Long-Term in Vitro Corrosion of Biodegradable WE43 Magnesium Alloy in DMEM
Abstract
:1. Introduction
2. Materials and Methods
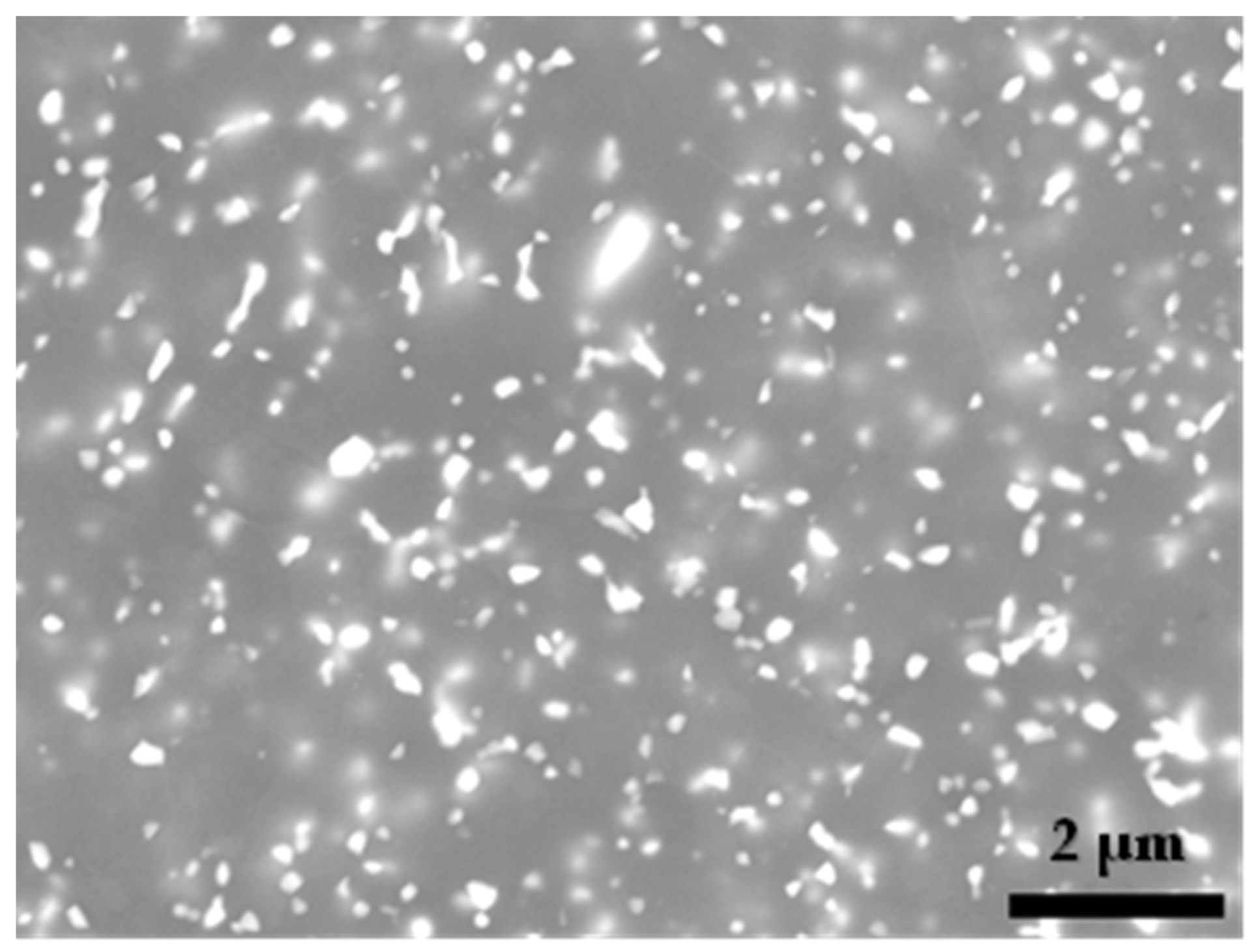
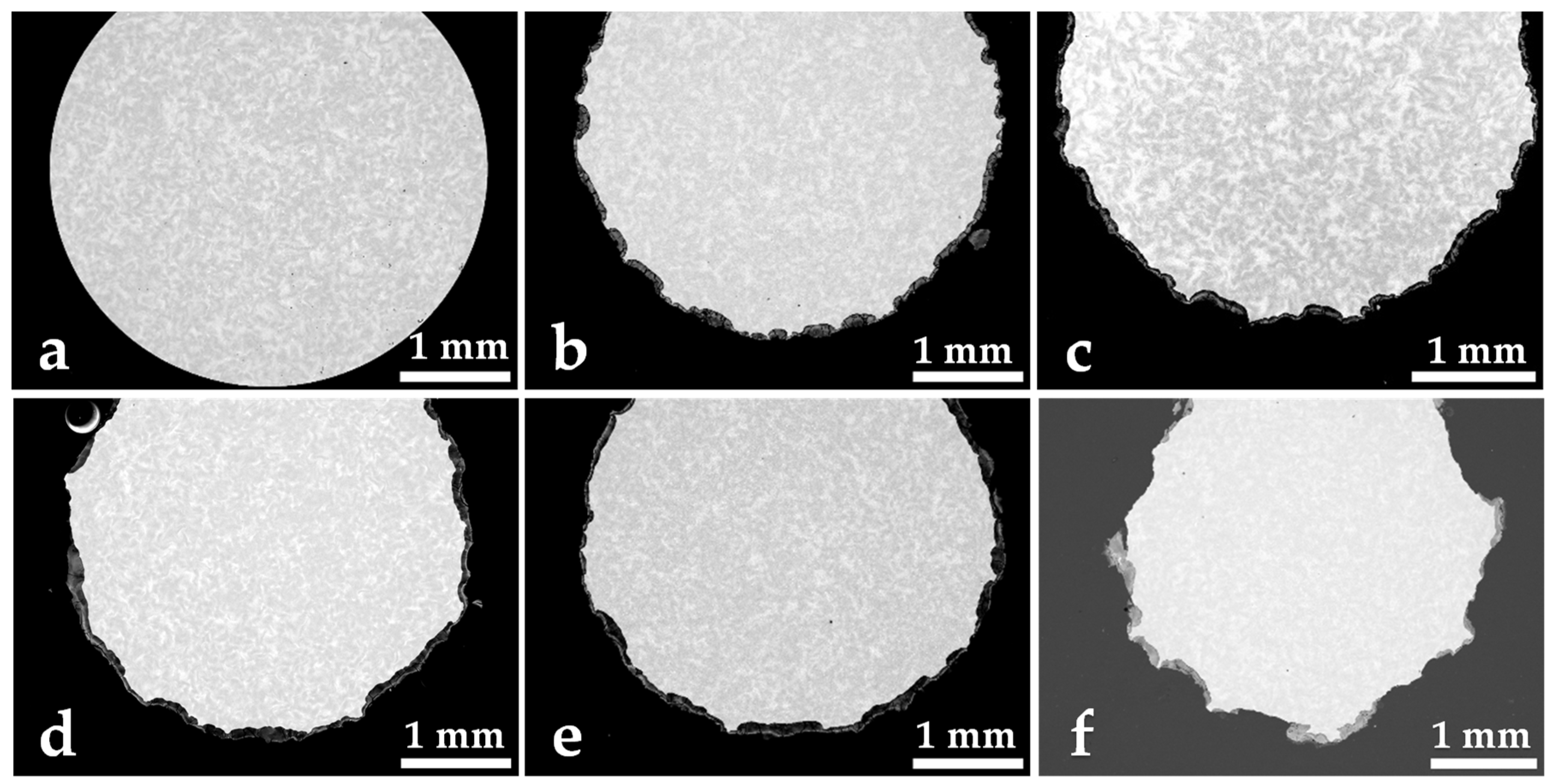
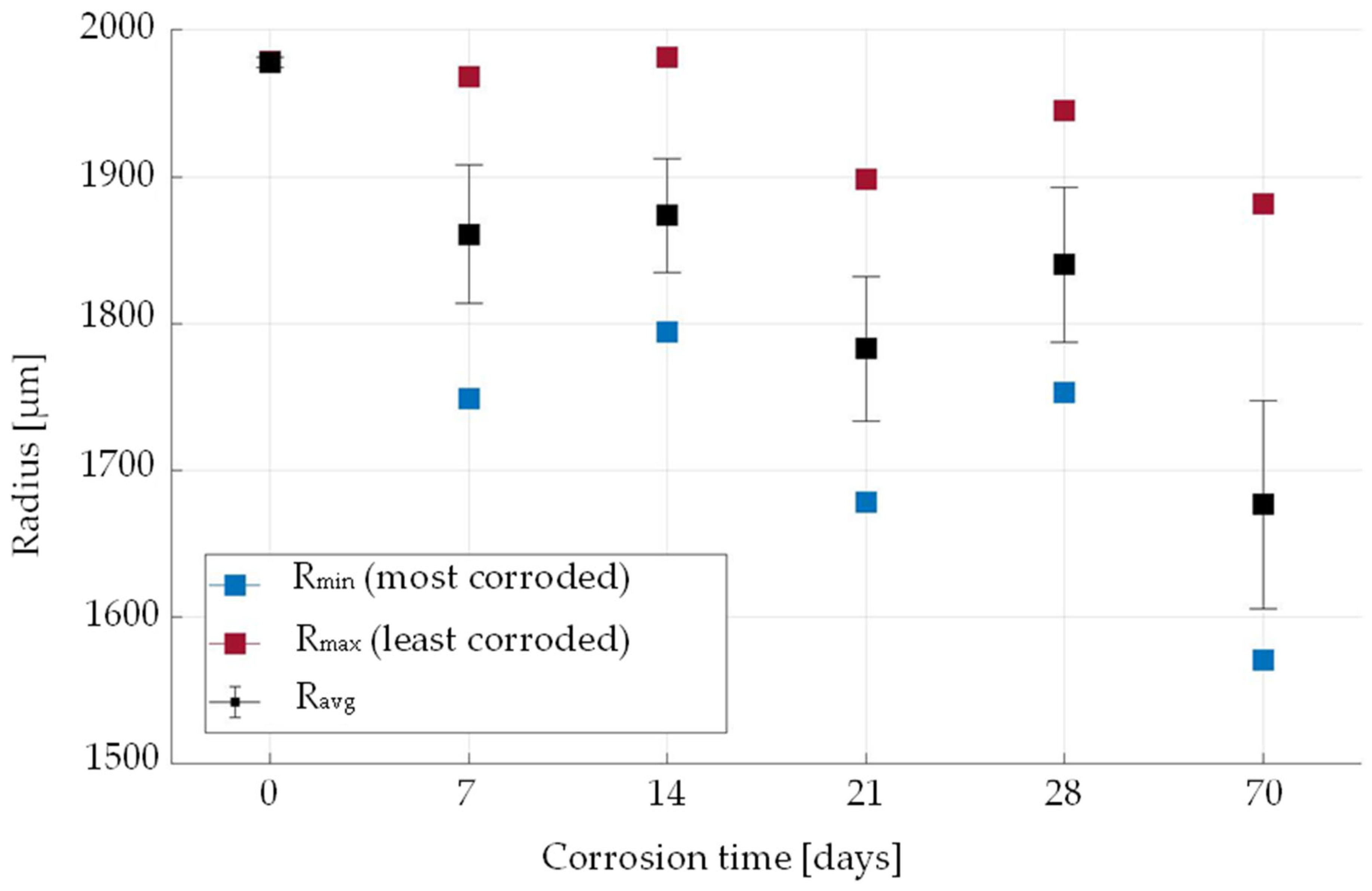
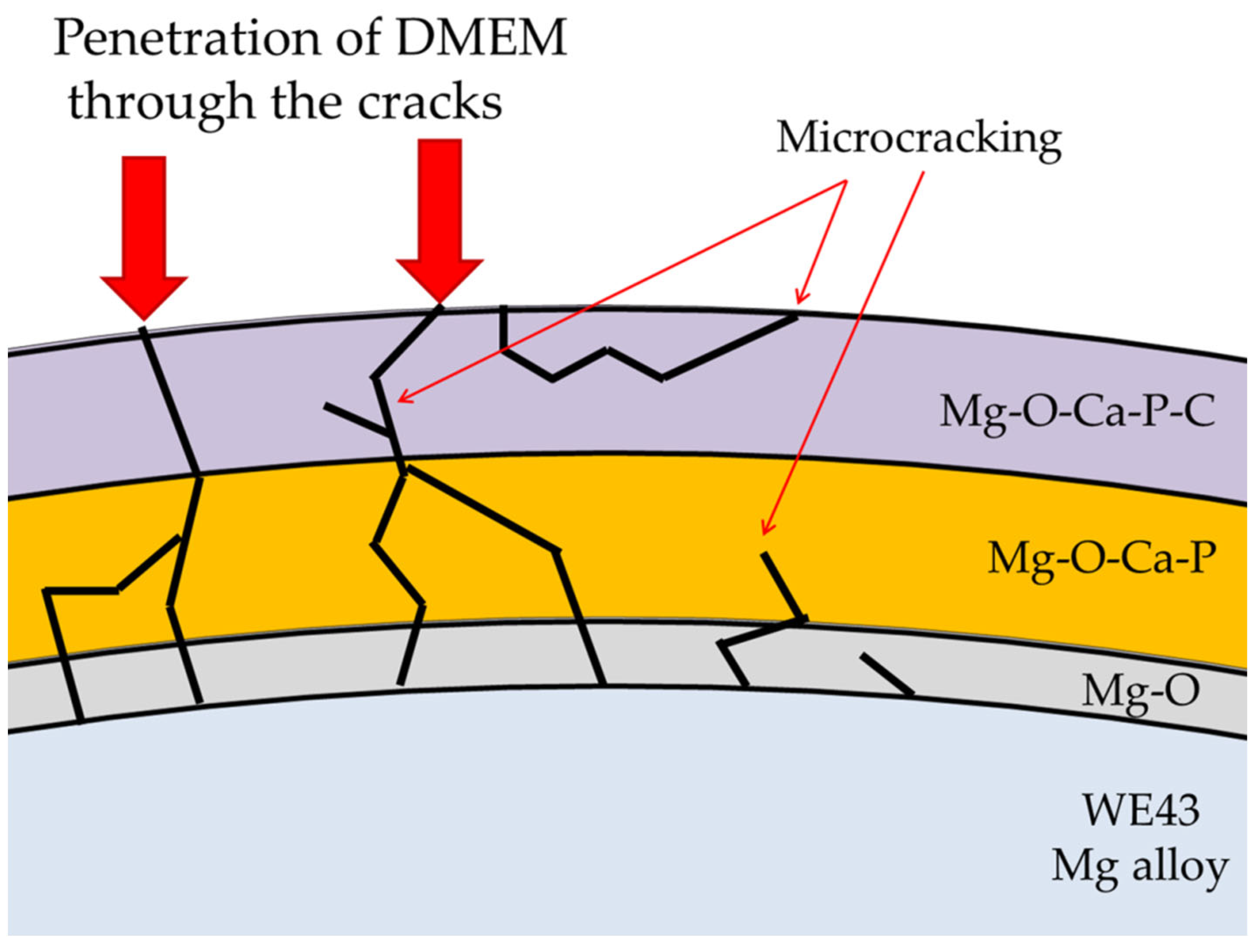
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waksman, R.; Pakala, R.; Kuchulakanti, P.K.; Baffour, R.; Hellinga, D.; Seabron, R.; Tio, F.O.; Wittchow, E.; Hartwig, S.; Harder, C.; et al. Safety and Efficacy of Bioabsorbable Magnesium Alloy Stents in Porcine Coronary Arteries. Catheter. Cardiovasc. Interv. 2006, 68, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, N.; Angrisani, N.; Reifenrath, J.; Lucas, A.; Thorey, F.; Bormann, D.; Meyer-Lindenberg, A. Biomechanical Testing and Degradation Analysis of MgCa0.8 Alloy Screws: A Comparative In Vivo Study in Rabbits. Acta Biomater. 2011, 7, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tao, H.; Zhang, Y.; Jiang, Y.; Zhang, S.; Zhao, C.; Li, J.; Zhang, B.; Song, Y.; Zhang, X. Biocompatibility of Bio-Mg-Zn Alloy within Bone with Heart, Liver, Kidney and Spleen. Chin. Sci. Bull. 2009, 54, 484–491. [Google Scholar] [CrossRef] [Green Version]
- Feyerabend, F.; Fischer, J.; Holtz, J.; Witte, F.; Willumeit, R.; Drücker, H.; Vogt, C.; Hort, N. Evaluation of Short-Term Effects of Rare Earth and Other Elements Used in Magnesium Alloys on Primary Cells and Cell Lines. Acta Biomater. 2010, 6, 1834–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In Vitro Corrosion and Biocompatibility of Binary Magnesium Alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In Vivo Corrosion of Four Magnesium Alloys and the Associated Bone Response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Castellani, C.; Lindtner, R.A.; Hausbrandt, P.; Tschegg, E.; Stanzl-Tschegg, S.E.; Zanoni, G.; Beck, S.; Weinberg, A.M. Bone-Implant Interface Strength and Osseointegration: Biodegradable Magnesium Alloy versus Standard Titanium Control. Acta Biomater. 2011, 7, 432–440. [Google Scholar] [CrossRef]
- Witte, F.; Eliezer, A. Biodegradable Metals. Degrad. Implant Mater. 2012, 9781461439, 93–109. [Google Scholar] [CrossRef]
- Gu, X.N.; Zheng, Y.F. A Review on Magnesium Alloys as Biodegradable Materials. Front. Mater. Sci. China 2010, 4, 111–115. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Viejo, F.; Skeldon, P.; Thompson, G.E. Corrosion Resistance of WE43 and AZ91D Magnesium Alloys with Phosphate PEO Coatings. Corros. Sci. 2008, 50, 1744–1752. [Google Scholar] [CrossRef]
- Gu, X.N.; Zhou, W.R.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Zhong, S.P.; Xi, T.F.; Chen, L.J. Corrosion Fatigue Behaviors of Two Biomedical Mg Alloys—AZ91D and WE43—In Simulated Body Fluid. Acta Biomater. 2010, 6, 4605–4613. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.B.; Dhakal, S.; Macreadie, I. A Toxic Synergy between Aluminium and Amyloid Beta in Yeast. Int. J. Mol. Sci. 2021, 22, 1835. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Singh, R.K. Neurotoxic Effects of Aluminium Exposure as a Potential Risk Factor for Alzheimer’s Disease. Pharmacol. Rep. 2022, 74, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.B.; Dietzel, W.; Blawert, C.; Atrens, A.; Lyon, P. Stress Corrosion Cracking of Rare-Earth Containing Magnesium Alloys ZE41, QE22 and Elektron 21 (EV31A) Compared with AZ80. Mater. Sci. Eng. A 2008, 480, 529–539. [Google Scholar] [CrossRef] [Green Version]
- Leleu, S.; Rives, B.; Bour, J.; Causse, N.; Pébère, N. On the stability of the oxides film formed on a magnesium alloy containing rare-earth elements. Electrochim. Acta 2018, 290, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Benn, F.; Derra, T.; Kröger, N.; Zinser, M.; Smeets, R.; Molina-Aldareguia, J.M.; Kopp, A.; LLorca, J. Microstructure, Mechanical Properties, Corrosion Resistance and Cytocompatibility of WE43 Mg Alloy Scaffolds Fabricated by Laser Powder Bed Fusion for Biomedical Applications. Mater. Sci. Eng. C 2021, 119, 111623. [Google Scholar] [CrossRef]
- Imwinkelried, T.; Beck, S.; Iizuka, T.; Schaller, B. Effect of a Plasmaelectrolytic Coating on the Strength Retention of In Vivo and In Vitro Degraded Magnesium Implants. Acta Biomater. 2013, 9, 8643–8649. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, G.; Lu, Q.; Wu, J.; Xu, R.; Yeung, K.W.K.; Chu, P.K. Improved Surface Corrosion Resistance of WE43 Magnesium Alloy by Dual Titanium and Oxygen Ion Implantation. Thin Solid Films 2013, 529, 407–411. [Google Scholar] [CrossRef]
- Guo, L.F.; Yue, T.M.; Man, H.C. Excimer Laser Surface Treatment of Magnesium Alloy WE43 for Corrosion Resistance Improvement. J. Mater. Sci. 2005, 40, 3531–3533. [Google Scholar] [CrossRef]
- Ye, C.H.; Zheng, Y.F.; Wang, S.Q.; Xi, T.F.; Li, Y.D. In Vitro Corrosion and Biocompatibility Study of Phytic Acid Modified WE43 Magnesium Alloy. Appl. Surf. Sci. 2012, 258, 3420–3427. [Google Scholar] [CrossRef]
- Li, M.; Cheng, Y.; Zheng, Y.F.; Zhang, X.; Xi, T.F.; Wei, S.C. Surface Characteristics and Corrosion Behaviour of WE43 Magnesium Alloy Coated by SiC Film. Appl. Surf. Sci. 2012, 258, 3074–3081. [Google Scholar] [CrossRef]
- Ascencio, M.; Pekguleryuz, M.; Omanovic, S. An Investigation of the Corrosion Mechanisms of WE43 Mg Alloy in a Modified Simulated Body Fluid Solution: The Influence of Immersion Time. Corros. Sci. 2014, 87, 489–503. [Google Scholar] [CrossRef]
- Mueller, W.D.; Lorenzo De Mele, M.F.; Nascimento, M.L.; Zeddies, M. Degradation of Magnesium and Its Alloys: Dependence on the Composition of the Synthetic Biological Media. J. Biomed. Mater. Res.—Part A 2009, 90, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Hiromoto, S. Effect of Inorganic Salts, Amino Acids and Proteins on the Degradation of Pure Magnesium In Vitro. Mater. Sci. Eng. C 2009, 29, 1559–1568. [Google Scholar] [CrossRef]
- Müller, L.; Müller, F.A. Preparation of SBF with Different HCO3- Content and Its Influence on the Composition of Biomimetic Apatites. Acta Biomater. 2006, 2, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Zhang, D.; Zhang, W.; Zhang, W. In Vitro Corrosion Study of Friction Stir Processed WE43 Magnesium Alloy in a Simulated Body Fluid. Materials 2016, 9, 542. [Google Scholar] [CrossRef] [Green Version]
- Ascencio, M.; Pekguleryuz, M.; Omanovic, S. An Investigation of the Corrosion Mechanisms of WE43Mg Alloy in a Modified Simulated Body Fluid Solution: The Effect of Electrolyte Renewal. Corros. Sci. 2015, 91, 297–310. [Google Scholar] [CrossRef]
- Kannan, M.B.; Raman, R.K.S. In Vitro Degradation and Mechanical Integrity of Calcium-Containing Magnesium Alloys in Modified-Simulated Body Fluid. Biomaterials 2008, 29, 2306–2314. [Google Scholar] [CrossRef]
- Wagener, V.; Virtanen, S. Influence of Electrolyte Composition (Simulated Body Fluid vs. Dulbecco’s Modified Eagle’s Medium), Temperature, and Solution Flow on the Biocorrosion Behavior of Commercially Pure Mg. Corrosion 2017, 73, 1413–1422. [Google Scholar] [CrossRef]
- Höhlinger, M.; Christa, D.; Zimmermann, V.; Heise, S.; Boccaccini, A.R.; Virtanen, S. Influence of Proteins on the Corrosion Behavior of a Chitosan-Bioactive Glass Coated Magnesium Alloy. Mater. Sci. Eng. C 2019, 100, 706–714. [Google Scholar] [CrossRef]
- Jin, W.; Wu, G.; Feng, H.; Wang, W.; Zhang, X.; Chu, P.K. Improvement of Corrosion Resistance and Biocompatibility of Rare-Earth WE43 Magnesium Alloy by Neodymium Self-Ion Implantation. Corros. Sci. 2015, 94, 142–155. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the Corrosion of Biodegradable Magnesium Implants: A Critical Review of Current Methodologies and Their Limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Milkereit, B.; Burgschat, L.; Kemsies, R.H.; Springer, A.; Schick, C.; Kessler, O. In Situ Differential Scanning Calorimetry Analysis of Dissolution and Precipitation Kinetics in Mg–Y–RE Alloy WE43. J. Magnes. Alloy. 2019, 7, 1–14. [Google Scholar] [CrossRef]
- Pereira, G.S.; Koga, G.Y.; Avila, J.A.; Bittencourt, I.M.; Fernandez, F.; Miyazaki, M.H.; Botta, W.J.; Bose Filho, W.W. Corrosion Resistance of WE43 Mg Alloy in Sodium Chloride Solution. Mater. Chem. Phys. 2021, 272, 124930. [Google Scholar] [CrossRef]
- Luo, K.; Zhang, L.; Wu, G.; Liu, W.; Ding, W. Effect of Y and Gd Content on the Microstructure and Mechanical Properties of Mg–Y–RE Alloys. J. Magnes. Alloy. 2019, 7, 345–354. [Google Scholar] [CrossRef]
- Leleu, S.; Rives, B.; Causse, N.; Pébère, N. Corrosion Rate Determination of Rare-Earth Mg Alloys in a Na2SO4 Solution by Electrochemical Measurements and Inductive Coupled Plasma-Optical Emission Spectroscopy. J. Magnes. Alloy. 2019, 7, 47–57. [Google Scholar] [CrossRef]
- Feng, B.; Liu, G.; Yang, P.; Huang, S.; Qi, D.; Chen, P.; Wang, C.; Du, J.; Zhang, S.; Liu, J. Different Role of Second Phase in the Micro-Galvanic Corrosion of WE43 Mg Alloy in NaCl and Na2SO4 Solution. J. Magnes. Alloy. 2021, 10, 1598–1608. [Google Scholar] [CrossRef]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg Alloys for Orthopedic Implants—A Review. J. Magnes. Alloy. 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Kalb, H.; Rzany, A.; Hensel, B. Impact of Microgalvanic Corrosion on the Degradation Morphology of WE43 and Pure Magnesium under Exposure to Simulated Body Fluid. Corros. Sci. 2012, 57, 122–130. [Google Scholar] [CrossRef]
- Schwarz, M.L.R.; Kowarsch, M.; Rose, S.; Becker, K.; Lenz, T.; Jani, L. Effect of Surface Roughness, Porosity, and a Resorbable Calcium Phosphate Coating on Osseointegration of Titanium in a Minipig Model. J. Biomed. Mater. Res.—Part A 2009, 89, 667–678. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed.; National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg-Zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]


| Y | Nd | Zr | Al | Mn | Fe | Mg |
|---|---|---|---|---|---|---|
| 3.8% | 2.6% | 0.02% | <0.01% | 0.0025% | 0.0065% | Bal. |
| Formulation | mg/L | Formulation | mg/L |
|---|---|---|---|
| Glycine | 30 | Magnesium Sulfate Anhydrous | 97.67 |
| L-Arginine Monohydrochloride | 84 | Potassium chloride | 400 |
| L-Cystine Dihydrochloride | 62.6 | Sodium Bicarbonate | 3700 |
| L-Histidine Monohydrochloride Monohydrate | 42 | Sodium Chloride | 6400 |
| L-Isoleucine | 105 | Sodium Phosphate Monobasic Anhydrous | 109 |
| L-Leucine | 105 | Choline Chloride | 4 |
| L-Lysine Monohydrochloride | 146 | D-Ca Pantothenate | 4 |
| L-Methionine | 30 | Folic Acid | 4 |
| L-Phenylalanine | 66 | Myo-Inositol | 7.2 |
| L-Serine | 42 | Nicotinamide | 4 |
| L-Threonine | 95 | Pyridoxal Hydrochloride | 4 |
| L-Tryptophan | 16 | Riboflavin | 0.4 |
| L-Tyrosine Disodium Salt Dihydrate | 103.79 | Thiamine Hydrochloride | 4 |
| L-Valine | 94 | D-Glucose Anhydrous | 4500 |
| Calcium Chloride Dihydrate | 4265 | Phenol Red Solution Salt | 15.9 |
| Ferric Nitrate Nonahydrate | 0.1 |
| Ion | ||||||||
|---|---|---|---|---|---|---|---|---|
| Blood Plasma | 142 | 5 | 1.5 | 2.5 | 103 | 27 | 1 | 0.5 |
| DMEM | 154 | 5.4 | 0.8 | 1.8 | 119 | 44 | 1 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nachtsheim, J.; Burja, J.; Ma, S.; Markert, B. Long-Term in Vitro Corrosion of Biodegradable WE43 Magnesium Alloy in DMEM. Metals 2022, 12, 2062. https://doi.org/10.3390/met12122062
Nachtsheim J, Burja J, Ma S, Markert B. Long-Term in Vitro Corrosion of Biodegradable WE43 Magnesium Alloy in DMEM. Metals. 2022; 12(12):2062. https://doi.org/10.3390/met12122062
Chicago/Turabian StyleNachtsheim, Julia, Jaka Burja, Songyun Ma, and Bernd Markert. 2022. "Long-Term in Vitro Corrosion of Biodegradable WE43 Magnesium Alloy in DMEM" Metals 12, no. 12: 2062. https://doi.org/10.3390/met12122062
APA StyleNachtsheim, J., Burja, J., Ma, S., & Markert, B. (2022). Long-Term in Vitro Corrosion of Biodegradable WE43 Magnesium Alloy in DMEM. Metals, 12(12), 2062. https://doi.org/10.3390/met12122062








