Abstract
S is a common corrosion medium for austenitic stainless steels. The severe intergranular fracture of austenitic stainless steels occurs in sulfur environments. In this paper, the permeation of S at different atomic positions for three symmetric tilt grain boundary types, i.e., Σ5(210), Σ5(310), and Σ9(114) have been computed using first-principles calculations. S has the strongest segregation tendency in the Σ5(210) grain boundary. A high content of S at the grain boundary indicates harm to the grain boundary. Sulfur segregation in the grain boundaries can weaken the strength of the metallic bond. When Mo and Cr are present at the Σ5(210) grain boundary, the sulfur-induced embrittlement is inhibited. With increased S concentration at the grain boundary, the coexistence of Mo and Cr can suppress the intergranular fracture of S on the grain boundary. The reason why high-Mo stainless steel has excellent sulfur-induced intergranular corrosion resistance is explained at the atomic level.
1. Introduction
The mechanical strength and corrosion resistance of metallic materials undergo drastic changes when impurity atoms, especially corrosive ones (such as sulfur), are present in the vicinity of grain boundaries. Super-austenitic stainless steels (SASSs) have the potential for use in extremely harsh service environments, such as flue-gas desulfurization, seawater desalination and the petrochemical industry, due to the large amounts of Cr, Mo, and N elements within them [1,2,3]. Steels belonging to the family of SASS usually contain 10–24 wt% Cr and 17–22 wt% Ni, as primary elements, plus 4–8 wt% Mo and 0.15–0.5 wt% N, as auxiliary elements. The pitting- and crevice corrosion resistance can be improved significantly by increasing the amounts of Cr and Mo in sulfur-containing environments and chloride-based media [4,5].
SCC is the abbreviation for stress corrosion cracking, which is a corrosion mechanism in which the combination of a susceptible alloy and a particular environment leads to cracking in the metal. SCC in sulfur-containing environments has always been a severe threat to the safety of materials [6,7]. The grain boundary (GB), as an important defect in the metal surface, has an effect on the corrosion and mechanical properties of metals [8]. The role of the GB in stress corrosion has been reported in previous work. Gesari et al. [9] studied the H-Fe interaction at the GB of body-centered cubic Fe and found that the GB region can provide a possible site for H accumulation. Additionally, some Fe-Fe bonds in the GB plane show a 60% decrease in the overlap population when H is present. Stefano et al. [10] studied the interaction between hydrogen atoms and Σ3(111) [1,2,3,4,5,6,7,8,9,10] and Σ5(210) [001] GBs of Ni, and found that the hydrogen atom can diffuse into the metal substrate easily, through the Σ5(210) [001] GB, with sparsely arranged atoms. This phenomenon is an important cause of SCC. Kart et al. [11] studied the sulfur-induced embrittlement of nickel Σ5(210) and the effect of the concentration of sulfur, and found that the extension of the nickel GB is a result of the repulsion of the segregated and neighboring sulfur.
Based on previous studies [12,13,14,15,16], it is know that Mo imparts pitting corrosion resistance to sulfur-ion-containing solutions. Furthermore, approximate amounts of Mo are added to high-Mo austenitic steels, to increase their resistance to pitting- and crevice corrosion mechanisms. Many theories have been proposed to explain the effect of Mo on pitting corrosion, but two mechanisms are the most accepted [14,15,16]. However, the detailed microscopic origin of the interaction between the grain boundaries and solute atoms still cannot be clarified, due to the limitations in experimental techniques. Therefore, the significance of the critical inter-granular concentration of sulfur in the GB remains unclear. First-principles methods can be used as an alternative approach, to bring some clarification to the critical questions that have been tackled, such as why, and how, sulfur atoms weaken the GB.
In the present work, the effect of Mo and Cr on S segregation and intergranular fracture at Σ5(210) GB in Fcc-Fe is determined using the first-principles method. The segregation behavior of S in the presence of Mo and Cr, and the effect of different S contents on GB structure, are studied. The effect of Mo and Cr on intergranular fractures, induced by S in high-Mo austenitic steels, is observed at the atomic level.
2. Computational Methods and Model
In this work the first-principles calculations are performed using the Cambridge Sequential Total Energy Package code (CASTEP) (2017), based on density functional theory (DFT) [17]. The Perdew-Burke-Ernzerhof (PBE) function is used, and correlation effects are treated using the generalized gradient approximation (GGA) [18]. A kinetic energy cutoff of 400 eV is used after carefully testing for convergence, and the Brillouin zone is sampled using Monkhorst--Pack k-point grid parameters: 7 × 3 × 2 for Σ5(210) GB, 7 × 2 × 2 for Σ5(310) GB, and 5 × 2 × 2 for Σ9(114) GB. An ultrasoft pseudopotential is used in the calculations. In the course of the self-consistent calculation, the convergence value of the total energy of the system is selected as 2.0 × 10−6 eV/atom, with the force on each atom lower than 0.3 eV/nm, and the stress deviation lower than 0.05 GPa. Considering that austenitic stainless steel is not magnetic, spin polarization is not considered in the calculation process. These GBs are modeled based on the optimized lattice parameter (3.446 Å) of γ-Fe, which is very close to the experimental results and the other calculated values for γ-Fe [19,20].
The binding energy of solute atoms in various atomic layers in each GB is calculated using the following equations [21]:
Here, (NFe, NR) refers to the calculated total energy of the GB unit cell, including NFe Fe atoms and NR (R = S, Mo and Cr) atoms. NR is the total energy of one solute atom, and is the number of Fe atoms in the pure GB model and bulk Fe unit cells. () is the total energy of the GB system without any impurities, and () is the total energy of the Fcc bulk Fe system, with the same number of pure GBs.
The diffuse tendency for the solute elements of different GB models can be calculated from the segregation energy [11]:
where (NR) is the binding energy when the bulk structure contains the same solute atoms.
The binding and segregation energies of S, Mo, and Cr in each GB structure are calculated to determine whether these elements can exist stably in different GB structures, and their occupancy tendency. The negative value of the segregation energy indicates that solute atoms tend to segregate at this plane.
Σ5(210), Σ5(310), and Σ9(114) symmetric tilt GBs, for calculation, are constructed on the basis of an optimized γ-Fe structure, to construct supercells (Figure 1). The dark gray dotted line is the plane of the GB. In these three GBs, numbers 1–11 represent the atomic layers parallel to the GB plane, and negative values represent the equivalent symmetrical atomic layers. Σ5(210) GB and Σ9(114) GB have 20 atomic layers, and Σ5(310) GB has 16 atomic layers. In the Σ5(210) GB structure, 0 represents a vacancy in the GB plane. On the basis of the optimization of the three GB structures, the binding energy and segregation energy of S and alloy compositions (Mo and Cr), at each atomic layer in the GBs, are calculated. Given the small atomic radius of S, the calculation results of its vacancies at the GB are also considered.
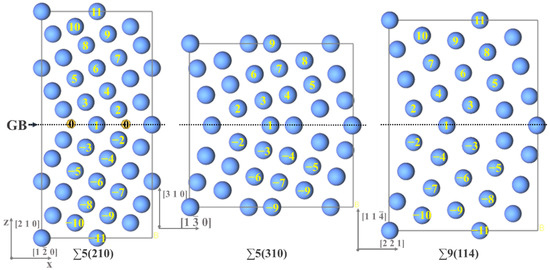
Figure 1.
The Σ5(210), Σ5(310), Σ9(114) grain boundary models of Fcc-Fe. The numbers in the figure represent different atomic layers at different GBs. The number 0 represents the possible interstitial site in the GB plane of the Σ5(210) grain boundary.
3. Results and Discussion
The binding energies and segregation energies for S at each atomic plane in the substitution system were calculated (as demonstrated in Figure 2), to analyze the occupancy tendency of S in the Σ5(210), Σ5(310), and Σ9(114) GBs. The blue dotted line shows the position of the GB plane. The binding energies of S at different positions in the three GBs are all negative, indicating that S can exist in the three GBs and form stable structures. Figure 2b shows the segregation energies of S at different planes in the three GB structures. S has the lowest segregation energy in the adjacent regions of the three GBs, that is, layers −2 and 2. The lowest segregation energy near the Σ5(210) GB is −1.77 eV. The lowest segregation energies of S in Σ5(310) GB and Σ9(114) GB structures are −1.29 eV and −1.26 eV, respectively. Sulfur is most likely to permeate the interface of the three GBs, and the segregation tendency in Σ5(210) GB is the strongest. The above calculation results show that S easily permeates the grain boundary. The segregation of S will produce embrittlement behavior, leading to intergranular fracture [22].
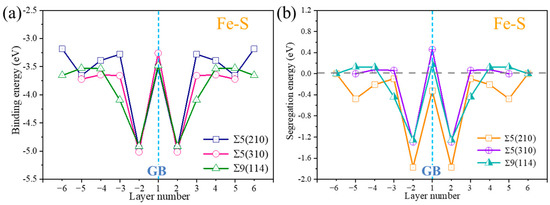
Figure 2.
(a) Binding energies of S in different layers of Σ5(210) GB, Σ5(310) GB and Σ9(114) GB, respectively. (b) Segregation energy of S in different layers of Σ5(210) GB, Σ5(310) GB and Σ9(114) GB.
Figure 3 shows the binding energies and segregation energies of Mo and Cr at different layers in the three GB structures. The binding energy results in Figure 3a,b show that Mo and Cr can also exist stably in the grain boundary structure. The segregation tendencies of the two elements in the three GBs are different, as shown in Figure 3c,d. The segregation energies of Mo at layer 1 (interface) of the three GBs are all negative. Mo is easy to segregate at the interface of the three GB structures. In the case of Cr, no evident differences of segregation tendency in each layer in the GBs are observed. The segregation energies of Cr in different atomic layers in the three GBs are all close to 0. This result indicates that Cr is basically uniformly distributed in Fcc-Fe. The above calculation results show that Mo easily exists at the GB in the Fcc-Fe. Previous studies have shown the presence of molybdenum-rich phases at grain boundaries in austenitic steels [23,24].
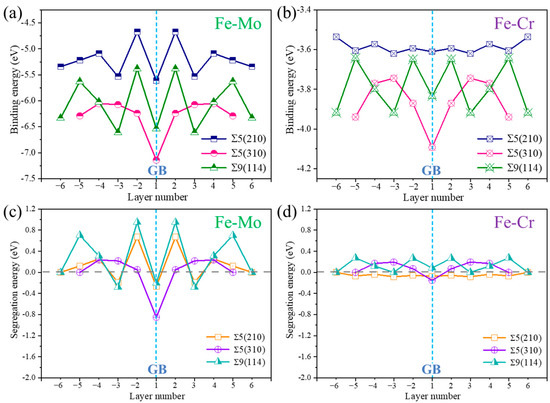
Figure 3.
Binding energies of (a) Mo and (b) Cr in different layers of Σ5(210) GB, Σ5(310) GB and Σ9(114) GB. Segregation energy of (c) Mo and (d) Cr in different layers of Σ5(210) GB, Σ5(310) GB and Σ9(114) GB.
S often produces intergranular fracture [25]. The Mo and Cr contents in SASS are extremely high, and their main function is to improve the intergranular corrosion resistance of stainless steel, especially the increased Mo content. Based on the above calculation results, sulfur has a strong segregation tendency and wide segregation range in the three GBs. Therefore, the Σ5(210) GB structure, as the strongest grain boundary for S segregation, is taken as a research object, to investigate the influence of Mo and Cr on the intergranular fracture of GBs by sulfur. The Σ5(210) GB structure has evident cavities at GBs, shown as the two 0 positions marked in Figure 1. The segregation energy of S at the interface is higher than that in the adjacent layers, in the Σ5(210) GB. Thus, the segregation energy of S at the interstitial sites in the GB is further analyzed. The 0 site is energetically favorable for S, with segregation energy −1.75 eV, which is similar to the segregation energy of S at the adjacent substitution position of Σ5(210) GB. Based on this calculation result, the case where S is at the 0 position of the GB is studied below.
The same S segregation sites at Σ5(210) GB, containing Mo or Cr at the interface (Fe-Mo-Σ5(210) GB and Fe-Cr-Σ5(210) GB), and Mo-Cr coexisting at the interface of Σ5(210) GBs (Fe-Mo-Cr-Σ5(210) GB) are considered simultaneously, to investigate the effect of Mo and Cr on S segregation, and segregation energies are also calculated (Figure 4). The segregation energy of S in the GB layer is the segregation energy of S at position 0. When S is located on the GB 0 layer and the second layer, Mo or Cr at the GB increases the segregation energy of S and has a certain inhibitory effect on the segregation of S. By comparison, the inhibitory effect of Mo at the GB is higher than that of Cr, which is consistent with the fact that high-Mo austenitic steel is widely used in sulfur-resistant corrosion environments [26,27,28]. The segregation energy of S on layer 0 in Fe-Mo-Cr-Σ5(210) GB is larger than that of S in other systems, which shows that the combined effect of Mo and Cr is higher than that of single Mo or Cr. When Mo and Cr are at the GB at the same time, the segregation energy of S at the grain interface gap is inhibited the most. Results show that Mo and Cr can inhibit the effect of sulfur on GBs.
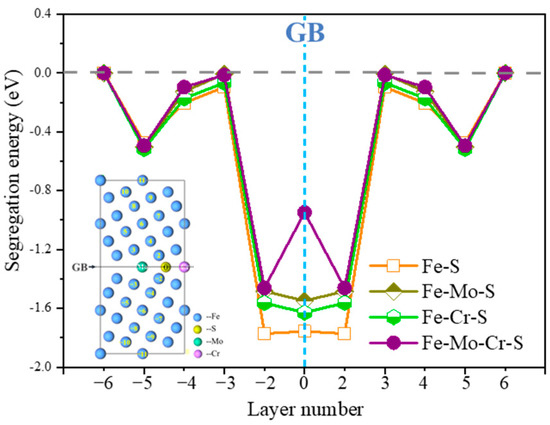
Figure 4.
The S segregation energies for various substitutional sites as a function of Mo, Cr at Σ5(210) GB interface.
The effect of the S aggregation in the crystal boundary on the structural stability of the crystal boundary is further analyzed. Figure 5 displays the variation in the GB structure when 1, 2, 4, 6, sulfur atoms are added to their favorable layers, and their corresponding S contents are 2.4, 4.8, 9.5 and 14 at.%. Model systems represented in Figure 5 are the optimized structures. Figure 5a shows the structure of pristine Σ5(210) GB (Fe-Σ5(210) GB), without S. Figure 5b has one sulfur atom in the 0 site and Figure 5c contains two sulfur atoms in two 0 sites. No significant change is observed in the mutual positions between the atomic layers and the structure containing two S atoms. Figure 5d shows a Σ5(210) GB with four sulfur atoms. Two of them are inserted in 0 sites, whereas the rest of the atoms are substituted for Fe atoms at layers −2 and 2. Similarly, Figure 5e includes 6 sulfur atoms: two in 0 sites, plus layers −2 and 2 each have two sulfur atoms. Increasing the number of S atoms gradually increases the distance between Fe atoms at the GB, particularly the region with closer distance between S atoms. The existence of S enlarges the interlayer space and causes the expansion of the GB, especially increasing the size in the z-axis direction. A large-distance cavity is formed between S atoms, and the interaction between Fe atoms decreases with the increasing number of sulfur atoms around the interface of the GB. The final result of aggregation of S in the GB region is that it causes GB cracking. Thus, the S aggregation in the GB weakens the bonding ability of the GB.
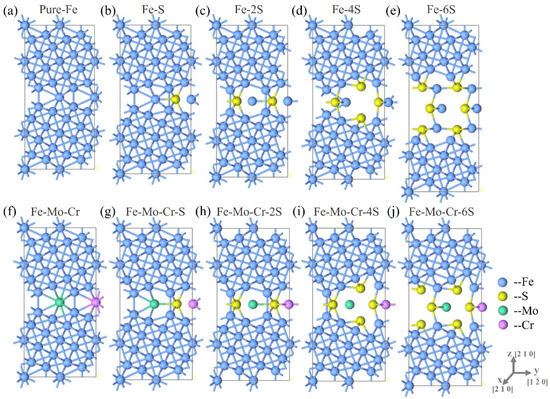
Figure 5.
(a–e) I Optimized models of different number of S atoms at Σ5(210) GB, (f–j) are the optimized models of different numbers of S atoms in Σ5(210) GB, containing Mo and Cr atoms.
In comparison with the S segregation at clean Σ5(210) GB, the presence of Mo and Cr at the interface is shown to restrain S segregation. The Fe-Mo-Cr-Σ5(210) GBs with different numbers of S atoms are optimized, to further analyze and verify the aggregation of S in the Σ5(210) GB, containing Mo and Cr at the atomic level. In optimized structures, the number of S atoms is increased in the GB region, and structural changes are analyzed (Figure 5f–i). Notably, in the Fe-Mo-Cr-Σ5(210) GB structural system, increasing the number of S atoms in the GB results in S still causing evident local distortion of the lattice. However, the change in the Fe-Mo-Cr-Σ5(210) GB, caused by S, is significantly weaker than that of clean Σ5(210) GB. Accordingly, the existence of Mo and Cr in the GBs can affect the deformation of the GB structure caused by the aggregation of S. When the number of S atoms is 2 or 4, the layer-to-layer and atom-to-atom expansion in the GB region of the Fe-Mo-Cr-Σ5(210) GB is significantly prevented, compared with those in clean Σ5(210) GB. As the number of S atoms increases to 6, the entire interface cracks.
To further investigate the effect of S on the lattice parameters of clean Σ5(210) GB and Fe-Mo-Cr-Σ5(210) GB, we calculate the expansion of the clean Σ5(210) GB and Fe-Mo-Cr-Σ5(210) GB in the z-axis direction, as a function of the number of S atoms segregated at the interface region (Figure 6). A high sulfur content results in high influence on the z-axis in the GB structure. When only one S atom is present in the structure, the existence of Mo or Cr in the interface region has a negligible effect on the expansion of the GB in the z-axis direction. When the number of S atoms is more than 2, the addition of the S reduces the expansion of the Fe-Mo-Cr-Σ5(210) GB in the z-axis direction, to significantly less than that of the clean Σ5(210) GB, and the deviation is 0.22 Å. This result suggests that Mo and Cr can inhibit the GB expansion caused by the aggregation of S in the GB region, and the inhibition effect becomes remarkable with increasing sulfur content.
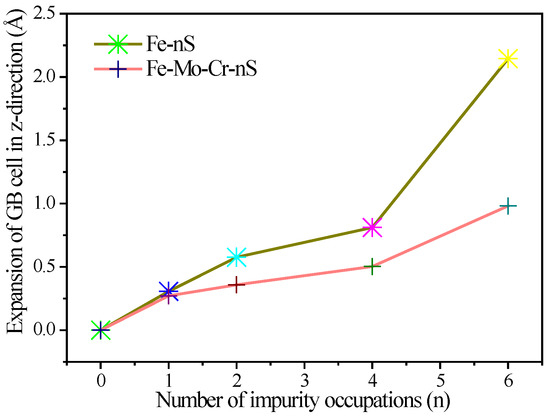
Figure 6.
Expansion of the clean Σ5(210) GB and Fe-Mo-Cr-Σ5(210) GB in the z-axis direction due to the increase of S concentration in the layers −2, 1 and 2.
In Figure 5, the addition of S at the GB has the most evident local effect on the interaction of the atoms in the first and third layers. Figure 7 shows the change in bond length between the Fe (or Mo, Cr) atoms in the first and third layers in the two GB structural systems, to enable understanding of the influence of the bonding characteristics between atoms in the adjacent S region, and determine the role of Mo and Cr. Figure 7 shows that, compared with GBs without S, when S is added in S-segregated sites, the bond lengths of Fe-Fe, Mo-Fe, and Cr-Fe increase. When Mo and Cr atoms exist in the interface, the bond-length expansion induced by S is weaker than the structure without Mo and Cr. For clean Σ5(210) GB, with increasing sulfur content in the GB region, the resultant increase in bond length between the first and third layers of Fe atoms is 0.104, 0.253, 0.264, and 0.33 Å, whereas the expansion of the Mo-Fe bond length in the Σ5(210) GB with Mo and Cr are 0.013, 0.108, 0.205, and 0.265 Å (Figure 7a). Figure 7b demonstrates that in the other group: Fe (Cr)-Fe, bond length increases with different sulfur content, where the resultant increases in the Fe-Fe bond length are 0.181, 0.252, 0.323 and 0.383 Å, and those in the Cr-Fe bond length are 0.179, 0.206, 0.284, 0.35 Å. The comparison shows that Mo and Cr in the interface has a significant inhibitory effect on the bond length between interlayer atoms in the region.
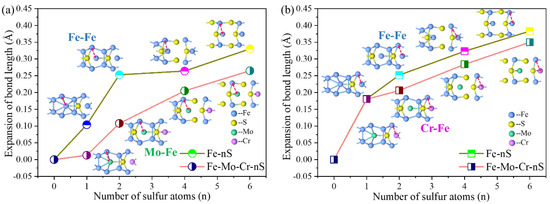
Figure 7.
(a) The bond-length variation of Fe-Fe and Mo-Fe in the Σ5(210) GB structure with and without Mo, Cr with different numbers of S atoms in the GB. (b) The bond-length variation of Fe-Fe and Cr-Fe in the Σ5(210) GB structure with and without Mo, Cr, with different numbers of S atoms in the GB. The red arrows indicate the positions of the measured bond lengths.
Meanwhile, we also compared the bond-length changes under different S atom numbers. When the number of S atoms is 1 or 2, Mo in the interface has an evident inhibitory effect on the increased bond length between atoms around it. However, when the number of S atoms is higher than 4, the inhibition of the bond length is slightly weakened. When only one sulfur is present, the bond length of Cr-Fe is slightly smaller than that of Fe-Fe without Mo or Cr, and the inhibition of the increase of the bond length around Cr is similar in other cases with different S content. With the segregation of sulfur, the bond length between atoms at the GB increases, which means that the bonding force of the GB is weakened. At the same sulfur content, the change in the Mo-Fe bond length, compared with that in the Fe-Fe bond length at the same position, is larger than that in the Cr-Fe bond length. The increase in S weakens the interatomic combination in the Σ5(210) GB region until cracking occurs, whereas Mo and Cr have a certain inhibitory effect on the GB.
To shed light on the role of S in the clean Σ5(210) GB and Fe-Mo-Cr-Σ5(210) GB, we further analyze the electronic properties of different structural systems. The charge density distribution can directly reflect the interactions between atoms at GBs. The calculated charge densities of Fe-Mo-Cr-Σ5(210) GB with different S contents in comparison with those of clean Σ5(210) GB are displayed in Figure 8. As shown in Figure 8a, the presence of 1 or 2 S atoms in the interstitial position in the GB is beneficial to the bonding between atoms at the GB. However, if four or more S atoms are added in the GB region, the charge densities in the GB area become much more diluted than those with minimal S content. Thus, the interatomic bonds of the GB with six S atoms are weak and almost negligible. This is consistent with the experimental results that sulfur segregates to be mainly responsible for intergranular corrosion in 304 stainless steels [25]. However, the existence of Mo and Cr elements can alleviate this phenomenon, which is conducive to improving the interaction between S and adjacent atoms and reducing the influence of S on the interface. Figure 8b shows that whether Σ5(210) GBs contain Mo and Cr, the distribution trends of the charge densities of the two GB structures, with increased number of S atoms, are consistent. However, when the number of S atoms is the same, the interatomic charge distribution of the Mo-Cr-Σ5(210) GB is close, that is, the atomic interaction is strong. Therefore, Mo and Cr can improve the adhesion of the GB structure with S segregation. These results are in good agreement with the findings that Cr and Mo can enhance the GB cohesion [22].
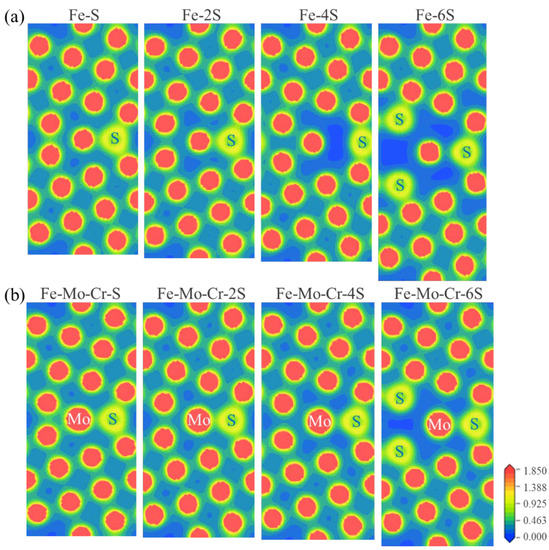
Figure 8.
The calculated charge density distributions for (a) clean Σ5(210) GBs with different numbers of S atoms in the GB region, (b) Mo-Cr-segregated Σ5(210) GBs with different numbers of S atoms in the GB region.
In the Σ5(210) GB structure, the presence of Mo and Cr in the interface remarkably affects S. The charge density of the Σ5(210) GB containing S shows that S weakens the charge interaction of its neighboring atoms. To explain this phenomenon, the total density of states and local density of clean Σ5(210) GB are calculated, where Σ5(210) GB contains an S atom at interface (Fe-S-Σ5(210) GB) and Mo-Cr-Σ5(210) GB contains an S atom at interface (Fe-Mo-Cr-S-Σ5(210) GB) (Figure 9). Figure 9a shows the total density of states diagram of three GB systems. The inclusion of these elements in clean Σ5(210) GB has little effect on the shape of the total density of states, position of each energy level, and strength of the GB system. Some differences in electron density remain at the Fermi energy level. A low value at the Fermi level indicates a stable structure [29]. In the local density of states at the Fermi level of Figure 9b, compared with that of the Fe-Σ5(210), the number of electrons at the Fermi energy of the Fe-S-Σ5(210) GB increases slightly, indicating that the electrochemical stability of the system is reduced. The electron number at the Fermi energy of Fe-Mo-Cr-S-Σ5(210) GB has a slight decrease in the number of electrons, compared with that of the clean Σ5(210) GB, indicating that the electrochemical stability of the GB system is improved. This result indicates that the Σ5(210) GB segregation of Mo and Cr is conducive to improving the electrochemical stability of the Fe-S-Σ5(210) GB structural system.
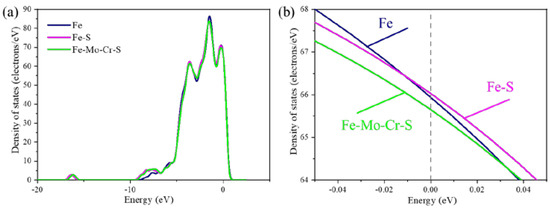
Figure 9.
The total density of state map (a) and the local density of state map (b) of the Fe-Σ5(210) GB, Fe-S-Σ5(210) GB, and Fe-Mo-Cr-S-Σ5(210) GB crystal boundary systems.
4. Conclusions
On the basis of the intergranular fracture induced by S in Fcc-Fe, three GB structures are constructed. The occupancy tendency of S at the GB is studied. The aggregation degree of S atom numbers in the Σ5(210) GB and the effect of Mo and Cr on the S-induced intergranular fracture of Fcc-Fe GB are studied. The following results are summarized:
- S tends to segregate in Σ5(210), Σ5(310), and Σ9(114) GBs. The segregation tendency of sulfur at the Σ5(210) GB is stronger than that at the two other grain boundaries. With increasing S atom numbers at the Σ5 (210) GB, the lattice distortion of the GB position leads to increasing interlayer spacing in the interface region, until the interface cracks.
- Mo or Cr in the GB has a certain inhibitory effect on S-induced intergranular fracture, and the effect of Mo is stronger than that of Cr. The combination of Mo and Cr more obviously inhibits the influence of S inside the GB. Mo and Cr can strengthen the bonding ability of GBs. These findings are helpful for understanding how S-induced intergranular fracture in Fcc-Fe is determined using Cr and Mo-segregation GBs in high-Mo austenitic stainless steel.
Author Contributions
S.L.: Methodology, Software, Formal analysis, Investigation, Visualization, Writing—Original Draft; Y.Z.: Software; J.R.: Visualization, Resources; N.D.: Resources; C.Z.: Resources; J.M.: Resources; Z.J.: Funding acquisition; H.L.: Resources; P.H.: Conceptualization, Writing—Review & Editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
The present work was financially supported by National Natural Science Foundation of China (Grant Nos. U1860204 and 51871159).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Jiang, Z.; Li, Z.; Wu, J.; Zhang, B.; Duan, F.; Feng, H.; Zhu, H. Influence of N on precipitation behavior, associated corrosion and mechanical properties of super austenitic stainless steel S32654. J. Mater. Sci. Technol. 2020, 42, 143–155. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, J.; Li, H.; Jiang, Z.; Geng, Y.; Feng, H.; Zhang, B.; Zhu, H. Refinement mechanism of cerium addition on solidification structure and sigma phase of super austenitic stainless steel S32654. J. Mater. Sci. Technol. 2022, 102, 105–114. [Google Scholar] [CrossRef]
- Willenbruch, R.D.; Clayton, C.R.; Oversluizen, M.; Kim, D.; Lu, Y. An XPS and electrochemical study of the influence of molybdenum and nitrogen on the passivity of austenitic stainless steel. Corros. Sci. 1990, 31, 179–190. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Jiang, Z.; Zhang, B.; Li, Z.; Wu, J.; Fan, S.; Feng, H.; Zhu, H. Effects of Cr and Mo on precipitation behavior and associated intergranular corrosion susceptibility of superaustenitic stainless steel S32654. Mater. Charact. 2019, 152, 141–150. [Google Scholar] [CrossRef]
- Shoemaker, L.E.; Crum, J.R. Experience in Effective Application of Metallic Materials for Construction of FGD Systems; Special Metals Corporation: New Hartford, NY, USA, 2010. [Google Scholar]
- Herda, W.; Rockel, M.; Grossmann, G.; Starke, K. High specialty stainless steels and nickel alloys for FGD dampers. In Proceedings of the Corrosion97, New Orleans, LA, USA, 9–14 March 1997. [Google Scholar]
- Liu, T.; Xia, S.; Bai, Q.; Zhou, B.; Zhang, L.; Lu, Y.; Shoji, T. Three-dimensional study of grain boundary engineering effects on intergranular stress corrosion cracking of 316 stainless steel in high temperature water. J. Nucl. Mater. 2018, 498, 290–299. [Google Scholar] [CrossRef]
- Gesari, S.B.; Pronsato, M.E.; Juan, A. The electronic structure and bonding of H pairs at Σ= 5 BCC Fe grain boundary. Appl. Surf. Sci. 2002, 187, 207–217. [Google Scholar] [CrossRef]
- Di Stefano, D.; Mrovec, M.; Elsässer, C. First-principles investigation of hydrogen trapping and diffusion at grain boundaries in nickel. Acta Mater. 2015, 98, 306–312. [Google Scholar] [CrossRef]
- Kart, H.H.; Uludogan, M.; Cagin, T. DFT studies of sulfur induced stress corrosion cracking in nickel. Comput. Mater. Sci. 2009, 44, 1236–1242. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Viejo, F.; Arrabal, R.; Matykina, E. Pitting corrosion behaviour of austenitic stainless steels–combining effects of Mn and Mo additions. Corros. Sci. 2008, 50, 1796–1806. [Google Scholar] [CrossRef]
- Kaneko, M.; Isaacs, H.S. Effects of molybdenum on the pitting of ferritic-and austenitic-stainless steels in bromide and chloride solutions. Corros. Sci. 2002, 44, 1825–1834. [Google Scholar] [CrossRef]
- Ameer, M.A.; Fekry, A.M.; Heakal, F.E. Electrochemical behaviour of passive films on molybdenum-containing austenitic stainless steels in aqueous solutions. Electrochim. Acta 2004, 50, 43–49. [Google Scholar] [CrossRef]
- Tobler, W.J.; Virtanen, S. Effect of Mo species on metastable pitting of Fe18Cr alloys—A current transient analysis. Corros. Sci. 2006, 48, 1585–1607. [Google Scholar] [CrossRef]
- Bastidas, J.M.; Torres, C.L.; Cano, E.; Polo, J.L. Influence of molybdenum on passivation of polarised stainless steels in a chloride environment. Corros. Sci. 2002, 44, 625–633. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.Y.; Wang, H.H. Gradient-corrected density functional calculation of elasticconstants of Fe, Co and Ni in bcc, fcc and hcp structures. Chin. J. Phys. 2000, 38, 949–961. [Google Scholar]
- Xie, H.; Zhao, N.; Shi, C.; He, C.; Liu, E. Effects of active elements on adhesion of the Al2O3/Fe interface: A first principles calculation. Comput. Mater. Sci. 2021, 188, 110226. [Google Scholar] [CrossRef]
- Hui, J.; Yang, G.; Liu, M.; Liu, W.; Wang, B. Effects of alloy compositions on hydrogen behaviors at a nickel grain boundary and a coherent twin boundary. Int. J. Hydrogen Energy 2020, 45, 10951–10961. [Google Scholar] [CrossRef]
- He, Y.; Zhao, X.; Yu, H.; Chen, C. Effect of S on H-induced grain-boundary embrittlement in γ-Fe by first-principles calculations. Int. J. Hydrogen Energy 2021, 46, 28346–28357. [Google Scholar] [CrossRef]
- Takahashi, J.; Ishikawa, K.; Kawakami, K.; Fujioka, M.; Kubota, N. Atomic-scale study on segregation behavior at austenite grain boundaries in boron-and molybdenum-added steels. Acta Mater. 2017, 133, 41–54. [Google Scholar] [CrossRef]
- Han, K.; Xin, Y.; Walsh, R.; Downey, S., II; Kalu, P.N. The effects of grain boundary precipitates on cryogenic properties of aged 316-type stainless steels. Mater. Sci. Eng. A 2009, 516, 169–179. [Google Scholar] [CrossRef]
- Wang, K.; Xu, T.; Shao, C.; Yang, C. Nonequilibrium grain boundary segregation of sulfur and its effect on intergranular corrosion for 304 stainless steel. J. Iron Steel Res. Int. 2011, 18, 61–66. [Google Scholar] [CrossRef]
- Saqlain Qurashi, M. Erosion and passivation of borated 254 SMO stainless steel in simulated flue gas desulfurization solution. Int. J. Electrochem. Sci. 2020, 15, 2987–3002. [Google Scholar] [CrossRef]
- Shah, M.; Ayob, M.T.M.; Rosdan, R.; Yaakob, N.; Embong, Z.; Othman, N.K. The effect of H2S pressure on the formation of multiple corrosion products on 316L stainless steel surface. Sci. World J. 2020, 2020, 3989563. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Li, Y.; Lu, J.; Wang, K.; Zhang, H. Effect of sulfide on corrosion behavior of stainless steel 316SS and Hastelloy C276 in sub/supercritical water. Int. J. Hydrogen Energy 2021, 46, 22222–22233. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, C.; Liu, J.; Li, Y.; Fang, X.; Li, J.; Han, P. First-principles study on the structural stability and segregation behavior of γ-Fe/Cr2N interface with alloying additives M (M= Mn, V, Ti, Mo, and Ni). Metals 2016, 6, 156. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).