Experimental Investigation of the Isothermal Section in the Al–Si–Y System at 773 K
Abstract
:1. Introduction
2. Experimental Procedure
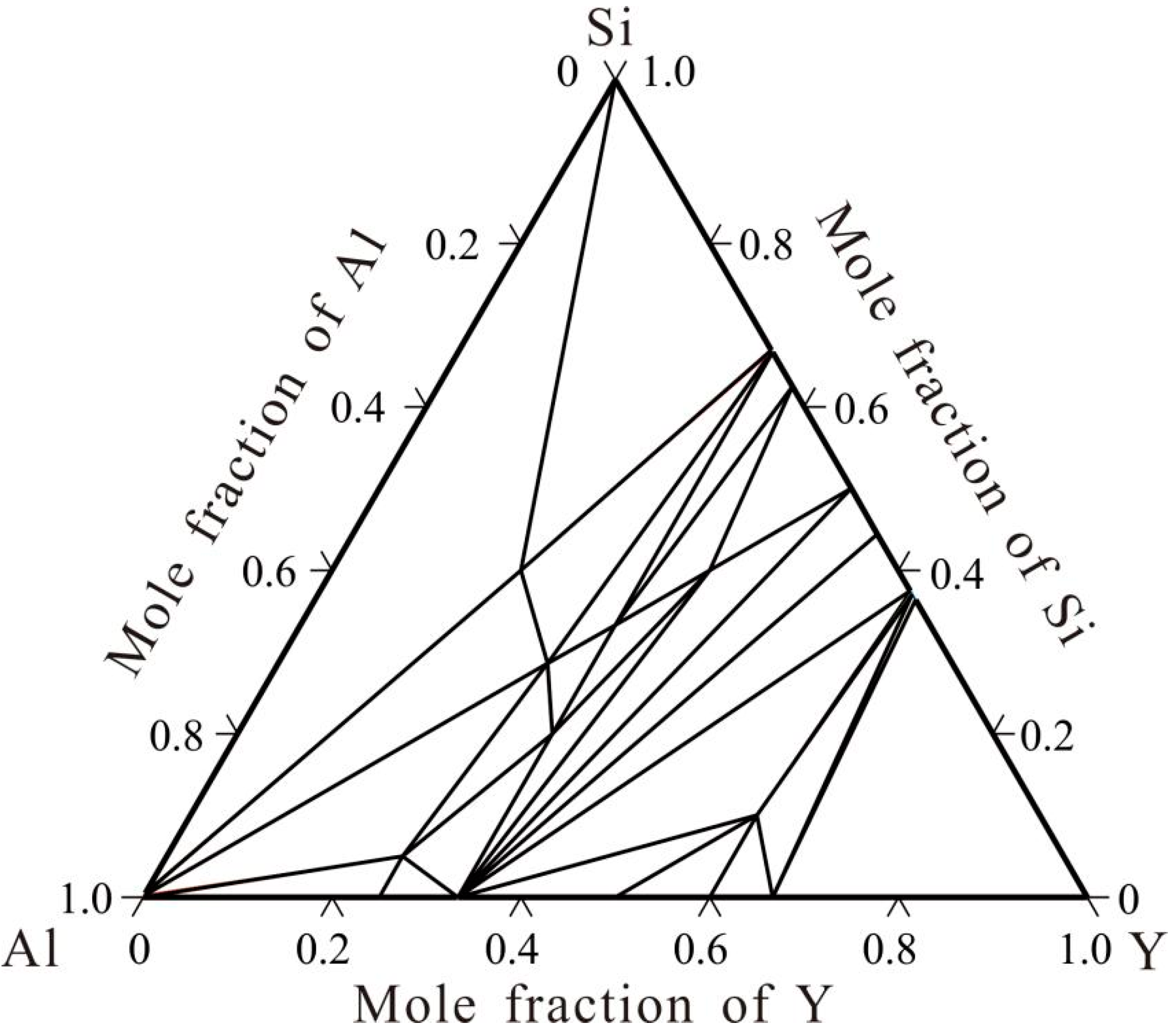
3. Results and Discussion
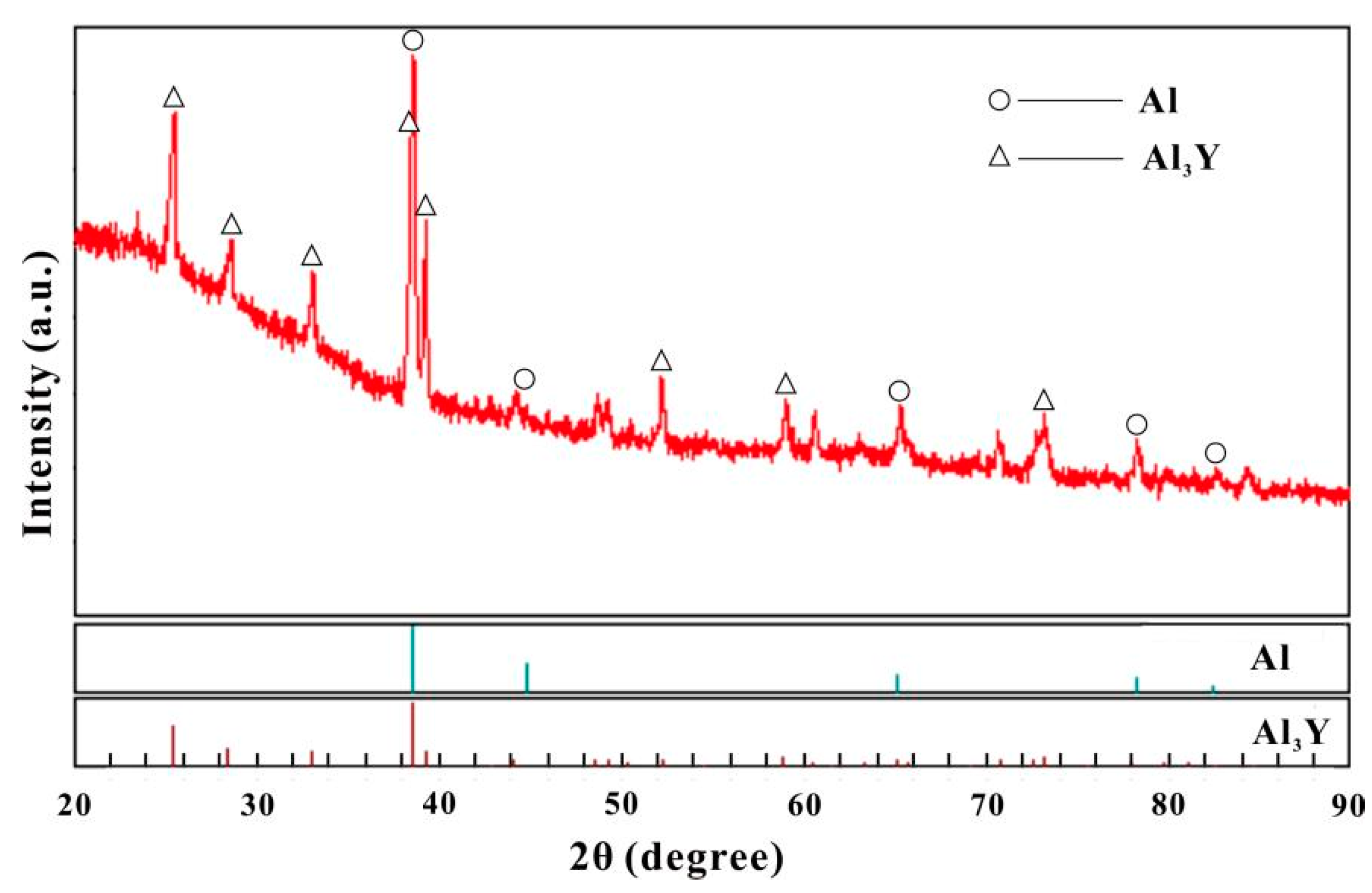
3.1. Aluminum-Rich Corner
3.2. Non-Aluminium-Rich Corner
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pukas, S.; Lasocha, W.; Gladyshevskii, R. Phase equilibria in the Er–Al–Si system at 873 K. Calphad 2009, 33, 23–26. [Google Scholar] [CrossRef]
- Rashno, S.; Reihanian, M.; Ranjbar, K. Tensile and creep properties of Al–7Si–0.3Mg alloy with Zr and Er addition. Mater. Sci. Technol. 2020, 36, 1603–1613. [Google Scholar] [CrossRef]
- Xu, K.; Chang, K.; Yu, M.; Zhou, D.; Du, Y.; Wang, L. Design of novel NiSiAlY alloys in marine salt-spray environment: Part II. Al-Ni-Si-Y thermodynamic dataset. J. Mater. Sci. Technol. 2021, 89, 186–198. [Google Scholar] [CrossRef]
- Magazu, S.; Migliardo, F.; Caccamo, M.T. Innovative wavelet protocols in analyzing elastic incoherent neutron scattering. J. Phys. Chem. B 2012, 116, 9417–9423. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Wen, M.; Chen, X.; Pan, F. Thermodynamic database of the phase diagrams in the Mg-Al-Zn-Y-Ce system. Rare Met. 2006, 25, 441–447. [Google Scholar] [CrossRef]
- Liu, S.; Du, Y.; Chen, H. A thermodynamic reassessment of the Al–Y system. Calphad 2006, 30, 334–340. [Google Scholar] [CrossRef]
- Liu, S.; Du, Y.; Xu, H.; He, C.; Schuster, J.C. Experimental investigation of the Al–Y phase diagram. J. Alloy. Compd. 2006, 414, 60–65. [Google Scholar] [CrossRef]
- Hennet, L.; Cristiglio, V. Aerodynamic levitation and laser heating. Eur. Phys. J. Spec. Top. 2011, 196, 151–165. [Google Scholar] [CrossRef]
- Bellissent, R.; Magazu, S.; Majolino, D.; Migliardo, P. Structural Properties of Amorphous Bulk Fe, CO and Fe-Co Binary Alloys. Phys. Scr. 1995, T57, 79. [Google Scholar] [CrossRef] [Green Version]
- Shukla, A.; Kang, Y.B.; Pelton, A.D. Thermodynamic assessment of the Ce–Si, Y–Si, Mg–Ce–Si and Mg–Y–Si systems. Int. J. Mater. Res. 2009, 100, 208–217. [Google Scholar] [CrossRef]
- Zhang, Z.; Chu, M.Y.; Zhao, X.S.; Li, K.; Shang, S.L.; Liu, Z.K.; Shen, J.Y. Thermodynamic modeling of the Si-Y system aided by first-principles and phonon calculations. Calphad 2019, 65, 282–290. [Google Scholar] [CrossRef]
- Xu, K.; Zou, H.; Chang, K.; Deng, Z.; Zhou, X.; Huang, Y.; Chen, L.; Huang, F.; Huang, Q. Thermodynamic description of the sintering aid system in silicon carbide ceramics with the addition of yttrium. J. Eur. Ceram. Soc. 2019, 39, 4510–4519. [Google Scholar] [CrossRef]
- Pan, Z.; Fabrichnaya, O.; Seifert, H.J.; Neher, R.; Brandt, K.; Herrmann, M. Thermodynamic evaluation of the Si-C-Al-Y-O system for LPS-SiC application. J. Phase Equilibria Diffus. 2010, 31, 238–249. [Google Scholar] [CrossRef]
- Xu, K.; Chang, K.; Du, Y.; Wang, L. Design of novel NiSiAlY alloys in marine salt-spray environment: Part I. Al-Si-Y and Ni-Si-Y subsystems. J. Mater. Sci. Technol. 2021, 88, 66–78. [Google Scholar] [CrossRef]
- Pukas, S.; Lutsyshyn, Y.; Manyako, M.; Gladyshevskii, E. Crystal structures of the RAlSi and RAlGe compounds. J. Alloys Compd. 2004, 367, 162–166. [Google Scholar] [CrossRef]
- Yin, T.; Ma, Z.; Chen, H.; Luo, Q.; Zhang, J.; Wu, G. Phase Equilibria of the Mg-Zn-Sm System in Mg-Rich Corner at 320 °C and 400 °C. Metals 2022, 12, 1553. [Google Scholar] [CrossRef]
- Huang, K.L.; Xiao, L.M.; Yang, Q.K.; Yang, L.; Li, Z.B.; Lu, Z.; Yao, Q.R.; Deng, J.Q.; Cheng, L.C.; Huang, C.M.; et al. Experimental Investigation of Isothermal Section in the La–Co–Ni System at 723 K. Metals 2022, 12, 1747. [Google Scholar] [CrossRef]
- Mezbahul-Islam, M.; Kevorkov, D.; Medraj, M. Experimental Study of the Mg-Ni-Y System at 673 K Using Diffusion Couples and Key Alloys. Metals 2015, 5, 1746–1769. [Google Scholar] [CrossRef] [Green Version]
- Magazu, S.; Migliardo, F.; Caccamo, M.T. Upgrading of Resolution Elastic Neutron Scattering (RENS). Adv. Mater. Sci. Eng. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhang, X.; Li, L.; Pan, Y.; Chen, Y.; Yang, S.; Lu, Y.; Han, J.; Liu, X. Phase Equilibria of the Co-Ti-Ta Ternary System. Metals 2018, 8, 958. [Google Scholar] [CrossRef]
- Magazu, S. IQENS-dynamic light scattering complementarity on hydrogenous systems. Phys. B Condens. Matter 1996, 226, 92–106. [Google Scholar] [CrossRef]
- Magazu, S. NMR, static and dynamic light and neutron scattering investigations on polymeric aqueous solutions. J. Mol. Struct. 2000, 523, 47–59. [Google Scholar] [CrossRef]
- Jannelli, M.P.; Migliardo, P.; Aliotta, F.; Tettamanti, E. Transport properties of liquid alcohols investigated by IQENS, NMR and DLS studies. J. Phys. Condens. Matter 1996, 8, 8157–8171. [Google Scholar] [CrossRef]
- Zhang, F.; Qin, A.; Liu, S.; Wang, J.; Du, Y. Phase equilibria and solidification characteristics of the Al–Sc–Si alloys. J. Mater. Sci. 2015, 51, 1644–1658. [Google Scholar] [CrossRef]
- Liu, X.J.; Wu, D.; Zhang, J.; Yang, M.; Zhu, J.; Li, L.; Chen, Y.; Yang, S.; Han, J.; Lu, Y.; et al. Experimental Investigation of Phase Equilibria in the Co-Re-Ta Ternary System. Metals 2018, 8, 911. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Du, Y.; Liu, S.; Cheng, K.; Zhang, Z. Phase equilibria and crystal structure of ternary compounds in Al-rich corner of Al-Er-Y system at 673 K and 873 K. J. Mater. Sci. Technol. 2021, 60, 128–138. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Zhang, L.; Tuo, B.; Bai, W.; Wu, D. Experimental Investigation of the Ti-Nb-Sn Isothermal Section at 1173 K. Metals 2016, 6, 60. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Li, Q.; Zhang, J.Y.; Chen, S.L.; Chou, K.C. Experimental investigation and thermodynamic calculation of the Al–Si–Ti system in Al-rich corner. J. Alloys Compd. 2014, 602, 58–65. [Google Scholar] [CrossRef]
- Schick, C. Differential scanning calorimetry (DSC) of semicrystalline polymers. Anal. Bioanal. Chem. 2009, 395, 1589–1611. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhang, L.; Wang, H.; Liu, L. Phase equilibria investigation of the Al–Ni–Er ternary system at 600 °C and 700 °C. Calphad 2021, 75, 102353. [Google Scholar] [CrossRef]




| Sample No. | Nominal Composition (at.%) | Treatment, Analysis |
|---|---|---|
| 1 | Al90Si9Y1 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 2 | Al90Si7.5Y2.5 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 3 | Al90Si5.5Y4.5 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 4 | Al90Si4Y6 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 5 | Al90Si2.5Y7.5 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 6 | Al90Si1Y9 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 7 | Al80Si19Y1 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 8 | Al80Si17Y3 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 9 | Al80Si15Y5 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 10 | Al80Si12.5Y7.5 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 11 | Al80Si11Y9 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 12 | Al80Si9Y11 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 13 | Al80Si7Y13 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 14 | Al80Si5Y15 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 15 | Al80Si2.5Y17.5 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 16 | Al80Si1Y19 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 17 | Al70Si29Y1 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 18 | Al70Si27Y3 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 19 | Al70Si24.5Y5.5 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 20 | Al70Si22.5Y7.5 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 21 | Al70Si20Y10 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 22 | Al70Si18Y12 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 23 | Al70Si16Y14 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 24 | Al70Si14Y16 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 25 | Al70Si12Y18 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 26 | Al70Si10Y20 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 27 | Al70Si8Y22 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 28 | Al70Si5Y25 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 29 | Al70Si4Y26 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 30 | Al70Si2Y28 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 31 | Al60Si39Y1 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 32 | Al60Si36Y4 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 33 | Al60Si32Y8 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 34 | Al60Si29Y11 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 35 | Al60Si26Y14 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 36 | Al60Si23Y17 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 37 | Al60Si19Y21 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 38 | Al60Si17Y23 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 39 | Al60Si14Y26 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 40 | Al60Si12Y28 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 41 | Al60Si9Y31 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 42 | Al60Si7Y33 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 43 | Al60Si4Y36 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 44 | Al60Si2Y38 | As-cast, 500 °C/6 weeks, DSC/XRD/EPMA |
| 45 | Al14.9Si65.3Y19.8 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 46 | Al8.4Si59.6Y32 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 47 | Al39.1Si33.6Y27.3 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 48 | Al30.7Si39.1Y30.2 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 49 | Al25Si44.4Y30.6 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 50 | Al20.3Si42.4Y37.3 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 51 | Al20Si38.1Y41.9 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 52 | Al14.1Si41.9Y44 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 53 | Al15.2Si39Y45.8 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 54 | Al9.8Si42.4Y48.8 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 55 | Al5.2Si42Y52.8 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 56 | Al10.6Si36.2Y53.2 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 57 | Al28Si19.6Y52.4 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 58 | Al17.1Si27.5Y55.4 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 59 | Al32.1Si11.6Y56.3 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 60 | Al23.5Si17.9Y58.6 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 61 | Al36.3Si4.1Y59.6 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 62 | Al29Si4.5Y66.5 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 63 | Al7.6Si23.4Y69 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 64 | Al17.1Si14Y68.9 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 65 | Al6.3Si52.6Y41.1 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 66 | Al41.9Si27.8Y30.2 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 67 | Al35.4Si29.4Y35.2 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 68 | Al23.9Si36.4Y39.7 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 69 | Al64Si5.9Y30.1 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 70 | Al44.6Si15.2Y40.2 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 71 | Al34.2Si22.2Y43.6 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 72 | Al23.3Si30.5Y46.2 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 73 | Al10Si41.8Y48.2 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 74 | Al9.7Si38.2Y52.1 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 75 | Al2.7Si45.5Y51.8 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 76 | Al8.3Si35.3Y56.4 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 77 | Al53.1Si4.5Y42.4 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 78 | Al46.6Si7.5Y45.9 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 79 | Al32Si9Y59 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| 80 | Al12.1Si23Y64.9 | As-cast, 500 °C/6 weeks, XRD/EPMA |
| Alloy No. | Sample Composition | Phase Composition (at.%) | Treatment, Analysis | |||
|---|---|---|---|---|---|---|
| Al | Si | Y | Phase | |||
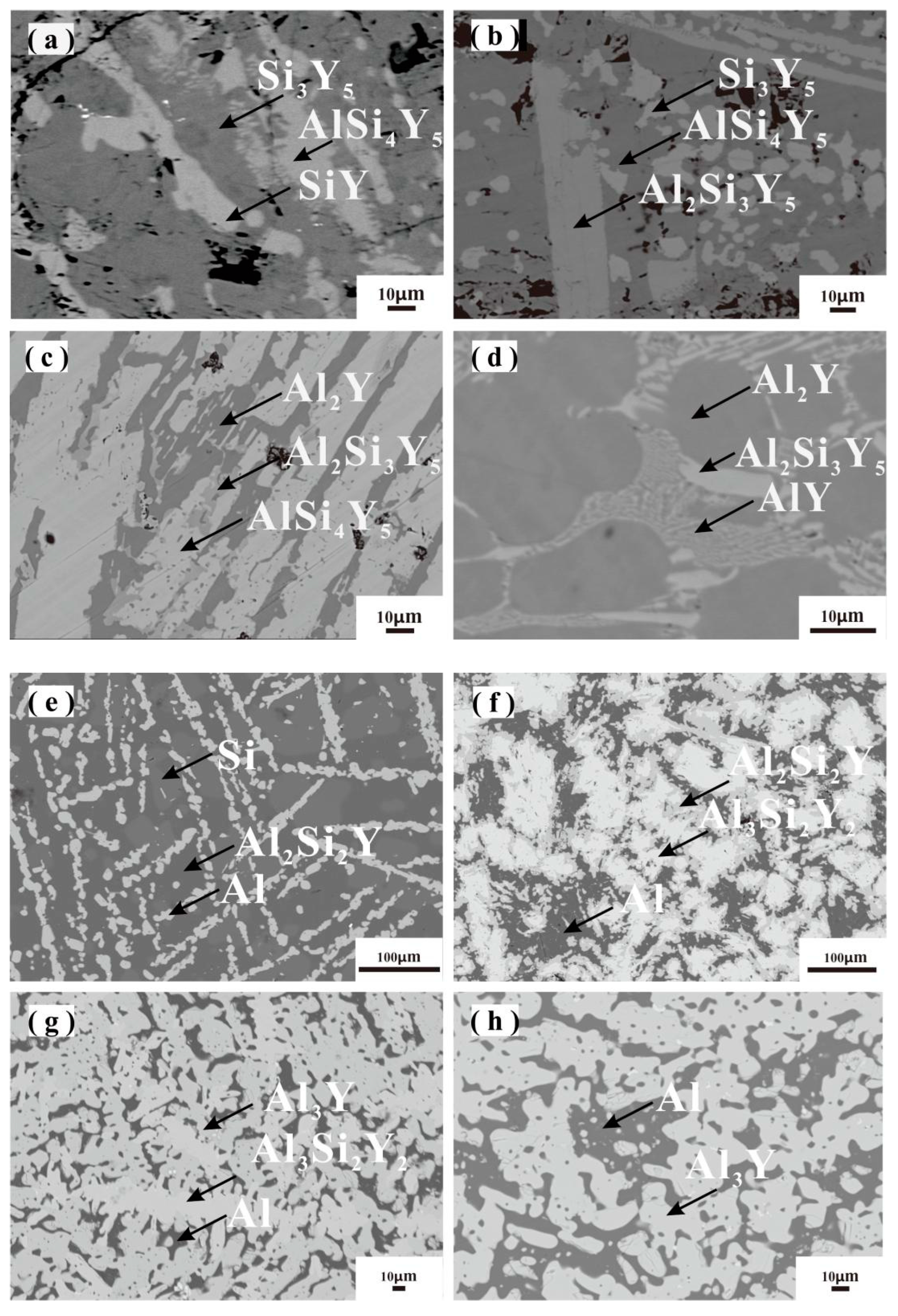
| 55 | Al5.2Si42Y52.8 | 0.5 | 36.7 | 62.8 | Si3Y5 | Annealed at 500 °C for 6 weeks |
| 10.7 | 37.9 | 51.4 | AlSi4Y5 | |||
| 3.3 | 50 | 46.7 | SiY | |||
| 56 | Al10.6Si36.2Y53.2 | 1.1 | 34.8 | 64.1 | Si3Y5 | Annealed at 500 °C for 6 weeks |
| 20.5 | 28.8 | 50.7 | Al2Si3Y5 | |||
| 9.2 | 40.5 | 50.3 | AlSi4Y5 | |||
| 72 | Al23.3Si30.5Y46.2 | 17.5 | 34.6 | 47.9 | Al2Si3Y5 | Annealed at 500 °C for 6 weeks |
| 9.7 | 39.8 | 50.5 | AlSi4Y5 | |||
| 65.8 | 0.6 | 33.6 | Al2Y | |||
| 43 | Al60Si4Y36 | 41.5 | 12.4 | 46.1 | AlY | Annealed at 500 °C for 6 weeks |
| 66 | 0.3 | 33.7 | Al2Y | |||
| 22.3 | 28.2 | 49.5 | Al2Si3Y5 | |||
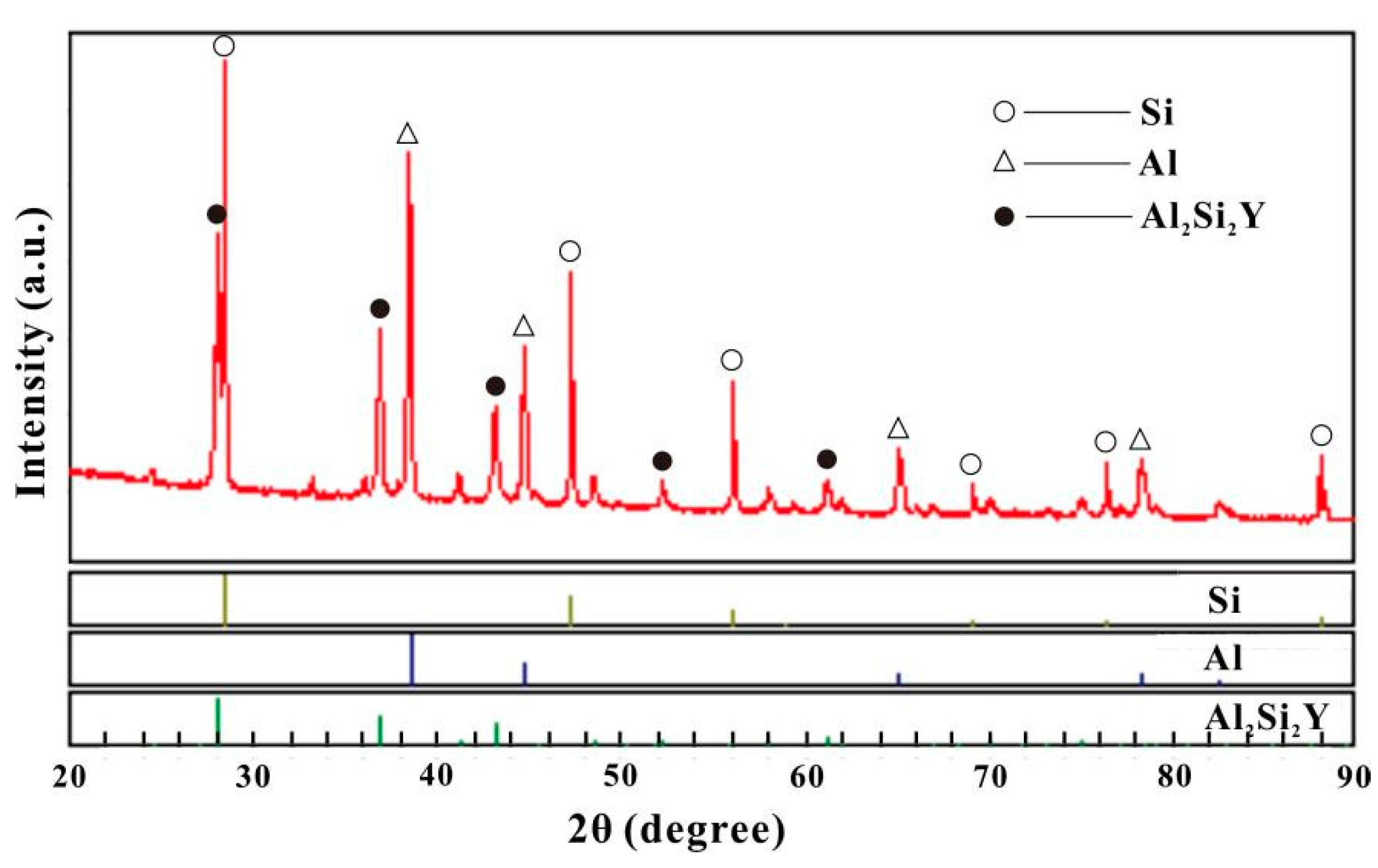
| 32 | Al60Si36Y4 | 41.8 | 38.1 | 20.1 | Al2Si2Y | Annealed at 500 °C for 6 weeks |
| 0.4 | 99.6 | 0 | Al | |||
| 98.8 | 1.2 | 0 | Si | |||
| 36 | Al60Si23Y17 | 41.5 | 38.8 | 19.7 | Al2Si2Y | Annealed at 500 °C for 6 weeks |
| 42.8 | 28.8 | 28.4 | Al3Si2Y2 | |||
| 99.2 | 0.8 | 0 | Al | |||
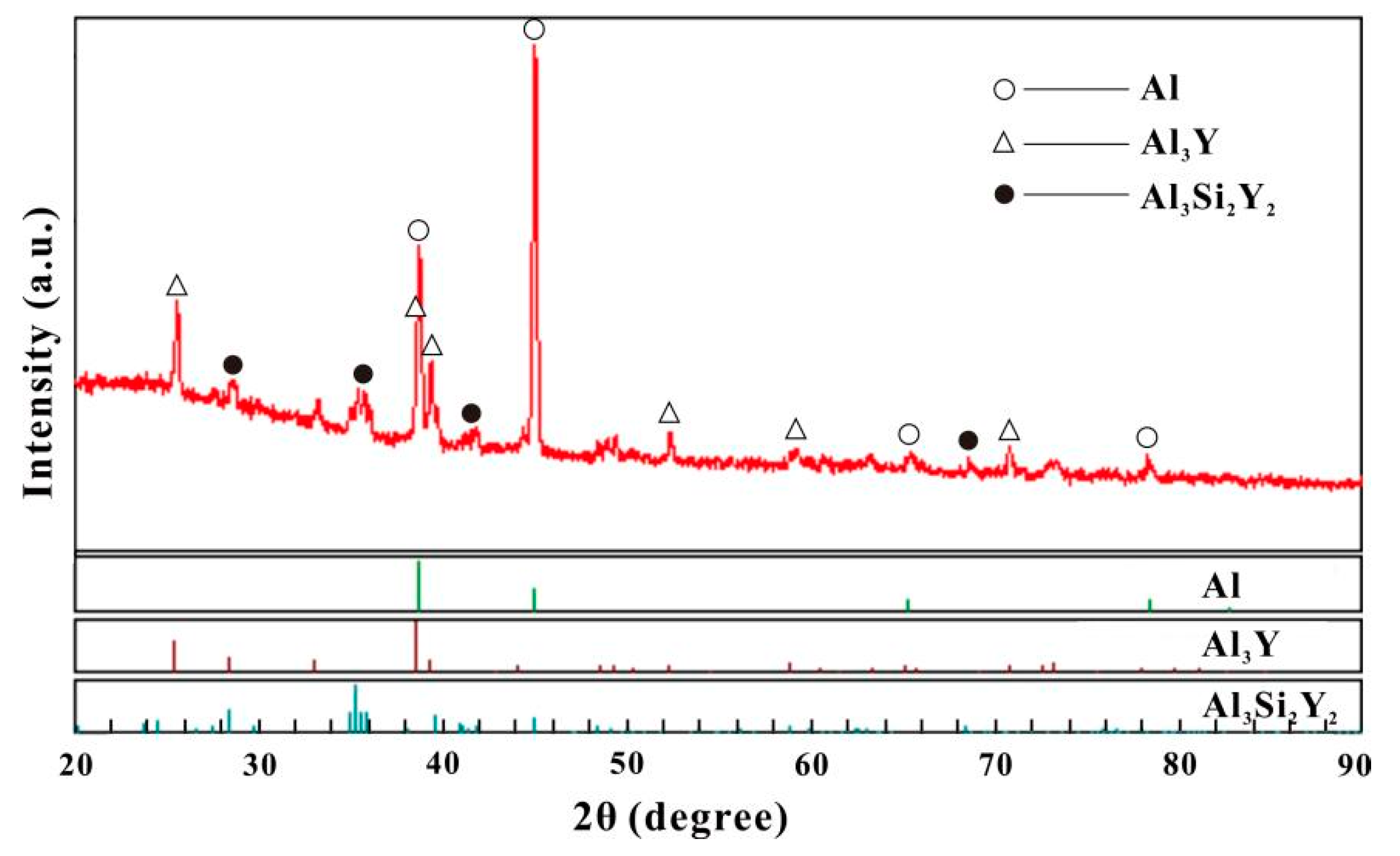
| 26 | Al70Si10Y20 | 44 | 27.2 | 28.8 | Al3Si2Y2 | Annealed at 500 °C for 6 weeks |
| 70.5 | 4.6 | 24.9 | Al3Y | |||
| 99.7 | 0.1 | 0.2 | Al | |||
| 15 | Al80Si2.5Y17.5 | 71 | 3.8 | 25.2 | Al3Y | Annealed at 500 °C for 6 weeks |
| 99.9 | 0.1 | 0 | Al | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Qin, H.; Yang, Q.; Huang, K.; Lu, Z.; Yao, Q.; Deng, J.; Cheng, L.; Huang, C.; Long, Q.; et al. Experimental Investigation of the Isothermal Section in the Al–Si–Y System at 773 K. Metals 2022, 12, 2020. https://doi.org/10.3390/met12122020
Yang L, Qin H, Yang Q, Huang K, Lu Z, Yao Q, Deng J, Cheng L, Huang C, Long Q, et al. Experimental Investigation of the Isothermal Section in the Al–Si–Y System at 773 K. Metals. 2022; 12(12):2020. https://doi.org/10.3390/met12122020
Chicago/Turabian StyleYang, Lu, Haiqing Qin, Qingkai Yang, Kailin Huang, Zhao Lu, Qingrong Yao, Jianqiu Deng, Lichun Cheng, Caimin Huang, Qianxin Long, and et al. 2022. "Experimental Investigation of the Isothermal Section in the Al–Si–Y System at 773 K" Metals 12, no. 12: 2020. https://doi.org/10.3390/met12122020
APA StyleYang, L., Qin, H., Yang, Q., Huang, K., Lu, Z., Yao, Q., Deng, J., Cheng, L., Huang, C., Long, Q., Wang, J., & Zhou, H. (2022). Experimental Investigation of the Isothermal Section in the Al–Si–Y System at 773 K. Metals, 12(12), 2020. https://doi.org/10.3390/met12122020






