Abstract
The solidification of heavy metal Cr has always been a challenge in the treatment of Cr-containing wastes, due to its high mobility in alkaline environments. In addition, the solidification mechanism of Cr has not been fully investigated. In this study, blast furnace slag (BFS)-based cementitious materials were used as binders for the immobilization of heavy metal Cr to investigate the solidification mechanism of Cr in different hydration products. From XRD, FTIR, XPS, and XANES analyses, it could be seen that SO42− in ettringite was replaced by Cr in the form of CrO42−, making SO42− re-dissolve in the liquid phase. The SO42− in the solution would compete with CrO42− ions, leading to the direct influence of SO42− content on the solidification efficiency of Cr. In ettringite, Cr mainly existed in the form of Cr6+, accounting for more than 84% however, Cr was solidified in Friedel’s salt under two coexisting valence states (Cr6+ and Cr3+). This resulted not only from the slow excitation rate of the BFS in the cementitious system that did not contain sulfate, but also from the existence of a certain amount of reducing substances in the BFS, such as Fe2+ and S2−, which could reduce some of Cr6+ to Cr3+. In Friedel’s salt, the residual Cr6+ replaced Cl− in the form of CrO42−, whereas the Cr3+ replaced Al3+. The binding energies of Cr 2p3/1 and Cr 2p3/2 decreased with the addition of Cr, indicating that the coordination numbers of Cr3+ and Cr6+ increased, and that the binding energies of Cr3+ and Cr6+ decreased after entering the structure of Friedel’s salt.
1. Introduction
Heavy metal Cr accumulates in plants and organisms, and then enters the human body through the food chain, causing skin damage, respiratory or gastrointestinal diseases, and even brain cell damage. The Cr usually exists in the state of Cr3+ and Cr6+. Cr3+, an essential trace ion necessary for human body, has weak mobility and low toxicity, whereas Cr6+ has strong mobility and high toxicity [1,2,3] Therefore, the treatment of Cr-containing waste is quite difficult. The free energy of oxidation of Cr3+ to Cr6+ is −459 kJ under alkaline conditions, and only −22.12 kJ under acidic conditions [4], indicating that an alkaline environment is favorable for the oxidation of Cr3+ to Cr6+ into CrO42− or HCrO4− anions. The Cr6+ presents a lower adsorption for negatively charged particles and hydration products than Cr3+, resulting in its strong solubility and mobility in alkaline environments. Thus, the existence of Cr6+ becomes a severe threat to the surrounding environment and human health and poses a great challenge to the solidification of Cr-containing wastes. The application of BFS-based material for the solidification of Cr was considered to be an effective method that could significantly reduce the possibility of Cr’s leaching into the environment. This application could be achieved in multiple ways, including physical encapsulation, double-replacement reactions to form precipitates, chemical bond adsorption, and the incorporation of Cr into mineral phase [5,6,7,8,9].
In recent years, much research has been conducted on the solidification mechanism and the existing state of Cr ions during the hydration reaction of BFS-based cementitious systems. Cr6+ mainly exists in the form of acid ions such as CrO42− and Cr2O72− in hydration products. Owing to the oxidation environment provided by the hydration reaction process of Cr6+, the solidification efficiency of Cr is only about 91%, which is quite low compared with other heavy metals [10,11]. The research of O. Yamaguch et al., demonstrated that the Cr6+ in hydration products, such as calcium sulfoaluminate, could be easily reduced to Cr3+ and form Cr(OH)3 precipitates under weak alkaline conditions [12]. The investigation conducted by M.R. Shatat et al. indicated that the Cr solidified as Ca2CrO5·3H2O and Cr doping changed the pore structure and reduced the early strength of the solidified material [13]. The study of M. Wazne et al. indicated that the solidification of Cr was mainly based on the lattice of the AFt phase at pH > 12.5 and adsorption at pH < 8. The Cr was almost completely converted to Cr6+ at pH < 5 [14]. A comparison between sulfate ettringite and chromate ettringite indicated that the chromate ettringite had a larger lattice and smaller crystalline shape due to the larger radius of CrO42− than SO42−, and displayed poor stability [15]. The solidification mechanism of different heavy metals was investigated, and it was found that the hydration products had a relatively low solidification rate of Cr compared with other heavy metals, at only about 50% [16]. The presence of Cr6+ would reduce the solidification efficiency of other heavy metal ions, and the Cr6+ would redissolve once the environmental conditions changed. The study of Zhang et al. demonstrated that Cr6+ mainly existed as CrO4-U phase in the hydration products in the absence of a reducing agent. The leaching test showed that the relationship between the leaching concentration of Cr6+ and total Cr was pH-dependent. The lower the pH of the reaction environment, the higher the reduction rate. The Cr mainly existed in the form of Cr6+ at pH 8.0~12.7, and Cr3+ at pH < 8. Most Cr exists in the form of CrO42−, replacing the SO42− in AFt phase lattice.
In the present study, BFS and flue gas desulfurization gypsum (FGDG) were applied for the solidification of K2CrO4. The solidification mechanism in different hydration products was investigated through X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Fourier-transform infrared spectroscopy (FTIR), and X-ray absorption near edge structure (XANES) to reveal the valence distribution and binding energy changes of Cr.
2. Experiments
2.1. Materials
The BFS and FGDG were collected from Hebei Xingtai Jintaicheng Environmental Resources Co., Ltd., Xingtai, China. These materials were dried at 35 °C for 72 h in an oven, and then stored separately in a sealed bag for subsequent use. In order to improve reactivity, the FGDG and BFS were ground into a fine powder in a ball mill (5 kg small ball mill, SMΦ500 × 500, Daoxu Donglin Laboratory Instrument Factory, Dongguan, China). K2CrO4 was of analytical pure grade.
2.2. Sample Preparation
The sample was prepared by weighing and thoroughly mixing and blending the raw materials and water. The paste mixture obtained after mixing was placed into a 30 × 30 × 50 mm mold, taken out after curing for 24 h, then stored at 35 °C and 90% humidity for further curing for 28 days. Then, the samples were crushed and soaked in anhydrous ethanol for 48 h to stop the hydration reaction, then they were dried in a vacuum oven at 35 °C for 72 h. The specific surface area of raw materials was analyzed using a CZB-9 BET Automatic Specific Surface Area Tester. The samples were finely ground to powder and then characterized by XRF (XRF-1800 sequential X-ray fluorescence spectrometer, Shenzhen, China), XRD (Rigaku Corporation, Shimadzu, Japan, in the range of 10–90°), XPS (Shimadzu-Kratos AXIS SUPRA X-ray photoelectron Spectrometer, Shimadzu, Japan), XANES (BL14W Silicon (111) crystal monochromator for beamline, Easyxafs Company, Washington, IL, USA), and FTIR (Nexus 70 FTIR spectrometer, Xiamen, China). The ion leaching concentration was determined by IC (Thermo Scientific Dionex Aquion IC system, Beijing, China).
2.3. The Solidification Mechanism of Cr
The solidification mechanism and existing state of Cr in ettringite were investigated with different analytical methods. Samples were prepared according to our previous studies (Wang et al., 2021, Wang et al., 2022), by adding CaO as an alkaline activator, with 80% BFS, 10% CaO, and 10% FGDG at a concentration of 80%. The samples were prepared by mixing K2CrO4 agents with 2, 4, and 6% of the total cementitious material, then cured for 28 days to investigate the solidification mechanism of Cr in ettringite, as listed in Table 1.

Table 1.
The samples investigated for solidification mechanism of Cr in ettringite.
The solidification mechanism of Cr in Friedel’s salt was investigated by different analytical methods. The samples were prepared by adding CaCl2 as the chlorine donor with 80% BFS, 10% CaO, and 10% CaCl2 at a concentration of 80%. The samples prepared by mixing 2, 4, and 6% K2CrO4 agents were compared after 28 days of curing, as listed in Table 2.

Table 2.
The samples investigated for solidification mechanism of Cr in Friedel’s salt.
3. Results and Discussion
3.1. Raw Materials
The treated BFS and FGDG were analyzed with a CZB-9 BET Automatic Specific Surface Area Analyzer, Beijing, China, and the specific surface areas were 500 and 450 m2/kg, respectively. The particle size of the raw materials was analyzed by a LMS-30 laser diffraction scattering particle size distribution analyzer, Beijing, China. As seen in Figure 1, the volume accumulative distribution (VAD) of 10, 50, and 90% of different raw materials was displayed on the upper left corner of the particle size distribution.
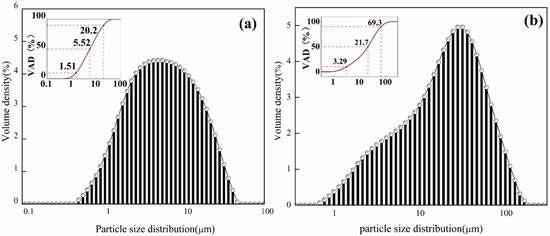
Figure 1.
Laser particle size analysis of (a) BFS and (b) FGDG.
The XRF test results of raw materials were analyzed by a XRF-1800 sequential X-ray fluorescence spectrometer, Shenzhen, China and is shown in Table 3. BFS was mainly composed of Ca, Si, Mg, and Al elements. As shown in Figure 2a, the XRD pattern of BFS displayed a distinct broad band, indicating the predominant amorphous nature of the BFS. In addition, a small contribution of crystalline CaCO3 was found. The performance indices of BFS could be calculated from chemical compositions based on Equations (1)–(3), for hydraulic activity, the activity coefficient, and alkalinity coefficient, respectively. The calculated values suggested that the BFS could be considered a high-quality slag with high activity and neutral alkalinity.

Table 3.
XRF results of BFS and FGDG (%).
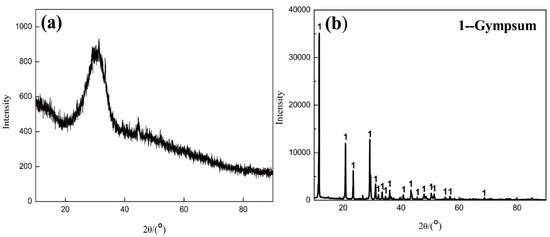
Figure 2.
XRD spectra of (a) BFS and (b) FGDG.
Hydraulic activity:
Activity coefficient:
Alkalinity coefficient:
where “W” represents the mass fraction.
3.2. The Solidification Mechanism of Cr in Ettringite
3.2.1. XRD Analysis of Ettringite
In order to determine the solidification mechanism of ettringite on Cr, XRD patterns of three different samples were analyzed to study the effects of K2CrO4 on the crystalline shape and lattice of ettringite. As shown in Figure 3, there was no significant difference in the XRD diffraction patterns of different samples, and the main hydration product was ettringite. However, with the increase in K2CrO4 content, the diffraction angle of ettringite slightly increased due to the replacement of SO42− by CrO42− in the liquid phase to form chromate ettringite. The binding ability of CrO42− to components, such as Ca2+, Al(OH)4− and OH−, was poor, leading to an increase in the phase distance of each product. However, as SO42− and CrO42− had the same valence state and similar particle sizes (2.3 Å and 2.4 Å) [17], the XRD patterns of each sample did not display many differences. In addition, the intensity of the derivative peaks of CaSO4 increased with the increase in K2CrO4 content, which was generated by the reaction of SO42− with Ca2+ in the solution. In order to verify the above results, the concentrations of SO42− and CrO42− in the leachate of different samples, after curing for 28 days, were determined by ion chromatography and spectrophotometry with colorimetric method.
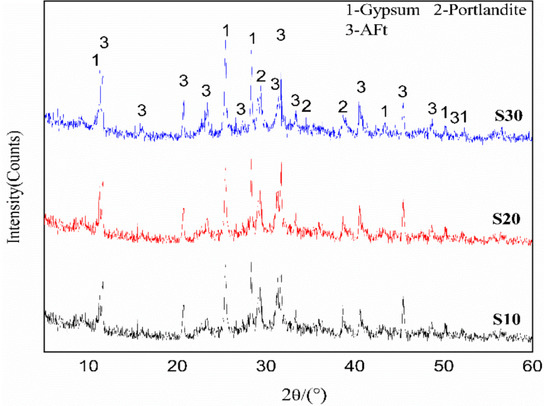
Figure 3.
XRD spectra of the samples (S10, S20, S30).
As shown in Table 4, as K2CrO4 content increased, the concentration of CrO42− in the leachate increased at a slow rate, indicating that ettringite displayed an excellent solidification effect on CrO42− and reduced the content of soluble Cr. Meanwhile, the concentration of SO42− in the leachate gradually increased, consistent with the addition of CrO42−, revealing that the replacement of SO42− in ettringite by CrO42− could redissolve SO42− into the liquid phase. As the equilibrium constants of chromate ettringite () were more than 3000 times higher than those of sulfate ettringite (), the chromate ettringite lattice displayed a low binding energy between components and poor binding capacity [18].
Note: 0 < X < 3.

Table 4.
Leaching concentrations of SO42− and CrO42− in the samples (ppm).
Table 4.
Leaching concentrations of SO42− and CrO42− in the samples (ppm).
| Samples | SO42− | CrO42− |
|---|---|---|
| S0 | 7.38 | ND |
| S10 | 15.19 | 3.54 |
| S30 | 25.33 | 8.77 |
Note: ND = Not detected.
As shown in Equation (4), when the solution contained a large amount of SO42− and no CrO42−, the sulfate ettringite could stably exist with only a small amount of Ca2+, Al(OH)3−, and OH− components dissolved. With the incorporation of CrO42−, the reaction of CrO42− with Ca2+, Al(OH)4−, and OH− in the solution caused a decrease in the saturation of the liquid phase, resulting in the further decomposition of the sulfate ettringite [19]. This decomposition and generation was continuous and directly related with the content of SO42− and CrO42− in the solution.
Therefore, the solidification effect of the samples on CrO42− was investigated by adding different amounts of FGDG, as listed in Table 5. The concentration of CrO42− in the leachate significantly increased with the increase in FGDG. This was because when SO42− content in the solution was sufficiently high, it would compete with CrO42−, leading to the release of a small number of components in the liquid phase and limiting CrO42− from participating in the reaction [20]. Therefore, the content of SO42− exerted a direct influence on the efficiency of Cr solidification.

Table 5.
Leaching concentration of CrO42− at different levels of FGDG doping.
3.2.2. FTIR Analysis of Ettringite
The effects of different amounts of K2CrO4 on the chemical bonding and molecular structure of the ettringite were investigated by FTIR analysis. As shown in Figure 4, with an increase in K2CrO4 content, the absorption bands centered around 3452 cm−1 and 1639 cm−1 were reduced, and the stretching vibrations of the S-O bond in ettringite near 1116 cm−1 significantly decreased.
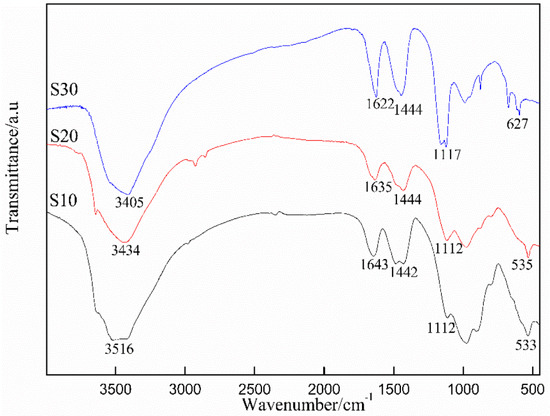
Figure 4.
FTIR spectra of the samples (S10, S20, S30).
The asymmetric stretching vibration peaks of H2O in chromate ettringite appeared around 3400 cm−1 and 1620 cm−1, indicating that SO42− in ettringite was replaced by CrO42−, and that the quantity and structure of crystal water in ettringite changed. Meanwhile, the crystallinity of ettringite was poor due to the relatively weak bonding strength of Cr-O. With an increase in CrO42− content, the intensity of the bending vibration peak located near 617 cm−1 in the spectrum of CaSO4 greatly increased, which verified the replacement of SO42− in ettringite by CrO42− and the generation of CaSO4 with Ca2+ in solution. The absorption band near 1426 cm−1 was ascribed to CO32− after the carbonization of samples, which remained unchanged with varying Cr content.
3.2.3. XPS Analysis of Ettringite
The valence distribution and binding energy change of Cr under different K2CrO4 doping contents were investigated by XPS analysis. Figure 5 presents the XPS curve-fitting of Cr. With an increase in Cr content, the main peaks of the binding energy of Cr 2p3/1 and Cr 2p3/2 increased and binding energy decreased, suggesting that the bond energy strength of Cr6+ became weakened by the doping of Cr. The main binding energy peaks of K2CrO4 were around 589.2 eV [21]. However, in the presence of Cr6+ in ettringite, its main binding energy peaks were located at 586.2 eV and 577.4 eV, respectively [22]. As shown in Figure 5, the main binding energy peaks of Cr in different samples ranged from 585 to 587.4 eV and 577.3 to 578 eV, indicating that Cr in different samples was dominated by Cr6+, accounting for more than 84%. These results further verified that the Cr of K2CrO4 entered the ettringite to generate chromate ettringite.
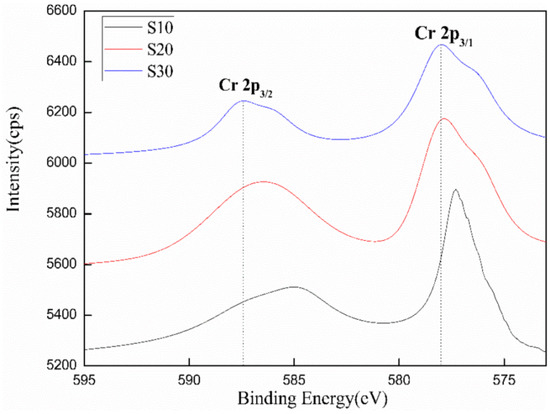
Figure 5.
XPS patterns of Cr in different samples (S10, S20, S30).
3.3. Study of the Solidification Mechanism of Cr in Friedel’s Salt
3.3.1. XRD Analysis of Friedel’s Salt
The XRD patterns of samples F10, F20, and F30 were tested to reveal the effect of K2CrO4 doping on the crystalline shape and lattice of Friedel’s salt, verifying its solidification mechanism of Cr. As shown in Figure 6, there was no distinct difference in the XRD patterns of different samples. With an increase in K2CrO4 content, the diffraction peak position of Friedel’s salt changed to a certain extent, but the intensity seemed to be unchanged, indicating that once the Cr entered the crystal of Friedel’s salt to make varied forms, the total amount was unchanged. The leaching concentrations of Cl− and CrO42− in the leachate of different samples were investigated by the horizontal vibration method, and the results are shown in Table 6. The concentration of CrO42− in the leachate slightly increased with the addition of K2CrO4, suggesting that part of the CrO42− entered the Friedel’s salt crystals for solidification and that the content of soluble Cr was reduced. Meanwhile, the Cl− concentration also slightly increased, but with no apparent regularity. These results suggest that an increase in CrO42− content led to the dissolution of partial Cl− from Friedel’s salt. This can be due to two reasons: (1) the Cl− was replaced and released into the solution, thus generating Friedel’s salt with more chlorine as Ca4Al2O6Cl2∙10H2O; (2) the Cr in Friedel’s salt were not only replacing Cl− with anions for solidification but were also reduced from Cr6+ to Cr3+ and solidified as cations.
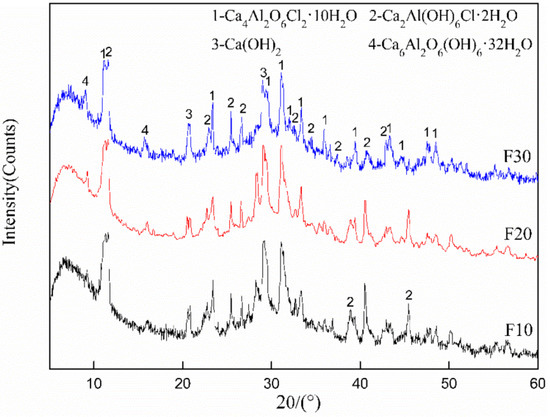
Figure 6.
XRD spectra of different samples (F10, F20, F30).

Table 6.
Leaching concentrations of Cl− and CrO42− in the samples (ppm).
3.3.2. FTIR Analysis of Friedel’s Salt
The effect of K2CrO4 addition on the chemical bonding and molecular structure changes of Friedel’s salt was investigated by FTIR analysis. As shown in Figure 7, as K2CrO4 content increased, the FTIR spectra of different samples hardly changed, and the absorption bands centered around 3450 cm−1 and 1637 cm−1 were basically unchanged with a slight reduction in the transmittance. This indicated that the doping of CrO42− exerted a slight effect on the generated species of Friedel’s salt, but the total amount of Friedel’s salt was basically unchanged, which was demonstrated in Section 3.3.1. This was due to the generation of Friedel’s salt with different atomic compositions. The weak absorption at 1112 cm−1 indicated that almost no ettringite was formed. In addition, there was no significant difference in the number and intensity of vibration peaks in the absorption bands of Friedel’s salt around 538 cm−1 and 425 cm−1. The weak absorption band located near 1488 cm−1 was also caused by the carbonization of CO2, and remained unchanged with the addition of Cr.
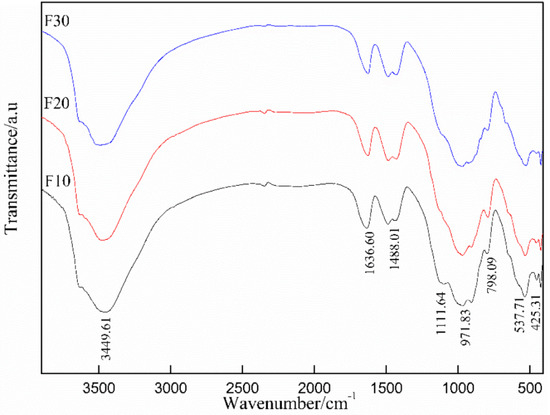
Figure 7.
FTIR spectra of the samples (F10, F20, F30).
3.3.3. XPS Analysis of Friedel’s Salt
The valence distribution and binding energy changes of Cr in Friedel’s salt at different levels of K2CrO4 doping were investigated by XPS analysis. The XPS curve-fittings of Cr for the split peaks are shown in Figure 8. The Cr in Friedel’s salt crystals coexisted in two valence states, and the binding energy peaks of both Cr6+ and Cr3+ increased with an increase in Cr content, indicating that the Cr content of both valence states increased. According to the calculation of the peak areas, the contents of Cr3+ and Cr6+ in Friedel’s salt were almost equal when the cementitious system was mixed with 2 and 4% of K2CrO4. This was because the potential activity of BFS displayed a slow excitation rate with the absence of SO42− in the cementitious system and reducing substances such as Fe2+ and S2− in the BFS could reduce some amount of Cr6+ to Cr3+. The residual Cr6+ would replace the Cl− in Friedel’s salt in the form of CrO42− to enter Friedel’s salt crystals, whereas the Cr3+ replaced the Al3+ to enter Friedel’s salt crystals. The reaction equation is shown as in Equation (5). The Cr3+ and Cr6+ in sample F30 with 6% K2CrO4 doping accounted for 39.2 and 60.8% of the total Cr, respectively. The percentage of Cr6+ significantly increased, whereas the percentage of Cr3+ showed a downward tendency. This was probably due to the depletion of almost all the reducing substances in the BFS and the increased proportion of solidified Cr in the form of CrO42− in Friedel’s salt.
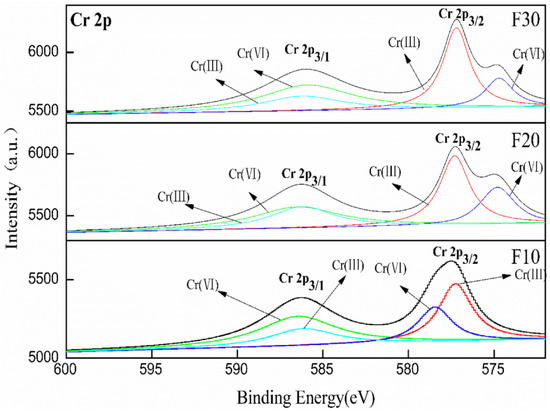
Figure 8.
XPS patterns of Cr in different samples (F10, F20, F30).
It can be seen from Figure 8 that the binding energies of Cr 2p3/1 and Cr 2p3/2 decreased with an increase in K2CrO4 content. As Cr was incorporated into the system, the binding energies of Cr3+ and Cr6+ became weakened after the partial reduction of Cr6+, and the bonds energy strengths of Cr3+ and Cr6+ became weaker. The above conclusion validated the results in Section 3.3.1, suggesting that the Cr in Friedel’s salt was solidified by replacing Al3+ and Cl−, and that Cr entered the crystal in the form of Cr3+ and CrO42−, which was different from the solidification mechanism of Cr in ettringite.
Note: M = Al3+ or Cr3+; X = 2Cl− or CrO42−.
3.4. XANES Analysis of Solidification Cr of Hydration Products
The Lm-edge XANES spectrum of Cr is quite sensitive to the atomic near-neighbor structure, and the information of near-neighbor structure of the Cr ions could be obtained by comparison of spectral lines. The more vacant orbitals there were that were suitable for leapfrogging, the stronger the characteristic peak of the Lm absorption edge with the electron transition to the vacant orbitals. Therefore, the energy calibration of the sample was carried out through a standard chromium foil, and the standard chromium foil was measured as a reference. The Cr-edge XANES spectra were mainly marked by three main peaks, namely leading edge, white line, and trailing edge. The XANES spectra of samples S0, S10, and S20 are shown in Figure 9a. The Cr spectrum displayed the characteristic peak of Cr3+ at 5993 eV, and Cr6+ at 6010 and 6025 eV [23,24]. Three samples showed similar patterns without a leading edge peak, suggesting their similar coordination environments and the absence of Cr3+ in ettringite. The high similarity of XANES spectra among the different samples and K2CrO4 reference indicated that the Cr in different samples basically existed in the state of Cr6+ in ettringite. The XANES spectra of samples F10, F20, and F30 are shown in Figure 9b. Compared with the spectra shown in Figure 9a, a front edge peak appeared in the spectrum of Friedel’s salt, which was very similar to the spectrum of Cr2O3 reference samples. In addition, three main peaks marked in the plots showed obvious characteristics, indicating that Cr3+ and Cr6+ coexisted in Friedel’s salt.
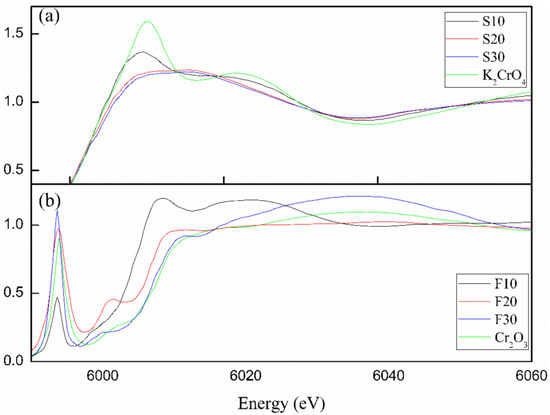
Figure 9.
XANES patterns of Cr in different samples (a) S10, S20, S30; (b) F10, F20, F30.
4. Discussion
In general, the solidification of Cr by the hydration products generated by slag-based cementitious materials was mainly in the following ways: (1) Cr6+ enters hydration product crystals to form chromate-bearing products by replacing SO42− in the form of CrO42−; (2) Cr6+ is reduced to Cr3+ by Fe2+ and S2− in BFS during hydration reactions, and then Cr3+ replaces Al3+ to enter hydration product crystals as cations; (3) after reacting with free Ca2+ in the solution to form CaCrO4 precipitate, CrO42− is attached to the surface of hydration products; (4) Cr forms CrO42− and is then physically adsorbed onto the surface of the hydration products by van der Waals forces [25].
Combined with the results of our previous studies, the pH of the leachate of the paste bulk was between 11 and 12. The CaCrO4 precipitation could not stably exist in the strongly alkaline environment, and the OH− provided by the alkaline material was the most competitive for the adsorption sites, which meant that the CrO42− released by the decomposition of CaCrO4 could not be solidified via adsorption; thus, it would re-dissolve into the liquid phase.
5. Conclusions
In this work, the solidification mechanism and final state of Cr in different hydration products were investigated by XRD, XPS, FTIR, and XANES. The ettringite demonstrated the excellent solidification effect on CrO42−, which reduced the content of soluble Cr. The replacement of SO42− by CrO42− in the ettringite crystals made SO42− become soluble in the liquid phase. When there was sufficient SO42− content in the solution, it would directly affect the solidification efficiency of Cr. The XPS pattern of Cr in ettringite showed that the main binding energy peaks of Cr in the different samples ranged from 585 to 587.4 eV and 577.3 to 578 eV, verifying that the major form of Cr was Cr6+, with a percentage above 84%.
The Cr in two valence states coexisted in Friedel’s salt, due to the slow excitation rate of the potential activity of BFS in the absence of SO42− in the system. Reducing substances, such as Fe2+ and S2−, in the BFS could reduce some amount of Cr6+ to Cr3+. Then, Cr3+ replaced Al3+, and the residual Cr6+ replaced Cl− in Friedel’s salt as CrO42− in the crystal. When Cr3+ and Cr6+ entered Friedel’s salt crystals, the coordination number increased and bond energy strength weakened with the decrease in the binding energies of Cr 2p3/1 and Cr 2p3/2.
The Cr XANES spectra of ettringite indicated that the coordination environments of the samples were similar without a leading edge peak, suggesting that Cr3+ was absent in ettringite and that the Cr in ettringite was basically in the state of Cr6+. The XANES spectrum of Cr in Friedel’s salt showed a leading edge peak similar to the Cr2O3 reference sample, and the three main peaks showed obvious characteristics that confirmed the existence of both Cr3+ and Cr6+ in cementitious system.
This study provides new ideas for the recovery and utilization of solid waste BFS and FGDG, as well as valuable solutions for solving the contamination problems of stockpiles and treating solid waste containing Cr. This research offers important guidelines for low cost and safe disposal of hazardous waste containing other heavy metals. Nevertheless, this study only focused on exploring the solidification mechanism of Cr in solid waste. Further investigation is needed for the solidification mechanism of Cr in wastewater.
Author Contributions
K.W.: formal analysis, investigation, validation, methodology, and writing—original draft; W.N.: resources and supervision; K.L.: funding acquisition and writing—review and editing; D.X.: investigation and validation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Department of Hebei Province, China [18273807D].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent for publication was obtained from all participants.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that they have no competing interests.
Abbreviations
BFS—blast furnace slag, FGDG—flue gas desulfurization gypsum.
References
- Li, W.; Sun, Y.; Huang, Y.; Shimaoka, T.; Wang, H.; Wang, Y.N.; Ma, L.; Zhang, D. Evaluation of chemical speciation and environmental risk levels of heavy metals during varied acid corrosion conditions for raw and solidified/stabilized MSWI fly ash. Waste Manag. 2019, 87, 407–416. [Google Scholar] [CrossRef]
- Wei, J.; Yao, J.; Yu, Q.; Bai, R. Chemical form of cr existing in hardened portland cement. J. Chin. Ceram. Soc. 2009, 37, 1118–1123. [Google Scholar]
- Wang, K.; Li, K.; Huang, X.; Ni, W.; Zhang, S. Leaching characteristics of Cr in municipal solid waste incineration fly ash solidified/stabilized using blast furnace slag-based cementitious materials. Int. J. Environ. Sci. Technol. 2021, 19, 7457–7468. [Google Scholar] [CrossRef]
- Li, D.; Gui, C.; Ji, G.; Hu, S.; Yuan, X. An interpretation to Cr(Ⅵ) leaching concentration rebound phenomenon with time in ferrous-reduced Cr(Ⅵ)-bearing solid matrices. J. Hazard. Mater. 2019, 378, 120734. [Google Scholar] [CrossRef]
- Wang, D.; He, W.; Wang, Q.; Wang, Z. Review on stabilization and leaching of heavy metals in cementitious materials. Kuei Suan Jen Hsueh Pao/J. Chin. Ceram. Soc. 2018, 46, 71–81. [Google Scholar]
- Zhou, X.; Zhou, M.; Wu, X.; Han, Y.; Geng, J.; Wang, T.; Wan, S.; Hou, H. Reductive solidification/stabilization of chromate in municipal solid waste incineration fly ash by ascorbic acid and blast furnace slag. Chemosphere 2017, 182, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, J.; Wang, J.; Zhan, L.; Ye, X.; Tan, H. Hydrothermal processing, characterization and leaching toxicity of Cr-added “fly ash-metakaolin” based geopolymer. Constr. Build. Mater. 2020, 251, 118931. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, W.; Gao, X. Solidification and immobilization of MSWI fly ash through aluminate geopolymerization: Based on partial charge model analysis. Waste Manag. 2016, 58, 270–279. [Google Scholar] [CrossRef]
- Richardson, I.G. The calcium silicate hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]
- Peysson, S.; Péra, J.; Chabannet, M. Immobilization of heavy metals by calcium sulfoaluminate cement. Cem. Concr. Res. 2005, 35, 2261–2270. [Google Scholar] [CrossRef]
- Huang, X.; Zhuang, R.; Muhammad, F.; Yu, L.; Shiau, Y.; Li, D. Solidification/stabilization of chromite ore processing residue using alkali-activated composite cementitious materials. Chemosphere 2017, 168, 300–308. [Google Scholar] [CrossRef]
- Yamaguchi, O.; Ida, M.; Uchiyama, Y.; Hanehara, S. A method for the determination of total Cr(VI) in cement. J. Eur. Ceram. Soc. 2006, 26, 785–790. [Google Scholar] [CrossRef]
- Shatat, M.R.; Ali, G.A.M.; Tantawy, M.A. Hydration characteristics and immobilization of Cr (VI) in slag cement-CKD pastes under hydrothermal treatment. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2015, 30, 1013–1019. [Google Scholar] [CrossRef]
- Wazne, M.; Jagupilla, S.C.; Moon, D.H.; Christodoulatos, C.; Koutsospyros, A. Leaching Mechanisms of Cr(VI) from Chromite Ore Processing Residue. J. Environ. Qual. 2008, 37, 2125. [Google Scholar] [CrossRef] [PubMed]
- Omotoso, O.E.; Ivey, D.G.; Mikula, R. Characterization of chromium doped tricalcium silicate using SEM/EDS, XRD and FTIR. J. Hazard. Mater. 1995, 42, 87–102. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, C.; Zhao, M.; Yang, K.; Shen, R.; Zheng, Y. Immobilization potential of Cr(VI) in sodium hydroxide activated slag pastes. J. Hazard. Mater. 2017, 321, 281–289. [Google Scholar] [CrossRef]
- Ang, K.; Li, K.; Huang, X.; Ni, W.; Zhang, S. Preparation of backfill materials by solidifying municipal solid waste incineration fly ash with slag-based cementitious materials. Int. J. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Lan, J.; Wang, Y. The differences between the characters of chromate ettringite and ettringite. Concrete 2004, 10, 8–11. [Google Scholar]
- Leisinger, S.M.; Lothenbach, B.; Le Saout, G.; Johnson, C.A. Thermodynamic Modeling of Solid Solutions Between Monosulfate and Monochromate 3CaO·Al2O3·Ca[(CrO4) × (SO4)1−x]·nH2O. Cem. Concr. Res. 2012, 42, 158–165. [Google Scholar] [CrossRef]
- Zhang, S.H.; Wu, M.F.; Tang, T.T.; Xing, Q.J.; Peng, C.Q.; Li, F.; Liu, H.; Luo, X.B.; Zou, J.P.; Min, X.B.; et al. Mechanism investigation of anoxic Cr(VI) removal by nano zero-valent iron based on XPS analysis in time scale. Chem. Eng. J. 2018, 335, 945–953. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Yanful, E.K.; Pratt, A.R. Chemical states in XPS and Raman analysis during removal of Cr(VI) from contaminated water by mixed maghemite–magnetite nanoparticles. J. Hazard. Mater. 2012, 235–236, 246–256. [Google Scholar] [CrossRef]
- Liu, Z.; Ni, W.; Li, Y.; Ba, H.; Li, N.; Ju, Y.; Zhao, B.; Jia, G.; Hu, W. The mechanism of hydration reaction of granulated blast furnace slag-steel slag-refining slag-desulfurization gypsum-based clinker-free cementitious materials. J. Build. Eng. 2021, 44, 103289. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Zhang, H.; Shen, K.; Qu, Y.; Jiang, Z. Wavelet analysis of extended X-ray absorption fine structure data: Theory, application. Phys. B Condens. Matter 2018, 542, 12–19. [Google Scholar] [CrossRef]
- Shao, Y. Mechanisms of Solidification and Stabilization of Waste Incineration Fly Ash with Slag-Based Cementitious Materials; Wuhan University: Wuhan, China, 2014. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).