High Temperature Oxidation Behaviors of Powder Metallurgical γ-TiAl Based Alloys: Effects of Surface Defects on Morphology of the Oxide Scale
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
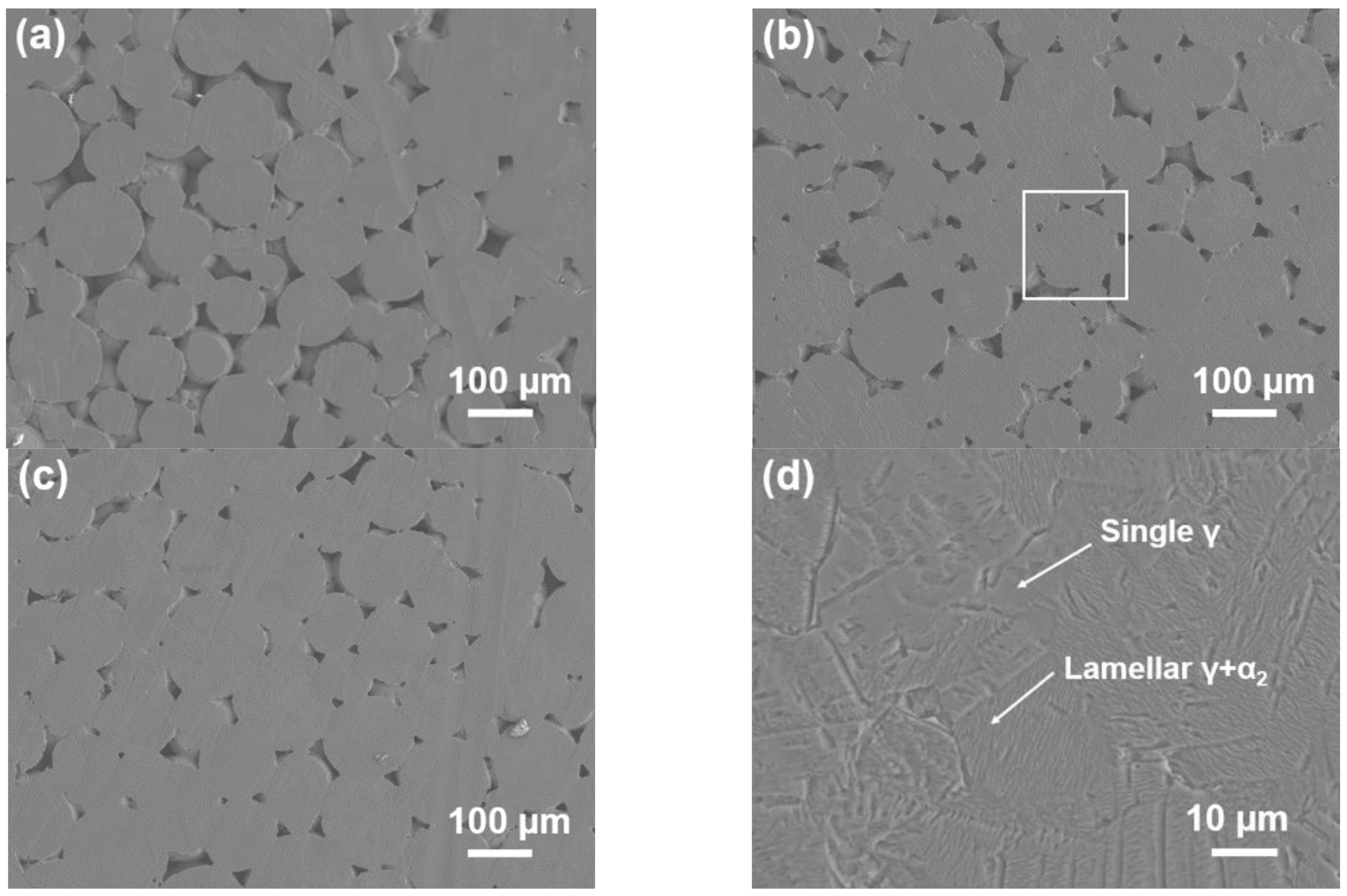
3.1. Alloys Microstructure
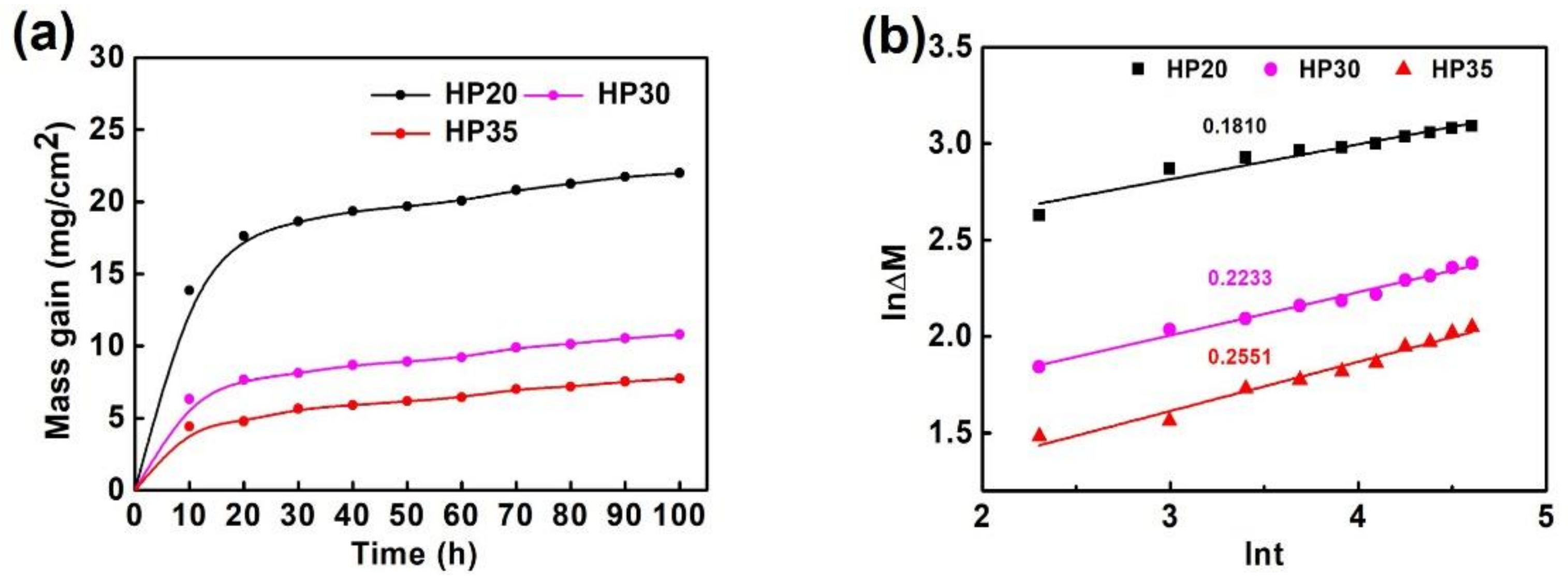
3.2. Oxidation Kinetics
3.3. Phase Composition of Oxide Scale
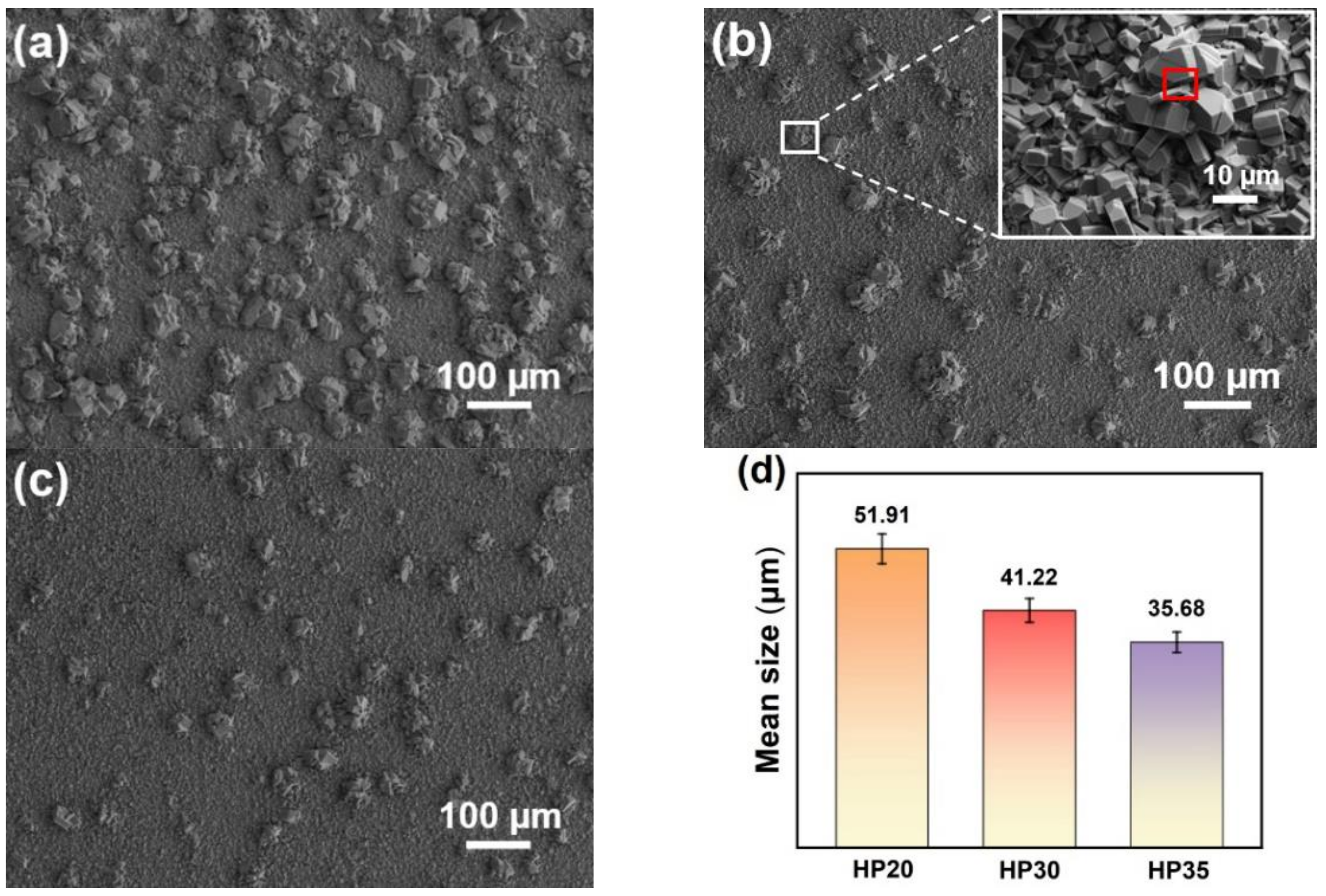
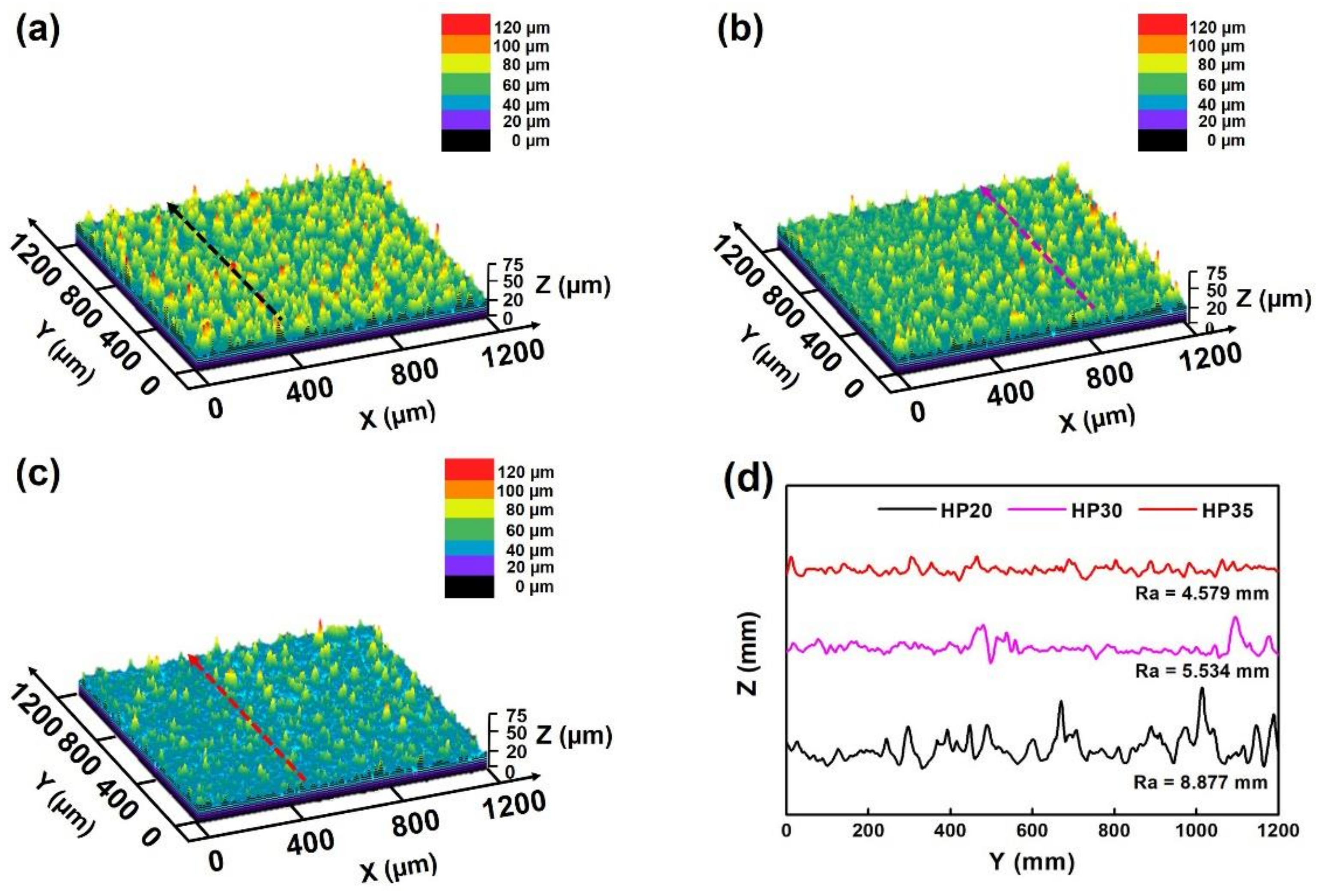
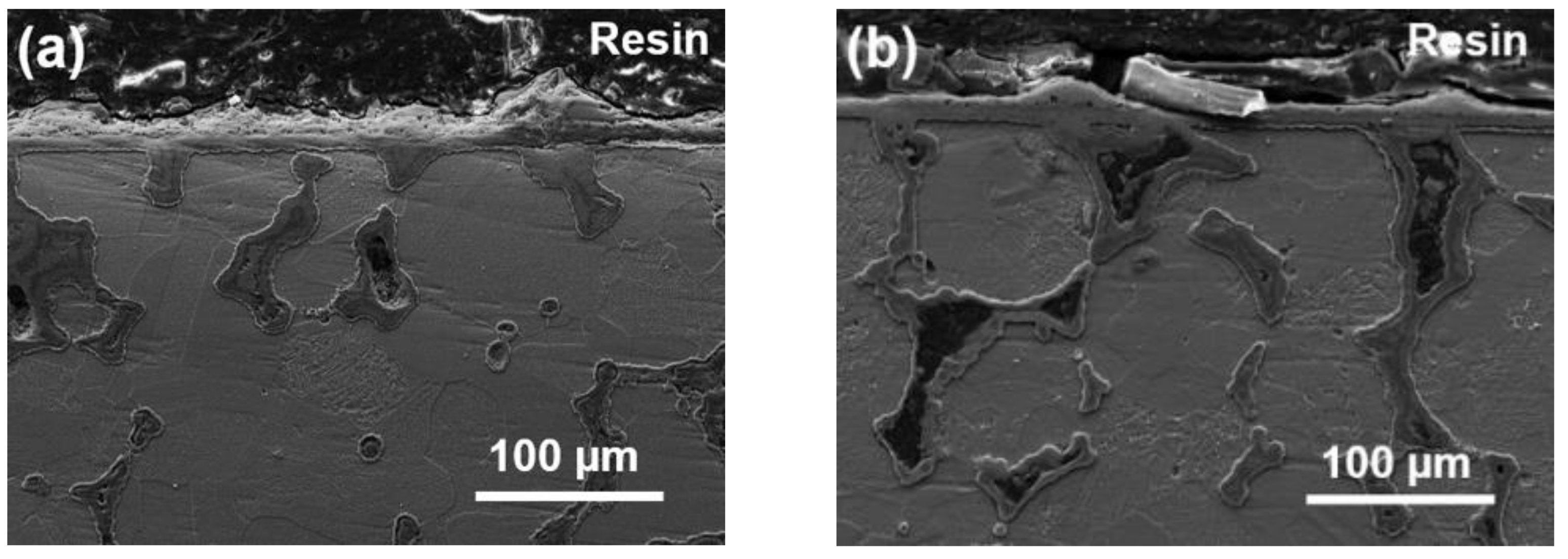
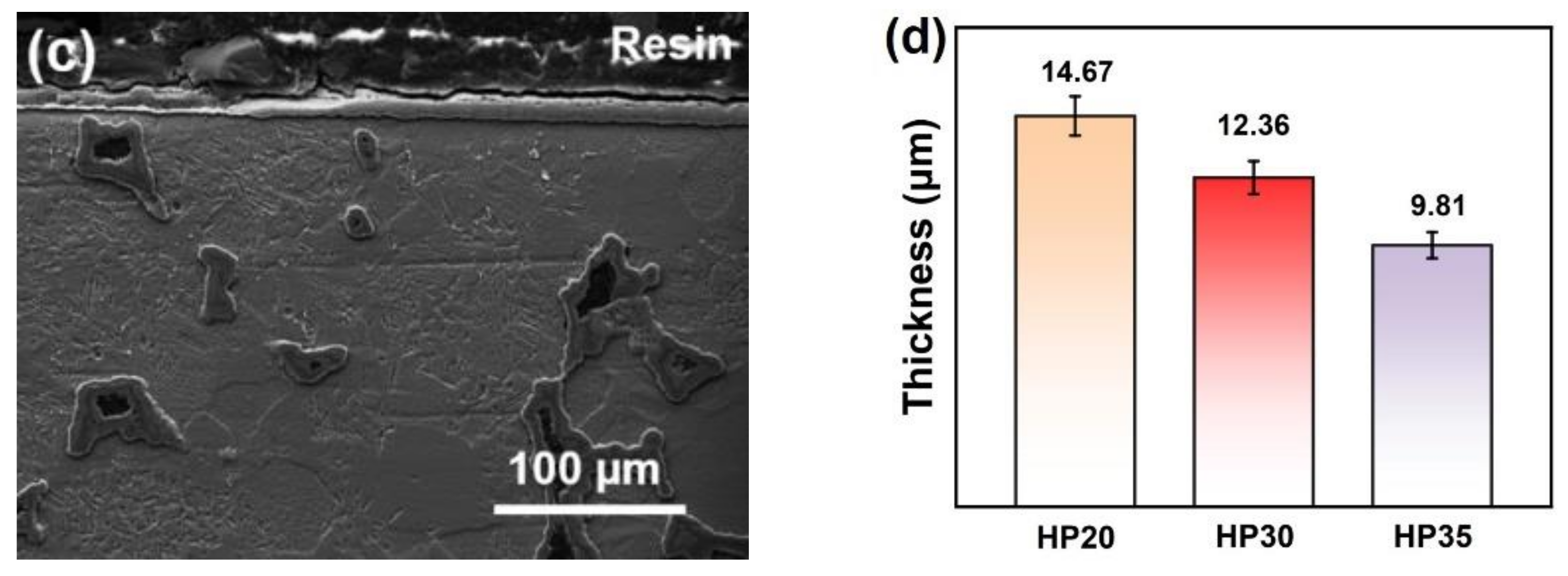
3.4. Morphology Feature of Oxide Scale
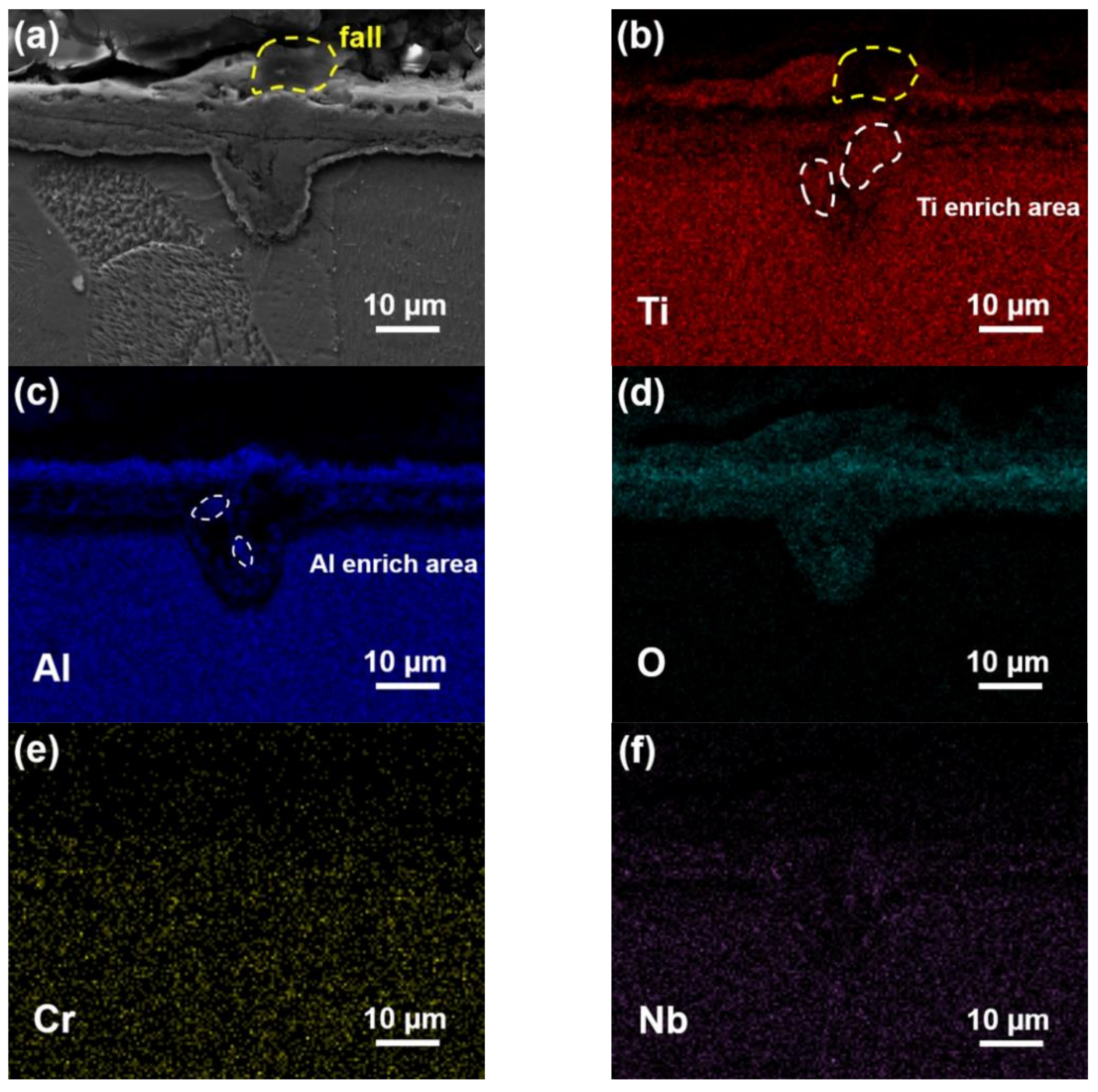
3.5. Chemical Composition of Oxide Scale
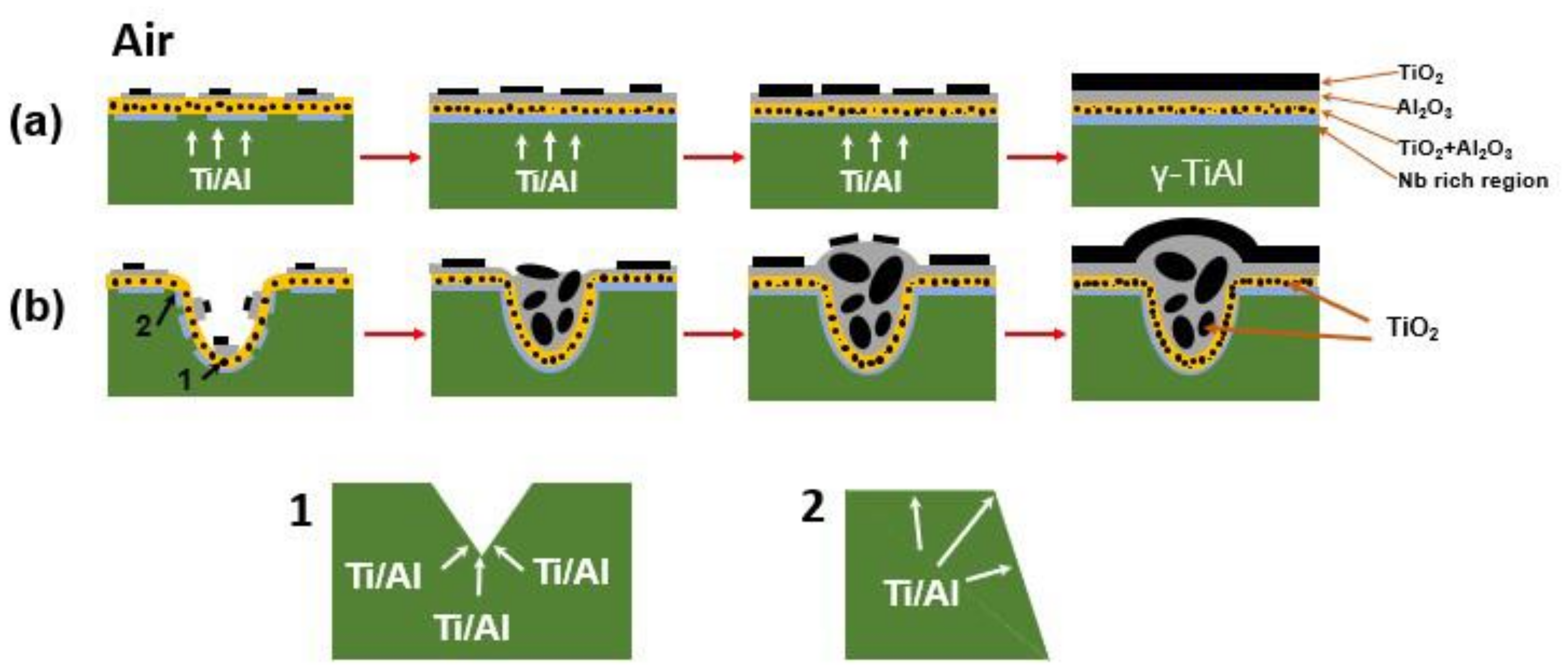
3.6. Formation Mechanism of Oxide Clusters
4. Conclusions
- (1)
- For alloys prepared at sintering pressures that increased from 20 to 35 MPa, the sintering necks tendered to grow; thus, the metallurgical defects decreased in amount and size unceasingly.
- (2)
- In the cyclic oxidation process, the existence of defects increased the actual contact area between the alloy and O. With the decrease of defects, the oxidation weight gain per unit area decreased continuously.
- (3)
- The oxide clusters normally formed at the positions of surface defects. When the number and size of the surface defects decreased, the number and sizes of the oxide clusters decreased accordingly.
- (4)
- Element diffusion to the inner face of the defects led to the formation of large TiO2 and Al2O3 particles, which should be the main reason for the formation of oxide clusters.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pradeep, N.B.; Hegde, M.M.R.; Rajendrachari, S.; Surendranathan, A.O. Investigation of Microstructure and Mechanical Properties of Microwave Consolidated Timgsr Alloy Prepared by High Energy Ball Milling. Powder Technol. 2022, 408, 117715. [Google Scholar] [CrossRef]
- Basavarajappa, P.N.; Hegde, M.M.R.; Rajendrachari, S.; Surendranathan, A.O. Investigation of Structural and Mechanical Properties of Nanostructured Timgsr Alloy for Biomedical Applications. Biointerface Res. Appl. Chem. 2022, 13, 118. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Z.; Wang, Q.; Zhao, L.; Zhang, X.; Gao, W.; Xu, J.; Song, Y.; Song, X.; Zhang, X.J.C. Optimization of Parameters in Laser Powder Bed Fusion TA15 Titanium Alloy Using Taguchi Method. Crystals 2022, 12, 1385. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Z.; Liu, W.; Ma, Y.; Zhang, X.; Zhao, L.; Yang, K.; Chen, Y.; Cai, Q.; Liang, C.; et al. Microstructure Evolution and Reaction Mechanism of Continuously Compositionally Ti/Al Intermetallic Graded Material Fabricated by Laser Powder Deposition. J. Mater. Res. Technol. 2022, 20, 4173–4185. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Wang, T.; Liu, W.; Ma, Y.J.M.S.; Treatment, H. Effects of Heat Treatment on the Microstructure and Properties of Graded-Density Powder Aluminum Alloys. Met. Sci. Heat Treat. 2022, 63, 590–598. [Google Scholar] [CrossRef]
- Kothari, K.; Radhakrishnan, R.; Wereley, N.M. Progress in Aerospace Sciences Advances in Gamma Titanium Aluminides and Their Manufacturing Techniques. Prog. Aerosp. Sci. 2012, 55, 1–16. [Google Scholar] [CrossRef]
- Appel, F.; Wagner, R. Microstructure and Deformation of Two-Phase γ-Titanium Aluminides. Mater. Sci. Eng. R Rep. 1998, 22, 187–268. [Google Scholar] [CrossRef]
- Zhang, S.; Tian, S.; Lv, X.; Yu, H.; Tian, N.; Jiao, Z.; Zhao, G.; Li, D. Deformation and Damage Behaviors of As-Cast TiAl-Nb Alloy during Creep. Prog. Nat. Sci. Mater. Int. 2018, 28, 618–625. [Google Scholar] [CrossRef]
- Duan, Z.; Han, Y.; Song, X.; Chen, H. Creep Behaviour of Equiaxed Fine-Grain γ-TiAl-Based Alloy Prepared by Powder Metallurgy. Mater. Sci. Technol. U. K. 2020, 36, 1457–1464. [Google Scholar] [CrossRef]
- Xuezheng, W.; Zhenxin, D.; Hua, C. High Temperature Mechanical Properties and Deformation Mechanism of Equiaxed Fine-Grained Ti-45 7 Nb Alloys. Rare Met. Mater. Eng. 2022, 51, 1543–1549. [Google Scholar]
- Dobbelstein, H.; Gurevich, E.L.; George, E.P.; Ostendorf, A.; Laplanche, G. Laser Metal Deposition of a Refractory TiZrNbHfTa High-Entropy Alloy. Addit. Manuf. 2018, 24, 386–390. [Google Scholar] [CrossRef]
- Wen, Z.; Song, X.; Chen, D.; Fan, T.; Liu, Y.; Cai, Q. Electrospinning Preparation and Microstructure Characterization of Homogeneous Diphasic Mullite Ceramic Nanofibers. Ceram. Int. 2020, 46, 12172–12179. [Google Scholar] [CrossRef]
- Song, X.; Zhang, K.; Song, Y.; Duan, Z.; Liu, Q.; Liu, Y. Morphology, Microstructure and Mechanical Properties of Electrospun Alumina Nanofibers Prepared Using Different Polymer Templates: A Comparative Study. J. Alloys Compd. 2020, 829, 154502. [Google Scholar] [CrossRef]
- Hu, M.; Wang, L.; Li, G.; Huang, Q.; Liu, Y.; He, J.; Wu, H.; Song, M.J.I. Investigations on Microstructure and Properties of Ti-Nb-Zr Medium-Entropy Alloys for Metallic Biomaterials. Intermetallics 2022, 145, 107568. [Google Scholar] [CrossRef]
- Zhao, Q.; Pan, Z.; Wang, X.; Luo, H.; Liu, Y.; Li, X. Corrosion and Passive Behavior of Alxcrfeni3-x (x = 0.6, 0.8, 1.0) Eutectic High Entropy Alloys in Chloride Environment. Corros. Sci. 2022, 208, 110666. [Google Scholar] [CrossRef]
- Pflumm, R.; Friedle, S.; Schütze, M. Oxidation Protection of γ-TiAl-Based Alloys—A Review. Intermetallics 2015, 56, 1–14. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, J.; Chen, C.; Weng, F. High Temperature Oxidation Behavior and Research Status of Modifications on Improving High Temperature Oxidation Resistance of Titanium Alloys and Titanium Aluminides: A Review. J. Alloys Compd. 2016, 685, 784–798. [Google Scholar] [CrossRef]
- Pflumm, R.; Donchev, A.; Mayer, S.; Clemens, H.; Schütze, M. High-Temperature Oxidation Behavior of Multi-Phase Mo-Containing γ-TiAl-Based Alloys. Intermetallics 2014, 53, 45–55. [Google Scholar] [CrossRef]
- Appel, F.; Paul, J.D.H.; Oehring, M. Gamma Titanium Aluminide Alloys Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 9783527322619. [Google Scholar]
- Jie, L. Isothermal Oxidation Behavior of TiAl-Nb-W-B-Y Alloys with Different Lamellar Colony Sizes. Rare Met. Mater. Eng. 2016, 45, 1695–1699. [Google Scholar] [CrossRef]
- Rakowski, J.M.; Meier, G.H.; Pettit, F.S.; Dettenwanger, F.; Schumann, E.; Rühle, M. The Effect of Surface Preparation on the Oxidation Behavior of Gamma TiA1-Base Intermetallic Alloys. Scr. Mater. 1996, 35, 1417–1422. [Google Scholar] [CrossRef]
- Copland, E.H.; Gleeson, B.; Young, D.J. Formation of Z-Ti50Al30O20 in the Sub-Oxide Zones of γ-TiAl-Based Alloys during Oxidation at 1000 °C. Acta Mater. 1999, 47, 2937–2949. [Google Scholar] [CrossRef]
- Paul, J.D.H.; Lorenz, U.; Oehring, M.; Appel, F. Up-Scaling the Size of TiAl Components Made via Ingot Metallurgy. Intermetallics 2013, 32, 318–328. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Z.; Liu, S.; Shi, K.; Bao, C.; Ning, Z.; Yan, P.; Wang, L.; Lou, Y. Elimination of Misrun and Gas Hole Defects of Investment Casting TiAl Alloy Turbocharger Based on Numerical Simulation and Experimental Study. China Foundry 2020, 17, 29–34. [Google Scholar] [CrossRef]
- Bao, C.; Zhang, S.; Ren, Y.; Zhang, Y.; Xie, H.; Zhao, J. Research Progress on Refractory Composition and Deformability of Shell Molds for TiAl Alloy Castings. China Foundry 2018, 15, 1–10. [Google Scholar] [CrossRef]
- Xia, Y.; Yu, P.; Schaffer, G.B.; Qian, M. Cobalt-Doped Ti-48Al-2Cr-2Nb Alloy Fabricated by Cold Compaction and Pressureless Sintering. Mater. Sci. Eng. A 2013, 574, 176–185. [Google Scholar] [CrossRef]
- Kayani, S.H.; Park, N.K. Effect of Cr and Nb on the Phase Transformation and Pore Formation of Ti-Al Base Alloys. J. Alloys Compd. 2017, 708, 308–315. [Google Scholar] [CrossRef]
- Correa, D.R.N.; Grandini, C.R.; Rocha, L.A.; Proenca, J.P.; Sottovia, L.; Cruz, N.C.; Rangel, E.C.; Hanawa, T. Effect of temperature on thermal oxidation behavior of biomedical ti-zr-mo alloys. J. Alloys Compd. 2022, 905, 164202. [Google Scholar] [CrossRef]
- Yue, H.; Peng, H.; Li, R.; Su, Y.; Zhao, Y.; Qi, K.; Chen, Y. Selective Electron Beam Melting of TiAl Alloy: Metallurgical Defects, Tensile Property, and Determination of Process Window. Adv. Eng. Mater. 2020, 22, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Feng, R.; Qiao, H.; Li, H.; Wang, M.; Qi, Y.; Lei, C. Effect of Cutting Crystal Directions on Micro-Defect Evolution of Single Crystal γ-TiAl Alloy with Molecular Dynamics Simulation. Metals 2019, 9, 1278. [Google Scholar] [CrossRef]
- Xu, Z.; Ouyang, W.; Liu, Y.; Jiao, J.; Liu, Y.; Zhang, W. Effects of Laser Polishing on Surface Morphology and Mechanical Properties of Additive Manufactured TiAl Components. J. Manuf. Process. 2021, 65, 51–59. [Google Scholar] [CrossRef]
- Cai, X.; Sun, D.; Li, H.; Meng, C.; Wang, L.; Shen, C. Dissimilar Joining of TiAl Alloy and Ni-Based Superalloy by Laser Welding Technology Using V/Cu Composite Interlayer. Opt. Laser Technol. 2019, 111, 205–213. [Google Scholar] [CrossRef]
- Wu, H.N.; Xu, D.S.; Wang, H.; Yang, R. Molecular Dynamics Simulation of Tensile Deformation and Fracture of γ-TiAl with and without Surface Defects. J. Mater. Sci. Technol. 2016, 32, 1033–1042. [Google Scholar] [CrossRef]
- Huang, Z.W.; Lin, J.P.; Zhao, Z.X.; Sun, H.L. Fatigue Response of a Grain Refined TiAl Alloy Ti-44Al-5Nb-1W-1B with Varied Surface Quality and Thermal Exposure History. Intermetallics 2017, 85, 1–14. [Google Scholar] [CrossRef]
- Patriarca, L.; Milano, P.; Pasquero, G.; Avio, S.A.; Sabbadini, S. Gt2012-69986 Assessment of Fatigue Sensitivity to Defects of a TiAl Alloy. In Turbo Expo: Power for Land, Sea, and Air; American Society of Mechanical Engineers: New York, NY, USA, 2012; pp. 1–6. [Google Scholar]
- Trzaska, Z.; Couret, A.; Monchoux, J.P. Spark Plasma Sintering Mechanisms at the Necks between TiAl Powder Particles. Acta Mater. 2016, 118, 100–108. [Google Scholar] [CrossRef]
- Duan, Z.; Song, X.; Han, Y.; Pei, W.; Chen, H. Enhancing High-Temperature Strength and Ductility of γ-TiAl Matrix Composites with Controllable Dual Alloy Structure. Mater. Sci. Eng. A 2021, 823, 141723. [Google Scholar] [CrossRef]
- Pilone, D.; Felli, F.; Brotzu, A. High Temperature Oxidation Behaviour of TiAl-Cr-Nb-Mo Alloys. Intermetallics 2013, 43, 131–137. [Google Scholar] [CrossRef]
- Lee, D.B. Effect of Cr, Nb, Mn, V, W and Si on High Temperature Oxidation of TiAl Alloys. Met. Mater. Int. 2005, 11, 141–147. [Google Scholar] [CrossRef]
- Qu, S.J.; Tang, S.Q.; Feng, A.H.; Feng, C.; Shen, J.; Chen, D.L. Microstructural Evolution and High-Temperature Oxidation Mechanisms of a Titanium Aluminide Based Alloy. Acta Mater. 2018, 148, 300–310. [Google Scholar] [CrossRef]
- Terner, M.; Biamino, S.; Baudana, G.; Penna, A.; Fino, P.; Pavese, M.; Ugues, D.; Badini, C. Initial Oxidation Behavior in Air of TiAl-2Nb and TiAl-8Nb Alloys Produced by Electron Beam Melting. J. Mater. Eng. Perform. 2015, 24, 3982–3988. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Zhu, Y.; Lin, J.; Ren, Z. Oxidation Behavior of γ-TiAl Based Alloys at 900 °C in Air. Mater. High Temp. 2016, 33, 234–240. [Google Scholar] [CrossRef]
- Małecka, J. Resistance to High-Temperature Oxidation of Ti-Al-Nb Alloys. Materials 2022, 15, 2137. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Renteria, A.F.; Weiß, S. Role of Alloying Elements during Thermocyclic Oxidation of β/γ-TiAl Alloys at High Temperatures. J. Alloys Compd. 2017, 691, 489–497. [Google Scholar] [CrossRef]
- Kim, D.; Seo, D.; Huang, X.; Sawatzky, T.; Saari, H.; Hong, J.; Kim, Y.W. Oxidation Behaviour of Gamma Titanium Aluminides with or without Protective Coatings. Int. Mater. Rev. 2014, 59, 297–325. [Google Scholar] [CrossRef]

| Samples | Sintered Density (g/cm3) | Porosity (%) |
|---|---|---|
| HP20 | 3.484 | 11.8 |
| HP30 | 3.600 | 8.9 |
| HP35 | 3.772 | 4.6 |
| Samples | n | kn (mgn·cm−2n·h−1) |
|---|---|---|
| HP20 | 5.5249 | 12.5524 |
| HP30 | 4.4782 | 5.9785 |
| HP35 | 3.9200 | 3.3241 |
| Composition (at%) | Deduced Phase | ||
|---|---|---|---|
| Ti | Al | O | |
| 29.45 | 1.06 | 69.49 | TiO2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Z.; Song, X.; Duan, Z.; Chen, H.; Huang, H.; Liu, Y.; Han, Y. High Temperature Oxidation Behaviors of Powder Metallurgical γ-TiAl Based Alloys: Effects of Surface Defects on Morphology of the Oxide Scale. Metals 2022, 12, 1743. https://doi.org/10.3390/met12101743
Su Z, Song X, Duan Z, Chen H, Huang H, Liu Y, Han Y. High Temperature Oxidation Behaviors of Powder Metallurgical γ-TiAl Based Alloys: Effects of Surface Defects on Morphology of the Oxide Scale. Metals. 2022; 12(10):1743. https://doi.org/10.3390/met12101743
Chicago/Turabian StyleSu, Ziteng, Xiaolei Song, Zhenxin Duan, Hua Chen, Haoxuan Huang, Yang Liu, and Ying Han. 2022. "High Temperature Oxidation Behaviors of Powder Metallurgical γ-TiAl Based Alloys: Effects of Surface Defects on Morphology of the Oxide Scale" Metals 12, no. 10: 1743. https://doi.org/10.3390/met12101743
APA StyleSu, Z., Song, X., Duan, Z., Chen, H., Huang, H., Liu, Y., & Han, Y. (2022). High Temperature Oxidation Behaviors of Powder Metallurgical γ-TiAl Based Alloys: Effects of Surface Defects on Morphology of the Oxide Scale. Metals, 12(10), 1743. https://doi.org/10.3390/met12101743






