Abstract
Graphite precipitation in the hot metal of a blast furnace (BF) has a significant effect on the low permeability zone of the deadman. In this work, the precipitation mechanisms of graphite in the hot metal of BF were investigated and discussed. Furthermore, the theoretical flame temperature of tuyere raceway, the center temperature of the deadman, and the critical temperature of graphite precipitated from the hot metal, were calculated and the graphitic carbon cycle and graphite enrichment mechanisms in the void of the deadman were analyzed. The results showed that the theoretical flame temperatures of the two BFs used in this study varied from 2100 °C to 2200 °C and the average center temperatures of the deadman in 4350 m3 and 1280 m3 BFs were 1329.08 °C and 1386.74, respectively. Moreover, graphite can precipitate from the hot metal and be enriched in the void of the deadman under certain conditions. It was assumed in this work that graphite is precipitated in the form of a 2 mm sphere and the precipitation rate of graphite in hot metal is approximately 1.01 × 10−8 kg/s. With variation in BF conditions, the precipitation–enrichment–dissolution process of graphite occurs continuously in the deadman of the BF.
1. Introduction
Hearth activity (representing the smoothness of liquid slag and iron flowing into the hearth and discharging freely from the hearth) is intensely related to the long campaign life of blast furnace (BF) [1,2,3]. Furthermore, the hearth activity state plays an important role in realizing high efficiency and longevity of BF. According to the erosion mechanisms of the BF layer lining and the low permeability zone in the deadman, analyzed by dissecting dozens of BFs with different volumes throughout the world [4,5,6,7,8,9], the low permeability zone in the deadman of BF is considered to be the critical factor affecting the hearth activity. During the smelting process of a BF, the main components of burden are iron ore and coke [10,11]. Studies [12,13] have found that the low permeability zone consists of graphite, fine coke particles, slag, and iron. Compared with other regions, the low permeability zone has a smaller porosity, worse air permeability, and lower hearth activity.
As the environment inside the BF is complicated, invisible, and changeable, there is relatively little research on the formation mechanism of the low permeability zone in the deadman and almost no research on graphite enrichment in the low permeability zone. It has been found [14,15] that when the center temperature of the deadman in the hearth of the BF is low, the carbon inside the hot metal can be precipitated in the form of crystal graphite, which together with residual ash, coke fines, and unburned pulverized coal in the hearth may block the void of the deadman. The relationship between the heat transfer coefficient and the BF bottom temperature, as well as the low permeability zone of the hearth, had been discussed by Sawa et al. [16]. Many studies have shown that temperature is one of the important factors influencing the precipitation of graphite from hot metal [17,18,19]. In the actual operation of a BF, the central temperature of the deadman (DMT) is difficult to be identified, while the theoretical flame temperature (TTT) of the raceway related to the DMT is easy to calculate. Consequently, to provide theoretical support for further investigation into the formation mechanism of the low permeability zone in the deadman, the DMT was calculated through the theoretical flame temperature and the mechanism of carbon precipitation in the hot metal was studied. Simultaneously, the graphite cycle and the mechanisms of graphite enrichment in the void of the deadman were further analyzed. This paper could provide a theoretical foundation for the formation of the low permeability zone in the deadman and the longevity of the BF.
2. Center Temperature of the Deadman in the BF Hearth
The BF is considered to be one of the most complex metallurgical reactors, and the BF smelting process cannot be observed directly. Via long-term research and exploration, it has been determined that the hearth activity state is important for estimating the actual running operation of the BF. Furthermore, the DMT is a significant parameter for identifying the hearth activity, which mainly depends on the TTT of the raceway, the bosh gas volume, the fuel rate, and the CO utilization efficiency at the furnace center.
2.1. Theoretical Flame Temperature of the BF
The TTT of the tuyere raceway is mainly used to evaluate the thermal state inside the BF, which can be applied to direct the BF operation and ensure the stability and smoothness of BF smelting. Currently, the TTT is commonly calculated by an empirical equation (Equation (1)) [20,21].
where TTT is the theoretical flame temperature (°C), Tf is the blast temperature (°C), M is the coal ratio (kg/t), MOj is the blast humidity (g/m3), and FO is the oxygen enrichment (%).
The TTT of the BF under a normal running state is reported between 2000 °C and 2200 °C [22]. Table 1 and Table 2 present the operation data of 4350 m3 and 1280 m3 BFs in steelworks, respectively. The TTTs of the two BFs were calculated according to Equation (1), as shown in Table 1 and Table 2. The TTT of the BF with a volume of 4350 m3 fluctuates between 2109.0 °C and 2191.6 °C, with an average value of approximately 2142.8 °C. In comparison to the 4350 m3 BF, the TTT variation range of the 1280 m3 BF is larger, from 2044.2 °C to 2198.1 °C. The average TTT of the 1280 m3 BF is approximately 2133.9 °C.

Table 1.
Operation data of the 4350 m3 BF in steelworks.

Table 2.
Operation data of the 1280 m3 BF in steelworks.
2.2. Center Temperature of the Deadman in the Hearth
The DMT in the hearth is a significant factor for estimating the hearth activity. A higher DMT indicates a better hearth activity. Raipala et al. [20,23] proposed a method for calculating the DMT, which is shown in Equation (2).
where DMT is the center temperature of the deadman (°C); Vbosh is the volume of the bosh gas inside the BF (m3/min); ηCO.C is the utilization efficiency of CO at the furnace center measured by a shaft probe (%); Dpcoke is the size of coke in the core of the deadman (mm); D is the hearth diameter (m); FR is the fuel ratio (kg/t); and Δt is the slag flow index, defined as the difference between the tapping temperature of the BF and the flow temperature of the slag in the BF (°C) [24].
As tuyere probing was carried out, the coke quality parameters and the operation parameters of two BFs with different volumes in steelworks were analyzed and listed in Table 3 and Table 4. The DMT is 1380~1450 °C with the normal production of the BF [25]. On the basis of Equation (2), the DMT of the 4350 m3 BF is 1270.72~1412.48 °C and the average value is approximately 1329.08 °C. In addition, the DMT of the 1280 m3 BF is between 1278.75 °C and 1494.01 °C and the average core temperature is about 1386.74 °C. With increasing the amount of hot metal flowing through the deadman, the DMT rises, indicating that the void of the deadman is large and gas permeability and liquid permeability are high. As a result, the hearth activity is favorable. However, when the DMT is lower than 1380 °C, even lower than the melting temperature of slag, the slag viscosity increases and the slag fluidity decreases. As a consequence, the hearth activity worsens and BF stability deteriorates.

Table 3.
Coke quality parameters and operation parameters of the 4350 m3 BF in steelworks.

Table 4.
Coke quality parameters and operation parameters of the 1280 m3 BF in steelworks.
3. Results and Discussion
3.1. Precipitation Temperature of Graphite in Hot Metal
At a certain temperature, carbon deposition reaction can occur when the carbon dissolved in hot metal is saturated. The reaction is as follows [26].
In the reaction, pure graphite is the reference state for [C] and the henry activity of the dissolved carbon is given by fC [%C].
The Gibbs free energy variation of the above-mentioned reaction can be generally expressed as follows:
where fC is the activity coefficient of carbon in hot metal and [%C] is the carbon concentration in hot metal. The logarithm of the activity coefficient of carbon at 1600 °C can be determined by Equation (5) [26].
where eij represents the interaction coefficient between elements i and j in hot metal and [%i] is the content of element i in hot metal. The interaction coefficient between other elements and carbon at 1600 °C is listed in Table 5, while Table 6 and Table 7 present the compositions of the hot metal derived from the production data of the above two BFs, respectively. Since it is difficult to actually measure the composition of the hot metal in the deadman, the composition of the metal inside the deadman is assumed to be the same as that in the taphole.

Table 5.
Interaction coefficient between other elements and carbon in hot metal at 1600 °C, data from [26].

Table 6.
Composition of hot metal derived from the 4350 m3 BF.

Table 7.
Composition of hot metal derived from the 1280 m3 BF.
Furthermore, the activity coefficient of carbon at different temperatures can be calculated by that at 1600 °C, as shown in Equation (6).
The critical temperature of graphite precipitation in hot metal is calculated according to Equations (3)–(6), together with the data in Table 5 and Table 6, and is found to be about 1382 °C for the 4350 m3 BF. From the above results, the DMT for this BF is between 1270.72 °C and 1412.48 °C. As a result, when the temperature of the hot metal is lower than 1382 °C, the graphite can be precipitated in the crystalline form and enriched in the void of the deadman. As the temperature is higher than 1382 °C, the precipitated graphite can be dissolved again in the hot metal. Therefore, the precipitation–enrichment–dissolution process of graphite is continuous in the hearth with variation in BF conditions.
The critical temperature of graphite precipitation in hot metal in the 1280 m3 BF can be calculated by Equations (3)–(6) and the data in Table 5 and Table 7, which is approximately 1359 °C. Consequently, when the temperature of the hot metal is lower than 1359 °C, the carbon deposition reaction can proceed in the hot metal. The DMT of this small BF is about 1278.75~1494.01 °C. As a result, when the temperature of the hot metal is below 1359 °C, the graphite can be precipitated in a crystalline form and be enriched in the void of the deadman. However, when the temperature of the hot metal is greater than 1359 °C, the precipitated carbon dissolves in the hot metal. With a variation in the BF conditions, the graphite constantly undergoes the precipitation-enrichment-dissolution process.
Therefore, what happens to the graphite in the hot metal can vary in BFs with different volumes since the DMT fluctuates due to the change in BF conditions. The critical temperature of graphite precipitation may also differ when the composition of the hot metal is changed, especially the content of carbon and sulfur in the hot metal.
3.2. Precipitation Rate of Graphite
The solubility of graphite in hot metal differs with temperature. At lower temperature of hot metal, graphite has lower solubility. When the temperature of the hot metal is lower than the precipitation critical temperature of graphite, the carbon in the hot metal will precipitate in the crystalline form, generating a boundary layer of graphite with a certain concentration in the void of the deadman, which contributes to the generation of a low liquid permeability zone. The precipitation rate of graphite can be presented as Equation (7) [26].
where Ms is the mass of precipitated carbon (kg), β is the mass transfer coefficient of melt component w(C) (m/s), A is the interface area between solid and melt (m2), ρ is the density of the graphite (kg/m3), w(C) is the carbon concentration in hot metal, and w(C)s is the saturated concentration of carbon at a certain temperature in hot metal.
The mass transfer coefficient of carbon in hot metal (β) is approximately 2.41 × 10−4 m/s, and the density of graphite (ρ) is about 2460 kg/m3 [26]. The graphite in the deadman between the taphole and the bottom surface of the BF is in the form of tiny particles, so it is assumed that the graphite is precipitated in the form of spheres and that the final radius (r) of the precipitated graphite is about 0.001 m [16]. So, the largest value of A is considered to be the surface area of the largest sphere (4πr2) [16]. When the average DMT is 1329.08 °C, the concentration of carbon in hot metal (w(C)) is approximately 4.72%. According to the above, the precipitation temperature of the graphite in the hot metal of the large BF is 1382 °C and the concentration of carbon in the hot metal (w(C)s) is approximately 4.85%. The maximum precipitation rate of graphite in hot metal is calculated to be approximately 1.01 × 10−8 kg/s, as follows:
The low permeability zone in the deadman of the BF may become large with increasing the precipitation amount of graphite according to the relationship between the precipitation of graphite and the permeability of the deadman. During the actual production of the BF, the BF condition can be regulated by adjusting the process parameters according to the graphite precipitation time. Thus, the precipitation time (t) required for the maximum contact area during the precipitation process of graphite is calculated by Equation (9). The precipitation time is approximately 17.7 min.
3.3. Circulation and Enrichment of Graphite in the Deadman
The precipitation and dissolution mechanisms of graphite in the hot metal of a BF hearth are demonstrated in Figure 1. With the variation in BF conditions, graphite in the hot metal undergoes a continuous cycle of precipitation, enrichment, and dissolution. When the hearth is inactive, the hot metal mainly circulates. Only a small amount of the hot metal flows through the bottom and the interior of the deadman, causing an increase in the temperature of the hearth sidewall and a decrease in the temperature inside the deadman as well as of the hearth bottom. According to the previous theoretical calculation, the precipitation temperature of graphite is just in the temperature range of the DMT. Therefore, when the temperature of the hot metal is lower than the precipitation temperature of graphite, the graphite can be continuously precipitated in the form of crystals and enriched in the void of the deadman. With passage of time, the amount of enriched graphite increases. As a result, the void of the deadman decreases, which can decrease the heat in hot metal as well as the flow rate of the hot metal through the deadman. Therefore, the temperature inside the deadman gradually decreases. In this case, the graphite can precipitate more easily in the void of the deadman, leading to the deterioration of the deadman and the formation of a low liquid permeability region.
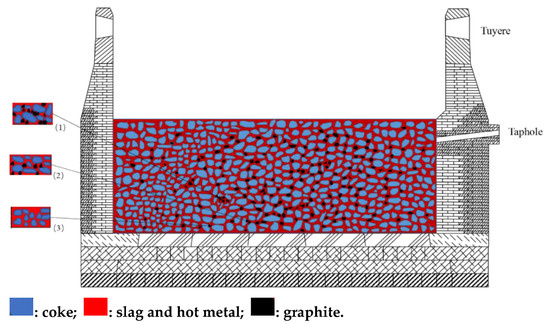
Figure 1.
Diagram of precipitation and disappearance of graphite in the BF hot metal. (1)  : Graphite is enriched in the deadman. (2)
: Graphite is enriched in the deadman. (2)  : Graphite is precipitated in hot metal. (3)
: Graphite is precipitated in hot metal. (3)  : Graphite is dissolved in hot metal.
: Graphite is dissolved in hot metal.
 : Graphite is enriched in the deadman. (2)
: Graphite is enriched in the deadman. (2)  : Graphite is precipitated in hot metal. (3)
: Graphite is precipitated in hot metal. (3)  : Graphite is dissolved in hot metal.
: Graphite is dissolved in hot metal.
When the hearth is active, most of the hot metal flows from the bottom of the deadman to the taphole, which can cause the temperature to increase at the bottom of the deadman and decrease at the hearth sidewall. The temperature of hot metal is higher than the precipitation temperature of graphite, and when the temperature of hot metal rises, the corresponding saturated solubility of carbon increases. As a result, graphite dissolves in the hot metal. According to the research of Sawa [16], most of the graphite precipitation in the inactive period is carried out by the slag at the initial stage when the hearth changes from inactive to active. Therefore, the graphite layer enriched in the void of the deadman will be partly dissolved in the hot metal with a high temperature and the graphite is gradually taken away from the void of the deadman by the hot metal.
BF ironmaking is complex and variable. The variations inside a BF can only be deduced from relevant data and are impossible to evaluate directly. However, what has been confirmed is that the DMT fluctuates constantly within a certain range and the graphite undergoes a continuous precipitation-enrichment-dissolution process in the void of the deadman.
4. Conclusions
In this paper, the precipitation process of graphite in hot metal was discussed and the relevant parameters of two BFs with different volumes were calculated. The following conclusions were drawn:
- For the 4350 m3 BF, the center temperature of the deadman is 1270.72~1412.48 °C and the critical temperature of the graphite precipitation in hot metal is about 1382 °C. The maximum rate of precipitation to form a 2 mm graphite sphere is 1.01 × 10−8 kg/s, and the time is approximately 17.7 min.
- As the furnace temperature varies, the graphite undergoes precipitation, enrichment, and dissolution in the void of the deadman. When the graphite is enriched, a low-permeability zone is formed in the deadman.
Author Contributions
Conceptualization, P.W.; methodology, Z.D. and H.W.; investigation, M.X. and Y.S.; writing—original draft preparation, M.X. and Y.S.; writing—review and editing, H.W. and M.H.; funding acquisition, Z.D. and H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (52004001) and the Projects of University Natural Science Research Project in Anhui Province (KJ2018A0045).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, H.; Wu, S.L.; Yu, X.B. New index of evaluating activity of blast furnace hearth. Iron Steel 2007, 42, 12–16. [Google Scholar]
- Zhang, J.L.; Jiao, K.X.; Liu, Z.J.; Yang, T.J. Comprehensive regulation technology for hearth protective layer of blast furnace longevity. Iron Steel 2017, 12, 1–7. [Google Scholar]
- Zhang, W.Z. Analysis and discussion on the activity of blast furnace hearth. Shandong Metall. 2022, 1, 21–22+26. [Google Scholar]
- Jiao, K.X.; Zhang, J.L.; Liu, Z.J.; Yang, T.J. Analysis of key issues on longevity of blast furnace hearth. Iron Steel 2020, 8, 193–198. [Google Scholar]
- Zhang, J.L.; Wang, Z.Y.; Jiao, K.X.; Wang, C.; Zhao, Y.A. Slag erosion resistance and hanging mechanism for the refractory in blast furnace hearth. Iron Steel 2015, 11, 27–31. [Google Scholar]
- Zhao, Q.Q.; Chen, T.; Zhao, G.L.; Wang, L.X. Design and practice of blast furnace longevity hearth. Tianjin Metall. 2020, 3, 5–7+26. [Google Scholar]
- Wang, G.; Li, A.F.; Liu, F.J.; Chen, G.Z.; Xu, J.; Liu, Z.B. Development and application of intelligent management system for blast furnace hearth longevity. China Metall. 2016, 4, 43–46. [Google Scholar]
- Wang, C.; Zhang, J.L.; Chen, W.; Li, X.L.; Jiao, K.X.; Pang, Z.P.; Wang, Z.Y.; Wang, T.S.; Liu, Z.J. Comparative analysis on the corrosion resistance to molten iron of four kinds of carbon bricks used in blast furnace hearth. Metals 2022, 12, 871. [Google Scholar] [CrossRef]
- Ni, A.; Li, C.; Zhang, W.; Xiao, Z.; Liu, D.; Xue, Z. Investigation of the hearth erosion of WISCO No.1 blast furnace based on the numerical analysis of iron flow and heat transfer in the hearth. Metals 2022, 12, 843. [Google Scholar] [CrossRef]
- He, K.; Wang, L.; Li, X.Y. Review of the energy consumption and production structure of China’s steel industry: Current situation and future development. Metals 2020, 10, 302. [Google Scholar] [CrossRef]
- Tang, H.Q.; Yun, Z.W.; Fu, X.F.; Du, S. Modeling and experimental study of ore-carbon briquette reduction under CO–CO2 atmosphere. Metals 2018, 4, 205. [Google Scholar] [CrossRef]
- Wang, X.L.; Jiao, K.X.; Qi, C.L.; Xiang, Z.Y. Formation mechanism and influencing factors of carbon brick protection layer in BF hearth. Ironmaking 2017, 5, 8–14. [Google Scholar]
- Wen, X.; Zou, Z.P.; Jiang, H.; Wang, W.; Zheng, S.B.; Yu, Z.D. Composition analysis and thermal conductivity measurement of iron skull in blast furnace hearth. J. Iron Steel Res. 2019, 9, 779–786. [Google Scholar]
- Zhang, L.L. Control of Side Wall Erosion of Hearth from the “Core”. 2014. Available online: http://www.csteelnews.com/ztbd/cmn/ (accessed on 29 July 2022).
- Zhang, W.; Hua, F.B.; Dai, J.; Xue, Z.L.; Ma, G.J.; Li, C.Z. Isothermal kinetic mechanism of coke dissolving in hot metal. Metals 2019, 9, 470. [Google Scholar] [CrossRef]
- Sawa, Y.; Takeda, K.; Taguchi, S.; Matsumoto, T.; Toshiyuki, W.; Kamano, H. Influence of low permeability zone in blast furnace hearth on temperature distribution in furnace bottom and on iron and slag tapping indices. Tetsu-to-Hagane 2009, 7, 1171–1178. [Google Scholar]
- Jiao, K.X.; Zhang, J.L.; Zuo, H.B.; Zhao, Y.A. Composition and formation mechanism of viscous layers in blast furnace hearth. J. Northeast Univ. (Nat. Sci.) 2014, 35, 987–991. [Google Scholar]
- Xie, R.J. Analysis of influencing factors on hot metal viscosity of blast furnace in MInguang Company of Quanzhou. Tianjin Metall. 2020, 6, 14–18+26. [Google Scholar]
- Xu, K. Study on Technology of High-Value Utilization of Carbon Precipitation at Ironmaking-Steelmaking Interface Due to Temperature Drop. Master’s Thesis, Northeastern University, Shenyang, China, 2019. [Google Scholar]
- Raipala, K. On Hearth Phenomena and Hot Metal Carbon Content in Blast Furnace; Helsinki University of Technology: Helsinki, Finland, 2003. [Google Scholar]
- Wang, Y.S.; Zhu, W.C. The discuss of theory flame temperature experience formula at the Shougang BFs. In Proceedings of the 2003 CSM Steel Congress, Beijing, China, 28–30 October 2003. [Google Scholar]
- Zhou, H.; Xu, K.; Yao, S.; Kou, M.Y.; Wu, S.L. Numerical simulation of injection of COREX decarbonized top gas to blast furnace. Iron Steel 2021, 2, 57–62. [Google Scholar]
- Raipala, K. Deadman and hearth phenomena in the blast furnace. Scand. J. Metall. 2000, 29, 39–46. [Google Scholar] [CrossRef]
- Dai, B.; Liang, K.; Wang, X.J.; Li, X.; Guo, Y.W. Development and practice of quantitative calculation models of blast furnace hearth activity. China Metall. 2015, 25, 45–49. [Google Scholar]
- Dai, B.; Liang, K.; Wang, X.J.; Hui, G.D.; Dong, H.; Sun, H.J. Fundamental research on hearth activity of blast furnace. In Proceedings of the 10th CSM Steel Congress and the 6th Baosteel Biennial Academic Conference, Shanghai, China, 21–23 October 2015. [Google Scholar]
- Jiao, K.X.; Zhang, J.L.; Liu, Z.J.; Liu, F.; Liang, L.S. Formation mechanism of the graphite-rich protective layer in blast furnace hearths. Int. J. Miner. Metall. Mater. 2016, 1, 16–24. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).