Preparation of Spiral Nitrogen-Doped Macroscopic Graphene Tube and Tuning the Activity of Oxygen Catalysis by Twisted Ferrum (Fe) Wires
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Preparation of Catalysts
2.3. Characterization
2.4. Electrochemical Measurements
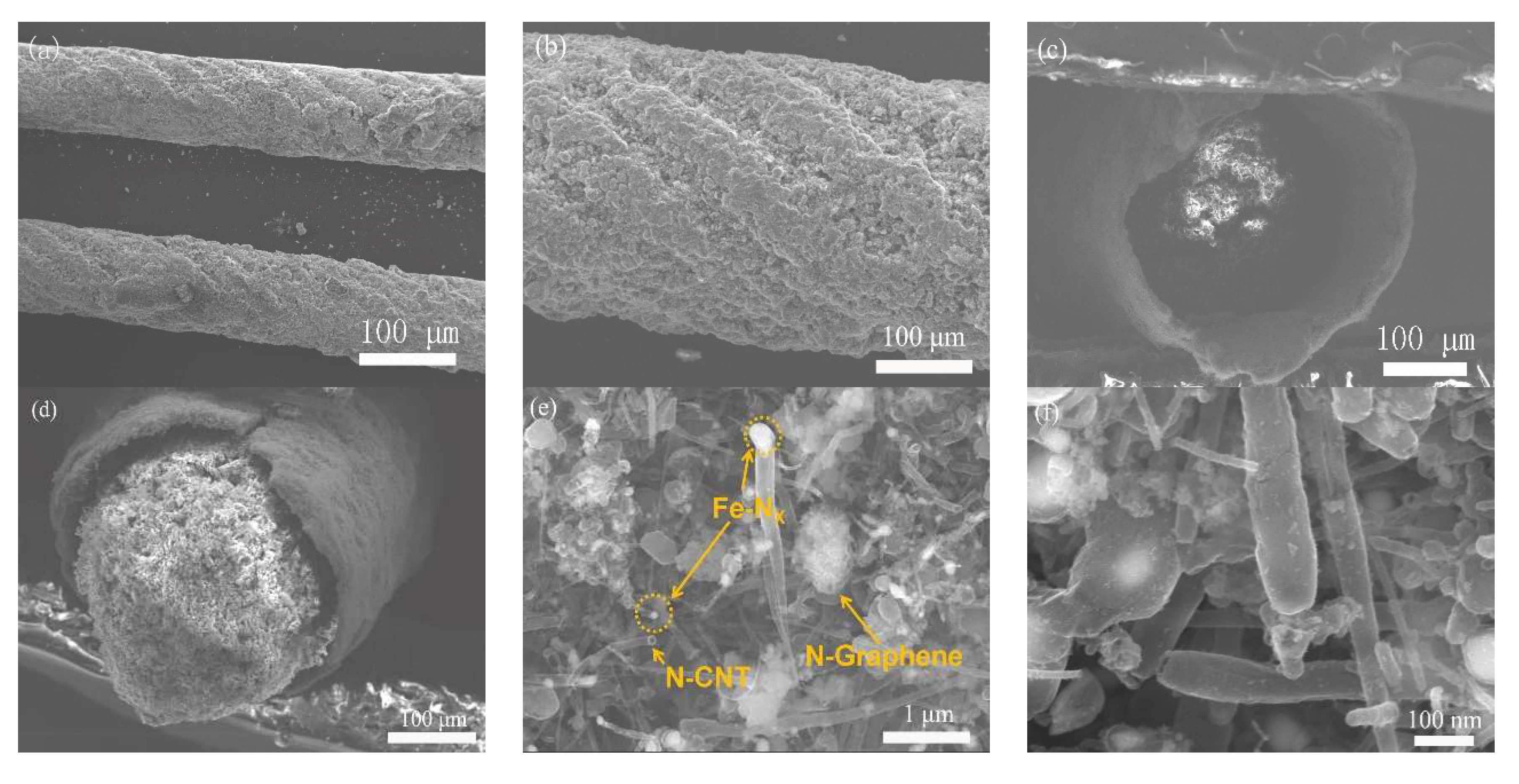
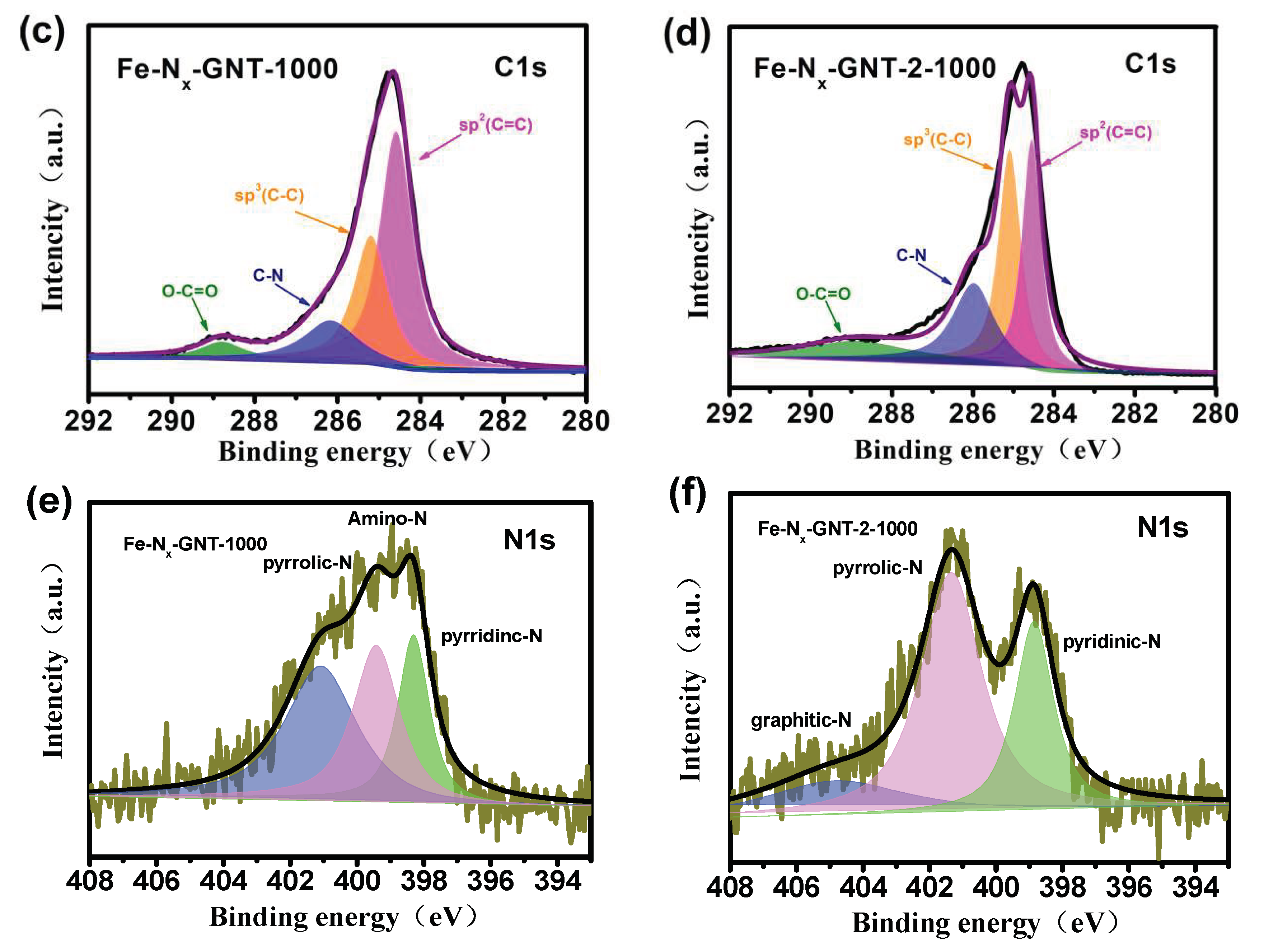
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ryu, J.; Jang, H.; Park, J.; Yoo, Y.; Park, M.; Cho, J. Seed-mediated atomic-scale reconstruction of silver manganate nanoplates for oxygen reduction towards high-energy aluminum-air flow batteries. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Ito, K.; Kubo, Y. Establishing the criteria and strategies to achieve high power during discharge of a Li-air battery. J. Mater. Chem. A 2019, 7, 23199–23207. [Google Scholar] [CrossRef]
- Wang, H.; Yin, F.X.; Liu, N.; Kou, R.H.; He, X.B.; Sun, C.J.; Chen, B.H.; Liu, D.J.; Yin, H.Q. Engineering Fe-Fe3C@Fe-N-C Active Sites and Hybrid Structures from Dual Metal-Organic Frameworks for Oxygen Reduction Reaction in H2-O2 Fuel Cell and Li-O2 Battery. Adv. Funct. Mater. 2019, 29, 1901531. [Google Scholar] [CrossRef]
- Papiya, F.; Nandy, A.; Mondal, S.; Kundu, P.P. Co/Al2O3-rGO nanocomposite as cathode electrocatalyst for superior oxygen reduction in microbial fuel cell applications: The effect of nanocomposite composition. Electrochim. Acta 2017, 254, 1–13. [Google Scholar] [CrossRef]
- Fichtner, J.; Garlyyev, B.; Watzele, S.; El-Sayed, H.A.; Schwämmlein, J.N.; Li, W.J.; Maillard, F.M.; Dubau, L.; Michalička, J.; Macak, J.M.; et al. Top-Down Synthesis of Nanostructured Platinum-Lanthanide Alloy Oxygen Reduction Reaction Catalysts: PtxPr/C as an Example. ACS Appl. Mater. Interfaces 2019, 11, 5129–5135. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Heine, T. Two-Dimensional Electrocatalyst with High Basal Plane Activity toward Oxygen Reduction Reaction. J. Am. Chem. Soc. 2018, 140, 12732–12735. [Google Scholar] [CrossRef]
- Serov, A.; Artyushkova, K.; Atanassov, P. Fe-N-C Oxygen Reduction Fuel Cell Catalyst Derived from Carbendazim: Synthesis, Structure, and Reactivity. Adv. Energy Mater. 2014, 10, 1301735. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, E.; Guo, Q.; Wang, Y.; Qiao, J.; Li, K.; Pei, P. N/S-Me (Fe, Co, Ni) doped hierarchical porous carbons for fuel cell oxygen reduction reaction with high catalytic activity and long-term stability. Appl. Energy 2016, 175, 468–478. [Google Scholar] [CrossRef]
- Li, P.; Wang, H.L. Recent advances in carbon-supported iron group electrocatalysts for the oxygen reduction reaction. New Carbon Mater. 2021, 36, 665–680. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.H.; Zhang, J.Q.; Zhi, L.J.; Wu, M.B. Boron and nitrogen co-doped carbon dots for boosting electrocatalytic oxygen reduction. New Carbon Mater. 2021, 36, 585–592. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.H.; Zhao, C.F.; Yin, X.P.; Zhao, Y.F. Co, N co-doped porous carbons as high-performance oxygen reduction electrocatalysts. New Carbon Mater. 2021, 36, 209–216. [Google Scholar] [CrossRef]
- Wang, Z.J.; Jia, R.R.; Zheng, J.F.; Zhao, J.G.; Li, L.; Song, J.L.; Zhu, Z.P. Nitrogen-Promoted Self-Assembly of N-Doped Carbon Nanotubes and Their Intrinsic Catalysis for Oxygen Reduction in Fuel Cells. ACS Nano 2011, 5, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Liu, X.; Tang, Y.; Lu, T. Electrostatic self-assembly of platinum nanochains on carbon nanotubes: A highly active electrocatalyst for the oxygen reduction reaction. Appl. Catal. B Environ. 2013, 140–141, 552–558. [Google Scholar] [CrossRef]
- Tan, Z.; Li, H.X.; Feng, Q.X.; Jiang, L.L.; Pan, H.Y.; Huang, Z.Y.; Zhou, Q.; Zhou, H.H.; Ma, S.; Kuang, Y.F. One-pot synthesis of Fe/N/S-doped porous carbon nanotubes for efficient oxygen reduction reaction. J. Mater. Chem. A 2019, 7, 1607–1615. [Google Scholar] [CrossRef]
- Li, Z.T.; Yang, T.T.; Zhao, W.N.; Xu, T.; Wei, L.Q.; Feng, J.Z.; Yang, X.J.; Ren, H.; Wu, M.B. Structural Modulation of Co Catalyzed Carbon Nanotubes with Cu-Co Bimetal Active Center to Inspire Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2019, 11, 3937–3945. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, G.W.; Gao, P.; Bi, S.F.; Tang, X.F.; Wang, D. High-performance supercapacitor of macroscopic graphene hydrogels by partial reduction and nitrogen doping of graphene oxide. Electrochim. Acta 2016, 221, 167–176. [Google Scholar] [CrossRef]
- Chen, T.; Dai, L.M. Macroscopic Graphene Fibers Directly Assembled from CVD-Grown Fiber-Shaped Hollow Graphene Tubes. Angew. Chem. Int. Ed. 2015, 54, 14947–14950. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.; Seo, E.K.; Kim, S.J.; Kim, W.; Park, M.G.; Yu, H.; Hwang, C. Electrical transport properties of graphene-covered-Cu wires grown by chemical vapor deposition. Curr. Appl. Phys. 2012, 12, 115–118. [Google Scholar] [CrossRef]
- Yang, W.X.; Liu, X.J.; Chen, L.L.; Liang, L.; Jia, J.B. A metal-organic framework devised Co-N doped carbon microsphere/nanofiber hybrid as a free-standing 3D oxygen catalyst. Chem. Commun. 2017, 53, 4034–4037. [Google Scholar] [CrossRef] [PubMed]
- Palaniselvam, T.; Irshad, A.; Unni, B.; Kurungot, S. Activity Modulated Low Platium Content Oxygen Reduction Electrocatalysts Prepared by Inducing Nano-Order Dislocations on Carbon Nanofiber through N-2-Doping. J. Phys. Chem. C 2012, 116, 14754–14763. [Google Scholar] [CrossRef]
- Cancellara, L.; Markurt, T.; Schulz, T.; Albrecht, M.; Hagedorn, S.; Walde, S.; Weyers, M.; Washiyama, S.; Collazo, R.; Sitar, Z. Role of oxygen diffusion in the dislocation reduction of epitaxial AlN on sapphire during high-temperature annealing. J. Appl. Phys. 2021, 130, 203101. [Google Scholar] [CrossRef]
- Wu, Y.P.; Hao, Y.F.; Jeong, H.Y.; Lee, Z.; Chen, S.S.; Jiang, W.; Wu, Q.Z.; Piner, R.D.; Kang, J.Y.; Ruoff, R.S. Crystal Structure Evolution of Individual Graphene Islands During CVD Growth on Copper Foil. Adv. Mater. 2013, 25, 6744–6751. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Pei, S.; Zhou, Z.; Chen, Z.; Xiong, X.; Sun, Y.; Zhang, Y. Fe, N-doped carbon spheres prepared by electrospinning method as high efficiency oxygen reduction catalyst. RSC Adv. 2019, 10, 779–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, F.Y.; Li, R.S.; Ge, S.Y.; Xu, W.R.; Zhang, Y.C. Nitrogen and phosphorus co-doped carbon networks derived from shrimp shells as an efficient oxygen reduction catalyst for microbial fuel cells. J. Power Sources 2020, 446, 227356. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Li, Y.F.; Yuan, S.X.; Chen, S.; Wang, C.W.; Li, X.M.; Su, F.Y.; Chen, C.M. Synthesis of 3D N, S dual-doped porous carbons with ultrahigh surface areas for highly efficient oxygen reduction reactions. ChemElectroChem 2018, 5, 3506–3513. [Google Scholar] [CrossRef]
- Xia, B.Y.; Yan, Y.; Li, N.; Wu, H.B.; Lou, X.W.; Wang, X. A metal-organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy 2016, 1, 15006. [Google Scholar] [CrossRef]
- Han, S.; Liu, H.; Zhou, C.; Ying, H.J. Growth of carbon nanotubes on graphene as 3D biocathode for NAD(+)/NADH balance model and high-rate production in microbial electrochemical synthesis from CO2. J. Mater. Chem. A 2019, 7, 1115–1123. [Google Scholar] [CrossRef]
- Pumera, M. Carbon nanotubes contain residual metal catalyst nanoparticles even after washing with nitric acid at elevated temperature because these metal nanoparticles are sheathed by several graphene sheets. Langmuir 2007, 23, 6453–6458. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.A.; Fernández, P.S.; de Lima, F.; Troiani, H.E.; Martins, M.E.; Arenillas, A.; Maia, G.; Camara, G.A. Remarkable electrochemical stability of one-step synthesized Pd nanoparticles supported on graphene and multi-walled carbon nanotubes. Nano Energy 2014, 9, 142–151. [Google Scholar] [CrossRef]
- Zhong, B.; Wang, C.J.; Wen, G.W.; Yu, Y.L.; Xia, L. Facile fabrication of boron and nitrogen co-doped carbon@Fe2O3/Fe3C/Fe nanoparticle decorated carbon nanotubes three-dimensional structure with excellent microwave absorption properties. Compos. Part B Eng. 2018, 132, 141–150. [Google Scholar] [CrossRef]
- Huang, B.; Yang, J.; Zou, Y.L.; Ma, L.L.; Zhou, X.Y. Sonochemical synthesis of SnO2/carbon nanotubes encapsulated in graphene sheets composites for lithium ion batteries with superior electrochemical performance. Electrochim. Acta 2014, 143, 63–69. [Google Scholar] [CrossRef]
- Miller, J.R.; Outlaw, R.A.; Holloway, B.C. Graphene Double-Layer Capacitor with ac Line-Filtering Performance. Science 2010, 329, 1637–1639. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.X.; San, H.; Hui, K.N. Carbon nanotube@manganese oxide nanosheet core-shell structure encapsulated within reduced graphene oxide film for flexible all-solid-state asymmetric supercapacitors. Carbon 2018, 132, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Du, M.; Chang, J.; Sun, J.; Gao, L. Supercapacitors with high capacitance based on reduced graphene oxide/carbon nanotubes/NiO composite electrodes. J. Mater. Chem. A 2014, 2, 3834–3840. [Google Scholar] [CrossRef]
- Liu, X.J.; Yang, W.X.; Chen, L.L.; Liu, Z.J.; Long, L.; Wang, S.Y.; Liu, C.Y.; Dong, S.J.; Jia, J.B. Graphitic Carbon Nitride (g-C3N4)-Derived Bamboo-Like Carbon Nanotubes/Co Nanoparticles Hybrids for Highly Efficient Electrocatalytic Oxygen Reduction. ACS Appl. Mater. Interfaces 2020, 12, 4463–4472. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yang, Z.W.; Li, H.J.; Wang, Y.Z.; Wang, X.M. Reduced graphene oxide encapsulated MnO microspheres as an anode for high-rate lithium ion capacitors. New Carbon Mater. 2021, 36, 573–581. [Google Scholar] [CrossRef]
- Wang, Z.; Ang, J.; Liu, J.; Ma, X.Y.D.; Kong, J.; Zhang, Y.; Yan, T.; Lu, X. FeNi alloys encapsulated in N-doped CNTs-tangled porous carbon fibers as highly efficient and durable bifunctional oxygen electrocatalyst for rechargeable zinc-air battery. Appl. Catal. B Environ. 2020, 263, 118344. [Google Scholar] [CrossRef]
- Chen, M.J.; Hwang, S.; Li, J.Z.; Karakalos, S.; Chen, K.; He, Y.H.; Mukherjee, S.; Su, D.; Wu, G. Pt alloy nanoparticles decorated on large-size nitrogen-doped graphene tubes for highly stable oxygen-reduction catalysts. Nanoscale 2018, 10, 17318–17326. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.T.; Chen, S.M.; Pei, Z.X.; Huang, Y.; Liang, G.J.; Mo, F.N.; Yang, Q.; Su, J.; Gao, Y.H.; Zapien, J.A.; et al. Single-Site Active Iron-Based Bifunctional Oxygen Catalyst for a Compressible and Rechargeable Zinc-Air Battery. ACS Nano 2018, 12, 1949–1958. [Google Scholar] [CrossRef]
- Li, Q.; Pan, H.; Higgins, D.; Cao, R.; Zhang, G.; Lv, H.; Wu, K.; Cho, J.; Wu, G. Metal-Organic Framework-Derived Bamboo-like Nitrogen-Doped Graphene Tubes as an Active Matrix for Hybrid Oxygen-Reduction Electrocatalysts. Small 2015, 11, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shaikh, A.U.; Tsui, E.Y.; Swager, T.M. Cobalt Porphyrin Functionalized Carbon Nanotubes for Oxygen Reduction. Chem. Mater. 2009, 21, 3234–3241. [Google Scholar] [CrossRef]
- Yang, G.; Choi, W.; Pu, X.; Yu, C. Scalable synthesis of bi-functional high-performance carbon nanotube sponge catalysts and electrodes with optimum C-N-Fe coordination for oxygen reduction reaction. Energy Environ. Sci. 2015, 8, 1799–1807. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Nguyen, M.H.; Bui, Q.B. A novel electrocatalyst based on Fe2Ni1 nanoparticles anchored nitrogen doped graphene nanosheets towards efficient oxygen reduction reaction. J. Alloys Compd. 2019, 780, 734–742. [Google Scholar] [CrossRef]
- Yu, Q.M.; Xu, J.X.; Wu, C.X.; Zhang, J.S.; Guan, L.H. MnO2 Nanofilms on Nitrogen-Doped Hollow Graphene Spheres as a High-Performance Electrocatalyst for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2016, 8, 35264–35269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, Y.J.; Zhou, K. Self-adjusting activity induced by intrinsic reaction intermediate in Fe–N–C single-atom catalysts. J. Am. Chem. Soc. 2019, 141, 14115–14119. [Google Scholar] [CrossRef]
- Xia, D.S.; Yang, X.; Xie, L.; Wei, Y.P.; Jiang, W.; Dou, M.; Li, X.N.; Li, J.; Gan, L.; Kang, F.Y. Direct growth of carbon nanotubes doped with single atomic Fe–N4 active sites and neighboring graphitic nitrogen for efficient and stable oxygen reduction electrocatalysis. Adv. Funct. Mater. 2019, 29, 1906174. [Google Scholar] [CrossRef]
- Yu, X.Z.; Lai, S.J.; Xin, S.S.; Chen, S.; Zhang, X.L.; She, X.L.; Zhan, T.R.; Zhao, X.L.; Yang, D.J. Coupling of iron phthalocyanine at carbon defect site via π-π stacking for enhanced oxygen reduction reaction. Appl. Catal. B Environ. 2021, 280, 119437. [Google Scholar] [CrossRef]
- Zheng, L.; Dong, Y.Y.; Chi, B.; Cui, Z.M.; Deng, Y.J.; Shi, X.D.; Du, L.; Liao, S.J. UIO-66-NH2-derived mesoporous carbon catalyst Co-doped with Fe/N/S as highly efficient cathode catalyst for PEMFCs. Small 2019, 15, 1803520. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, T.; Hu, G.; Jia, X.; Wågberg, T. Formation of Active Sites for Oxygen Reduction Reactions by Transformation of Nitrogen Functionalities in Nitrogen-Doped Carbon Nanotubes. ACS Nano 2012, 6, 8904–8912. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, C.; Liu, F.; Hou, Y. Hybrid of Iron Nitride and Nitrogen-Doped Graphene Aerogel as Synergistic Catalyst for Oxygen Reduction Reaction. Adv. Funct. Mater. 2014, 24, 2930–2937. [Google Scholar] [CrossRef]
- Yang, D.S.; Bhattacharjya, D.; Song, M.Y.; Razmjooei, F.; Ko, J.; Yang, Q.H.; Yu, J.S. Nitrogen-Doped Ordered Mesoporous Carbon with Different Morphologies for the Oxygen Reduction Reaction: Effect of Iron Species and Synergy of Textural Properties. ChemCatChem 2015, 7, 2882–2890. [Google Scholar] [CrossRef]
- Yasin, G.; Ibrahim, S.; Ajmal, S.; Ibraheem, S.; Ali, S.; Nadda, A.K.; Zhang, G.X.; Kaur, J.; Maiyalagan, T.; Gupta, R.K.; et al. Tailoring of electrocatalyst interactions at interfacial level to benchmark the oxygen reduction reaction. Coordin. Chem. Rev. 2022, 469, 214669. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, Y.; Chen, S.; Li, X.; Ma, S. Preparation of Spiral Nitrogen-Doped Macroscopic Graphene Tube and Tuning the Activity of Oxygen Catalysis by Twisted Ferrum (Fe) Wires. Metals 2022, 12, 2050. https://doi.org/10.3390/met12122050
Li Y, Liu Y, Chen S, Li X, Ma S. Preparation of Spiral Nitrogen-Doped Macroscopic Graphene Tube and Tuning the Activity of Oxygen Catalysis by Twisted Ferrum (Fe) Wires. Metals. 2022; 12(12):2050. https://doi.org/10.3390/met12122050
Chicago/Turabian StyleLi, Yongfeng, Yanzhen Liu, Shuai Chen, Xiaoming Li, and Shengguo Ma. 2022. "Preparation of Spiral Nitrogen-Doped Macroscopic Graphene Tube and Tuning the Activity of Oxygen Catalysis by Twisted Ferrum (Fe) Wires" Metals 12, no. 12: 2050. https://doi.org/10.3390/met12122050
APA StyleLi, Y., Liu, Y., Chen, S., Li, X., & Ma, S. (2022). Preparation of Spiral Nitrogen-Doped Macroscopic Graphene Tube and Tuning the Activity of Oxygen Catalysis by Twisted Ferrum (Fe) Wires. Metals, 12(12), 2050. https://doi.org/10.3390/met12122050




