Microstructure, Mechanical Properties, and Damping Capacity of AlxCoCrFeNi High-Entropy Alloys Prepared by Spark Plasma Sintering
Abstract
1. Introduction
2. Materials and Methods
2.1. AlxCoCrFeNi High-Entropy Alloy Prepared by SPS
2.2. Microstructural and Mechanical Property Characterization
3. Results and Discussion
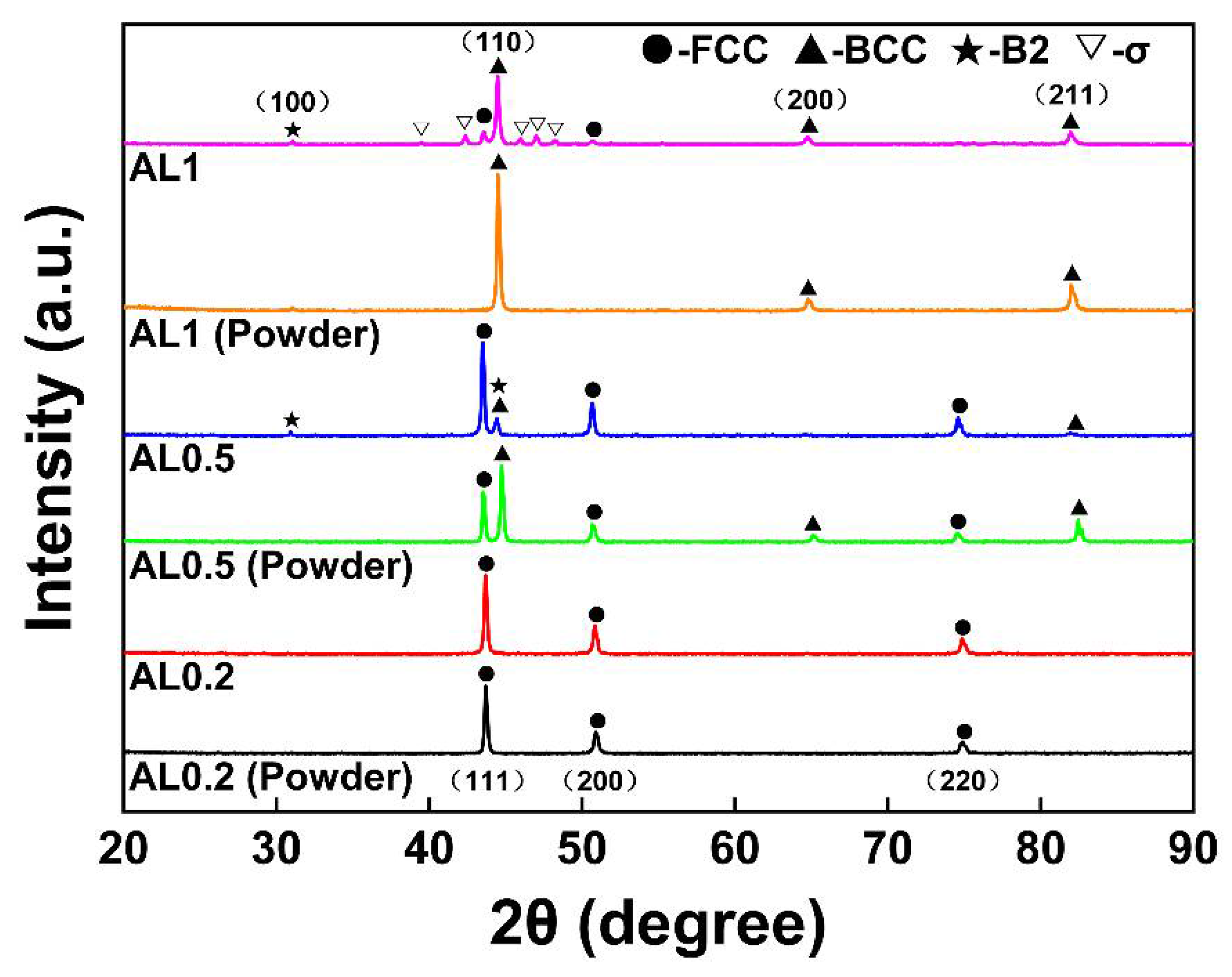
3.1. XRD
3.2. Microstructure
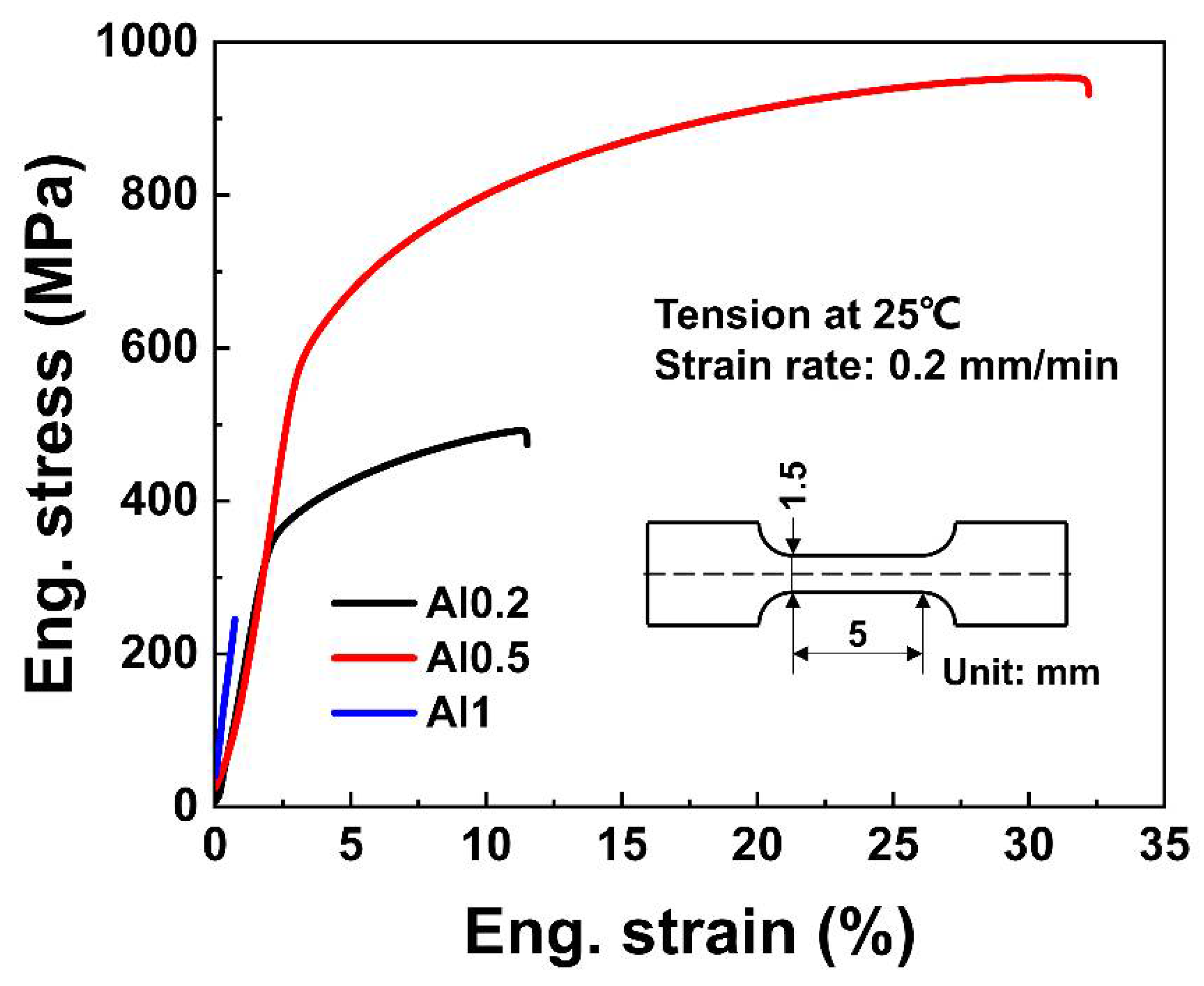
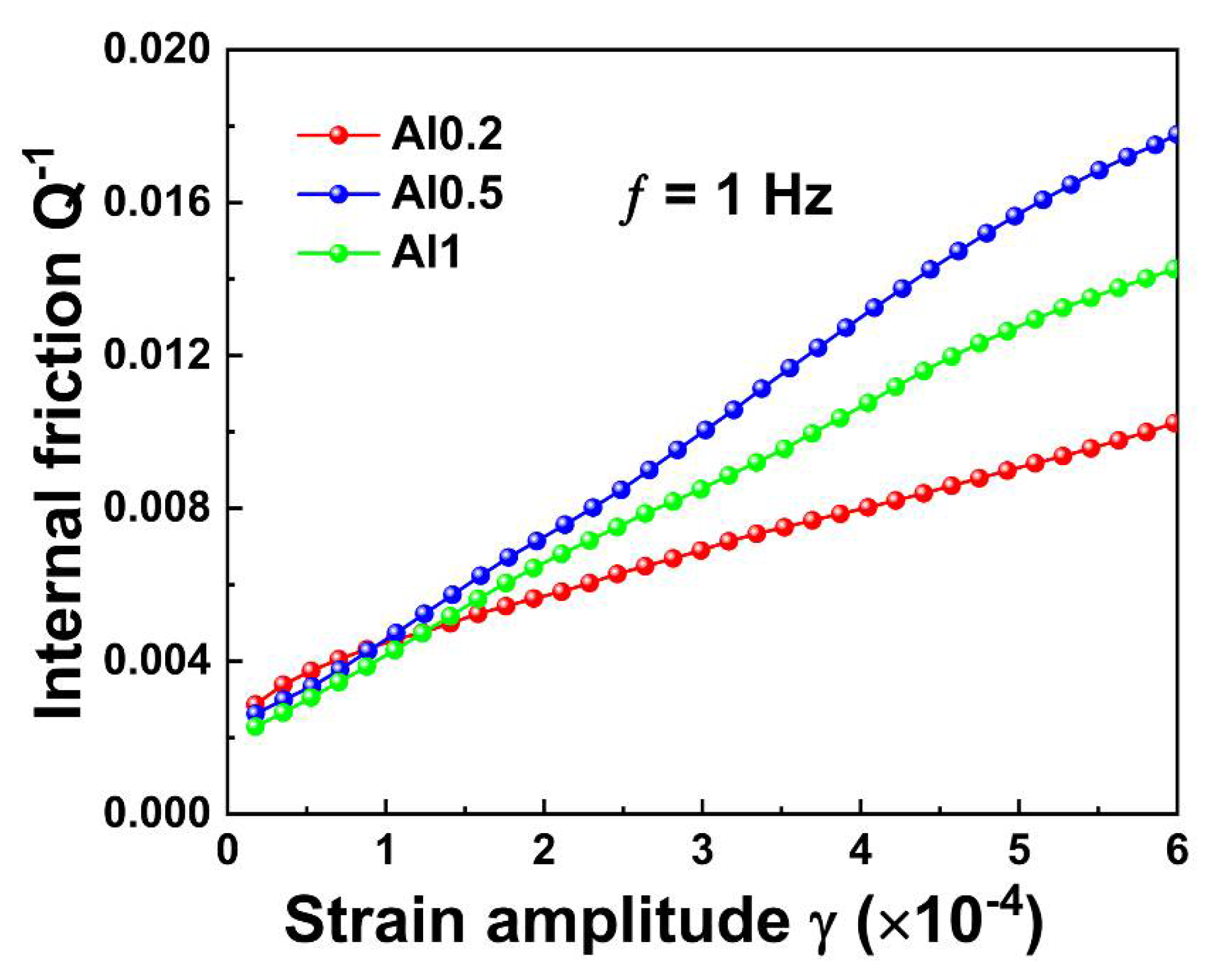
3.3. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shang, C.; Axinte, E.; Ge, W.; Zhang, Z.; Wang, Y. High-entropy alloy coatings with excellent mechanical, corrosion resistance and magnetic properties prepared by mechanical alloying and hot pressing sintering. Surf. Interfaces 2017, 9, 36–43. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Yan, S.; Yu, G.; Chen, J.; He, J.; Yin, F. Microstructure evolution and mechanical properties of atmosphere plasma sprayed AlCoCrFeNi high-entropy alloy coatings under post-annealing. J. Alloys Compd. 2021, 872, 159607. [Google Scholar] [CrossRef]
- Gawel, R.; Rogal, Ł.; Dąbek, J.; Wójcik-Bania, M.; Przybylski, K. High temperature oxidation behaviour of non-equimolar AlCoCrFeNi high entropy alloys. Vacuum 2021, 184, 109969. [Google Scholar] [CrossRef]
- Wang, W.-R.; Wang, W.-L.; Wang, S.-C.; Tsai, Y.-C.; Lai, C.-H.; Yeh, J.-W. Effects of al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Chen, T.-J.; Chen, S.-K.; Yeh, J.-W. Microstructure and mechanical property of as-cast,-homogenized, and-deformed AlxCoCrFeNi (0 ≤ x ≤ 2) high-entropy alloys. J. Alloys Compd. 2009, 488, 57–64. [Google Scholar] [CrossRef]
- Jien-Wei, Y. Recent progress in high entropy alloys. Ann. Chim. Sci. Mat. 2006, 31, 633–648. [Google Scholar]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Senkov, O.; Senkova, S.; Miracle, D.; Woodward, C. Mechanical properties of low-density, refractory multi-principal element alloys of the Cr–Nb–Ti–V–Zr system. Mater. Sci. Eng. A 2013, 565, 51–62. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.; Scott, J.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Butler, T.M.; Weaver, M.L. Investigation of the phase stabilities in AlCoCrFeNi high entropy alloys. J. Alloys Compd. 2017, 691, 119–129. [Google Scholar] [CrossRef]
- Zhou, P.; Xiao, D.; Wu, Z.; Song, M. Microstructure and mechanical properties of AlCoCrFeNi high entropy alloys produced by spark plasma sintering. Mater. Res. Express 2019, 6, 0865e7. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, X.; Ma, T.; Wen, L.; Hu, L.; Hu, M. Effect of al on characterization and properties of AlxCoCrFeNi high entropy alloy prepared via electro-deoxidization of the metal oxides and vacuum hot pressing sintering process. J. Alloys Compd. 2021, 864, 158717. [Google Scholar] [CrossRef]
- Sourav, A.; Yebaji, S.; Thangaraju, S. Structure-property relationships in hot forged AlxCoCrFeNi high entropy alloys. Mater. Sci. Eng. A 2020, 793, 139877. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Zhao, D.; Chen, Y.; Guan, S.; Liu, Y.; Liu, L.; Peng, S.; Kong, F.; Poplawsky, J.D.; et al. Strong yet ductile nanolamellar high-entropy alloys by additive manufacturing. Nature 2022, 608, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-F.; Chen, S.-K.; Chen, T.-J.; Chu, P.-C.; Yeh, J.-W.; Lin, S.-J. Electrical, magnetic, and hall properties of AlxCoCrFeNi high-entropy alloys. J. Alloys Compd. 2011, 509, 1607–1614. [Google Scholar] [CrossRef]
- Cieslak, J.; Tobola, J.; Berent, K.; Marciszko, M. Phase composition of AlxFeNiCrCo high entropy alloys prepared by sintering and arc-melting methods. J. Alloys Compd. 2018, 740, 264–272. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Xiao, H.; Li, L.; Kou, H.; Li, J. A novel strategy for enhancing mechanical performance of Al0.5CoCrFeNi high-entropy alloy via high magnetic field. Mater. Lett. 2019, 240, 250–252. [Google Scholar] [CrossRef]
- Zhao, C.; Li, J.; He, Y.; Wang, J.; Wang, W.Y.; Kou, H.; Wang, J. Effect of strong magnetic field on the microstructure and mechanical-magnetic properties of AlCoCrFeNi high-entropy alloy. J. Alloys Compd. 2020, 820, 153407. [Google Scholar] [CrossRef]
- Uporov, S.; Bykov, V.; Pryanichnikov, S.; Shubin, A.; Uporova, N. Effect of synthesis route on structure and properties of alcocrfeni high-entropy alloy. Intermetallics 2017, 83, 1–8. [Google Scholar] [CrossRef]
- Wang, W.-R.; Wang, W.-L.; Yeh, J.-W. Phases, microstructure and mechanical properties of AlxCoCrFeNi high-entropy alloys at elevated temperatures. J. Alloys Compd. 2014, 589, 143–152. [Google Scholar] [CrossRef]
- Muthupandi, G.; Lim, K.R.; Na, Y.S.; Park, J.; Lee, D.; Kim, H.; Park, S.; Choi, Y.S. Pile-up and sink-in nanoindentation behaviors in AlCoCrFeNi multi-phase high entropy alloy. Mater. Sci. Eng. A 2017, 696, 146–154. [Google Scholar] [CrossRef]
- Li, Y.; Lee, J.; Kang, B.; Hong, S.H. Microstructure and elevated-temperature mechanical properties of refractory AlMo0.5NbTa0.5TiZr high entropy alloy fabricated by powder metallurgy. arXiv 2017, arXiv:1801.00263. [Google Scholar]
- Ji, W.; Wang, W.; Wang, H.; Zhang, J.; Wang, Y.; Zhang, F.; Fu, Z. Alloying behavior and novel properties of CoCrFeNiMn high-entropy alloy fabricated by mechanical alloying and spark plasma sintering. Intermetallics 2015, 56, 24–27. [Google Scholar] [CrossRef]
- Zhang, M.; Li, R.; Yuan, T.; Feng, X.; Xie, S. Effect of low-melting-point sintering aid on densification mechanisms of boron carbide during spark plasma sintering. Scr. Mater. 2019, 163, 34–39. [Google Scholar] [CrossRef]
- Deng, S.; Li, R.; Yuan, T.; Xie, S.; Zhang, M.; Zhou, K.; Cao, P. Direct current-enhanced densification kinetics during spark plasma sintering of tungsten powder. Scr. Mater. 2018, 143, 25–29. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, T.; Li, R.; Xie, S.; Wang, M.; Weng, Q. Densification mechanisms and microstructural evolution during spark plasma sintering of boron carbide powders. Ceram. Int. 2018, 44, 3571–3579. [Google Scholar] [CrossRef]
- Zhou, P.; Xiao, D.; Yuan, T. Microstructure, mechanical and corrosion properties of AlCoCrFeNi high-entropy alloy prepared by spark plasma sintering. Acta Metall. Sin. (Engl. Lett.) 2020, 33, 937–946. [Google Scholar] [CrossRef]
- Xie, S.; Li, R.; Yuan, T.; Zhou, L.; Zhang, M.; Wang, M.; Niu, P.; Cao, P.; Chen, C. Effect of heating rate on microstructure and mechanical properties of AlCoCrFeNi high entropy alloy produced by spark plasma sintering. Mater. Charact. 2019, 154, 169–180. [Google Scholar] [CrossRef]
- Rogal, Ł.; Szklarz, Z.; Bobrowski, P.; Kalita, D.; Garzeł, G.; Tarasek, A.; Kot, M.; Szlezynger, M. Microstructure and mechanical properties of Al–Co–Cr–Fe–Ni base high entropy alloys obtained using powder metallurgy. Met. Mater. Int. 2019, 25, 930–945. [Google Scholar] [CrossRef]
- Hillel, G.; Natovitz, L.; Salhov, S.; Haroush, S.; Pinkas, M.; Meshi, L. Understanding the role of the constituting elements of the AlCoCrFeNi high entropy alloy through the investigation of quaternary alloys. Metals 2020, 10, 1275. [Google Scholar] [CrossRef]
- Shivam, V.; Shadangi, Y.; Basu, J.; Mukhopadhyay, N.K. Evolution of phases, hardness and magnetic properties of alcocrfeni high entropy alloy processed by mechanical alloying. J. Alloys Compd. 2020, 832, 154826. [Google Scholar] [CrossRef]
- Mohanty, S.; Maity, T.N.; Mukhopadhyay, S.; Sarkar, S.; Gurao, N.P. Powder metallurgical processing of equiatomic AlCoCrFeNi high entropy alloy: Microstructure and mechanical properties. Mater. Sci. Eng. A 2017, 679, 299–313. [Google Scholar] [CrossRef]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-entropy alloys by mechanical alloying: A review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Kunce, I.; Polanski, M.; Karczewski, K.; Plocinski, T.; Kurzydlowski, K.J. Microstructural characterisation of high-entropy alloy AlCoCrFeNi fabricated by laser engineered net shaping. J. Alloys Compd. 2015, 648, 751–758. [Google Scholar] [CrossRef]
- Xie, S.; Li, R.; Yuan, T.; Zhang, M.; Cao, P. Effect of phase transformation on densification kinetics and properties of spark plasma sintered Al0.7CoCrFeNi high-entropy alloy. Mater. Charact. 2019, 160, 110098. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Zhao, M.; Jiang, Q. Effect of aluminum contents on microstructure and properties of AlxCoCrFeNi alloys. J. Alloys Compd. 2010, 504, S515–S518. [Google Scholar] [CrossRef]
- Yang, T.F.; Xia, S.Q.; Liu, S.; Wang, C.X.; Liu, S.S.; Zhang, Y.; Xue, J.M.; Yan, S.; Wang, Y.G. Effects of al addition on microstructure and mechanical properties of AlxCoCrFeNi high-entropy alloy. Mater. Sci. Eng. A 2015, 648, 15–22. [Google Scholar] [CrossRef]
- Gangireddy, S.; Gwalani, B.; Soni, V.; Banerjee, R.; Mishra, R.S. Contrasting mechanical behavior in precipitation hardenable AlxCoCrFeNi high entropy alloy microstructures: Single phase FCC vs. Dual phase FCC-BCC. Mater. Sci. Eng. A 2019, 739, 158–166. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Annasamy, M.; Cizek, P.; Kamaraj, M.; Muralikrishna, G.M.; Hodgson, P.; Fabijanic, D.; Murty, B. Evolution of phase constitution with mechanical alloying and spark plasma sintering of nanocrystalline AlxCoCrFeNi (x = 0, 0.3, 0.6, 1 mol) high-entropy alloys. J. Mater. Res. 2022, 37, 959–975. [Google Scholar] [CrossRef]
- Liu, Y.X.; Cheng, C.Q.; Shang, J.L.; Rui, W.; Peng, L.; Jie, Z. Oxidation behavior of high-entropy alloys AlxCoCrFeNi (x = 0.15, 0.4) in supercritical water and comparison with HR3C steel. Trans. Nonferr. Met. Soc. China 2015, 25, 1341–1351. [Google Scholar] [CrossRef]
- Niu, S.-Z.; Kou, H.-C.; Wang, J.; Li, J.-S. Improved tensile properties of Al0.5CoCrFeNi high-entropy alloy by tailoring microstructures. Rare Met. 2021, 40, 1–6. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Gao, P.; Xiang, Q.; Qu, Y.; Cheng, J.; Ren, Y.; Yu, B.; Qiu, K. AlxCrFeNi medium entropy alloys with high damping capacity. J. Alloys Compd. 2021, 876, 159991. [Google Scholar] [CrossRef]
- Shi, Y.; Collins, L.; Feng, R.; Zhang, C.; Balke, N.; Liaw, P.K.; Yang, B. Homogenization of AlxCoCrFeNi high-entropy alloys with improved corrosion resistance. Corros. Sci. 2018, 133, 120–131. [Google Scholar] [CrossRef]
- Joseph, J.; Hodgson, P.; Jarvis, T.; Wu, X.; Stanford, N.; Fabijanic, D.M. Effect of hot isostatic pressing on the microstructure and mechanical properties of additive manufactured AlxCoCrFeNi high entropy alloys. Mater. Sci. Eng. A 2018, 733, 59–70. [Google Scholar] [CrossRef]
- Rao, J.; Diao, H.; Ocelík, V.; Vainchtein, D.; Zhang, C.; Kuo, C.; Tang, Z.; Guo, W.; Poplawsky, J.; Zhou, Y. Secondary phases in AlxCoCrFeNi high-entropy alloys: An in-situ tem heating study and thermodynamic appraisal. Acta Mater. 2017, 131, 206–220. [Google Scholar] [CrossRef]
- Aizenshtein, M.; Strumza, E.; Brosh, E.; Hayun, S. Microstructure, kinetics and thermodynamics of hea Al0.5CoCrFeNi at t ≥ 800 °C. Mater. Charact. 2021, 171, 110738. [Google Scholar] [CrossRef]
- Aizenshtein, M.; Priel, E.; Hayun, S. Effect of pre-deformation and b2 morphology on the mechanical properties of Al0.5CoCrFeNi HEA. Mater. Sci. Eng. A 2020, 788, 139575. [Google Scholar] [CrossRef]
- Meshi, L.; Linden, Y.; Munitz, A.; Salhov, S.; Pinkas, M. Retardation of the σ phase formation in the alcocrfeni multi-component alloy. Mater. Charact. 2019, 148, 171–177. [Google Scholar] [CrossRef]
- Lin, C.M.; Tsai, H.L. Evolution of microstructure, hardness, and corrosion properties of high-entropy Al0.5CoCrFeNi alloy. Intermetallics 2011, 19, 288–294. [Google Scholar] [CrossRef]
- Ghaderi, A.; Moghanni, H.; Dehghani, K. Microstructural evolution and mechanical properties of Al0.5CoCrFeNi high-entropy alloy after cold rolling and annealing treatments. J. Mater. Eng. Perform. 2021, 30, 7817–7825. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, M.C.; Diao, H.; Yang, T.; Liu, J.; Zuo, T.; Zhang, Y.; Lu, Z.; Cheng, Y.; Zhang, Y. Aluminum alloying effects on lattice types, microstructures, and mechanical behavior of high-entropy alloys systems. JOM 2013, 65, 1848–1858. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Munitz, A.; Salhov, S.; Hayun, S.; Frage, N. Heat treatment impacts the micro-structure and mechanical properties of alcocrfeni high entropy alloy. J. Alloys Compd. 2016, 683, 221–230. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z. Microstructure and properties of AlxCoCrFeNi high-entropy alloys prepared by plasma cladding. Int. Mater. Rev. 2018, 32, 589–592. [Google Scholar]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Xu, D.; Chen, K.; Chen, Y.; Chen, S. Evolution of the second-phase particles and their effect on tensile fracture behavior of 2219 Al-xCu alloys. Metals 2020, 10, 197. [Google Scholar] [CrossRef]
- Wang, H.; Wang, F.; Liu, H.; Pan, D.; Pan, Q.; Liu, Y.; Xiao, J.; Zhang, P. Influence of alloy elements (Mo, Nb, Ti) on the strength and damping capacity of Fe-Cr based alloy. Mater. Sci. Eng. A 2016, 667, 326–331. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, X.-P.; Tang, Y.; You, W.-X.; Xu, Y.-G. Microstructure and properties of mn-cu-based damping alloys prepared by ball milling and hot-press sintering. J. Mater. Eng. Perform. 2019, 28, 2641–2648. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, F.; Wu, b.; Xu, R.; Zhang, J.; Zhao, D.; Luo, F. Effect of sintering temperature on properties of M2052 damping alloy prepared by powder metallurgy. Funct. Mater. 2016, 47, 9211–9215. [Google Scholar]
- Xu, X.-Q.; Xu, Y.-G. Higher damping capacity induced by negative magnetostriction of Fe-16Cr-2.5Mo-0∼0.2V alloys. J. Alloys Compd. 2021, 852, 156673. [Google Scholar] [CrossRef]
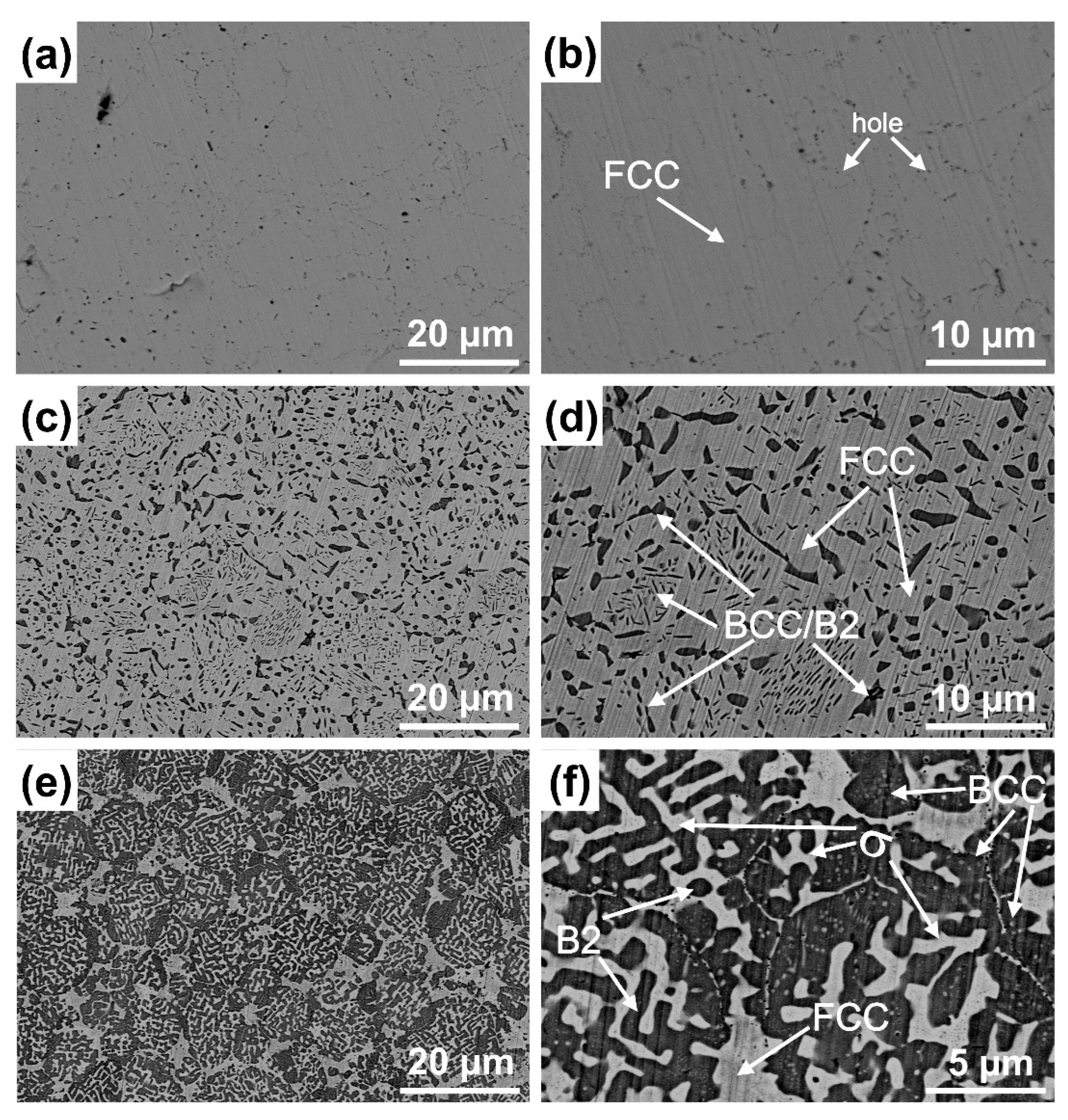



| AlxCoCrFeNi | Regions | Phase | Chemical Composition (at. %) | ||||
|---|---|---|---|---|---|---|---|
| Al | Co | Cr | Fe | Ni | |||
| Al0.2 | White matrix | FCC | 4.08 | 24.06 | 24.27 | 24.06 | 23.53 |
| Al0.5 | White matrix | FCC | 7.83 | 22.80 | 25.13 | 24.04 | 20.20 |
| Irregular bulk | B2/BCC | 25.71 | 17.42 | 13.12 | 14.68 | 29.07 | |
| Needle-like | B2/BCC | 25.15 | 17.83 | 11.85 | 14.32 | 30.85 | |
| Al1 | Gray | FCC | 6.29 | 25.45 | 23.56 | 26.56 | 18.14 |
| Precipitates | BCC | 21.48 | 19.84 | 18.26 | 18.75 | 21.67 | |
| Black | B2 | 27.86 | 12.06 | 15.74 | 18.65 | 25.69 | |
| Bright-white | σ | 8.19 | 18.17 | 37.63 | 28.34 | 7.67 | |
| AlxCoCrFeNi | YS (MPa) | UTS (MPa) | Elongation (%) | Hardness (HV) | Compactness (%) |
|---|---|---|---|---|---|
| Al0.2 | 347.5 ± 3 | 491.9 ± 5 | 11.5 ± 0.3 | 168.2 ± 4 | 92.2 ± 0.6 |
| Al0.5 | 557.7 ± 5 | 954.4 ± 9 | 32.2 ± 0.5 | 280.8 ± 5 | 99.2 ± 0.2 |
| Al1 | - | 245.3 ± 3 | 0.7 ± 0.1 | 585.4 ± 9 | 98.8 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, K.; Huang, L.; Wang, X.; Yu, L.; Feng, W. Microstructure, Mechanical Properties, and Damping Capacity of AlxCoCrFeNi High-Entropy Alloys Prepared by Spark Plasma Sintering. Metals 2022, 12, 2058. https://doi.org/10.3390/met12122058
Xiong K, Huang L, Wang X, Yu L, Feng W. Microstructure, Mechanical Properties, and Damping Capacity of AlxCoCrFeNi High-Entropy Alloys Prepared by Spark Plasma Sintering. Metals. 2022; 12(12):2058. https://doi.org/10.3390/met12122058
Chicago/Turabian StyleXiong, Ke, Lin Huang, Xiaofeng Wang, Lin Yu, and Wei Feng. 2022. "Microstructure, Mechanical Properties, and Damping Capacity of AlxCoCrFeNi High-Entropy Alloys Prepared by Spark Plasma Sintering" Metals 12, no. 12: 2058. https://doi.org/10.3390/met12122058
APA StyleXiong, K., Huang, L., Wang, X., Yu, L., & Feng, W. (2022). Microstructure, Mechanical Properties, and Damping Capacity of AlxCoCrFeNi High-Entropy Alloys Prepared by Spark Plasma Sintering. Metals, 12(12), 2058. https://doi.org/10.3390/met12122058




