Effect of Heat Input on Hydrogen Embrittlement of TIG Welded 304 Austenitic Stainless Steel
Abstract
1. Introduction
2. Experimental Details
3. Results and Discussion
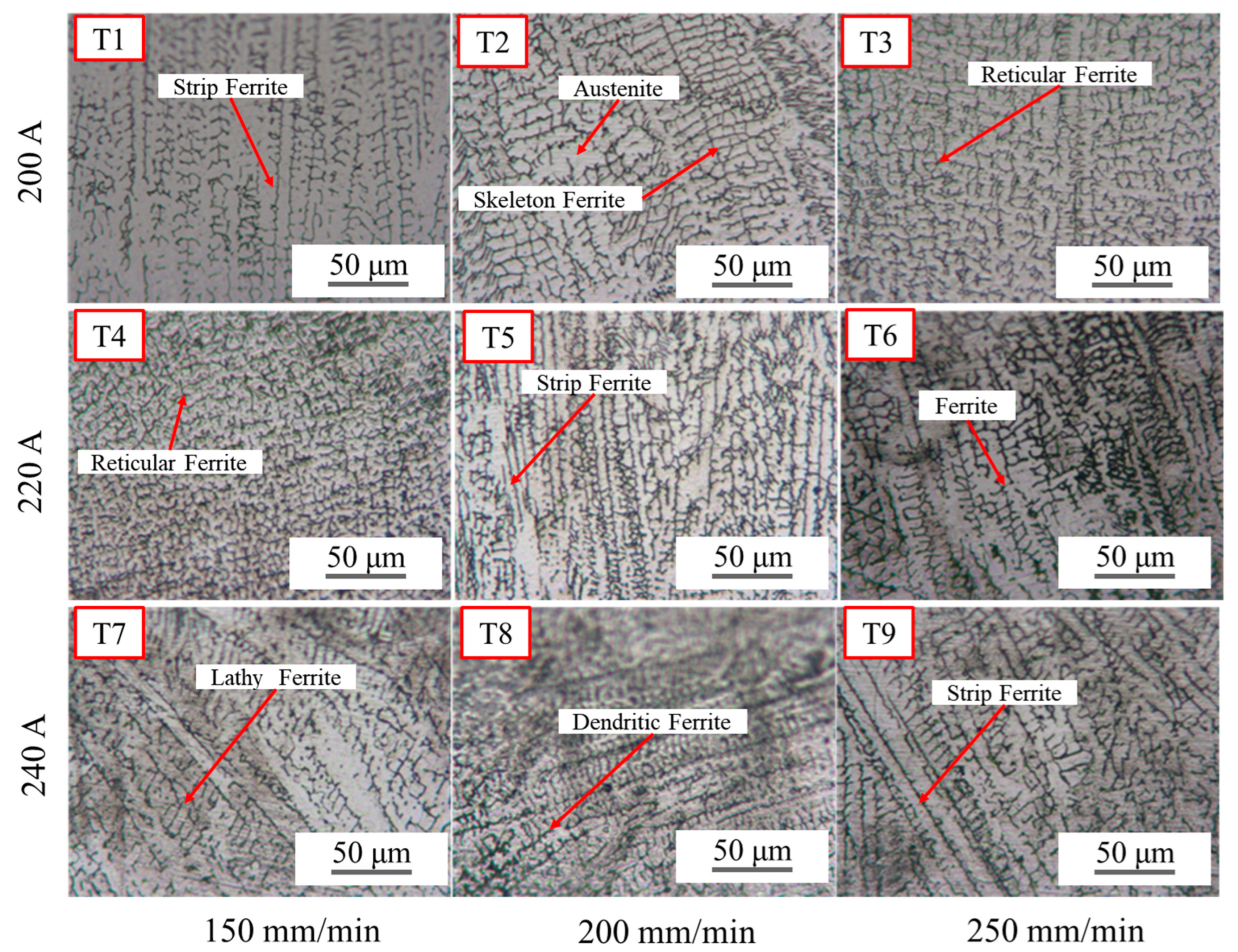
3.1. Influence of Heat Input on Weld Microstructure
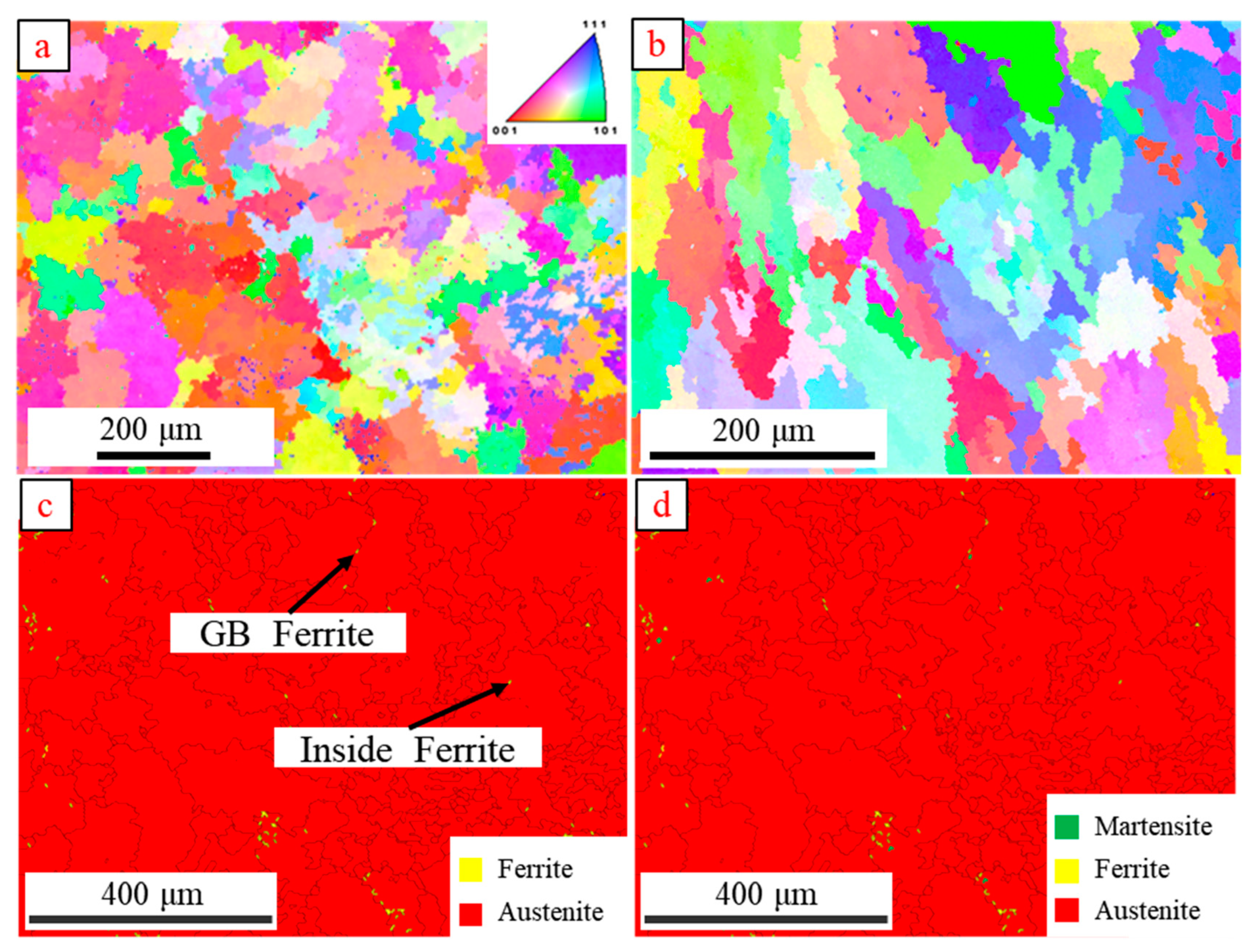
3.2. Microstructure Evolution
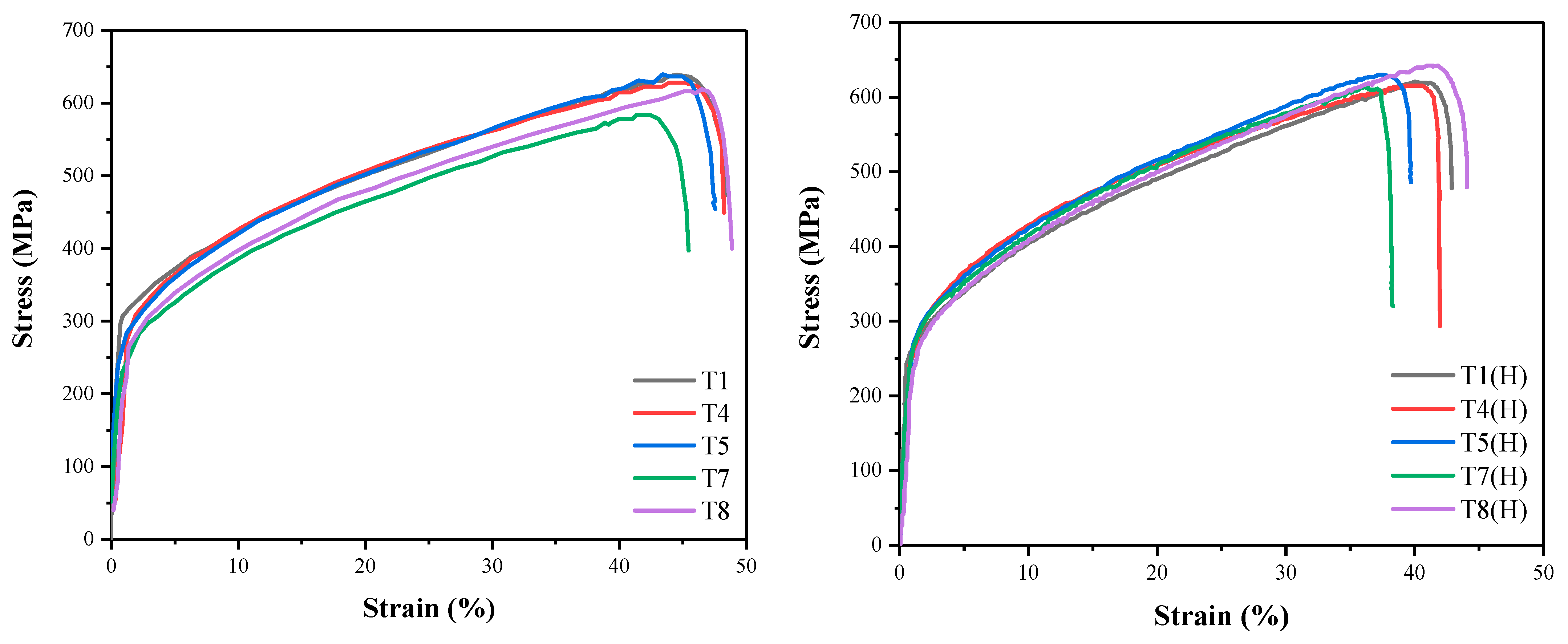
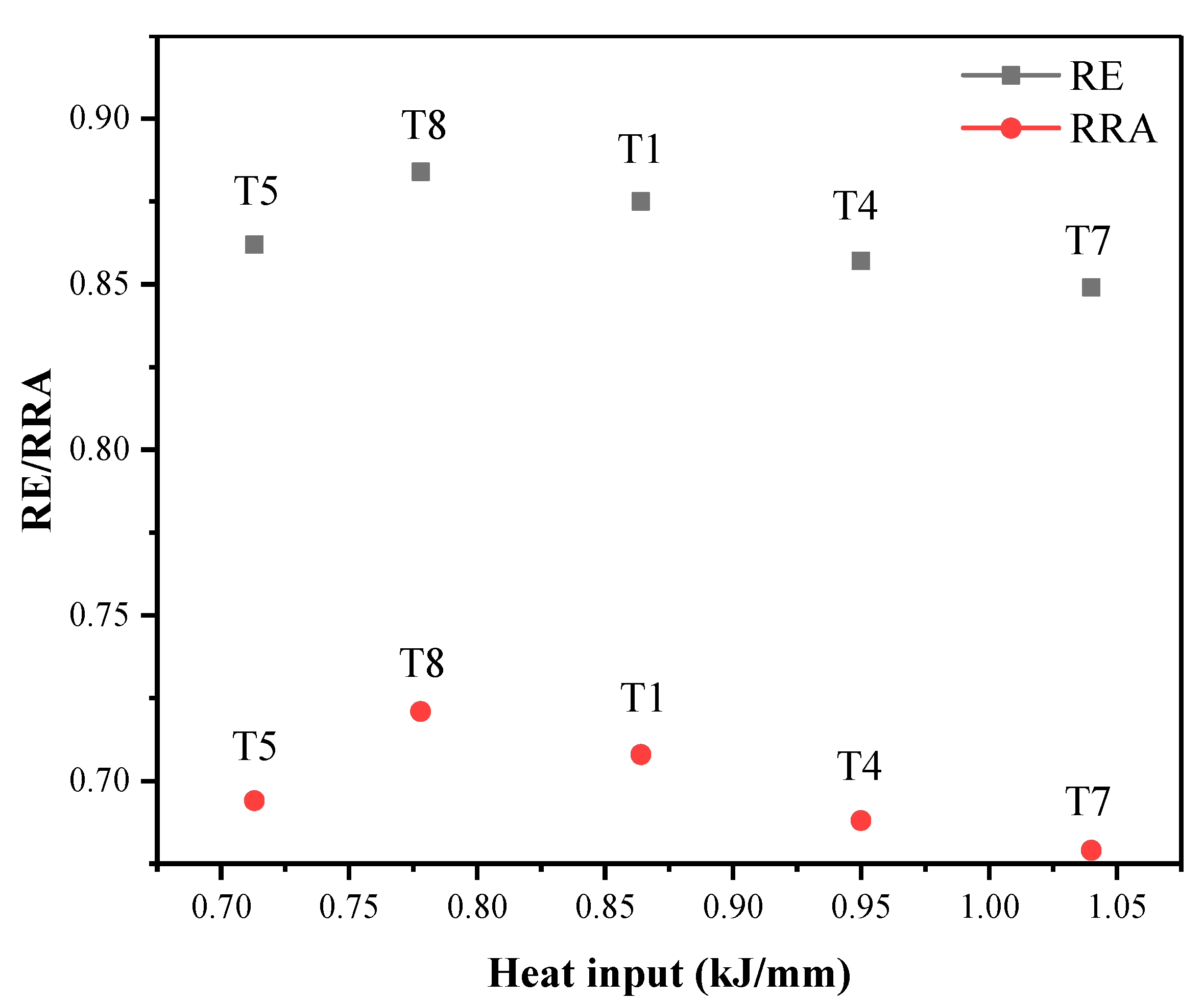
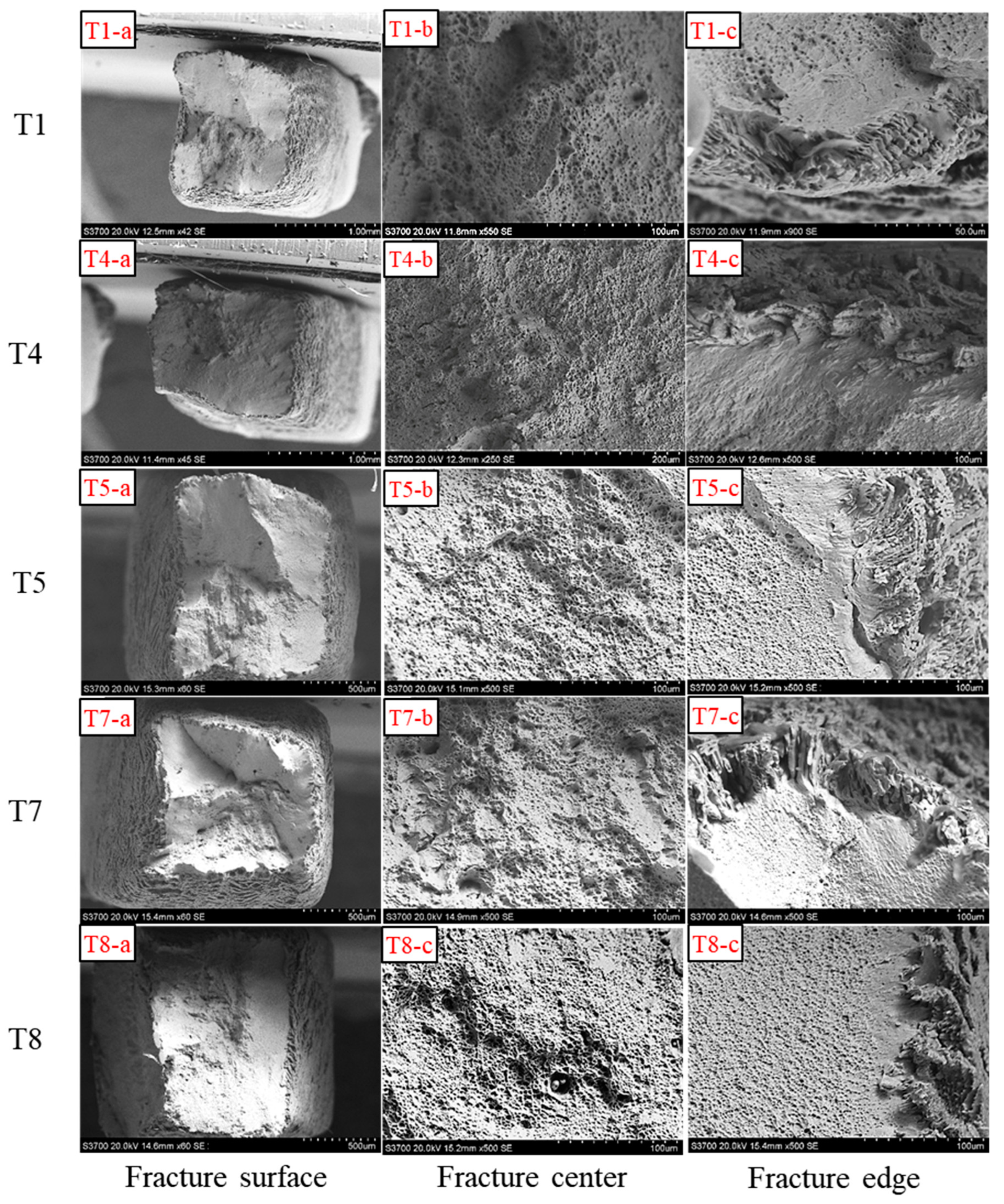
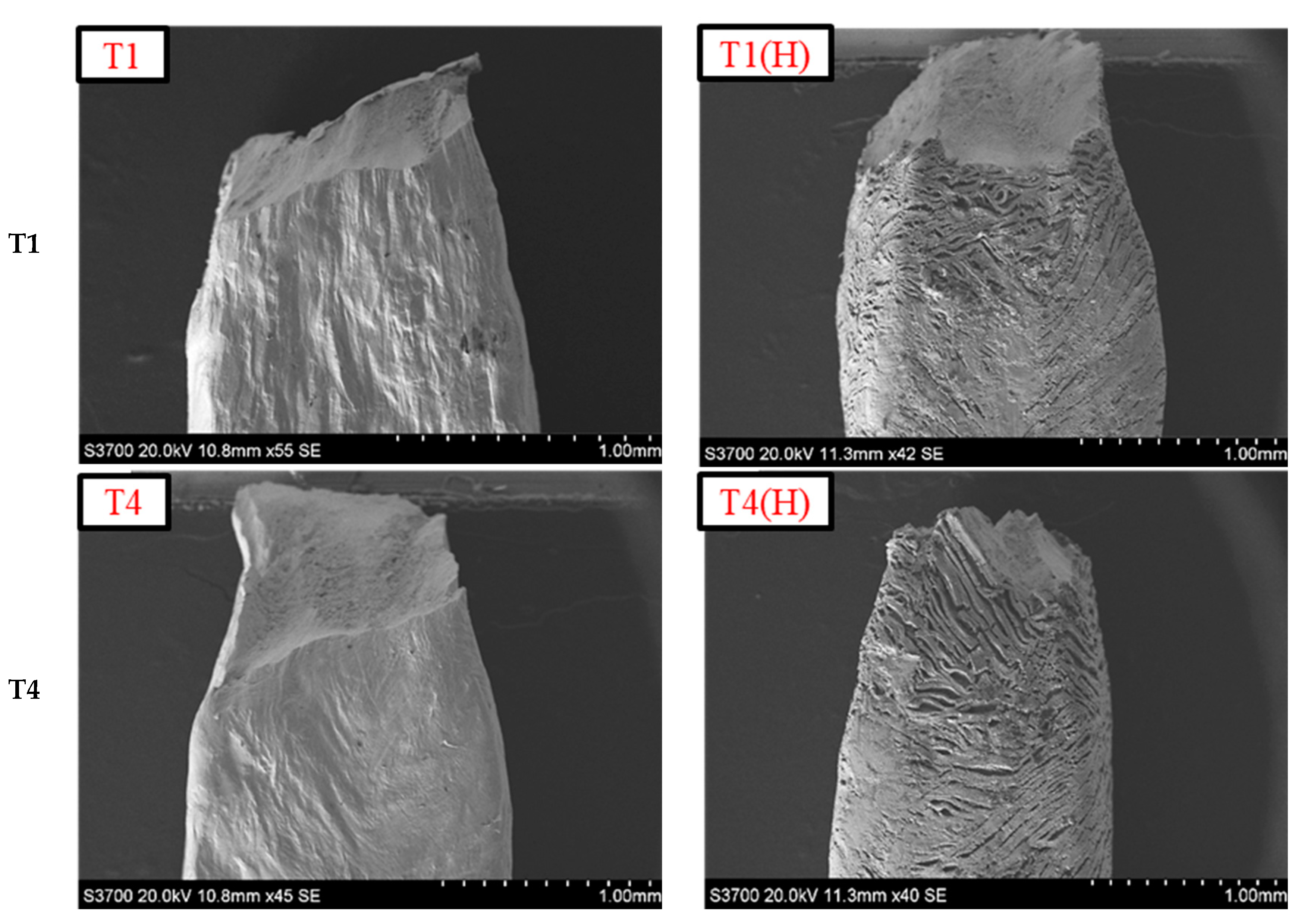
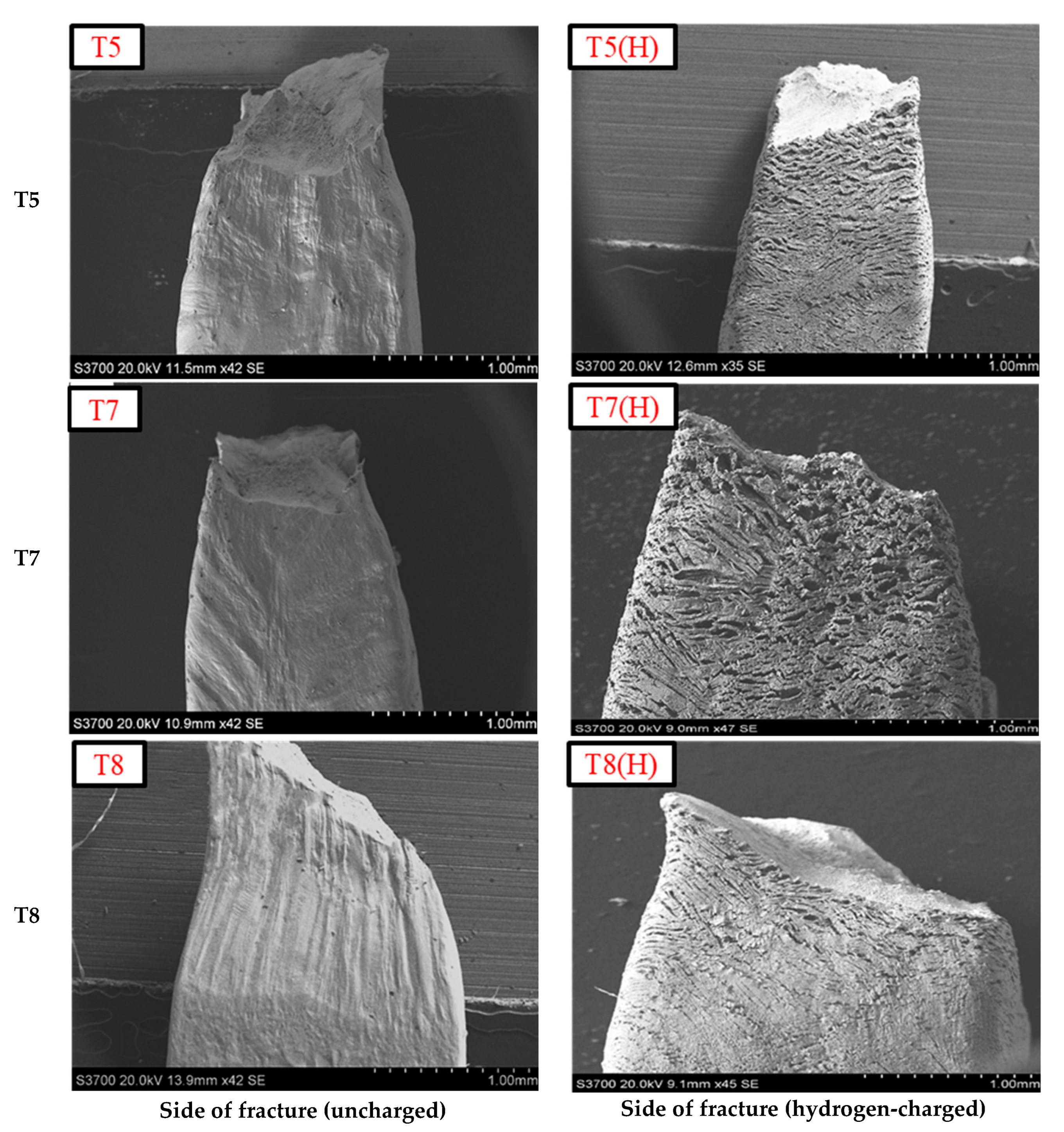
3.3. Influence of Heat Input on HE of Welds
4. Conclusions
- (1)
- After TIG welding, weld grain size significantly increased compared to the base metal. In addition, TIG weld tissue characteristics are closely related to the welding heat input. With an increase in TIG welding heat input, the ferrite content in the weld shows a tendency to first increase and then decrease. In the perspective of morphology, it first changes from lathy ferrite and strip ferrite to dendritic ferrite, and finally becomes reticular ferrite and lathy ferrite.
- (2)
- Through the study of the microstructure evolution pattern of 304 austenitic stainless steel TIG welds before and after hydrogen charging, it was found that a BCC martensitic phase with a content of 0.367% was generated in the weld after hydrogen charging. The leftward shift of the diffraction peak angle of the TIG weld after hydrogen charging indicates a slight increase in the lattice size of the weld.
- (3)
- With the increase in welding heat input, the resistance of 304 austenitic stainless steel TIG welds to HE first increases and then decreases. The resistance of TIG welds to HE at a heat input of 0.778 kJ/mm is better; the relative elongation (RE) is 0.884, and the relative reduction of area (RRA) is 0.721.
- (4)
- Continuous dendritic ferrite can improve the resistance of 304 stainless steel welds to HE. Therefore, in order to improve the resistance of the weld to HE in practice, the welding heat input should be changed to increase the continuous ferrite content.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Guo, T.; Xu, L.; Chen, L.; Jiang, B.; Wang, X.; Wu, H.; Qiao, L. Promotion of pitting corrosion at hydrogen-enriched α/γ phase boundaries in austenitic stainless steel weld joints. Acta Mater. 2022, 227, 117728. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, X.; Zhang, Y.; Wu, H.; Yang, Y. Numerical study on effect of inclusions on hydrogen segregation in steel under stress conditions. Int. J. Hydrogen Energy 2022, 47, 20310–20322. [Google Scholar] [CrossRef]
- Kim, K.S.; Kang, J.H.; Kim, S.J. Nitrogen effect on hydrogen diffusivity and hydrogen embrittlement behavior in austenitic stainless steels. Scr. Mater. 2020, 184, 70–73. [Google Scholar] [CrossRef]
- Landowski, M.; Swierczynska, A.; Rogalski, G.; Fydrych, D. Autogenous Fiber Laser Welding of 316L Austenitic and 2304 Lean Duplex Stainless Steels. Materials 2020, 13, 2930. [Google Scholar] [CrossRef] [PubMed]
- Hyza, B.; Jh, D.; Xmm, B.; Ying, S.B.; Tao, W.A.; Wjl, A.; Qxsa, C.; Jymc, E.; Dyz, F.; Wei, L. Effect of deformation microstructures on hydrogen embrittlement sensitivity and failure mechanism of 304 austenitic stainless steel: The significant role of rolling temperature. J. Mater. Res. Technol. 2022, 17, 2831–2846. [Google Scholar]
- Thakare, J.G.; Pandey, C.; Mahapatra, M.M.; Mulik, R.S. An assessment for mechanical and microstructure behavior of dissimilar material welded joint between nuclear grade martensitic P91 and austenitic SS304 L steel. J. Manuf. Process. 2019, 48, 249–259. [Google Scholar] [CrossRef]
- Ogawa, Y.; Takakuwa, O.; Okazaki, S.; Okita, K.; Matsuoka, S. Pronounced transition of crack initiation and propagation modes in the hydrogen-related failure of a Ni-based superalloy 718 under internal and external hydrogen conditions. Corros. Sci. 2019, 161, 108186. [Google Scholar] [CrossRef]
- Izawa, C.; Wagner, S.; Deutges, M.; Martin, M.; Weber, S.; Pargeter, R.; Michler, T.; Uchida, H.H.; Gemma, R.; Pundt, A. Relationship between hydrogen embrittlement and M-d30 temperature: Prediction of low-nickel austenitic stainless steel’s resistance. Int. J. Hydrogen Energy 2019, 44, 25064–25075. [Google Scholar] [CrossRef]
- Singh, S.; Altstetter, C. Effects of hydrogen concentration on slow crack growth in stainless steels. Metall. Mater. Trans. A 1982, 13, 1799–1808. [Google Scholar] [CrossRef]
- Perng, T.P.; Altstetter, C.J. Comparison of hydrogen gas embrittlement of austenitic and ferritic stainless steels. Metall. Mater. Trans. A 1987, 18, 123–134. [Google Scholar] [CrossRef]
- Gavriljuk, V.G.; Stein, G.; Berns, H. Structural Stability of Austenitic CrMn(Mo)N Steels for High-Strength, Corrosion-Resistant Retaining Rings. Steel Res. Int. 2003, 74, 444–452. [Google Scholar] [CrossRef]
- Shivanyuk, V. On a role of hydrogen-induced ɛ-martensite in embrittlement of stable austenitic steel. Scr. Mater. 2003, 49, 601–606. [Google Scholar] [CrossRef]
- Teus, S.M.; Shyvanyuk, V.N.; Gavriljuk, V.G. Hydrogen-induced γ→ɛ transformation and the role of ɛ-martensite in hydrogen embrittlement of austenitic steels. Mater. Sci. Eng. A 2008, 497, 290–294. [Google Scholar] [CrossRef]
- Martin, M.; Weber, S.; Izawa, C.; Wagner, S.; Pundt, A.; Theisen, W. Influence of machining-induced martensite on hydrogen-assisted fracture of AISI type 304 austenitic stainless steel. Int. J. Hydrogen Energy 2011, 36, 11195–11206. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Li, X.; Wu, W.; Gong, J. Combined effects of prior plastic deformation and sensitization on hydrogen embrittlement of 304 austenitic stainless steel. Int. J. Hydrogen Energy 2019, 44, 7014–7031. [Google Scholar] [CrossRef]
- Gulenc, B.; Develi, K.; Kahraman, N.; Durgutlu, A. Experimental study of the effect of hydrogen in argon as a shielding gas in MIG welding of austenitic stainless steel. Int. J. Hydrogen Energy 2005, 30, 1475–1481. [Google Scholar] [CrossRef]
- Reccagni, P.; Rnek, C.; Engelberg, D.L.; Lu, Q.; Gittos, M. Assessment of the Influence of Hydrogen on the Microstructure of TIG Welded Grade 2205 Duplex Stainless Steel. In Proceedings of the 2016 European Corrosion Congress, Montpellier, France, 11–15 September 2016. [Google Scholar]
- Chen, X.Y.; Ma, L.L.; Zhou, C.S.; Hong, Y.J.; Tao, H.M.; Zheng, J.Y.; Zhang, L. Improved resistance to hydrogen environment embrittlement of warm-deformed 304 austenitic stainless steel in high-pressure hydrogen atmosphere. Corros. Sci. 2019, 148, 159–170. [Google Scholar] [CrossRef]
- Luppo, M.I.; Hazarabedian, A.; Ovejero-García, J. Effects of delta ferrite on hydrogen embrittlement of austenitic stainless steel welds. Corros. Sci. 1999, 41, 87–103. [Google Scholar] [CrossRef]
- Zhou, C.; Dai, P.; Wu, H.; He, M.; Liu, X.; Chu, P.K. Effect of the ferrite morphology on hydrogen embrittlement of MAG welded 304 austenitic stainless steel. Appl. Surf. Sci. 2022, 606, 154866. [Google Scholar] [CrossRef]
- Fu, Z.H.; Yang, B.J.; Shan, M.L.; Li, T.; Zhu, Z.Y.; Ma, C.P.; Zhang, X.; Gou, G.Q.; Wang, Z.R.; Gao, W. Hydrogen embrittlement behavior of SUS301L-MT stainless steel laser-arc hybrid welded joint localized zones. Corros. Sci. 2020, 164, 108337. [Google Scholar] [CrossRef]
- Fu, Z.H.; Li, T.; Shan, M.L.; Gou, G.Q.; Zhu, Z.Y.; Ma, C.P.; Gao, W.; Hu, Y.C. Hydrogen atoms on the SCC behavior of SUS301L-MT stainless steel laser-arc hybrid welded joints. Corros. Sci. 2019, 148, 272–280. [Google Scholar] [CrossRef]
- Cao, R.; Xu, L.; Jiang, B.; Gao, M.; Qiao, L. Coupling effect of microstructure and hydrogen absorbed during service on pitting corrosion of 321 austenitic stainless steel weld joints. Corros. Sci. 2019, 164, 108339. [Google Scholar] [CrossRef]
- Takeda, S.; Ohara, Y.; Uchimoto, T.; Enoki, H.; Iijima, T.; Tokuda, E.; Yamada, T.; Nagatomo, Y. Characterization of fatigue crack of hydrogen-charged austenitic stainless steel by electromagnetic and ultrasonic techniques. Int. J. Hydrogen Energy 2022, 47, 32223–32234. [Google Scholar] [CrossRef]
- Michler, T.; Naumann, J. Hydrogen environment embrittlement of austenitic stainless steels at low temperatures. Int. J. Hydrogen Energy 2008, 33, 2111–2122. [Google Scholar] [CrossRef]
- Fukuyama, S.; Sun, D.S.; Zhang, L.; Wen, M.; Yokogawa, K. Effect of temperature on hydrogen environment embrittlement of type 316 series austenitic stainless seels at low temperatures. J. Jpn. I Met. 2003, 67, 456–459. [Google Scholar] [CrossRef]
- Han, Y.D.; Jing, H.Y.; Xu, L.Y. Welding heat input effect on the hydrogen permeation in the X80 steel welded joints. Mater. Chem. Phys. 2012, 132, 216–222. [Google Scholar] [CrossRef]
- GB/T 24511-2017; Stainless Steel and Heat Resisting Steel Plate, Sheet and Strip for Pressure Equipments. Chinese Standard Press: Beijing, China, 2017.
- GB/T 983-2012; Covered Electrodes for Manual Metal Arc Welding of Stainless and Heat-Resisting Steels. Chinese Standard Press: Beijing, China, 2012.
- GB/T 13298-2015; Inspection Methods of Microstructure for Metals. Chinese Standard Press: Beijing, China, 2015.
- ISO/TR 17671-1:2002(E); Welding—Recommendations for Welding of Metallic Materials—Part 1: General Guidance for Arc Welding. ISO: Geneva, Switzerland, 2002.
- Lippold, J.C. Solidification of Austenitic Stainless Steel Weldments. Weld. J. 1979, 58, 362S–374S. [Google Scholar]
- Hu, H.; Zhao, M.; Chen, S.; Rong, L. Effect of grain boundary character distribution on hydrogen embrittlement in Fe–Ni based alloy. Mater. Sci. Eng. A 2020, 780, 139201. [Google Scholar] [CrossRef]
- Mundra, K.; Blackburn, J.M.; Debroy, T. Absorption and transport of hydrogen during gas metal arc welding of low alloy steel. Sci. Technol. Weld. Join. 1997, 2, 174–184. [Google Scholar] [CrossRef]
- Wessman, S. Evaluation of the WRC 1992 diagram using computational thermodynamics. Welding in the World 2013, 57, 305–313. [Google Scholar] [CrossRef]
- Saluja, R.; Moeed, K. Experimental Investigation of Solidification-Mode and Response Surface Modeling of Ferrite-Content in Grade 304L Pulse GMA Welded Plates. Mater. Today Proc. 2019, 18, 3876–3890. [Google Scholar] [CrossRef]
- Chuaiphan, W.; Srijaroenpramong, L. Optimization of TIG welding parameter in dissimilar joints of low nickel stainless steel AISI 205 and AISI 216. J. Manuf. Process. 2020, 58, 163–178. [Google Scholar] [CrossRef]
- Kotecki, D.J. WRC-1992 Constitution Diagram for Stainless Steel Weld Metals. Weld. J. 2017, 96, 22–24. [Google Scholar]
- Zhao, Q. Effect of Hydrogen on Internal Friction of NiTi—based alloys. Master’s Thesis, Nanjing Tech University, Nanjing, China, 2014. (In Chinese). [Google Scholar]
- Mine, Y.; Koga, K.; Kraft, O.; Takashima, K. Mechanical characterisation of hydrogen-induced quasi-cleavage in a metastable austenitic steel using micro-tensile testing. Scr. Mater. 2016, 113, 176–179. [Google Scholar] [CrossRef]
- Yang, Q.; Qiao, L.J.; Chiovelli, S.; Luo, J.L. Critical hydrogen charging conditions for martensite transformation and surface cracking in type 304 stainless steel. Scr. Mater. 1999, 40, 1209–1214. [Google Scholar] [CrossRef]
- Hoelzel, M.; Danilkin, S.A.; Ehrenberg, H.; Toebbens, D.M.; Udovic, T.J.; Fuess, H.; Wipf, H. Effects of high-pressure hydrogen charging on the structure of austenitic stainless steels. Mater. Sci. Eng. A 2004, 384, 255–261. [Google Scholar] [CrossRef]
- Li, X.G.; Gong, B.M.; Deng, C.Y.; Li, Y.Z. Effect of pre-strain on microstructure and hydrogen embrittlement of K-TIG welded austenitic stainless steel. Corros. Sci. 2019, 149, 1–17. [Google Scholar] [CrossRef]
- El-Tahawy, M.; Um, T.; Nam, H.S.; Choe, H.; Gubicza, J. The effect of hydrogen charging on the evolution of lattice defects and phase composition during tension in 316L stainless steel. Mater. Sci. Eng. A 2019, 739, 31–36. [Google Scholar] [CrossRef]
- Zheng, Y.; Tan, Y.; Zhou, C.; Chen, G.; Zhang, G. A review on effect of hydrogen on rubber seals used in the high-pressure hydrogen infrastructure. Int. J. Hydrogen Energy 2020, 45, 23721–23738. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Z.; Xu, X.; Li, Y.; Du, C.; Liu, X. Interaction between hydrogen and cyclic stress and its role in fatigue damage mechanism. Corros. Sci. 2019, 157, 146–156. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.Y.; Zheng, J.Y.; Zhao, Y.Z.; Xu, P.; Zhou, C.L.; Li, X. Effect of strain-induced martensite on hydrogen embrittlement of austenitic stainless steels investigated by combined tension and hydrogen release methods. Int. J. Hydrogen Energy 2013, 38, 8208–8214. [Google Scholar] [CrossRef]
- Dong, L.; Han, E.H.; Peng, Q.; Ke, W.; Wang, L. Environmentally assisted crack growth in 308L stainless steel weld metal in simulated primary water. Corros. Sci. 2017, 117, 1–10. [Google Scholar] [CrossRef]
- Robinson, K. The influence of lattice hydrogen content on the hydrogen-assisted cracking of high strength steel. Corros. Sci. 1986, 26, 705–717. [Google Scholar]
- Rozenak, P.; Eliezer, D. Effects of metallurgical variables on hydrogen embrittlement in AISI type 316, 321 and 347 stainless steels. Mater. Sci. Eng. A 1983, 61, 31–41. [Google Scholar] [CrossRef]
- Boiler, A. ASME Boiler and Pressure Vessel Code: An International Code; American Society of Mechanical Engineers: New York, NY, USA, 2007. [Google Scholar]
- Se-Hwan , C.H.I.; Kim, G.C.; Hong, J.H.; Shin, Y.K.; Kim, Y.J. The influence of delta (δ) ferrite on the irradiation effects in type-304 stainless steel weldment. In Proceedings of the Korean Nuclear Society Spring Meeting, Cheju, Korea, 18 May 2001. [Google Scholar]
- Buckley, J.R.; Hardie, D. The effect of pre-straining and δ-ferrite on the embrittlement of 304L stainless steel by hydrogen. Corros. Sci. 1993, 34, 93–107. [Google Scholar] [CrossRef]
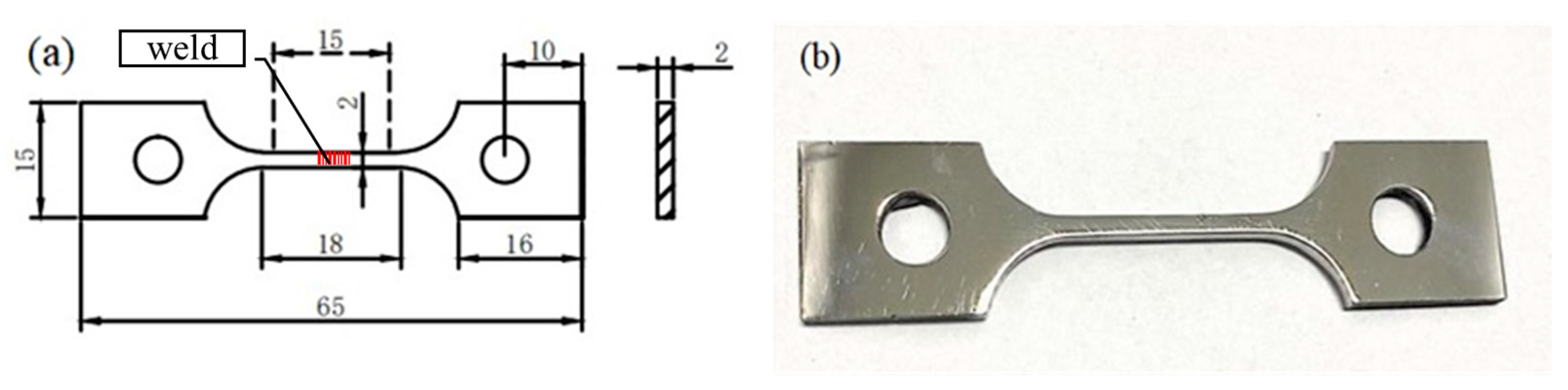



| Material | C | Si | Mn | P | S | Cr | Ni | N | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 304 (wt. %) | 0.078 | 0.584 | 1.10 | 0.02 | 0.012 | 19.05 | 8.08 | 0.080 | Bal. |
| E308 (wt. %) | 0.074 | 0.835 | 0.67 | 0.031 | 0.019 | 18.36 | 9.34 | - | Bal. |
| Material | Data Source | Tensile Strength (MPa) | Yield Strength (MPa) | Elongation (%) |
|---|---|---|---|---|
| 304 | Measured | 733 | 315 | 68.2 |
| standard [24] | ≥520 | ≥220 | ≥40 |
| Weld | Welding Current (A) | Welding Voltage (V) | Welding Speed (mm/min) | Heat Input (kJ/mm) |
|---|---|---|---|---|
| T1 | 200 | 18 | 150 | 0.864 |
| T2 | 200 | 18 | 200 | 0.648 |
| T3 | 200 | 18 | 250 | 0.518 |
| T4 | 220 | 18 | 150 | 0.950 |
| T5 | 220 | 18 | 200 | 0.713 |
| T6 | 220 | 18 | 250 | 0.570 |
| T7 | 240 | 18 | 150 | 1.04 |
| T8 | 240 | 18 | 200 | 0.778 |
| T9 | 240 | 18 | 250 | 0.622 |
| Solidification Mode | Reaction Process | Microstructure | Ferrite Content Range (%) |
|---|---|---|---|
| A | L → L + A → A | Full austenite, regular solidification structure | 0 |
| AF | L → L + A → L + A + (A + F) → F + A | Ferrites exist at cell and dendrite boundaries | 0~4 |
| FA | L → L + F → L + F + → F + A + A (F) | Skeletonized and lathy ferrite formed after ferrite-to-austenite transformation | 4~20 |
| F | L → L + F → F → F + A | Acicular ferrite or ferrite parent phase with grain boundaries in the form of austenite and Widmanstatten | 20 or more |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, J.; Wu, H.; Zhou, C.; Zhang, Y.; He, M.; Yan, X.; Xie, H.; Yan, R.; Yin, Y. Effect of Heat Input on Hydrogen Embrittlement of TIG Welded 304 Austenitic Stainless Steel. Metals 2022, 12, 1943. https://doi.org/10.3390/met12111943
Xue J, Wu H, Zhou C, Zhang Y, He M, Yan X, Xie H, Yan R, Yin Y. Effect of Heat Input on Hydrogen Embrittlement of TIG Welded 304 Austenitic Stainless Steel. Metals. 2022; 12(11):1943. https://doi.org/10.3390/met12111943
Chicago/Turabian StyleXue, Jinxin, Hao Wu, Chilou Zhou, Yuanming Zhang, Mohan He, Xinrui Yan, Huiyu Xie, Rui Yan, and Yansheng Yin. 2022. "Effect of Heat Input on Hydrogen Embrittlement of TIG Welded 304 Austenitic Stainless Steel" Metals 12, no. 11: 1943. https://doi.org/10.3390/met12111943
APA StyleXue, J., Wu, H., Zhou, C., Zhang, Y., He, M., Yan, X., Xie, H., Yan, R., & Yin, Y. (2022). Effect of Heat Input on Hydrogen Embrittlement of TIG Welded 304 Austenitic Stainless Steel. Metals, 12(11), 1943. https://doi.org/10.3390/met12111943









