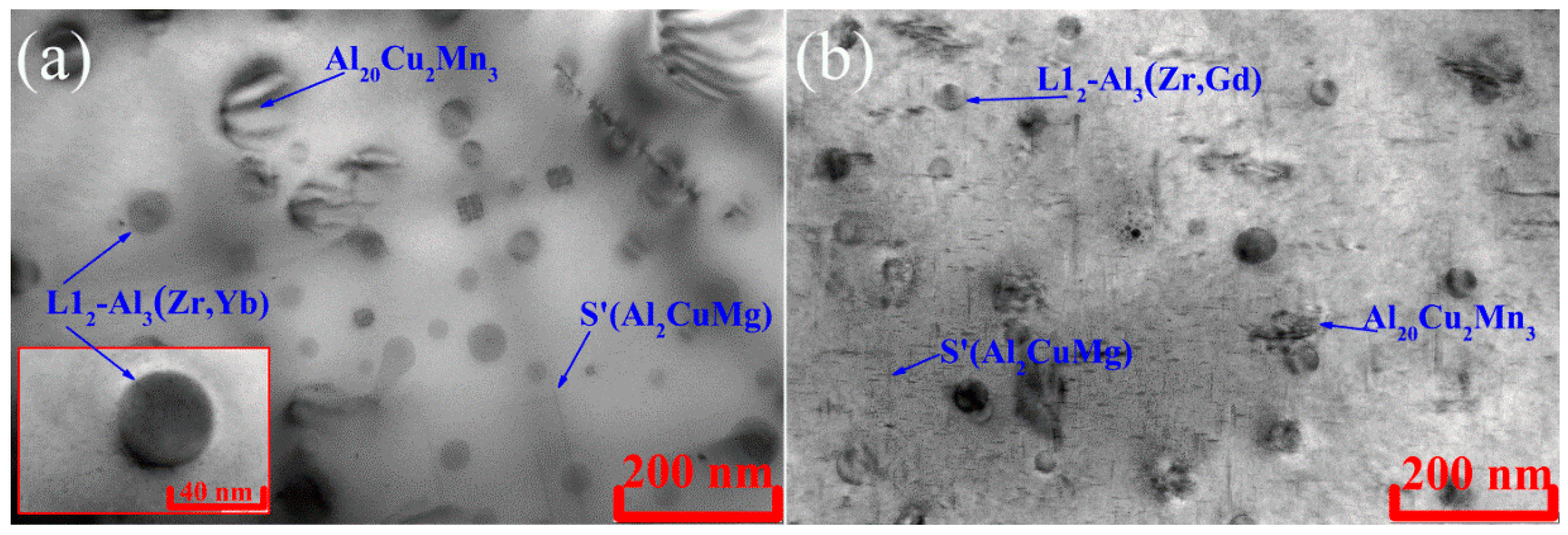
- The L12-Al3(Zr,Yb) or L12-Al3(Zr,Gd) and Al20Cu2Mn3 phase precipitates were nucleated during solution treatment. The average sizes of the L12-Al3(Zr,Yb) and L12-Al3(Zr,Gd) are 28 ± 6 nm and 32 ± 4 nm, respectively. Al20Cu2Mn3 phase precipitates formed with a finer size 100–200 nm.
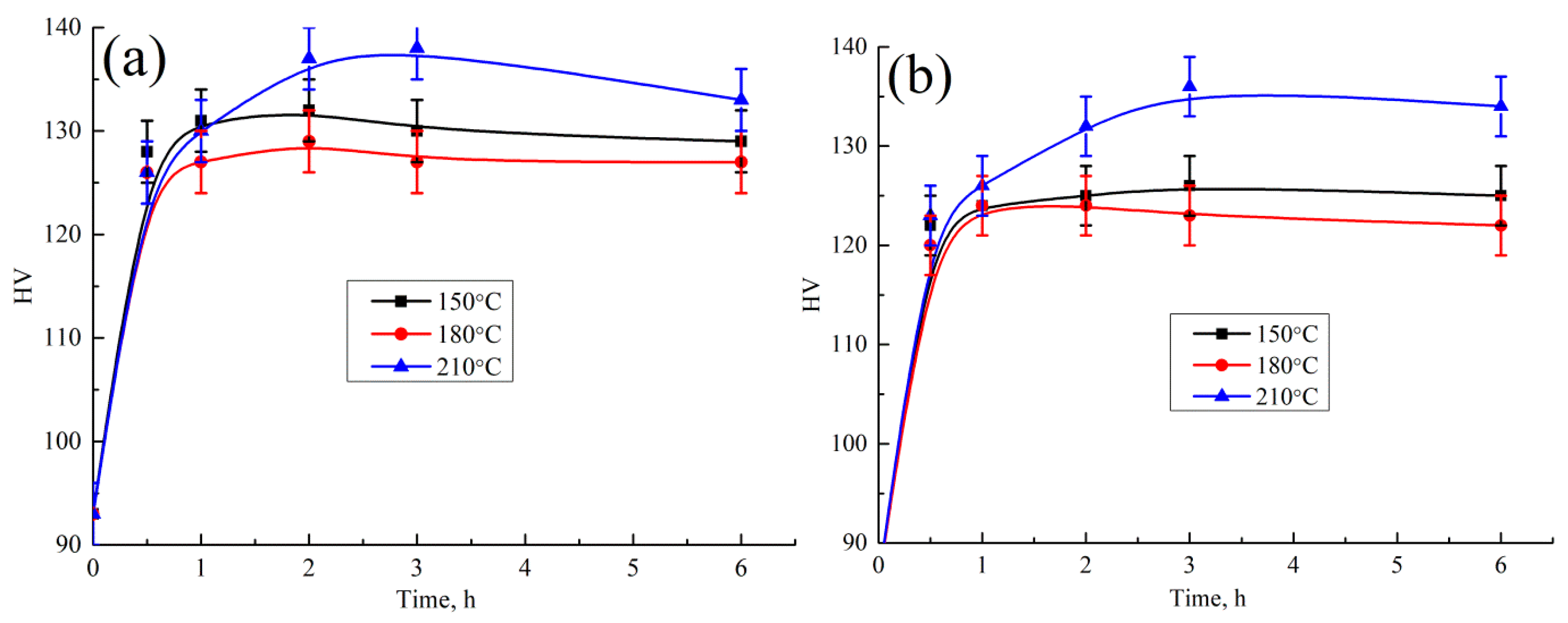
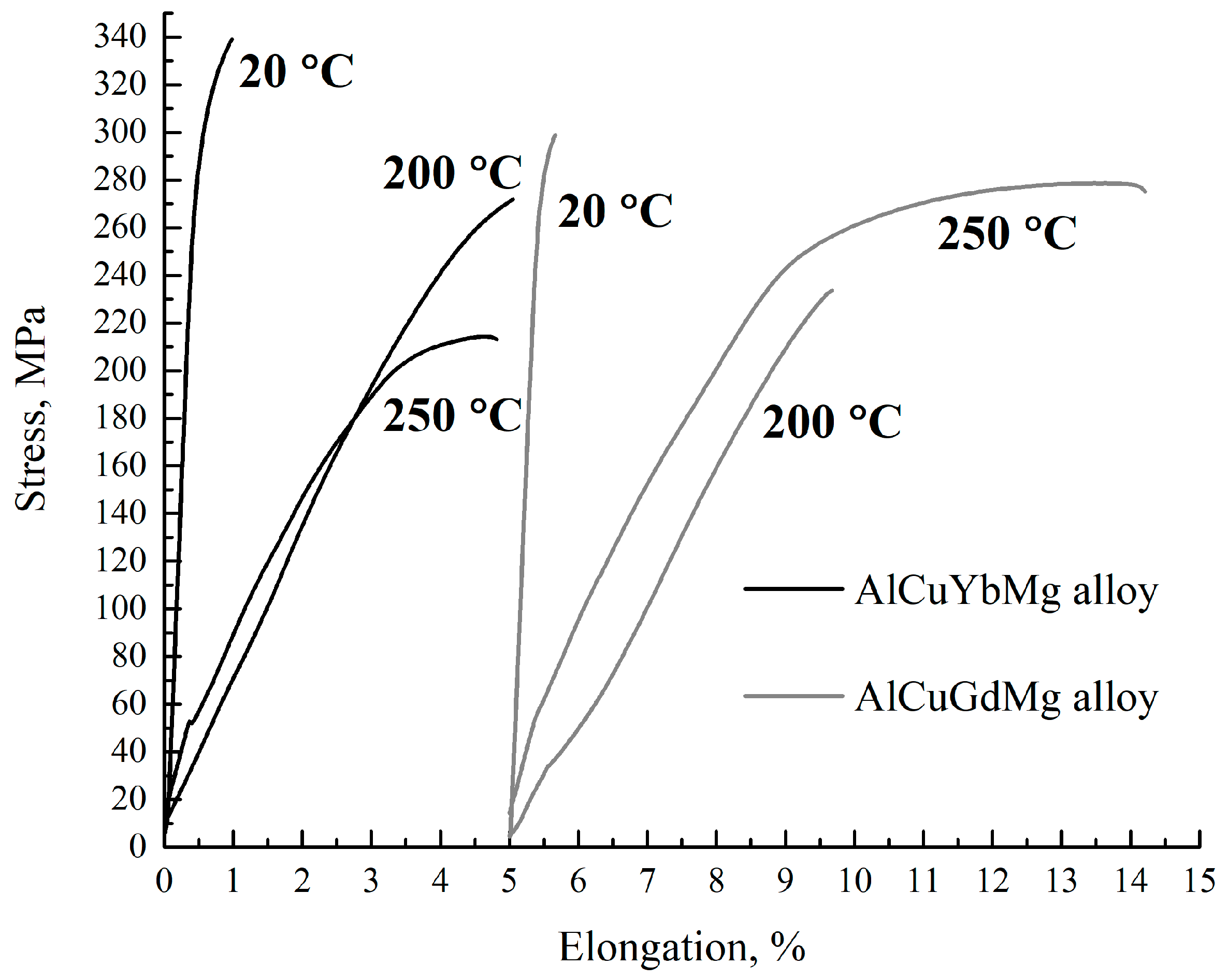
- The highest hardening effect was achieved after 3 h of aging at 210 °C in both alloys due to S’(Al2CuMg) precipitates. Typical disk-shaped precipitates were identified in the TEM. The UTS of the AlCuYbMg and AlCuGdMg alloys at room temperature are 338 and 299 MPa, respectively. The UTS decreases to 220–272 MPa when increasing the temperature of the tensile test to 200–250 °C. At the same time the elongation significantly increases. The rupture stress at 250 °C for 100 h under stress is 111–113 MPa.
- The contribution from different structure parts in the yield strength was calculated. The main strengthening effects of 54–60 MPa and 138–153 MPa were achieved from L12 and S’ precipitates, respectively. The calculated values of YS are consistent with the experimental data.
Author Contributions
Funding
Conflicts of Interest
References
- AIH Committee. ASM Handbook Vol. 2: Properties and Selection—Nonferrous Alloys and Special-Purpose Materials; ASM International: Materials Park, OH, USA, 2001; ISBN 0871700077. [Google Scholar]
- Zolotorevsky, V.S.; Belov, N.A.; Glazoff, M.V. Casting Aluminum Alloys; Alcoa Technical Center: New Kensington, PA, USA, 2007; ISBN 9780080453705. [Google Scholar]
- Eskin, D.G.; Suyitno; Katgerman, L. Mechanical properties in the semi-solid state and hot tearing of aluminium alloys. Prog. Mater. Sci. 2004, 49, 629–711. [Google Scholar] [CrossRef]
- Zolotorevskii, V.S.; Pozdnyakov, A.V.; Churyumov, A.Y. Search for promising compositions for developing new multiphase casting alloys based on Al-Cu-Mg matrix using thermodynamic calculations and mathematic simulation. Phys. Met. Metallogr. 2012, 113, 1052–1060. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Zolotorevskiy, V.S. Determining hot cracking index of Al–Si–Cu–Mg casting alloys calculated using effective solidification range. Int. J. Cast Met. Res. 2014, 27, 193–198. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Wei, Z.; Zhu, Z. The effect of Y on the hot-tearing resistance of Al–5wt.% Cu based alloy. Mater. Des. 2010, 31, 2483–2487. [Google Scholar] [CrossRef]
- Krachan, T.; Stel’makhovych, B.; Kuz’ma, Y. The Y–Cu–Al system. J. All. Comp. 2003, 349, 134–139. [Google Scholar] [CrossRef]
- Zhang, L.; Masset, P.J.; Tao, X.; Huanga, G.; Luo, H.; Liu, L.; Jin, Z. Thermodynamic description of the Al–Cu–Y ternary system. CALPHAD Comput. Coupling Phase Diagr. Thermochem. 2011, 35, 574–579. [Google Scholar] [CrossRef]
- Zhang, L.G.; Liu, L.B.; Huang, G.X.; Qi, H.Y.; Jia, B.R.; Jin, Z.P. Thermodynamic assessment of the Al–Cu–Er system. CALPHAD Comput. Coupling Phase Diagr. Thermochem. 2008, 32, 527–534. [Google Scholar] [CrossRef]
- Zhang, L.; Masset, P.J.; Cao, F.; Meng, F.; Liu, L.; Jin, Z. Phase relationships in the Al-rich region of the Al–Cu–Er system. J. All. Comp. 2011, 509, 3822–3831. [Google Scholar] [CrossRef]
- Huang, G.; Liu, L.; Zhang, L.; Jin, Z. Thermodynamic description of the al-cu-yb ternary system supported by first-principles calculations. J. Min. Metall. Sect. B Metall. 2016, 52, 177–183. [Google Scholar] [CrossRef]
- Belov, N.A.; Khvan, A.V.; Alabin, A.N. Microstructure and phase composition of Al-Ce-Cu alloys in the Al-rich corner. Mater. Sci. Forum 2006, 519, 395–400. [Google Scholar] [CrossRef]
- Belov, N.A.; Khvan, A.V. The ternary Al-Ce-Cu phase diagram in the aluminum-rich corner. Acta Mater. 2007, 55, 5473–5482. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Barkov, R.Y. Microstructure and materials characterisation of the novel Al–Cu–Y alloy. Mater. Sci. Technol. 2018, 34, 1489–1496. [Google Scholar] [CrossRef]
- Pozdnyakov, A.V.; Barkov, R.Y.; Sarsenbaev, Z.; Amer, S.M.; Prosviryakov, A.S. Evolution of Microstructure and Mechanical Properties of a New Al–Cu–Er Wrought Alloy. Phys. Met. Metallogr. 2019, 120, 614–619. [Google Scholar] [CrossRef]
- Amer, S.M.; Barkov, R.Y.; Yakovtseva, O.A.; Pozdniakov, A.V. Comparative Analysis of Structure and Properties of Quasibinary Al–6.5Cu–2.3Y and Al–6Cu–4.05Er Alloys. Phys. Met. Metallogr. 2020, 121, 476–482. [Google Scholar] [CrossRef]
- Amer, S.; Barkov, R.; Pozdniakov, A. Microstructure and mechanical properties of novel quasibinary al-cu-yb and al-cu-gd alloys. Metals 2021, 11, 476. [Google Scholar] [CrossRef]
- Amer, S.M.; Barkov, R.Y.; Yakovtseva, O.A.; Loginova, I.S.; Pozdniakov, A.V. Effect of Zr on microstructure and mechanical properties of the Al–Cu–Er alloy. Mater. Sci. Technol. 2020, 36, 453–459. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Barkov, R.Y.; Amer, S.M.; Levchenko, V.S.; Kotov, A.D.; Mikhaylovskaya, A.V. Microstructure, mechanical properties and superplasticity of the Al–Cu–Y–Zr alloy. Mater. Sci. Eng. A 2019, 758, 28–35. [Google Scholar] [CrossRef]
- Amer, S.M.; Mikhaylovskaya, A.V.; Barkov, R.Y.; Kotov, A.D.; Mochugovskiy, A.G.; Yakovtseva, O.A.; Glavatskikh, M.V.; Loginova, I.S.; Medvedeva, S.V.; Pozdniakov, A.V. Effect of Homogenization Treatment Regime on Microstructure, Recrystallization Behavior, Mechanical Properties, and Superplasticity of Al-Cu-Er-Zr Alloy. JOM 2021, 73, 3092–3101. [Google Scholar] [CrossRef]
- Mamzurina, O.I.; Amer, S.M.; Loginova, I.S.; Glavatskikh, M.V.; Mochugovskiy, A.G.; Barkov, R.Y.; Pozdniakov, A.V. Effect of Zr on Microstructure and Mechanical Properties of the Al–Cu–Yb and Al–Cu–Gd Alloys. Metals 2022, 12, 479. [Google Scholar] [CrossRef]
- Amer, S.M.; Barkov, R.Y.; Pozdniakov, A.V. Effect of Mn on the Phase Composition and Properties of Al–Cu–Y–Zr Alloy. Phys. Met. Metallogr. 2020, 121, 1227–1232. [Google Scholar] [CrossRef]
- Amer, S.; Yakovtseva, O.; Loginova, I.; Medvedeva, S.; Prosviryakov, A.; Bazlov, A.; Barkov, R.; Pozdniakov, A. The Phase Composition and Mechanical Properties of the Novel Precipitation-Strengthening Al-Cu-Er-Mn-Zr Alloy. Appl. Sci. 2020, 10, 5345. [Google Scholar] [CrossRef]
- Amer, S.M.; Mamzurina, O.I.; Loginova, I.S.; Glavatskikh, M.V.; Barkov, R.Y.; Pozdniakov, A.V. Effect of Mn Addition on the Phase Composition and Strengthening Behavior of AlCuYbZr and AlCuGdZr Alloys. JOM 2022, 74, 3646–3654. [Google Scholar] [CrossRef]
- Amer, S.M.; Barkov, R.Y.; Prosviryakov, A.S.; Pozdniakov, A.V. Structure and Properties of New Heat-Resistant Cast Alloys Based on the Al–Cu–Y and Al–Cu–Er Systems. Phys. Met. Metallogr. 2021, 122, 908–914. [Google Scholar] [CrossRef]
- Amer, S.M.; Barkov, R.Y.; Prosviryakov, A.S.; Pozdniakov, A.V. Structure and Properties of New Wrought Al–Cu–Y- and Al–Cu–Er-Based Alloys. Phys. Met. Metallogr. 2021, 122, 915–922. [Google Scholar] [CrossRef]
- Xiao, D.; Huang, B. Effect of Yb addition on precipitation and microstructure of Al-Cu-Mg-Ag alloys. Trans. Nonferrous Met. Soc. 2007, 17, 1181–1185. [Google Scholar] [CrossRef]
- Chen, K.H.; Fang, H.C.; Zhang, Z.l.; Chen, X.; Liu, G. Effect of of Yb, Cr and Zr additions on recrystallization and corrosion resistance of Al–Zn–Mg–Cu alloys Author links open overlay panel. Mater. Sci. Eng. A 2008, 497, 426–431. [Google Scholar] [CrossRef]
- Fang, H.C.; Luo, F.H.; Chen, K.H. Effect of intermetallic phases and recrystallization on the corrosion and fracture behavior of an Al-Zn-Mg-Cu-Zr-Yb-Cr alloy. Mater. Sci. Eng. A 2017, 648, 480–490. [Google Scholar] [CrossRef]
- Fang, H.C.; Chen, K.H.; Chen, X.; Chao, H.; Peng, G.S. Effect of Cr, Yb and Zr additions on localized corrosion of Al–Zn–Mg–Cu alloy. Cor. Sci. 2009, 51, 2872–2877. [Google Scholar] [CrossRef]
- Zhang, X.G.; Mei, F.Q.; Zhang, H.Y.; Wang, S.H.; Fang, C.F.; Hao, H. Effects of Gd and Y additions on microstructure and properties of Al–Zn–Mg–Cu–Zr alloys. Mater. Sci. Eng. A 2012, 552, 230–235. [Google Scholar] [CrossRef]
- Peng, G.; Chen, K.; Fang, H.; Chen, S. Effect of Cr and Yb additions on microstructure and properties of low copper Al–Zn–Mg–Cu–Zr alloy. Mater. Des. 2012, 36, 279–283. [Google Scholar] [CrossRef]
- Li, J.H.; Suetsugu, S.; Tsunekawa, Y.; Schumacher, P. Refinement of Eutectic Si Phase in Al-5Si Alloys with Yb Additions. Metall. Mater. Trans. A. 2013, 44, 669–681. [Google Scholar] [CrossRef]
- Jia, K.; Yu, W.-B.; Yao, J.-M.; Zhang, S.; Wu, H. Al–9.00%Si–0.25%Mg alloys modified by ytterbium. Rare Met. 2017, 36, 95–100. [Google Scholar] [CrossRef]
- Zhiming, S.H.I.; Qiang, W.A.N.G.; Yuting, S.H.I.; Ge, Z.H.A.O.; Zhang, R. Microstructure and mechanical properties of Gd-modified A356 aluminum alloys. J. Rare Earths 2015, 33, 1004–1009. [Google Scholar] [CrossRef]
- Peng, G.; Chen, K.; Fang, H.; Chen, S. A study of nanoscale Al3(Zr,Yb) dispersoids structure and thermal stability in Al–Zr–Yb alloy. Mater. Sci. Eng. A 2012, 535, 311–315. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, W.; Gao, H.; Han, Y.; Wang, K.; Wang, J.; Sun, B.; Gu, S.; You, W. Precipitation evolution of Al–Zr–Yb alloys during isochronal aging. Scr. Mater. 2013, 69, 477–480. [Google Scholar] [CrossRef]
- Wen, S.P.; Gao, K.Y.; Huang, H.; Wang, W.; Nie, Z.R. Role of Yb and Si on the precipitation hardening and recrystallization of dilute Al–Zr alloys. J. Alloy. Compd. 2014, 599, 65–70. [Google Scholar] [CrossRef]
- Cacciamani, G.; De Negri, S.; Saccone, A.; Ferro, R. The Al–R–Mg (R=Gd, Dy, Ho) systems. Part I: Experimental investigation. Intermetallics 2003, 11, 1125–1134. [Google Scholar] [CrossRef]
- Van Dalen, M.E.; Dunand, D.C.; Seidman, D.N. Microstructural evolution and creep properties of precipitation-strengthened Al–0.06Sc–0.02Gd and Al–0.06Sc–0.02Yb (at.%) alloys. Acta Mater. 2011, 59, 5224–5237. [Google Scholar] [CrossRef]
- Hao, H.L.; Ni, D.R.; Zhang, Z.; Wang, D.; Xiao, B.L.; Ma, Z.Y. Microstructure and mechanical properties of Al-Mg-Er sheets jointed by friction stir welding. Mater. Des. 2013, 52, 706–712. [Google Scholar] [CrossRef]
- Yang, D.; Li, X.; He, D.; Huang, H. Effect of minor Er and Zr on microstructure and mechanical properties of Al-Mg-Mn alloy (5083) welded joints. Mater. Sci. Eng. A 2013, 561, 226–231. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Yarasu, V.; Barkov, R.Y.; Yakovtseva, O.A.; Makhov, S.V.; Napalkov, V.I. Microstructure and mechanical properties of novel Al-Mg-Mn-Zr-Sc-Er alloy. Mater. Lett. 2017, 202, 116–119. [Google Scholar] [CrossRef]
- Barkov, M.V.; Mamzurina, O.I.; Glavatskikh, M.V.; Barkov, R.Y.; Pozdniakov, A.V. Structure and Properties of Al–Cu–Yb Alloy with Iron and Silicon Impurities. Russ. J. Non-Ferrous Met. 2022, 63, 434–440. [Google Scholar] [CrossRef]
- Barkov, M.V.; Mamzurina, O.I.; Glavatskikh, M.V.; Barkov, R.Y.; Pozdniakov, A.V. The Effects of Impurities on the Phase Composition and the Properties of the Al–Cu–Gd Alloy. Phys. Met. Metallogr. 2022, 123, 604–608. [Google Scholar] [CrossRef]
- Starink, M.J.; Wang, S.C. A model for the yield strength of overaged Al–Zn–Mg–Cu alloys. Acta Mater. 2003, 51, 5131–5150. [Google Scholar] [CrossRef] [Green Version]
- Deschamps, A.; Brechet, Y. Influence of predeformation and ageing of an Al–Zn–Mg alloy—II. Modeling of precipitation kinetics and yield stress. Acta Mater. 1998, 47, 293–305. [Google Scholar] [CrossRef]
- Ma, K.; Wen, H.; Hu, T.; Topping, T.D.; Isheim, D.; Seidman, D.N.; Lavernia, E.J.; Schoenung, J.M. Mechanical behavior and strengthening mechanisms in ultrafine grain precipitation-strengthened aluminum alloy. Acta Mater. 2014, 62, 141–155. [Google Scholar] [CrossRef]
- Dong, J.; Gao, N.; Chen, Y.; Cao, L.; Song, H.; Fröck, H.; Milkereit, B.; Starink, M.J. Achieving ultra-high strength of Al-Cu-Li alloys by the combination of high pressure torsion and age-hardening. Mater. Sci. Eng. A 2022, 832, 142504. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, N.; Sha, G.; Ringer, S.P.; Starink, M.J. Microstructural evolution, strengthening and thermal stability of an ultrafine-grained Al–Cu–Mg alloy. Acta Mater. 2016, 109, 202–212. [Google Scholar] [CrossRef] [Green Version]
- Weakley-Bollin, S.C.; Donlon, W.; Donlon, W.; Wolverton, C.; Allison, J.E.; Jones, J.W. Modeling the age-hardening behavior of Al-Si-Cu alloys. Metall. Mater. Trans. A 2004, 35, 2407–2418. [Google Scholar] [CrossRef]

| Alloy | Al | Cu | Yb or Gd | Mg | Mn | Zr | Fe | Si | Ti |
|---|---|---|---|---|---|---|---|---|---|
| AlCuYbMg | bal. | 4.1 | 2.0 | 1 | 0.8 | 0.3 | 0.15 | 0.15 | 0.15 |
| AlCuGdMg | bal. | 4.5 | 2.7 | 1.1 | 0.8 | 0.3 | 0.15 | 0.15 | 0.15 |
| Alloy | Al | Cu | Mg | Yb or Gd | Zr | Mn |
|---|---|---|---|---|---|---|
| AlCuYbMg | bal. | 1.3–1.4 | 0.6–0.8 | 0.1–0.3 | 0.3–0.5 | 0.6–0.8 |
| AlCuGdMg | bal. | 1.2–1.4 | 0.8–0.9 | 0.1–0.3 | 0.4 | 0.6–0.8 |
| Alloy | Al | Cu | Mg | Yb or Gd | Zr | Mn |
|---|---|---|---|---|---|---|
| AlCuYbMg | bal. | 2.5–2.6 | 1.0–1.1 | 0.1–0.3 | 0.3–0.5 | 0.6–0.8 |
| AlCuGdMg | bal. | 2.0–2.2 | 1.1–1.2 | 0.1–0.3 | 0.4 | 0.6–0.8 |
| Alloy | 20 °C | 200 °C | 250 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| YS, MPa | UTS, MPa | El., % | YS, MPa | UTS, MPa | El., % | YS, MPa | UTS, MPa | El., % | |
| AlCuYbMg | 312 ± 3 | 338 ± 1 | 0.6 ± 0.1 | 258 ± 10 | 270 ± 2 | 0.4 ± 0.2 | 206 ± 6 | 219 ± 6 | 1.4 ± 0.1 |
| AlCuGdMg | 298 ± 4 | 299 ± 4 | 0.2 ± 0.1 | 228 ± 10 | 234 ± 11 | 0.4 ± 0.1 | 235 ± 10 | 270 ± 5 | 4.7 ± 0.2 |
| Equation | Structure Parameters | AlCuYbMg | AlCuGdMg |
|---|---|---|---|
| [34,35,36] | , k = 0.065 MPa/m−2 | 18.4 | 16.5 |
| [34] | = 109 sm−2 [2] | 21.2 | 21.2 |
| = 13.8CCu+18.6CMg [46] | CCu = 0.12%, CMg = 0.1% | 3.5 | 3.5 |
(Orovan equation [46]) | r = 750 nm, f = 0.08 | 8.6 | 8.6 |
(Orovan equations for spherical [46] and disc shaped particles [50]) | L12 (rYb = 14 nm, rGd = 16 mn, f = 0.007) | 60.2 | 54.4 |
| Al20Cu2Mn3 (r = 150 nm, fYb = 0.0054, fGd = 0.004) | 14 | 12 | |
| S’(Al2CuMg) (dYb = 200 nm, dGd = 100 nm, h = 1.5 nm, fYb = 0.04, fGd = 0.037 | 153.9 | 138.6 | |
| , MPa | 279.8 | 254.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamzurina, O.I.; Amer, S.M.; Glavatskikh, M.V.; Barkov, R.Y.; Loginova, I.S.; Pozdniakov, A.V. Microstructure and Mechanical Properties of Novel Heat Resistant Cast Al-Cu-Yb(Gd)-Mg-Mn-Zr Alloys. Metals 2022, 12, 2079. https://doi.org/10.3390/met12122079
Mamzurina OI, Amer SM, Glavatskikh MV, Barkov RY, Loginova IS, Pozdniakov AV. Microstructure and Mechanical Properties of Novel Heat Resistant Cast Al-Cu-Yb(Gd)-Mg-Mn-Zr Alloys. Metals. 2022; 12(12):2079. https://doi.org/10.3390/met12122079
Chicago/Turabian StyleMamzurina, Olga I., Sayed M. Amer, Maria V. Glavatskikh, Ruslan Yu. Barkov, Irina S. Loginova, and Andrey V. Pozdniakov. 2022. "Microstructure and Mechanical Properties of Novel Heat Resistant Cast Al-Cu-Yb(Gd)-Mg-Mn-Zr Alloys" Metals 12, no. 12: 2079. https://doi.org/10.3390/met12122079
APA StyleMamzurina, O. I., Amer, S. M., Glavatskikh, M. V., Barkov, R. Y., Loginova, I. S., & Pozdniakov, A. V. (2022). Microstructure and Mechanical Properties of Novel Heat Resistant Cast Al-Cu-Yb(Gd)-Mg-Mn-Zr Alloys. Metals, 12(12), 2079. https://doi.org/10.3390/met12122079









