Influence of Vanadium Extraction Converter Process Optimization on Vanadium Extraction Effect
Abstract
:1. Introduction
2. Experimental Equipment and Methods
2.1. Water Model Experiment
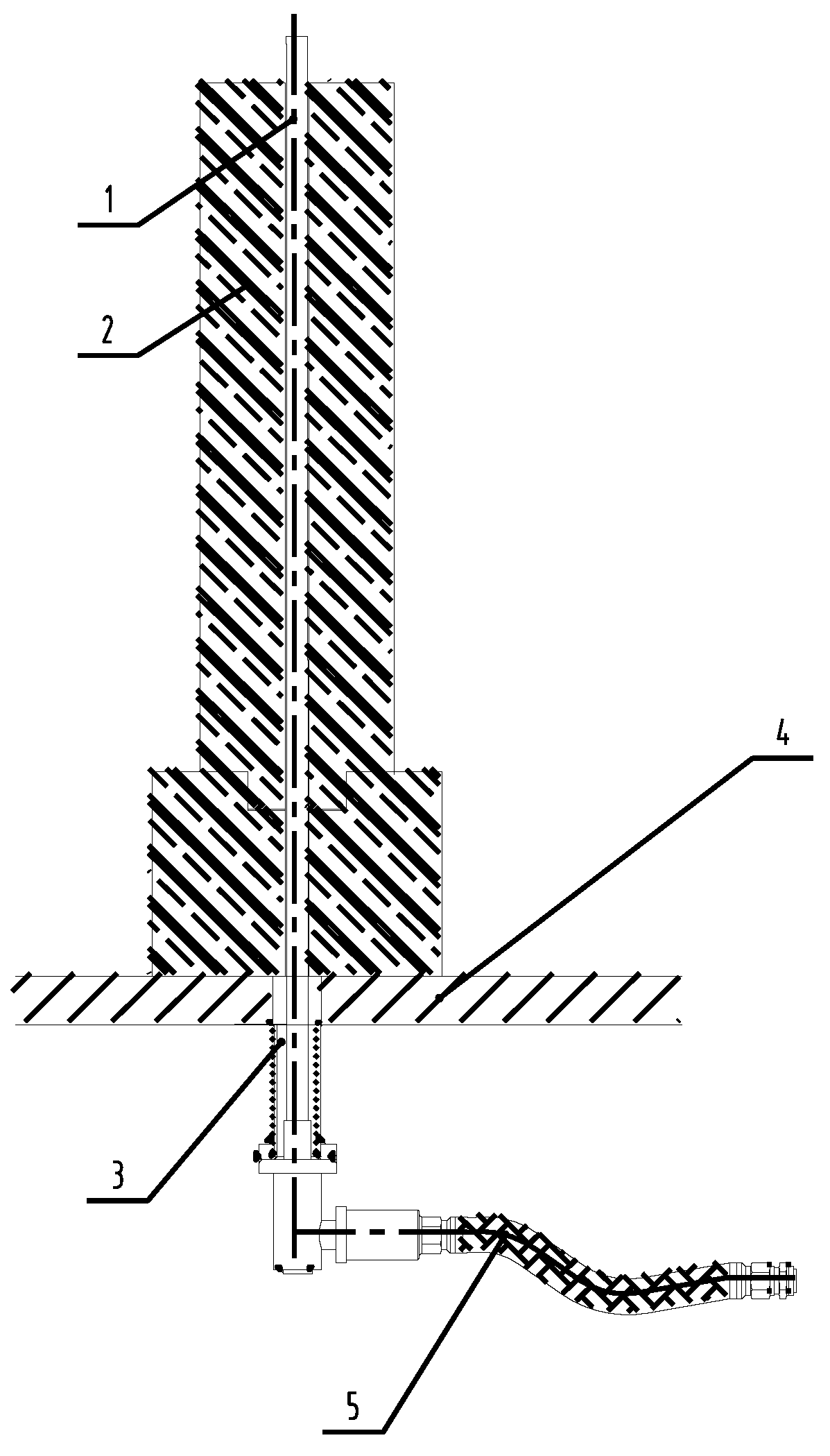
2.1.1. Experimental Equipment
2.1.2. Experimental Parameters
2.1.3. Experimental Method
2.2. Industrial Test
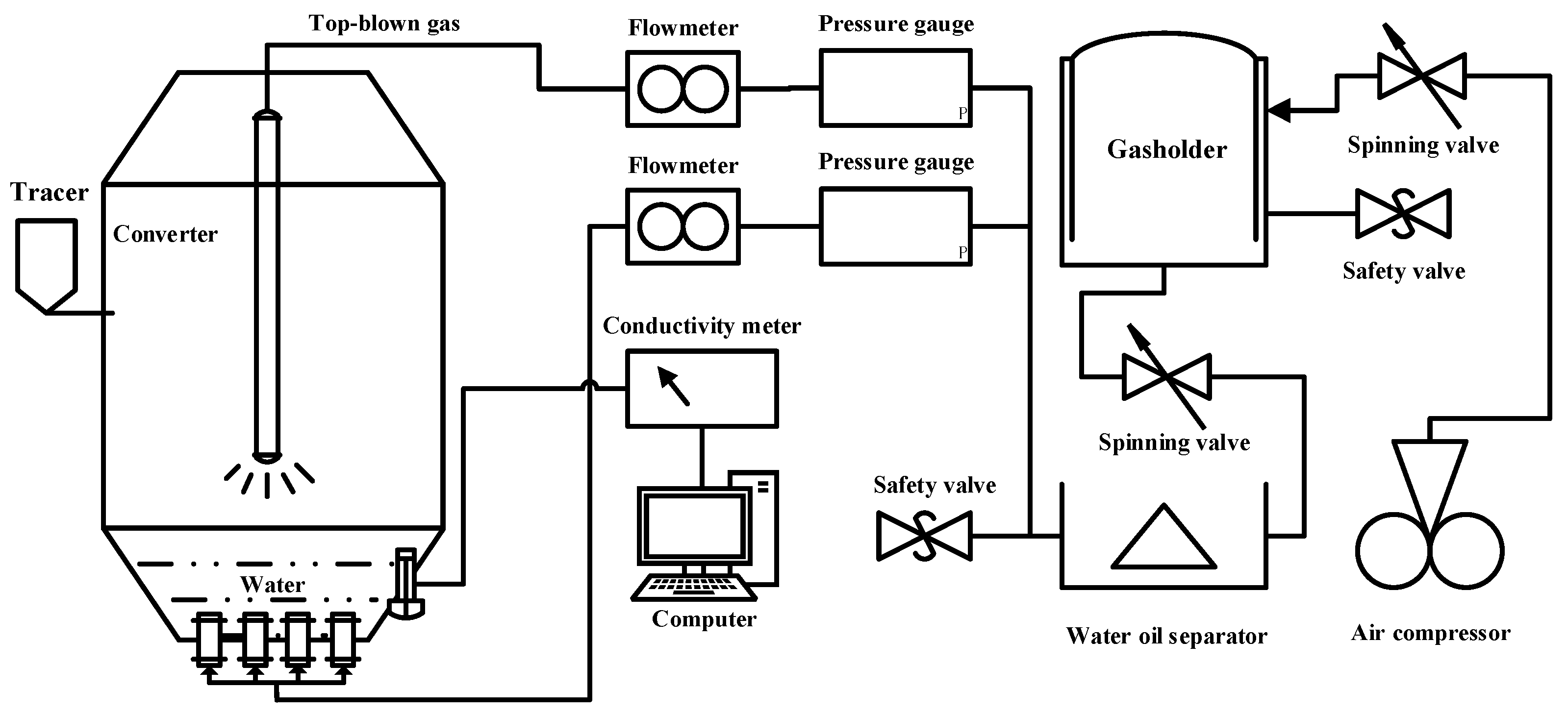
2.2.1. Test Equipment and Process
2.2.2. Test Scheme
2.2.3. Test Methods
3. Results
3.1. Determination of the Arrangement of Bottom Blowing Elements on the Bottom of the Converter
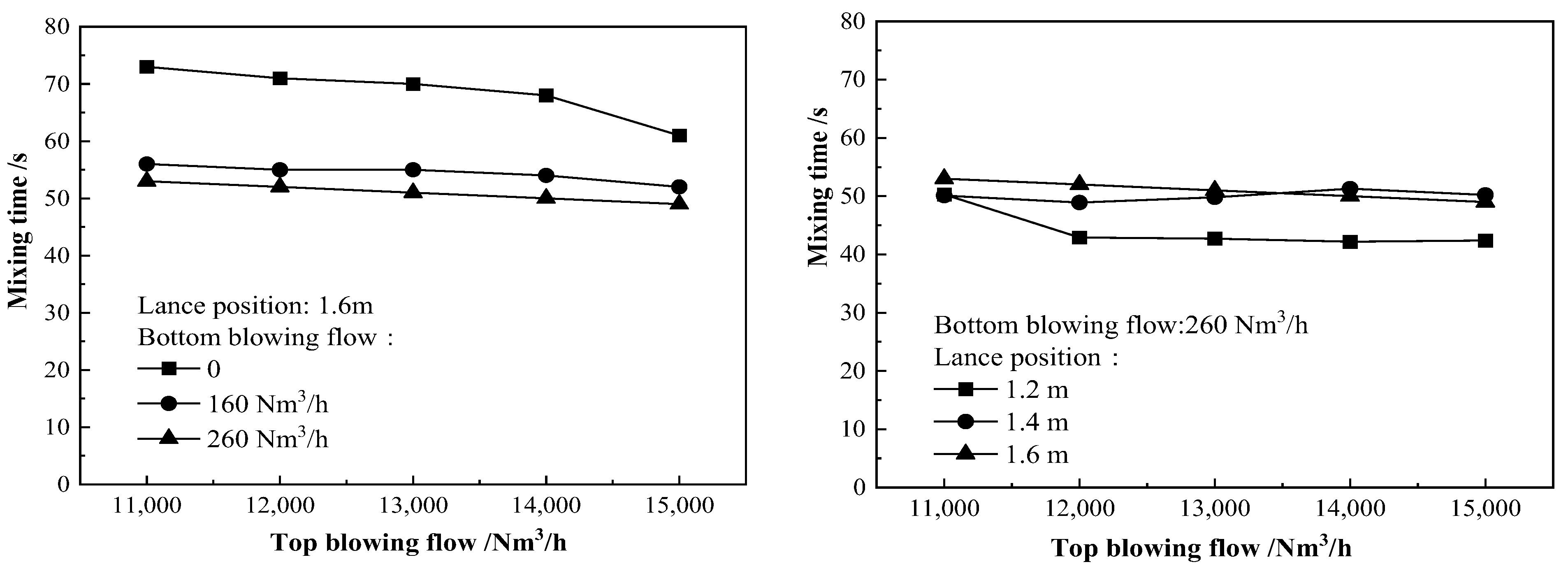
3.2. Influence of Top Blowing Gas Flow, Oxygen Lance Position and Bottom Blowing Gas Flow on the Stirring Effect of Molten Bath
3.3. Effect of Vanadium Extraction from Hot Metal before and after Process Optimization
4. Discussion
4.1. Variation of Molten Bath Stirring Energy after Process Optimization
4.2. Analysis of Factors Affecting the Distribution Ratio of Vanadium
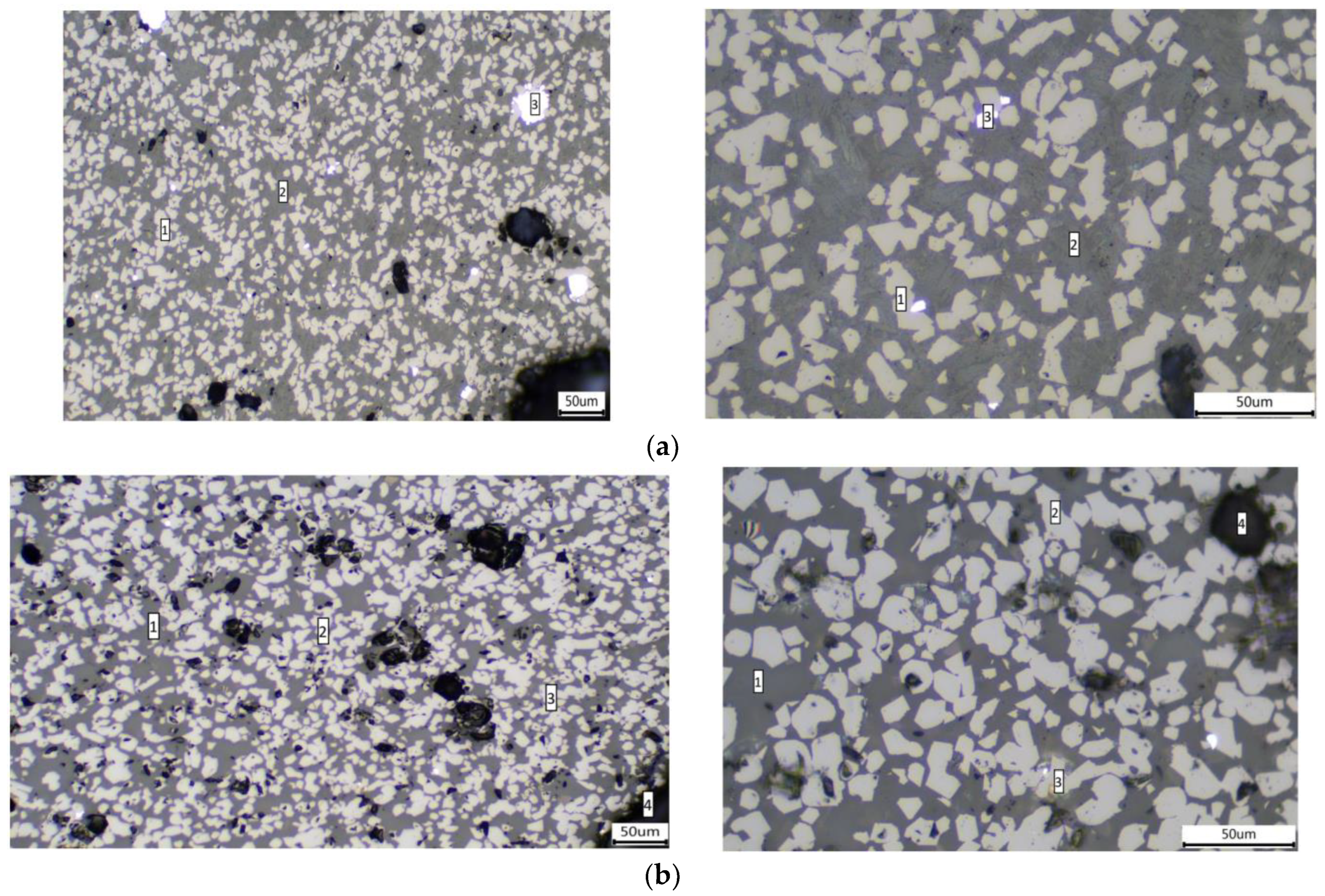
4.3. Changes in Slag Mineral Phase Composition before and after Process Optimization
5. Conclusions
- The arrangement of the bottom blowing element was determined by the water model experiment. When the bottom blowing gas element was located at 0.45D (D was the diameter of the furnace bottom), the best stirring effect could be obtained.
- The best blowing process parameters in the blowing process were determined: in the early stage of blowing, the top blowing gas flow was 14,000 Nm3/h, the oxygen lance position was from 1.4 to 1.5 m, and the bottom blowing gas flow was 160 Nm3/h. In the middle stage of blowing, the top blowing gas flow was from 12,000 to 13,000 Nm3/h, the oxygen lance position was 1.55 m, and the bottom blowing gas flow was 260 Nm3/h. In the later stage of blowing, the top blowing gas flow was from 13,000 to 13,500 Nm3/h, the oxygen lance position was 1.3 m and the bottom blowing gas flow was 260 Nm3/h.
- After process optimization, the average value of vanadium content of semi-steel and metal iron content of vanadium slag were 0.033 wt% and 22.39 wt%, respectively, which were 0.003 wt% and 5.25 wt% lower than those before process optimization. The average vanadium oxide content of vanadium slag was 18.54 wt%, which was 0.2 wt% higher than that before process optimization. This shows that after the process optimization, the kinetic conditions of the molten bath were good, and the vanadium oxygen reaction was more sufficient.
Author Contributions
Funding
Conflicts of Interest
References
- Shushi, Z.; Zhenyang, W.; Peng, H.; Jiating, R.; Jianliang, Z.; Jing, P. Distribution behavior of vanadium and titanium between hot metal and high titanium slag relevant to HIsmelt smelting condition. J. Mater. Res. Technol. 2022, 19, 4517–4524. [Google Scholar]
- Deng, R.; Xiao, H.; Xie, Z.; Liu, Z.; Yu, Q.; Chen, G.; Tao, C. A novel method for extracting vanadium by low temperature sodium roasting from converter vanadium slag. Chin. J. Chem. Eng. 2020, 28, 2208–2213. [Google Scholar] [CrossRef]
- Rui, Y.; Shaolong, L.; Yusi, C.; Jilin, H.; Jianxun, S.; Bin, Y. A critical review on extraction and refining of vanadium metal. Int. J. Refract. Met. Hard Mater. 2021, 101, 105696. [Google Scholar]
- Lindvall, M.; Berg, M.; Sichen, D. The effect of Al2O3, CaO and SiO2 on the Phase relationship in FeO-SiO2 based slag with 20 mass% vanadium. J. Sustain. Metall. 2017, 3, 289–299. [Google Scholar] [CrossRef] [Green Version]
- Diao, J.; Qiu, Y.-Y.; Lei, J.; Zhang, Q.; Tan, W.-F.; Li, H.-Y.; Xie, B. Study on Saturated Solubility of MgO in Converter Vanadium Slag. JOM 2020, 73, 999–1003. [Google Scholar] [CrossRef]
- Chen, L.; Diao, J.; Wang, G.; Xie, B. Assessment of Dephosphorization During Vanadium Extraction Process in Converter. JOM 2018, 70, 963–968. [Google Scholar] [CrossRef]
- Tatjana, J.; Elena, Y.; Klaus, H.; to Baben, M.; Guixuan, W.; Michael, M. Addition of V2O5 and V2O3 to the CaO–FeO–Fe2O3–MgO–SiO2 database for vanadium distribution and viscosity calculations. Calphad 2021, 74, 102284. [Google Scholar]
- Song, W.-C.; Li, H.; Zhu, F.-X.; Li, K.; Zheng, Q. Extraction of vanadium from molten vanadium bearing slag by oxidation with pure oxygen in the presence of CaO. Trans. Nonferrous Met. Soc. China 2014, 24, 2687–2694. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Song, W.-C.; Li, H. Vanadium extraction and dephosphorization from V-bearing hot metal with fluxes containing CaO. J. Cent. South Univ. 2015, 22, 2887–2893. [Google Scholar] [CrossRef]
- Li, X.-S.; Xie, B. Extraction of vanadium from high calcium vanadium slag using direct roasting and soda leaching. Int. J. Miner. Metall. Mater. 2012, 19, 595–601. [Google Scholar] [CrossRef]
- Zhenyu, Z.; Ping, T. Optimization on Temperature Strategy of BOF Vanadium Extraction to Enhance Vanadium Yield with Minimum Carbon Loss. Metals 2021, 11, 906. [Google Scholar] [CrossRef]
- Liu, F.; Sun, D.; Zhu, R.; Dong, K.; Bai, R. Effect of Side-blowing Arrangement on Flow Field and Vanadium Extraction Rate in Converter Steelmaking Process. ISIJ Int. 2018, 58, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Yu, S.; Shen, X.; Xu, L.; Wang, N.; Chen, M. Kinetic study on the oxidation of elements in hot metal during vanadium extraction process. Steel Res. Int. 2016, 87, 1228–1237. [Google Scholar] [CrossRef]
- Du, W.-T.; Jiang, Q.; Chen, Z.; Liang, X.-P.; Wang, Y. Experimental Characterization of CO2 and CaCO3 Used in a Pyrometallurgical Vanadium-Extraction Process. JOM 2019, 71, 4925–4930. [Google Scholar] [CrossRef]
- Guo, Z.-L.; Wang, Y.; Qi, L.; Wang, S.-C. Study on the Effect of Different CO2–O2 Mixture Gas Blowing Modes on Vanadium Oxidation. In 10th International Symposium on High-Temperature Metallurgical Processing; Springer: Berlin/Heidelberg, Germany, 2019; pp. 787–794. [Google Scholar]
- Lindvall, M.; Tikka, J.; Berg, M.; Ye, G.; Sichen, D. Vanadium Extraction from a Fe–V (2.0 Mass%)–P (0.1 Mass%) Melt and Investigation of the Phase Relations in the Formed FeO–SiO2 -Based Slag with 20 Mass% V. J. Sustain. Metall. 2017, 3, 808–822. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, Y.-C.; Pan, S.-M. Research on Large Converter Combined Blowing and Slag Splashing Technology. China Metall. 2013, 23, 1–4. [Google Scholar]
- Gao, Q.; Wu, W.; Zhao, J.-X. Cold Simulation of the Effect of Self-Rotating Oxygen Lance on the Mixing Time of Molten bath. J. Iron Steel Res. 2020, 32, 945–950. [Google Scholar]
- Zhang, H.-S.; Xiao, Z.-Q. Influence of slag/steel mixing state on mass transport rate. Iron Steel 1987, 22, 24–28. [Google Scholar]
- Kim, S.-H.; Fruehan, R.-J.; Guthrie, R.-I. Physical Model Studies of Slag/Metal Reactions in Gas Stirred Ladles—Determination of Critical Gas Flow Rate. Iron Steel Mak. 1993, 20, 71–76. [Google Scholar]
- Kohtani, T. On the metallurgical and blowing characteristics of LD-OB process. Steelmak. Process 1982, 65, 211–220. [Google Scholar]






| Name | Prototype | Model |
|---|---|---|
| molten bath diameter/mm | 3540 | 753 |
| Converter height/mm | 7140 | 1519 |
| Converter mouth diameter/mm | 2220 | 472 |
| Name | Gas Density /kg∙m−3 | Liquid Density /kg·m−3 | Nozzle Throat Diameter/mm | Nozzle Outlet Diameter/mm | Molten Bath Depth/mm |
|---|---|---|---|---|---|
| Prototype | 1.43 | 7000 | 33.0 | 41.8 | 1131 |
| Model | 1.29 | 1000 | 7.02 | 8.89 | 240 |
| Scheme Code | A1 | A2 | A3 | A4 | A5 |
|---|---|---|---|---|---|
| Top blowing gas flow in prototype/Nm3∙h−1 | 11,000 | 12,000 | 13,000 | 14,000 | 15,000 |
| Top blowing gas flow in the model/Nm3∙h−1 | 93.99 | 102.53 | 111.07 | 119.61 | 128.16 |
| Scheme code | B1 | B2 | B3 | B4 | |
| Bottom gas element arrangement | 0.40D | 0.45D | 0.53D | 0.60D | |
| Scheme code | C1 | C2 | |||
| Bottom blowing gas flow in the prototype/Nm3∙h−1 | 160 | 260 | |||
| Bottom blow gas flow in the model/Nm3∙h−1 | 1.25 | 1.63 | |||
| Scheme code | D1 | D2 | D3 | ||
| Oxygen lance position in prototype/mm | 1200 | 1400 | 1600 | ||
| Oxygen lance position in the model/mm | 255 | 233 | 340 |
| Name | Bottom Blowing Gas Supply Element | Bottom Blowing Gas Flow | Top Blowing Gas Flow and Oxygen Lance Position | Coolant Addition Time |
|---|---|---|---|---|
| Before optimization | The capillary bricks | The bottom blowing flow of the whole process was 160 Nm3/h | The top blowing flow was from 11,000 to 15,000 Nm3/h, and the oxygen lance position was from 1.2 to 1.6 m | Iron balls and pellets were added in two times, every 2 min, and the addition is completed within 4 min. |
| after optimization | The circular seam bottom blowing gas supply element | 0–220 s, bottom blowing flow was 160 Nm3/h; 220–280 s, the bottom blowing flow was 260 Nm3/h | 0–120 s, the top blowing flow was 14,000 Nm3/h, and the oxygen lance position was from 1.4 to 1.5 m; 120–220 s, the top blowing flow was from 12,000 to 13,000 Nm3/h, and the oxygen lance position was 1.55 m; 220–280 s, the top blowing flow was from 12,000 to 13,000 Nm3/h, the gun position was 1.3 m | Iron balls and pellets were added in three times, every 1 min, and the addition was completed within 3.5 min. |
| Item | Value | Before Process Optimization | After Process Optimization |
|---|---|---|---|
| (V2O5) in vanadium slag | average value/wt% | 18.34 | 18.54 |
| variation range/wt% | 15.4–20.18 | 15.7–20.2 | |
| metallic iron in vanadium slag | average value/wt% | 27.63 | 22.39 |
| variation range/wt% | 22.2–35.06 | 17.04–35.28 | |
| [C] in semi-steel | average value/wt% | 3.28 | 3.36 |
| variation range/wt% | 2.45–3.99 | 2.38–4.16 | |
| [V] in semi-steel | average value/wt% | 0.036 | 0.033 |
| variation range/wt% | 0.012–0.087 | 0.014–0.066 | |
| Semi-steel temperature | average value/℃ | 1358 | 1351 |
| variation range/℃ | 1277–1458 | 1261–1449 |
| Stage | Top Blowing Gas Flow/Nm3/h | Oxygen Lance Position/m | Bottom Blowing Gas Flow/Nm3/h | Mixing Time/s | Stirring Energy/W/m3 |
|---|---|---|---|---|---|
| Early stage of blowing (0–120 s) | 14,000 | 1.4–1.5 | 160 | 42 | 39.45–42.15 |
| Middle stage of blowing (120–220 s) | 12,000–13,000 | 1.55 | 260 | 50 | 25.78–32.02 |
| Later stage of blowing (220–280 s) | 13,000–13,500 | 1.3 | 260 | 38 | 37.65–41.83 |
| Name | CaO | SiO2 | MgO | FeO | MnO | V2O5 | M.Fe |
|---|---|---|---|---|---|---|---|
| Before optimization | 4.78 | 12.38 | 1.24 | 31.26 | 8.0 | 18.56 | 17.28 |
| After optimization | 4.57 | 11.78 | 1.44 | 24.31 | 7.8 | 21.66 | 15.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Wu, W.; Zhao, B.; Li, X.; Xiao, F. Influence of Vanadium Extraction Converter Process Optimization on Vanadium Extraction Effect. Metals 2022, 12, 2061. https://doi.org/10.3390/met12122061
Zhao J, Wu W, Zhao B, Li X, Xiao F. Influence of Vanadium Extraction Converter Process Optimization on Vanadium Extraction Effect. Metals. 2022; 12(12):2061. https://doi.org/10.3390/met12122061
Chicago/Turabian StyleZhao, Jinxuan, Wei Wu, Bo Zhao, Xiangchen Li, and Feng Xiao. 2022. "Influence of Vanadium Extraction Converter Process Optimization on Vanadium Extraction Effect" Metals 12, no. 12: 2061. https://doi.org/10.3390/met12122061
APA StyleZhao, J., Wu, W., Zhao, B., Li, X., & Xiao, F. (2022). Influence of Vanadium Extraction Converter Process Optimization on Vanadium Extraction Effect. Metals, 12(12), 2061. https://doi.org/10.3390/met12122061






