Fabrication and Characterization of Aluminum-Graphene Nano-Platelets—Nano-Sized Al4C3 Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- -
- Aluminum powder with a chemical purity of 99.5%. The average particle size was measured by us to be 37 µm.
- -
- Graphene nano-platelets produced by io-li-tech Company with thickness 6–8 nm and purity 99.5%.
2.2. Experimental Procedure
2.3. Research Methodology
3. Results
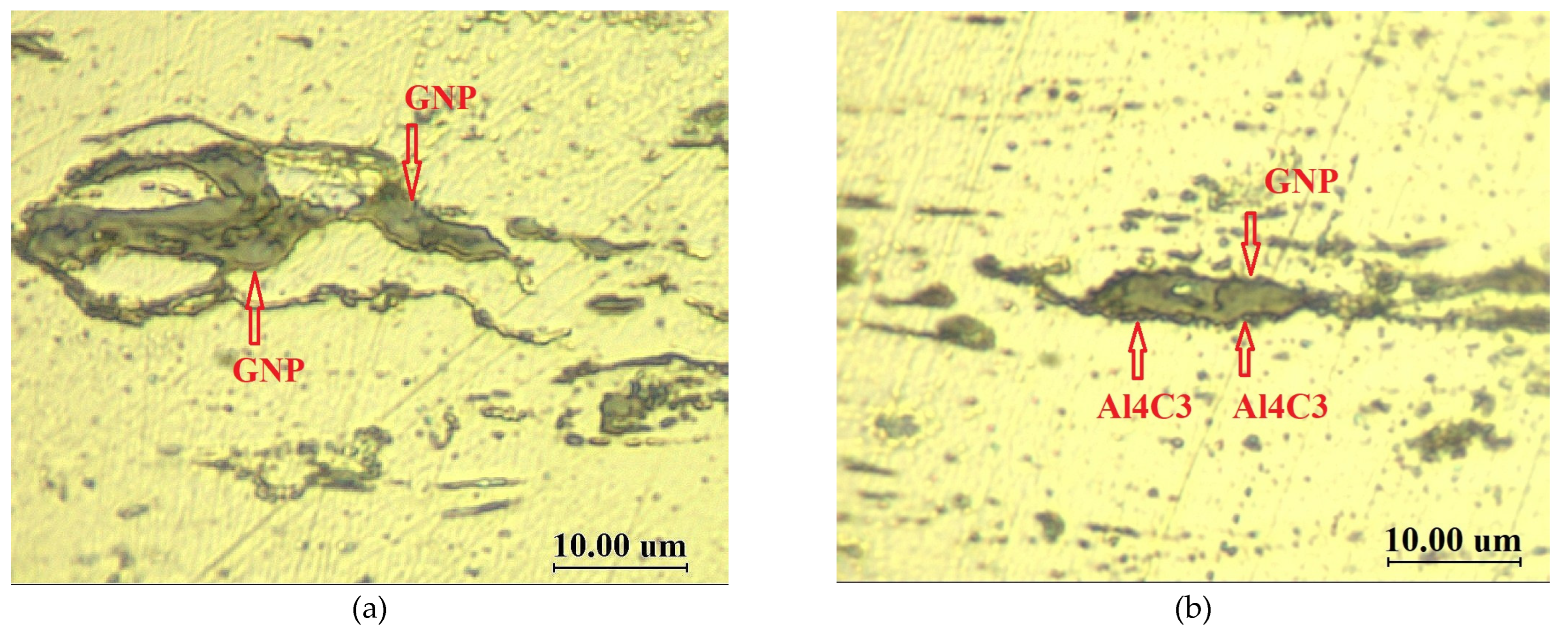
3.1. Light Microscopy
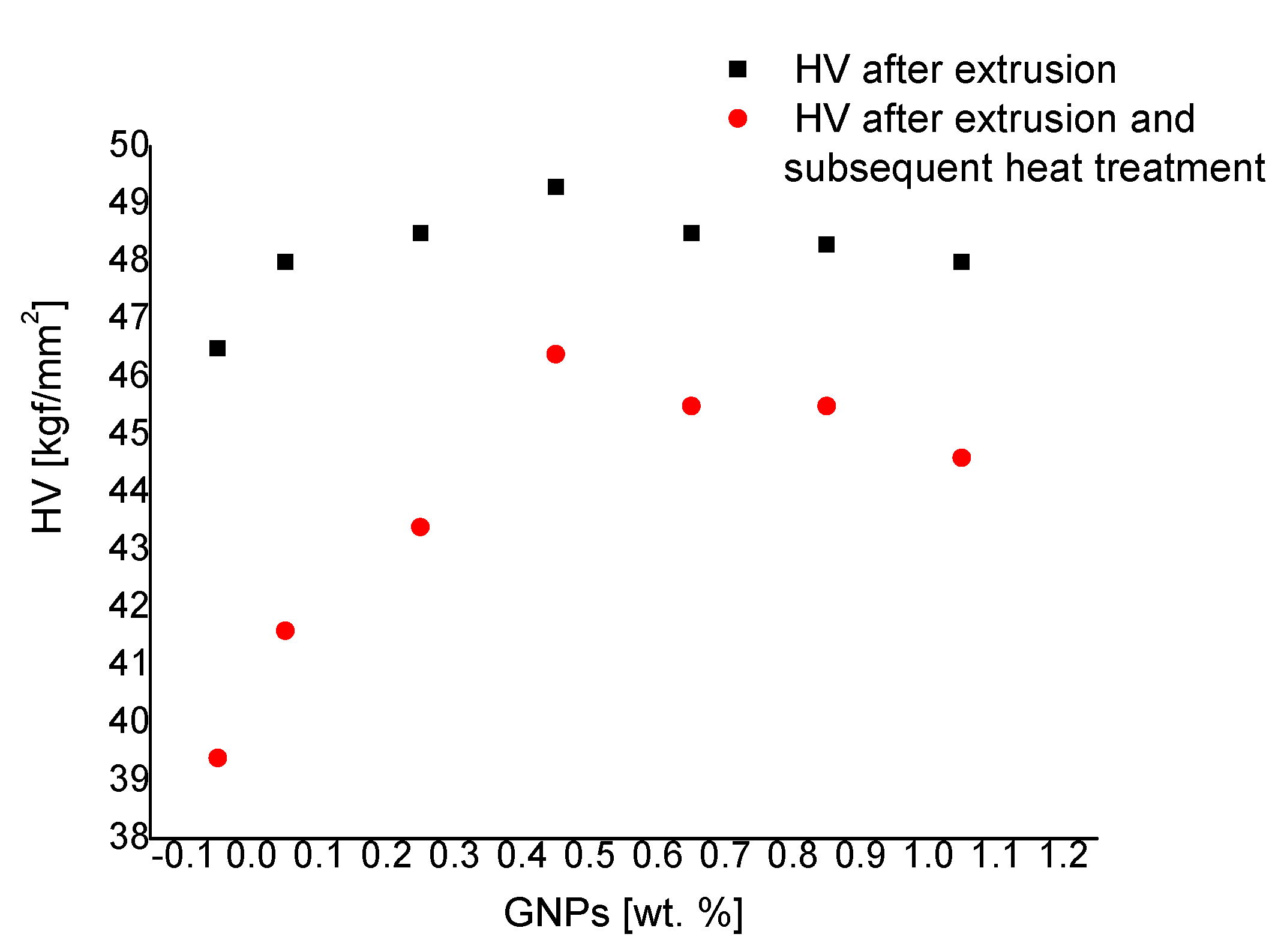
3.2. Microhardness
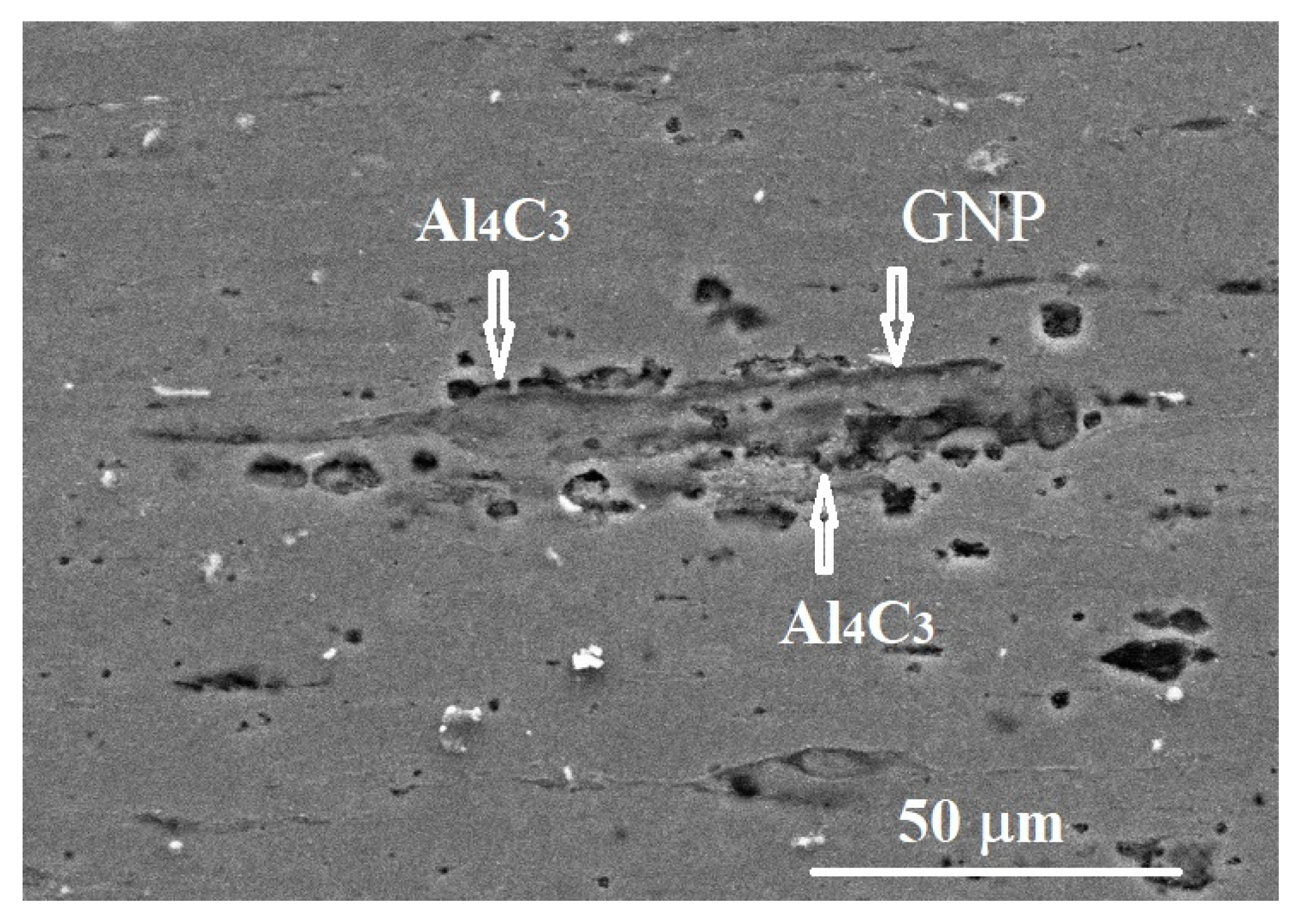
3.3. SEM-EDS
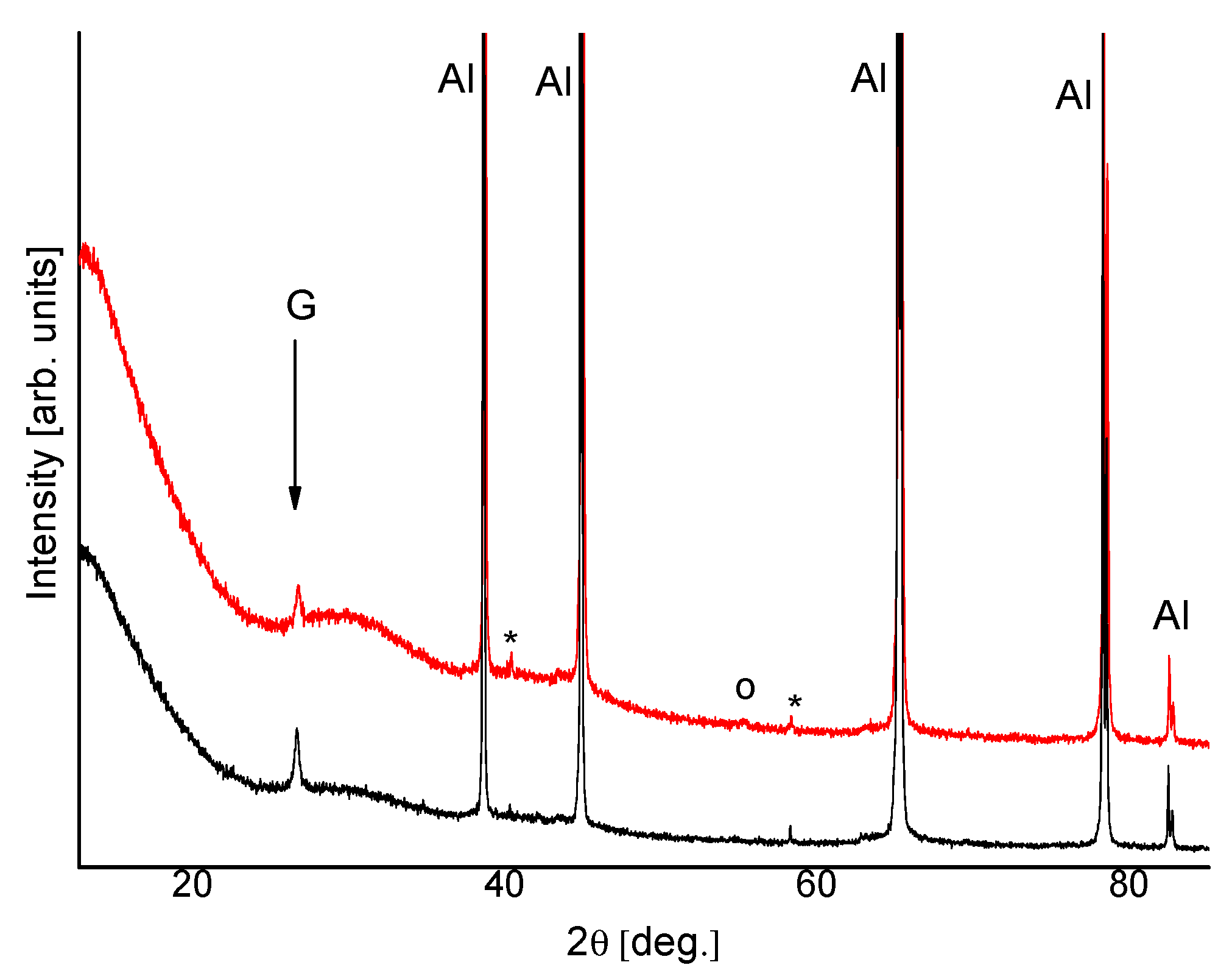
3.4. XRD
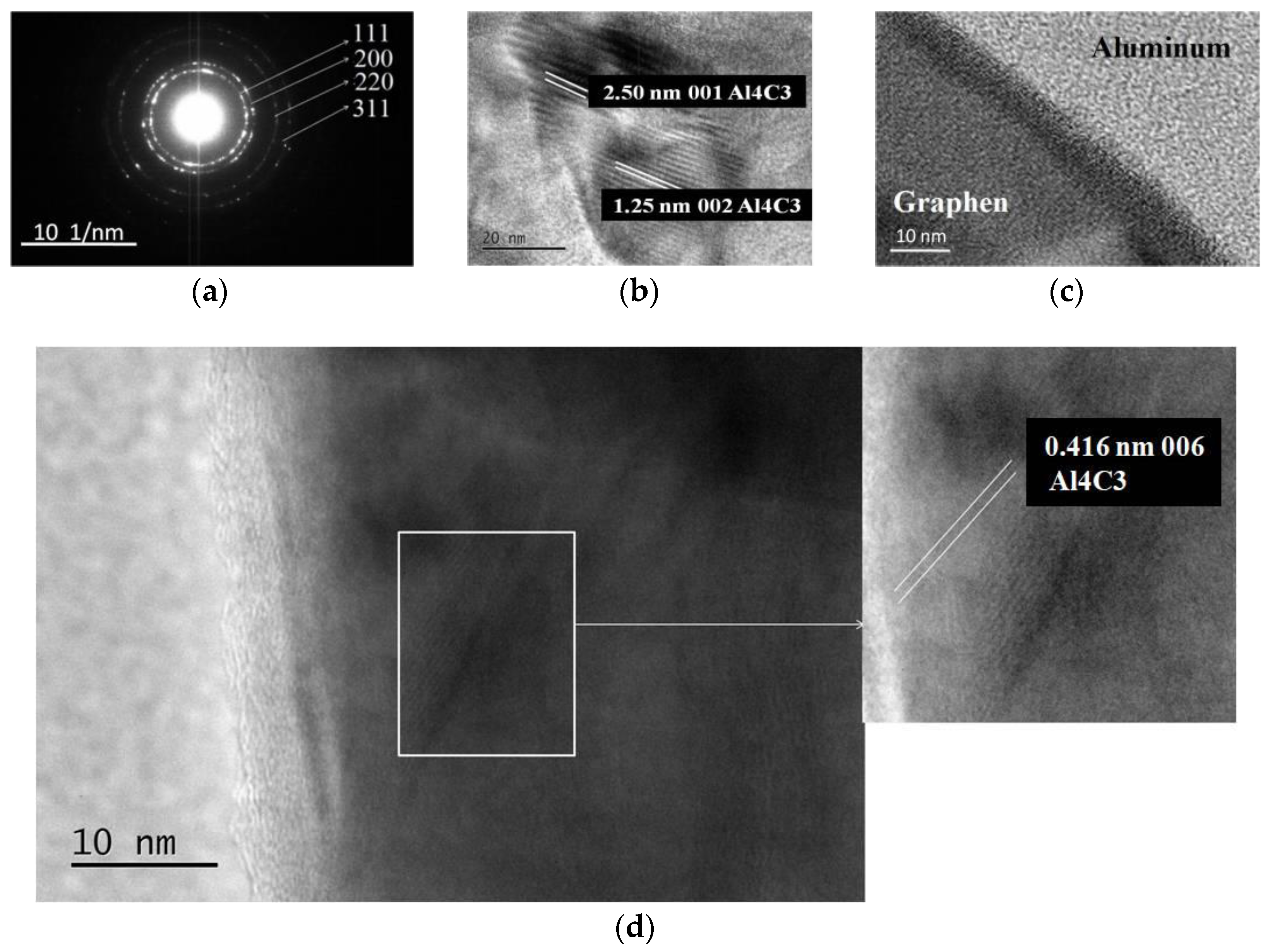
3.5. TEM and HRTEM
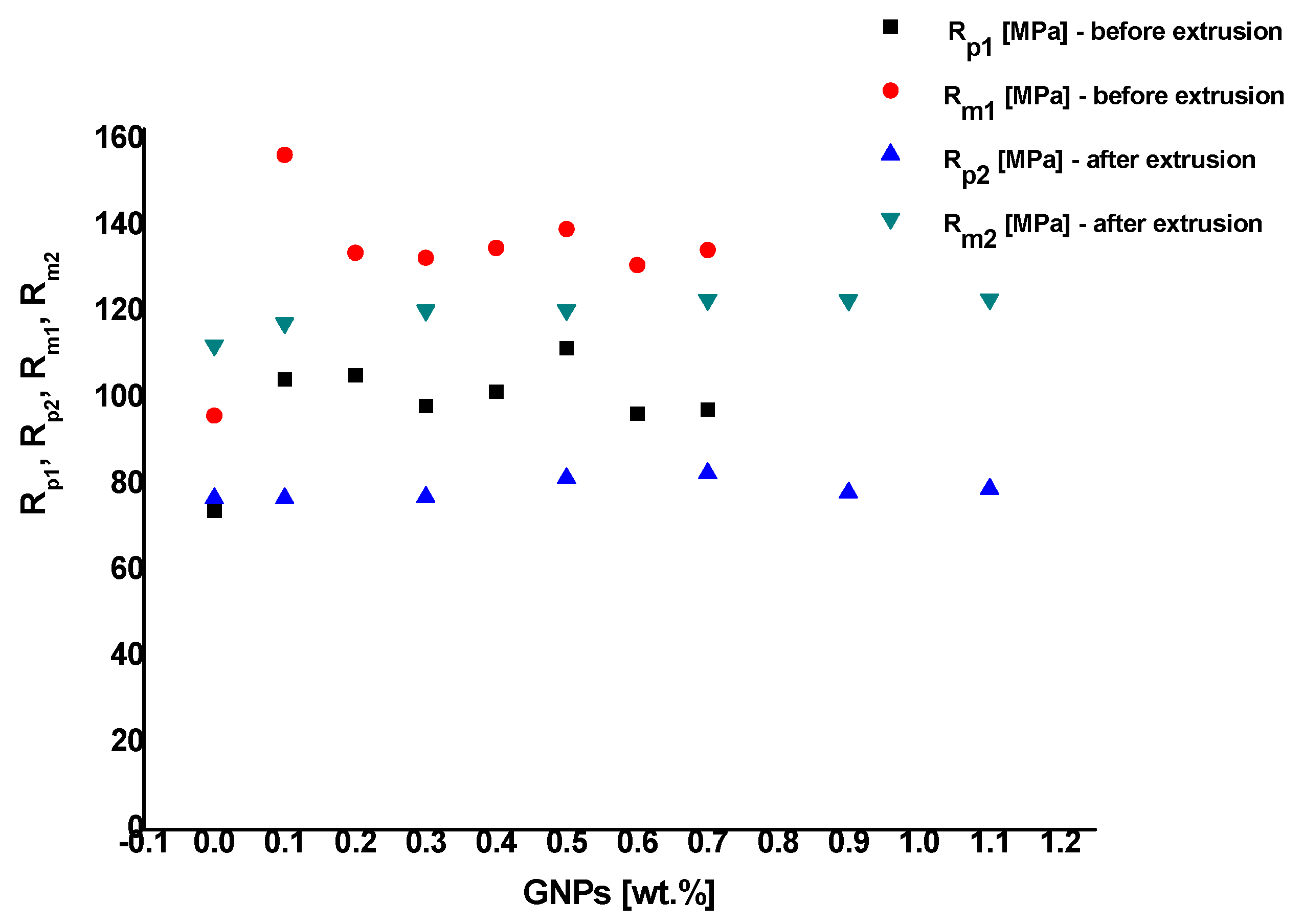
3.6. Tensile Testing
4. Discussion
5. Conclusions
- (1)
- Aluminum-based composites with graphene nano-platelets from 0.1 to 1.1 wt.% and nano-sized carbides at the aluminum-graphene interface can be successfully produced by powder metallurgy via extrusion and subsequent annealing at 610 °C and holding time of 3 h.
- (2)
- The presence of nano-sized carbides type Al4C3 can be most conclusively proved, and their dimensions determined by HRTEM. They are around 20 nm.
- (3)
- The microhardness and the strength properties of extruded samples, whose microstructure constituents are aluminum matrix and GNPs, are the highest. The microhardness and the strength properties of extruded and annealed samples, whose microstructure constituents are aluminum matrix, GNPs, and nano-sized carbides, are lower. This is due to the greater strength-reducing of the material, which occurs as a result of annealing, than the strengthening, which is due to the presence of graphene nano-platelets and nano-sized carbides. The microhardness and the strength properties of pure aluminum in the respective state are the lowest.
- (4)
- GNPs and A4C3 fracture and semi-pulled out or semi-slipped Al4C3 from the matrix are observed as a result of the chemical bond existing between GNPs and aluminum matrix.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Güler, Ö.; Bağcı, N. A short review on mechanical properties of graphene reinforced metal matrix composites. J. Mater. Res. Technol. 2020, 9, 6808–6833. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Wang, Z.; Song, H.; Yu, Z.-Z.; Koratkar, N. Enhanced Mechanical Properties of Nanocomposites at Low Graphene Content. ACS Nano 2009, 3, 3884–3890. [Google Scholar] [CrossRef] [PubMed]
- Şenel, M.C.; Gürbüz, M.; Koç, E. Effect of Graphene Content on Tensile Strength and Microstructure of Aluminum Matrix Composites. Univers. J. Mater. Sci. 2018, 6, 79–84. [Google Scholar] [CrossRef]
- Şenel, M.C.; Gürbüz, M.; Koç, E. An Investigation into the Mechanical Properties and Microstructures of Graphene Reinforced Aluminum Composites. Intl. J. Res. Chem. Metall. Civ. Engg. 2018, 5, 5–8. [Google Scholar] [CrossRef]
- Li, J.L.; Xiong, Y.C.; Wang, X.D.; Yan, S.J.; Yang, C.; He, W.W.; Chen, J.Z.; Wang, S.Q.; Zhang, X.Y.; Dai, S.L. Microstructure and tensile properties of bulk nanostructured aluminum/graphene composites prepared via cryomilling. Mater Sci. Eng. A 2015, 626, 400–405. [Google Scholar] [CrossRef]
- Gao, X.; Yue, H.; Guo, E.; Zhang, H.; Lin, X.; Yao, L.; Wang, B. Preparation and tensile properties of homogeneously dispersed graphene reinforced aluminum matrix composites. Mater. Des. 2016, 94, 54–60. [Google Scholar] [CrossRef]
- Yan, S.J.; Yang, C.; Hong, Q.H.; Chen, J.Z.; Liu, D.B.; Dai, S. Research of graphene-reinforced aluminum matrix nanocomposites. J. Mater. Eng. 2014, 1, 1–6. [Google Scholar]
- Wang, J.; Guo, L.-N.; Lin, W.-M.; Chen, J.; Liu, C.-L.; Chen, S.-D.; Zhang, S.; Zhen, T.-T. Effect of the graphene content on the microstructures and properties of graphene/aluminum composites. New Carbon Mater. 2019, 34, 275–285. [Google Scholar] [CrossRef]
- Du, X.; Zheng, K.; Liu, F. Microstructure and mechanical properties of graphene-reinforced aluminum-matrix composites. Mater. Teh. 2018, 52, 763–768. [Google Scholar] [CrossRef]
- Du, X.M.; Chen, R.Q.; Liu, F.G. Investigation of Graphene Nanosheets Reinforced Aluminum Matrix Composites. Dig. J. Nanomater. Biostructures 2017, 12, 37–45. [Google Scholar]
- Saboori, A.; Dadkhah, M.; Fino, P.; Pavese, M. An Overview of Metal Matrix Nanocomposites Reinforced with Graphene Nanoplatelets; Mechanical, Electrical and Thermophysical Properties. Metals 2018, 8, 423. [Google Scholar] [CrossRef]
- Bartolucci, S.F.; Paras, J.; Rafiee, M.A.; Rafiee, J.; Lee, S.; Kapoor, D.; Koratkar, N. Graphene–aluminum nanocomposites. Mater. Sci. Eng. A 2011, 528, 7933–7937. [Google Scholar] [CrossRef]
- Li, G.; Xiong, B. Effects of graphene content on microstructures and tensile property of graphene-nanosheets/aluminum composites. J. Alloys Compd. 2017, 697, 31–36. [Google Scholar] [CrossRef]
- Xiong, B.; Liu, K.; Xiong, W.; Wu, X.; Sun, J. Strengthening effect induced by interfacial reaction in graphene nano-platelets reinforced aluminum matrix composites. J. Alloys Compd. 2020, 845, 156282. [Google Scholar] [CrossRef]
- Zhou, W.; Mikulova, P.; Fan, Y.; Kikuchi, K.; Nomura, N.; Kawasaki, A. Interfacial reaction induced efficient load transfer in few-layer graphene reinforced Al matrix composites for high-performance conductor. Compos. Part B Eng. 2019, 167, 93–99. [Google Scholar] [CrossRef]
- Saboori, A.; Pavese, M.; Badini, C.F.; Fino, P. Microstructure and Thermal Conductivity of Al–Graphene Composites Fabricated by Powder Metallurgy and Hot Rolling Techniques. Acta Met. Sin. 2017, 30, 675–687. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Geng, L. Effect of heat treatment on interfacial bonding and strengthening efficiency of graphene in GNP/Al composites. Compos. Part A Appl. Sci. Manuf. 2019, 121, 487–498. [Google Scholar] [CrossRef]
- Chen, W.; Yang, T.; Dong, L.; Elmasry, A.; Song, J.; Deng, N.; Elmarakbi, A.; Liu, T.; Lv, H.B.; Fu, Y.Q. Advances in graphene reinforced metal matrix nanocomposites: Mechanisms, processing, modelling, properties and applications. Nanotechnol. Precis. Eng. 2020, 3, 189–210. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Zhao, J.; Jiang, Z. Microstructure and mechanical properties of aluminium-graphene composite powders produced by mechanical milling. Mech. Adv. Mater. Mod. Process. 2018, 4, 4. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Tang, A.; Asif, M. Effect of Graphene Nano-platelets addition on mechanical properties of pure aluminum using a semi-powder method. Prog. Nat. Sci. Mater. Int. 2014, 24, 101–108. [Google Scholar] [CrossRef]
- Khan, M.; Din, R.U.; Basit, M.A.; Wadood, A.; Husain, S.W.; Akhtar, S.; Aune, R.E. Study of microstructure and mechanical behaviour of aluminium alloy hybrid composite with boron carbide and graphene nano-platelets. Mater. Chem. Phys. 2021, 271, 124936. [Google Scholar] [CrossRef]
- Li, M.; Gao, H.; Liang, J.; Gu, S.; You, W.; Shu, D.; Wang, J.; Sun, B. Microstructure evolution and properties of graphene nano-platelets reinforced aluminum matrix composites. Mater. Charact. 2018, 140, 172–178. [Google Scholar] [CrossRef]
- Lazarova, R.; Mourjeva, Y.; Petkov, V.; Anestiev, L.; Marinov, M.; Dimitrova, R.; Shuleva, D. Microstructure and Mechanical Properties of Aluminum: Graphene Composites Produced by Powder Metallurgical Method. J. Mater. Eng. Perform. 2022, 31, 1–9. [Google Scholar] [CrossRef]
- Knych, T.; Mamala, A.; Kwaśniewski, P.; Kiesiewicz, G.; Smyrak, B.; Gniełczyk, M.; Kawecki, A.; Korzeń, K.; Sieja-Smaga, E. New graphene composites for power engineering. Materials 2022, 15, 715. [Google Scholar] [CrossRef]
- Gilman, P.S.; Benjamin, J.S. Mechanical alloying. Ann. Rev. Mater. Sci. 1983, 13, 279–300. [Google Scholar] [CrossRef]
- Guo, B.; Chen, B.; Zhang, X.; Cen, X.; Wang, X.; Song, M.; Ni, S.; Yi, J.; Shen, T.; Du, Y. Exploring the size effects of Al4C3 on the mechanical properties and thermal behaviors of Al-based composites reinforced by SiC and carbon nanotubes. Carbon 2018, 135, 224–235. [Google Scholar] [CrossRef]
- Liu, X.; Liu, E.; Li, J.; He, C.; Zhao, N. Investigation of the evolution and strengthening effect of aluminum carbide for in-situ preparation of carbon nanosheets/aluminum composites. Mater. Sci. Eng. A 2019, 764, 138139. [Google Scholar] [CrossRef]
- Xu, W.; Chenchong, W.; Zhichao, Z.; Ping, L.; Yanhua, S.; Guofu, Z. Interfacial microstructure and growth mechanism of Al4C3 in Grf/Alcomposites fabricated by liquid pressure method. Micron 2014, 65, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yang, W.; Zhou, C.; Zhang, N.; Chao, Z.; Liu, H.; Cao, Y.; Sun, Y.; Shao, P.; Wu, G. Effect of ball milling time on graphene nanosheets reinforced Al6063 composite fabricated by pressure infiltration method. Carbon 2019, 141, 25–39. [Google Scholar] [CrossRef]
- David, B.; Williams, C. Barry Carter, Transmission Electron Microscopy, A Textbook for Materials Science; Part 1 Basics, Part 2 Diffraction, Part 3 Imaging, Part 4 Spectrometry; Springer Science + Business Media, LLC: Berlin/Heidelberg, Germany, 2009; 757p. [Google Scholar]
- Fultz, B.; Howe, J. Transmission Electron Microscopy and Diffractometry of Materials, 3rd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2008; 745p. [Google Scholar]
- Mihaylov, L.; Inoue, A.; Lyubenova, L.; Nihtianova, D.; Spassov, T. Nanoporous metallic structures by de-alloying bulk glass forming Zr-based alloys. Intermetallics 2018, 98, 148–153. [Google Scholar] [CrossRef]
- Mihaylov, L.; Lyubenova, L.; Gerdjikov, T.; Nihtianova, D.; Spassov, T. Selective dissolution of amorphous Zr–Cu–Ni–Al alloys. Corros. Sci. 2015, 94, 350–358. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarova, R.; Mourdjeva, Y.; Nihtianova, D.; Stefanov, G.; Petkov, V. Fabrication and Characterization of Aluminum-Graphene Nano-Platelets—Nano-Sized Al4C3 Composite. Metals 2022, 12, 2057. https://doi.org/10.3390/met12122057
Lazarova R, Mourdjeva Y, Nihtianova D, Stefanov G, Petkov V. Fabrication and Characterization of Aluminum-Graphene Nano-Platelets—Nano-Sized Al4C3 Composite. Metals. 2022; 12(12):2057. https://doi.org/10.3390/met12122057
Chicago/Turabian StyleLazarova, Rumyana, Yana Mourdjeva, Diana Nihtianova, Georgi Stefanov, and Veselin Petkov. 2022. "Fabrication and Characterization of Aluminum-Graphene Nano-Platelets—Nano-Sized Al4C3 Composite" Metals 12, no. 12: 2057. https://doi.org/10.3390/met12122057
APA StyleLazarova, R., Mourdjeva, Y., Nihtianova, D., Stefanov, G., & Petkov, V. (2022). Fabrication and Characterization of Aluminum-Graphene Nano-Platelets—Nano-Sized Al4C3 Composite. Metals, 12(12), 2057. https://doi.org/10.3390/met12122057






