Influence of Nonmetallic Interstitials on the Phase Transformation between FCC and HCP Titanium: A Density Functional Theory Study
Abstract
1. Introduction
2. Method
3. Results and Discussion
3.1. Stable Interstitial Site
3.2. Stability against Interstitial Content
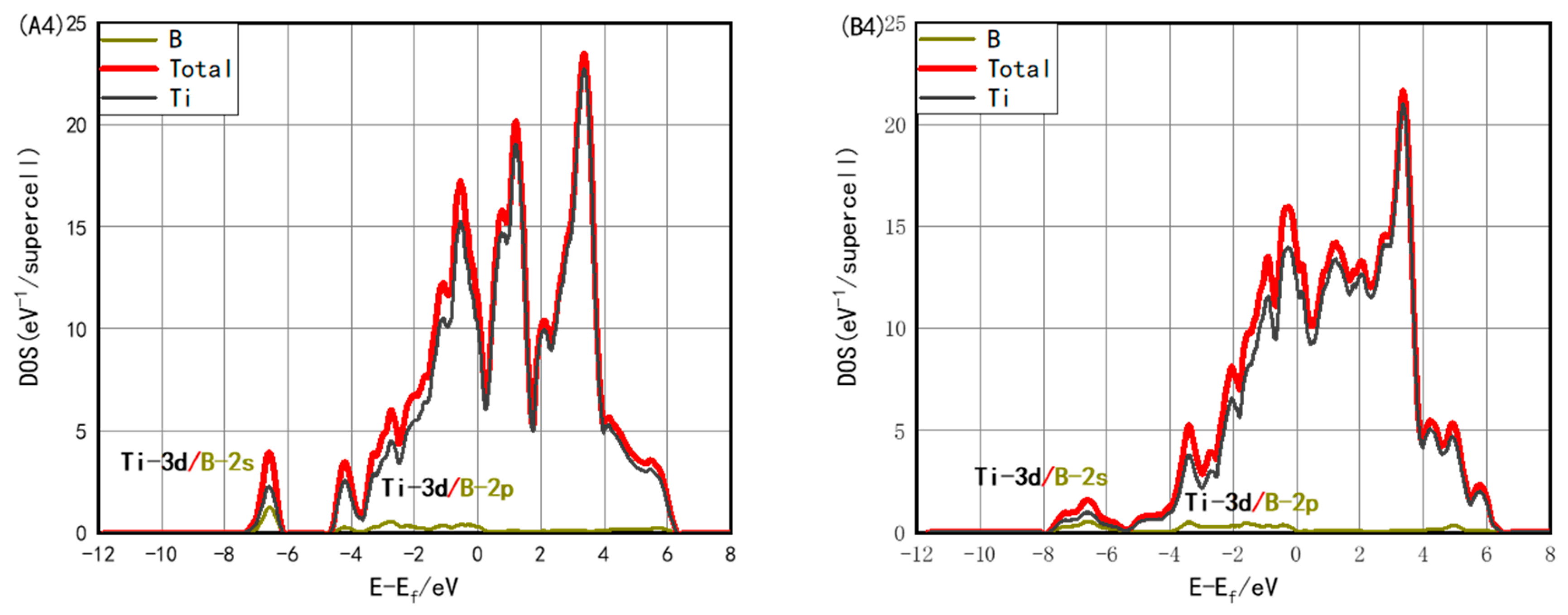
3.3. Electronic Structure with Interstitials
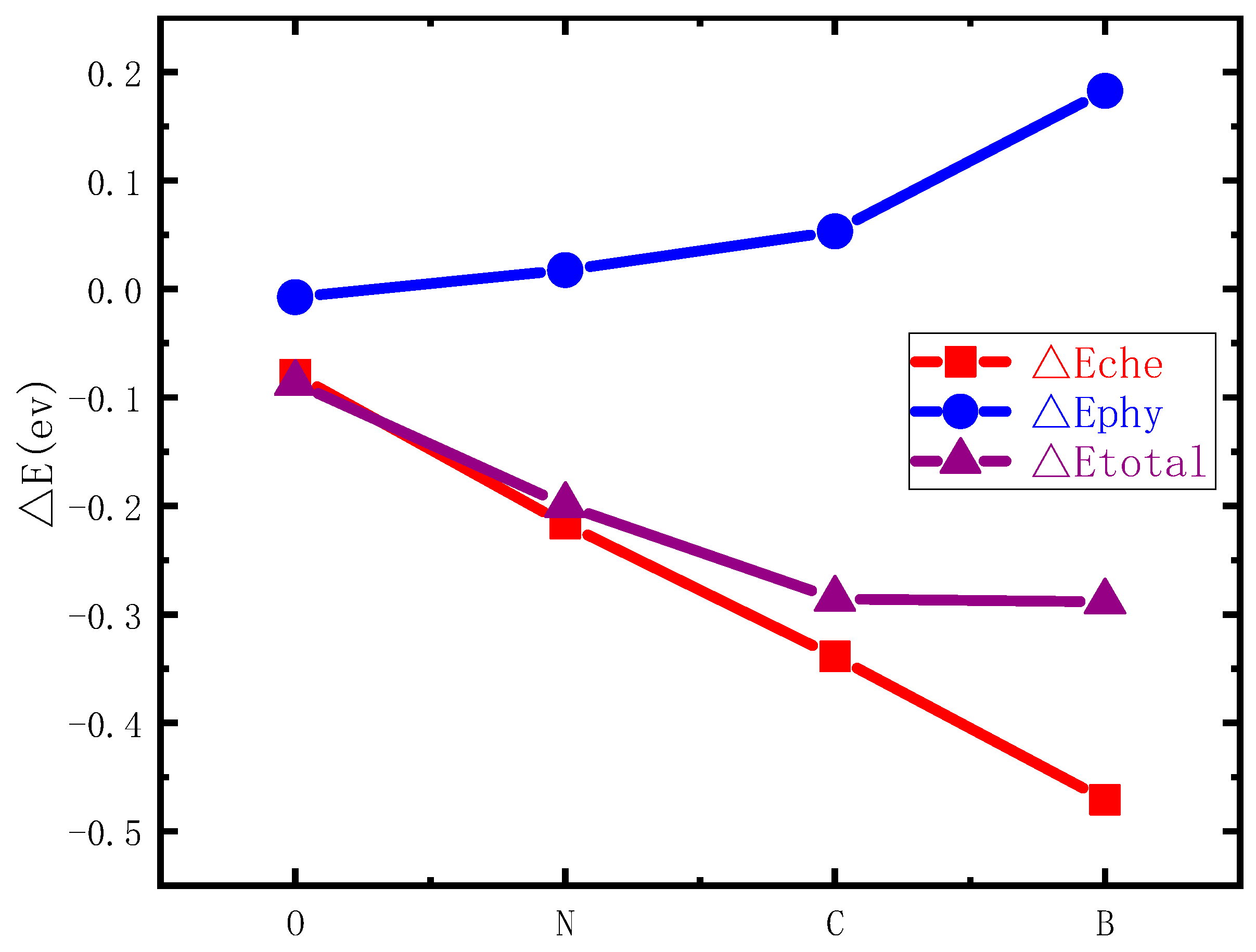
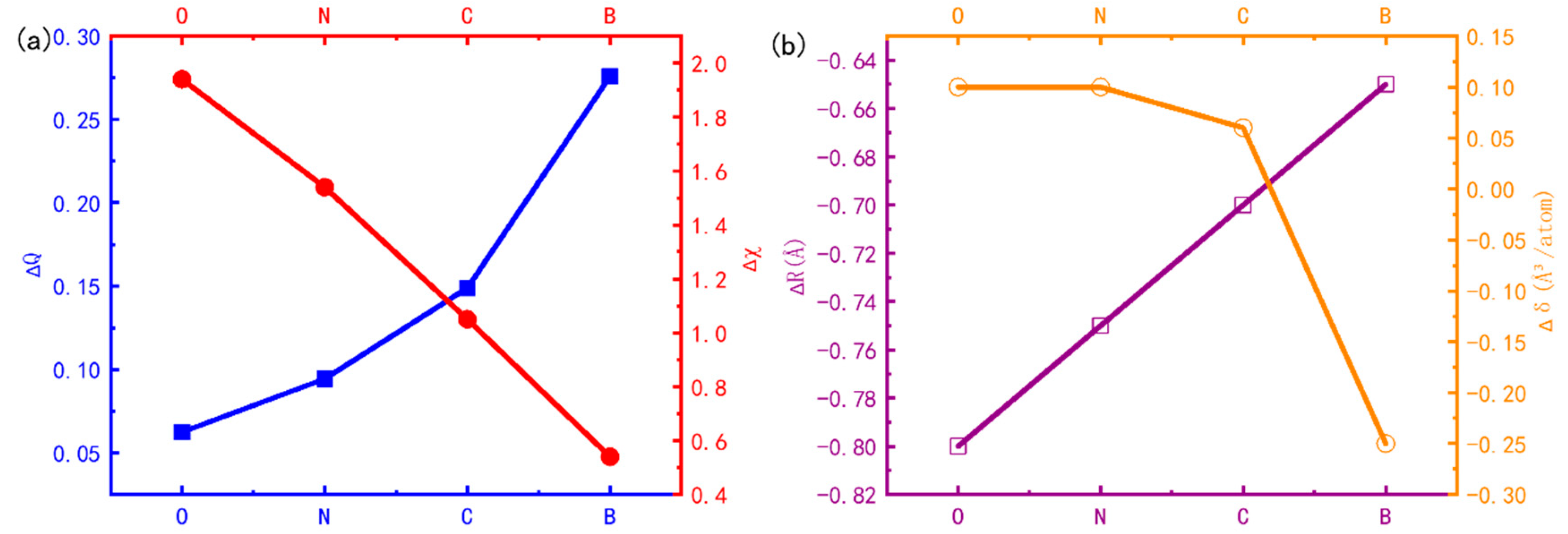
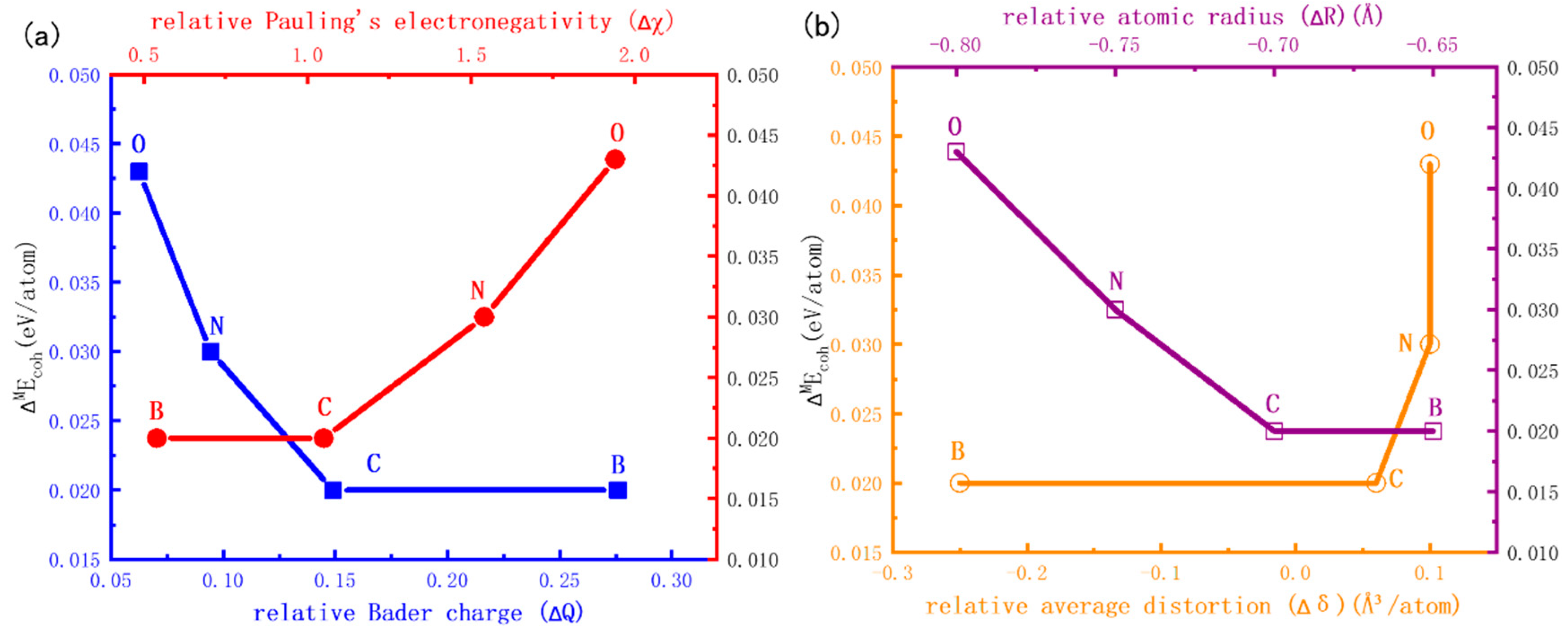
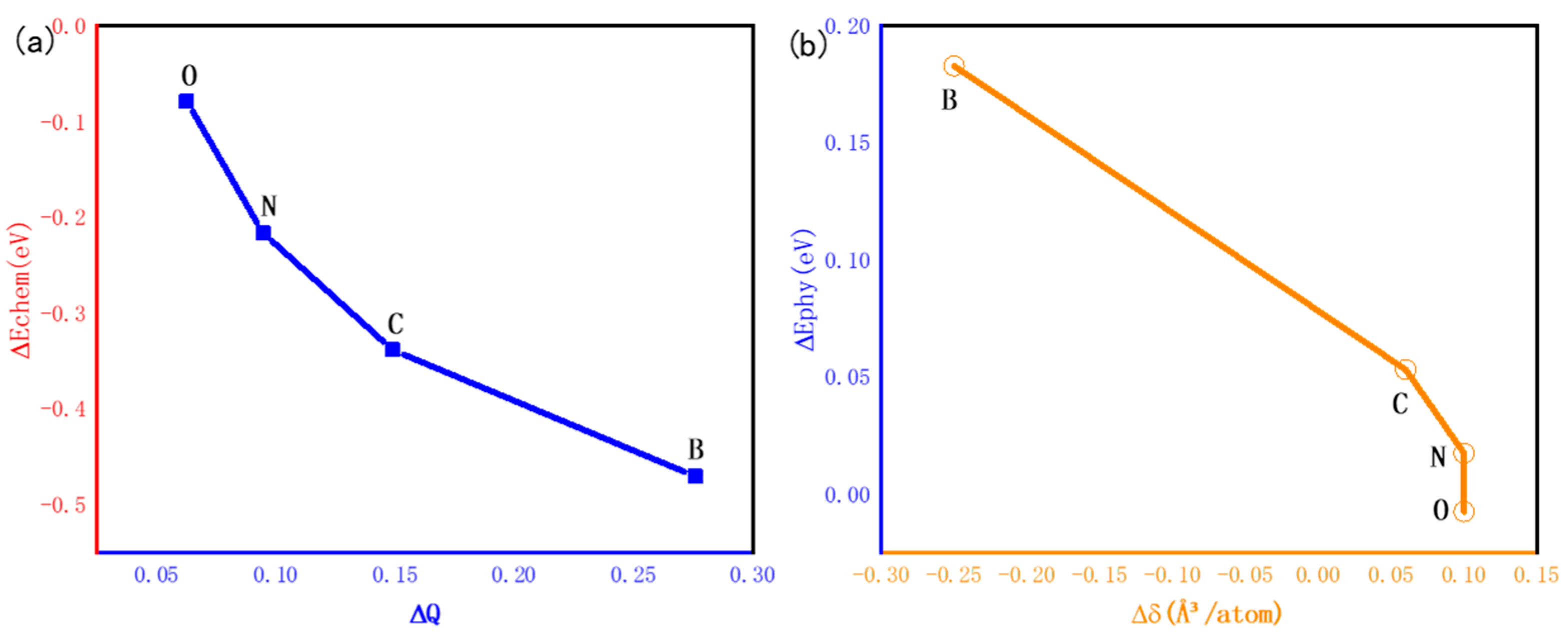
3.4. Influencing Factors of HCP-FCC Phase Transition
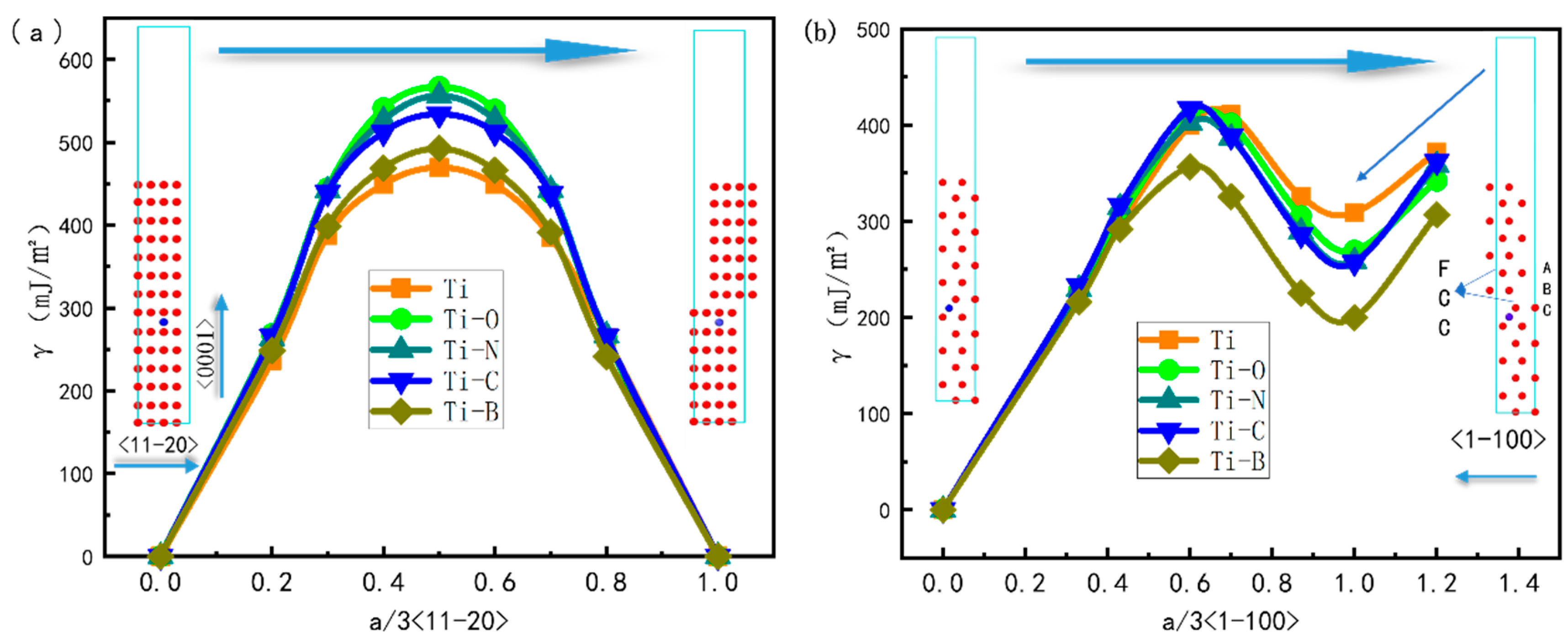
3.5. Stacking Fault Energy
4. Conclusions
- In both HCP-Ti and FCC-Ti, the nonmetallic atoms B, C, N and O favor the octahedral interstitial site rather than the tetrahedral site. The octahedral B, C, N and O interstitials decrease the energy gap between FCC-Ti and HCP-Ti, and the gap further decreases with the increase in the interstitial content.
- The relative stability of the interstitial-containing HCP-Ti and FCC-Ti is strongly relevant to the electronic structure, while it is weakly relevant to the lattice distortion. The relative stability of FCC-Ti decreases with the relative Pauling’s electronegativity, while it increases with the relative Bader charge.
- The existence of interstitial atoms reduces the intrinsic stacking fault energy of HCP-Ti, which is conducive to the decomposition of perfect dislocation into partial dislocations and is further beneficial to the formation of FCC-Ti.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hama, T.; Kobuki, A.; Takuda, H. Crystal-plasticity finite-element analysis of anisotropic deformation behavior in a commercially pure titanium Grade 1 sheet. Int. J. Plast. 2017, 91, 77–108. [Google Scholar] [CrossRef]
- Kazemi, M.; Ahangarani, S.; Esmailian, M.; Shanaghi, A. Investigation on the corrosion behavior and biocompatibility of Ti-6Al-4V implant coated with HA/TiN dual layer for medical applications. Surf. Coat. Technol. 2020, 397, 126044. [Google Scholar] [CrossRef]
- Davids, W.J.; Chen, H.; Nomoto, K.; Wang, H.; Babu, S.; Primig, S.; Liao, X.; Breen, A.; Ringer, S.P. Phase transformation pathways in Ti-6Al-4V manufactured via electron beam powder bed fusion. Acta Mater. 2021, 215, 117131. [Google Scholar] [CrossRef]
- Grünbaum, E.; Schwarz, R. Epitaxial Growth of Titanium Films on Mica. J. Appl. Phys. 1969, 40, 3364–3369. [Google Scholar] [CrossRef]
- Bonevich, D.v.H.J.; Josell, D. Face-centered-cubic titanium: An artifact in titanium/aluminum multilayers. J. Mater. Res. Technol. 1999, 14, 1977–1981. [Google Scholar] [CrossRef]
- Prasanthi, T.N.; Sudha, C.; Ravikirana; Saroja, S. Formation and reversion of metastable fcc phase in a Ti–5Ta–2Nb explosive clad. Mater. Charact. 2016, 116, 24–32. [Google Scholar] [CrossRef]
- Wu, H.C.; Kumar, A.; Wang, J.; Bi, X.F.; Tomé, C.N.; Zhang, Z.; Mao, S.X. Rolling-induced Face Centered Cubic Titanium in Hexagonal Close Packed Titanium at Room Temperature. Sci. Rep. 2016, 6, 24370. [Google Scholar] [CrossRef]
- Yu, Q.; Kacher, J.; Gammer, C.; Traylor, R.; Samanta, A.; Yang, Z.; Minor, A.M. In situ TEM observation of FCC Ti formation at elevated temperatures. Scr. Mater. 2017, 140, 9–12. [Google Scholar] [CrossRef]
- Chen, C.; Qian, S.; Wang, S.; Niu, L.; Liu, R.; Liao, B.; Zhong, Z.; Lu, P.; Li, P.; Cao, L.; et al. The microstructure and formation mechanism of face-centered cubic Ti in commercial pure Ti foils during tensile deformation at room temperature. Mater. Charact. 2018, 136, 257–263. [Google Scholar] [CrossRef]
- Han, G.; Lu, X.; Xia, Q.; Lei, B.; Yan, Y.; Shang, C. Face-centered-cubic titanium—A new crystal structure of Ti in a Ti-8Mo-6Fe alloy. J. Alloy. Compd. 2018, 748, 943–952. [Google Scholar] [CrossRef]
- Ma, X.; Guo, X.; Fu, M.; Qiao, Y. In-situ TEM observation of hcp-Ti to fcc-Ti phase transformation in Nb-Ti-Si based alloys. Mater. Charact. 2018, 142, 332–339. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, S.; Liebscher, C.H.; Dye, D.; Ponge, D.; Scheu, C.; Dehm, G.; Raabe, D.; Gault, B.; Lu, W. Could face-centered cubic titanium in cold-rolled commercially-pure titanium only be a Ti-hydride? Scr. Mater. 2020, 178, 39–43. [Google Scholar] [CrossRef]
- Traylor, R.; Zhang, R.; Kacher, J.; Douglas, J.O.; Bagot, P.A.; Minor, A.M. Impurity and texture driven HCP-to-FCC transformations in Ti-X thin films during in situ TEM annealing and FIB milling. Acta Mater. 2020, 184, 199–210. [Google Scholar] [CrossRef]
- Jiang, S.; Huang, L.; Gao, X.; Liu, G.; Zhang, R.; Jiao, Y.; Peng, S.; An, Q.; Wang, S.; Geng, L. Interstitial carbon induced FCC-Ti exhibiting ultrahigh strength in a Ti37Nb28Mo28-C7 complex concentrated alloy. Acta Mater. 2021, 203, 116456. [Google Scholar] [CrossRef]
- Tian, X.; Zhu, Y.; Lim, C.V.S.; Williams, J.; Boyer, R.; Wu, X.; Zhang, K.; Huang, A. Isotropic and improved tensile properties of Ti-6Al-4V achieved by in-situ rolling in direct energy deposition. Addit. Manuf. 2021, 46, 102151. [Google Scholar] [CrossRef]
- Liu, C.; Li, G.; Gu, H.; Yuan, F.; Han, F.; Ali, M.; Zhang, Y.; Guo, W. Observation of FCC-Zr phase in as-cast Zircaloy-4 alloy. Mater. Lett. 2020, 267, 127551. [Google Scholar] [CrossRef]
- Tao, B.; Qiu, R.; Liu, Y.; Tan, X.; Liu, Q. FCC phase transformation of Zr alloy during air cooling and aging. J. Nucl. Mater. 2021, 551, 152989. [Google Scholar] [CrossRef]
- Banerjee, D.; Williams, J.C. Perspectives on Titanium Science and Technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Cho, K.; Morioka, R.; Harjo, S.; Kawasaki, T.; Yasuda, H.Y. Study on formation mechanism of {332}<113> deformation twinning in metastable β-type Ti alloy focusing on stress-induced α” martensite phase. Scr. Mater. 2020, 177, 106–111. [Google Scholar] [CrossRef]
- Dumas, O.; Malet, L.; Hary, B.; Prima, F.; Godet, S. Crystallography and reorientation mechanism upon deformation in the martensite of an α-α’ Ti-6Al-4V dual-phase microstructure exhibiting high work-hardening rate. Acta Mater. 2021, 205, 116530. [Google Scholar] [CrossRef]
- Niessen, F.; Gazder, A.A.; Mitchell, D.R.G.; Pereloma, E.V. In-situ observation of nucleation, growth and interaction of deformation-induced α″ martensite in metastable Ti–10V–2Fe–3Al. Mater. Sci. Eng. A 2021, 802, 140237. [Google Scholar] [CrossRef]
- Kumar, K.N.C.; Suresh, K. Reduction in anisotropy of mechanical properties of Ti-6Al-4V through martensite decomposition. Mater. Lett. 2022, 306, 130903. [Google Scholar] [CrossRef]
- Bai, F.; Yin, L.; Zhao, W.; Zhou, H.; Song, M.; Liu, Y.; Liu, X. Deformational behavior of face-centered cubic (FCC) phase in high-pure titanium. Mater. Sci. Eng. A 2021, 800, 140287. [Google Scholar] [CrossRef]
- Yang, J.X.; Zhao, H.L.; Gong, H.R.; Song, M.; Ren, Q.Q. Proposed mechanism of HCP→FCC phase transition in titianium through first principles calculation and experiments. Sci. Rep. 2018, 8, 1992. [Google Scholar] [CrossRef]
- Chen, P.; Wang, F.; Li, B. Transitory phase transformations during {101¯2} twinning in titanium. Acta Mater. 2019, 171, 65–78. [Google Scholar] [CrossRef]
- Zheng, X.; Gong, M.; Xiong, T.; Ge, H.; Yang, L.; Zhou, Y.; Zheng, S.; Wang, J.; Ma, X. Deformation induced FCC lamellae and their interaction in commercial pure Ti. Scr. Mater. 2019, 162, 326–330. [Google Scholar] [CrossRef]
- He, M.; Shen, Y.; Jia, N.; Liaw, P. C and N doping in high-entropy alloys: A pathway to achieve desired strength-ductility synergy. Appl. Mater. 2021, 25, 101162. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B Condens. Matter 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Yu, H.; Cao, S.; Youssef, S.S.; Ma, Y.-J.; Lei, J.-F.; Qi, Y.; Hu, Q.-M.; Yang, R. Generalized stacking fault energies and critical resolved shear stresses of random α-Ti-Al alloys from first-principles calculations. J. Alloy. Compd. 2021, 850, 156314. [Google Scholar] [CrossRef]
- Bercegeay, C.; Bernard, S. First-principles equations of state and elastic properties of seven metals. Phys. Rev. B 2005, 72, 214101. [Google Scholar] [CrossRef]
- De Jong, M.; Chen, W.; Angsten, T.; Jain, A.; Notestine, R.; Gamst, A.; Sluiter, M.; Ande, C.K.; Van Der Zwaag, S.; Plata, J.J.; et al. Charting the complete elastic properties of inorganic crystalline compounds. Sci. Data 2015, 2, 150009. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Kwasniak, P.; Muzyk, M.; Garbacz, H.; Kurzydlowski, K.J. Influence of C, H, N, and O interstitial atoms on deformation mechanism in titanium—First principles calculations of generalized stacking fault energy. Mater. Lett. 2013, 94, 92–94. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- You, D.; Ganorkar, S.; Kim, S.; Kang, K.; Shin, W.-Y.; Lee, D. Machine learning-based prediction models for formation energies of interstitial atoms in HCP crystals. Scr. Mater. 2020, 183, 1–5. [Google Scholar] [CrossRef]
- You, D.; Ganorkar, S.; Joo, M.; Park, D.; Kim, S.; Kang, K.; Lee, D. Ab initio study of H, B, C, N, O, and self-interstitial atoms in hcp-Zr. J. Alloy. Compd. 2019, 787, 631–637. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Duan, Y.; Wu, Y.; Peng, M.; Qi, H. The interstitial diffusion behaviors and mechanisms of boron in α-Ti and β-Ti: A first-principles calculation. Comput. Mater. Sci. 2020, 184, 109866. [Google Scholar] [CrossRef]
- Shitara, K.; Yoshiya, M.; Umeda, J.; Kondoh, K. Substantial role of charge transfer on the diffusion mechanism of interstitial elements in α-titanium: A First-principles study. Scr. Mater. 2021, 203, 114065. [Google Scholar] [CrossRef]
- Connétable, D.; David, M. Diffusion of interstitial species (H and O atoms) in fcc systems (Al, Cu, Co, Ni and Pd): Contribution of first and second order transition states. J. Alloy. Compd. 2019, 772, 280–287. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, G.-P.; Li, M. The effects of short-range chemical and structural ordering related to oxygen interstitials on mechanical properties of CrCoFeNi high-entropy alloys: A first-principles study. J. Alloy. Compd. 2020, 843, 156060. [Google Scholar] [CrossRef]
- Genç, A.; Banerjee, R.; Hill, D.; Fraser, H. Structure of TiB precipitates in laser deposited in situ, Ti-6Al-4V–TiB composites. Mater. Lett. 2006, 60, 859–863. [Google Scholar] [CrossRef]
- Chen, C.L.; Lu, W.; Lin, J.P.; He, L.L.; Chen, G.L.; Ye, H.Q. Orientation relationship between TiB precipitate and γ-TiAl phase. Scr. Mater. 2007, 56, 441–444. [Google Scholar] [CrossRef]
- Cui, Y.; Aoyagi, K.; Zhao, Y.; Yamanaka, K.; Hayasaka, Y.; Koizumi, Y.; Fujieda, T.; Chiba, A. Manufacturing of a nanosized TiB strengthened Ti-based alloy via electron beam powder bed fusion. Addit. Manuf. 2020, 36, 101472. [Google Scholar] [CrossRef]
- Steinberg, S.; Dronskowski, R. The Crystal Orbital Hamilton Population (COHP) Method as a Tool to Visualize and Analyze Chemical Bonding in Intermetallic Compounds. Crystals 2018, 8, 225. [Google Scholar] [CrossRef]
- Allen, L.C. ElectronegativityIs the Average One-Electron Energy of the Valence-Shell Electrons in Ground-State Free Atoms. J. Am. Chem. Soc. 1989, 111, 9003–9014. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Slater, J.C. Atomic Radii in Crystals. J. Chem. Phys. 1964, 41, 3199–3204. [Google Scholar] [CrossRef]
- Achmad, T.L.; Fu, W.; Chen, H.; Zhang, C.; Yang, Z.-G. First-principles calculations of generalized-stacking-fault-energy of Co-based alloys. Comput. Mater. Sci. 2016, 121, 86–96. [Google Scholar] [CrossRef]
- Liang, L.; Hardouin Duparc, O.B.M. Ab initio reappraisal of the dislocation-associated stacking faults in hcp titanium: A new dissociation mechanism. Philos. Mag. Lett. 2016, 97, 19–26. [Google Scholar] [CrossRef]
- Wu, X.; Wang, R.; Wang, S. Generalized-stacking-fault energy and surface properties for HCP metals: A first-principles study. Appl. Surf. Sci. 2010, 256, 3409–3412. [Google Scholar] [CrossRef]
- Hu, Q.-M.; Yang, R. Basal-plane stacking fault energy of hexagonal close-packed metals based on the Ising model. Acta Mater. 2013, 61, 1136–1145. [Google Scholar] [CrossRef]
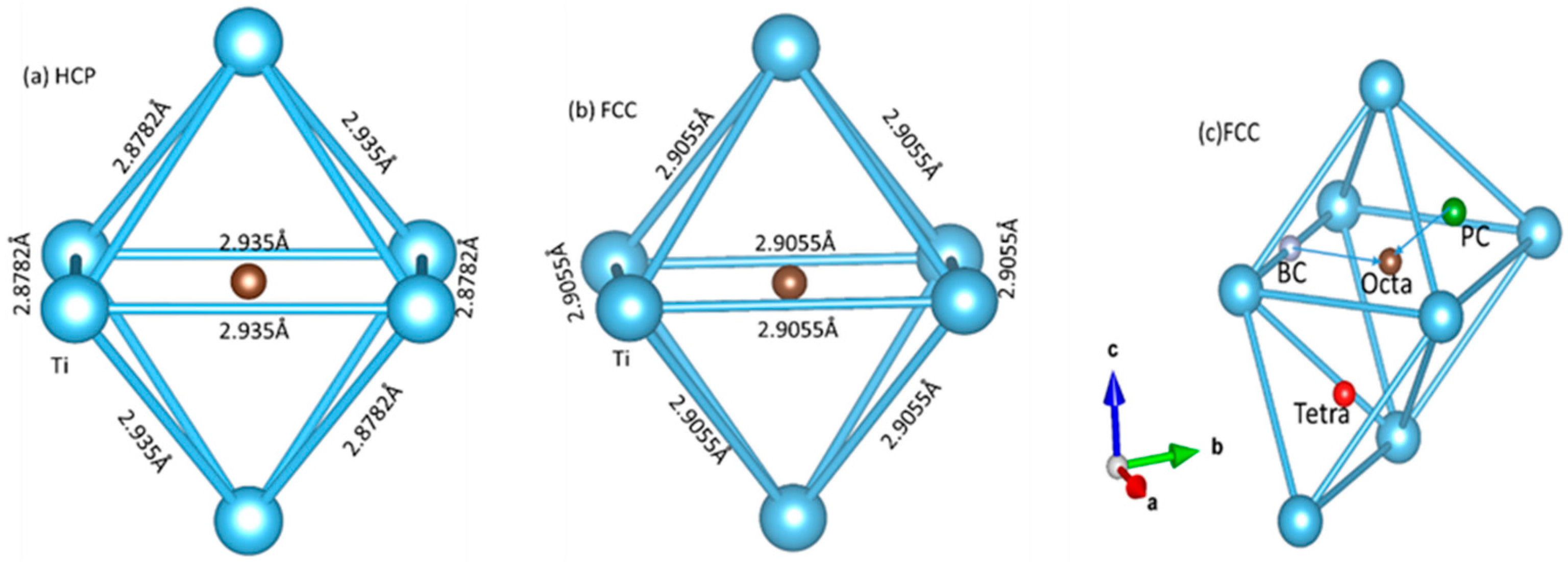
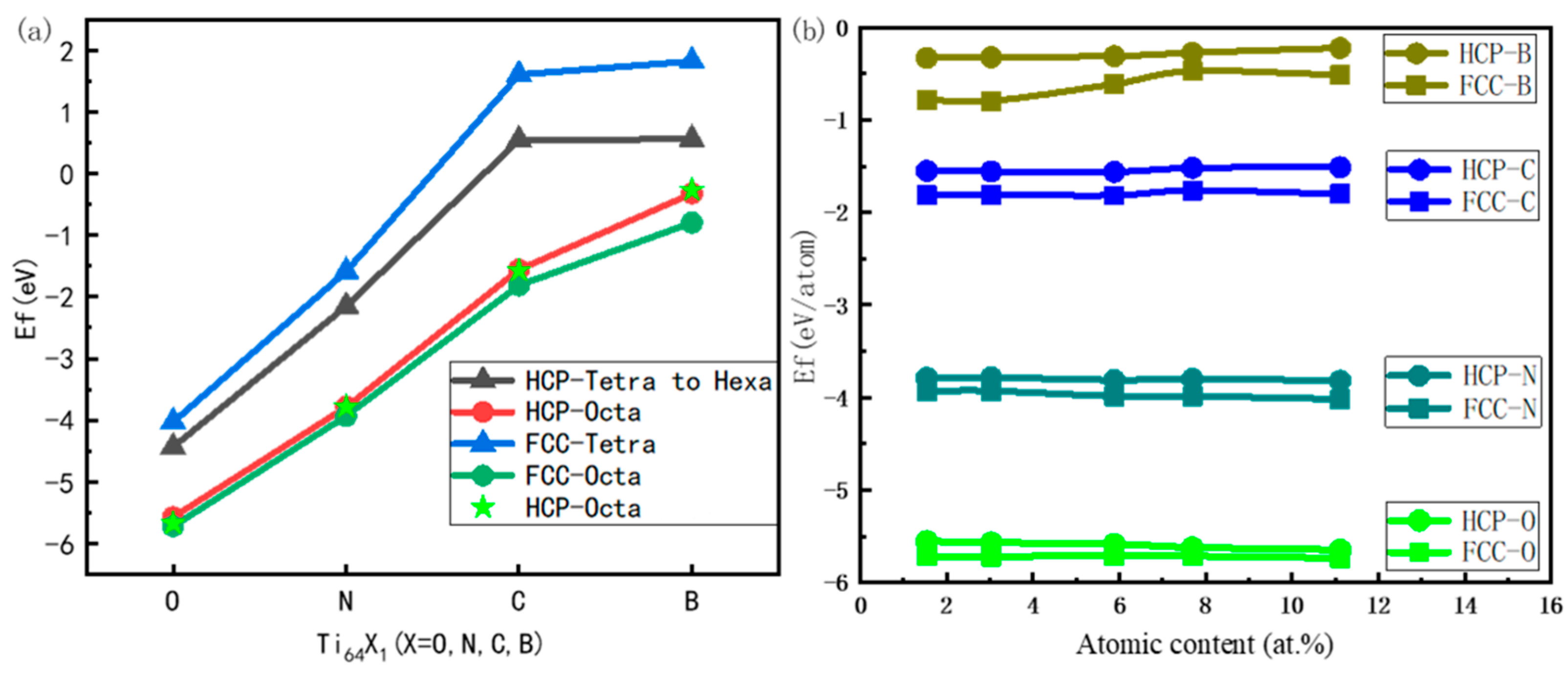
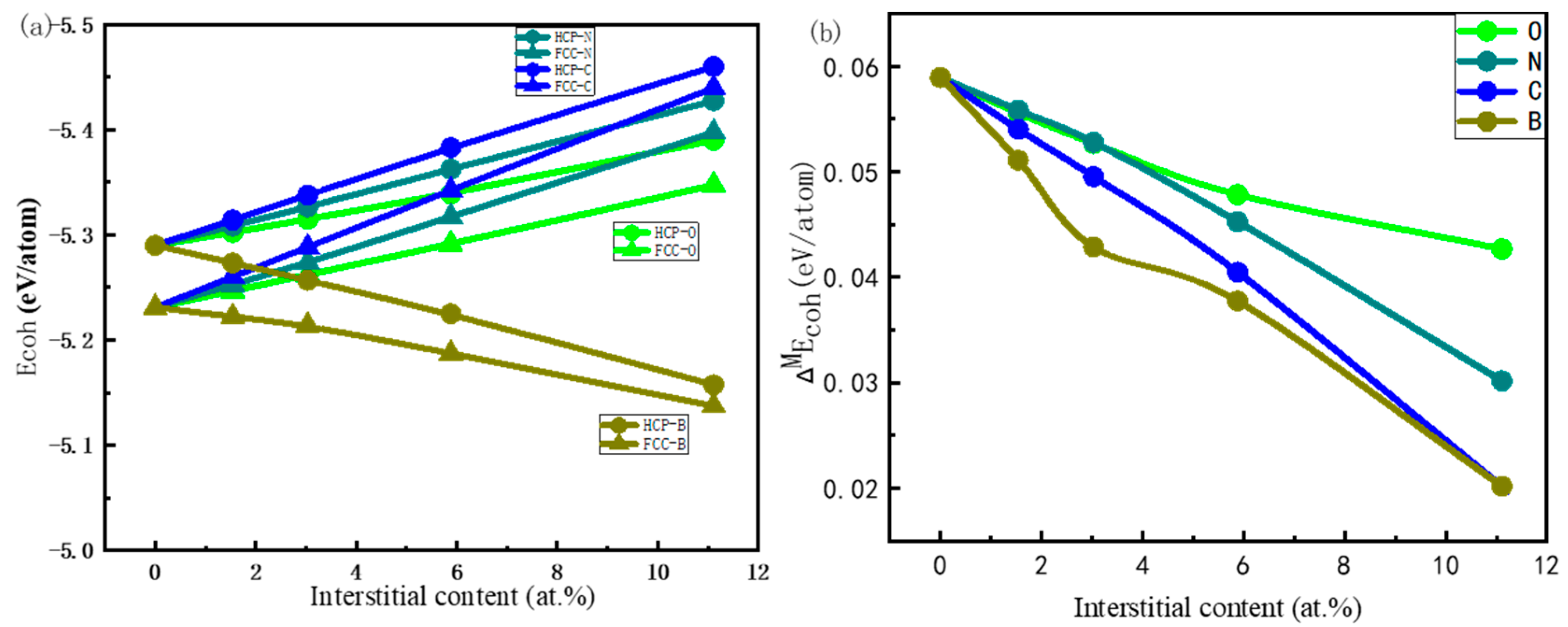
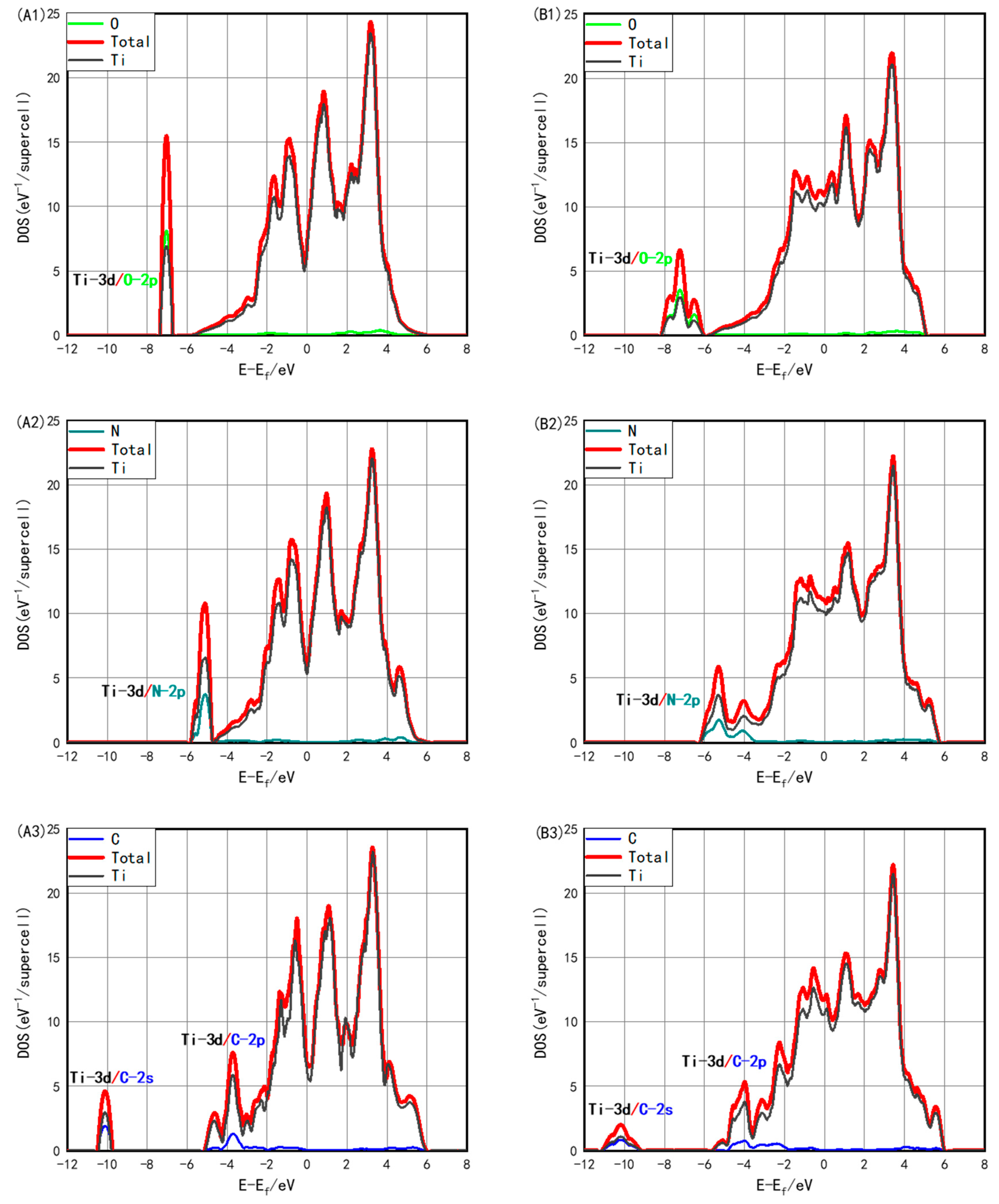
| Phase | a (Å) | c/a | C11 (GPa) | C12 (GPa) | C13 (GPa) | C33 (GPa) | C44 (GPa) |
|---|---|---|---|---|---|---|---|
| HCP a | 2.935 | 1.585 | 175 | 87.9 | 76.8 | 189 | 42.0 |
| FCC a | 4.109 | / | 127 | 101 | / | / | 60.3 |
| HCP b | 2.931 | 1.584 | 177 | 84.5 | 77.0 | 190 | 41.5 |
| HCP c | 2.930 | / | 172 | 85.0 | 78.6 | 188 | 39.0 |
| FCC d | 4.109 | / | 123 | 99.0 | / | / | 55.0 |
| System | Ti-O | Ti-N | Ti-C | Ti-B |
|---|---|---|---|---|
| ICOHPFCC(eV) | −2.338 | −2.864 | −3.156 | −3.450 |
| ICOHPHCP(eV) | −2.000 | −2.674 | −2.999 | −3.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Hu, J.; Cao, S.; Feng, G.; Yang, Y.; Liu, R.; Li, S.; Zhao, F.; Feng, A.; Hu, Q.; et al. Influence of Nonmetallic Interstitials on the Phase Transformation between FCC and HCP Titanium: A Density Functional Theory Study. Metals 2022, 12, 1607. https://doi.org/10.3390/met12101607
Yang M, Hu J, Cao S, Feng G, Yang Y, Liu R, Li S, Zhao F, Feng A, Hu Q, et al. Influence of Nonmetallic Interstitials on the Phase Transformation between FCC and HCP Titanium: A Density Functional Theory Study. Metals. 2022; 12(10):1607. https://doi.org/10.3390/met12101607
Chicago/Turabian StyleYang, Mengmeng, Jianan Hu, Shuo Cao, Guang Feng, Yi Yang, Renci Liu, Shujun Li, Fu Zhao, Aihan Feng, Qingmiao Hu, and et al. 2022. "Influence of Nonmetallic Interstitials on the Phase Transformation between FCC and HCP Titanium: A Density Functional Theory Study" Metals 12, no. 10: 1607. https://doi.org/10.3390/met12101607
APA StyleYang, M., Hu, J., Cao, S., Feng, G., Yang, Y., Liu, R., Li, S., Zhao, F., Feng, A., Hu, Q., Huang, A., & Wang, H. (2022). Influence of Nonmetallic Interstitials on the Phase Transformation between FCC and HCP Titanium: A Density Functional Theory Study. Metals, 12(10), 1607. https://doi.org/10.3390/met12101607








