Microstructure and Properties of Laser Surface Remelting AlCoCrFeNi2.1 High-Entropy Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
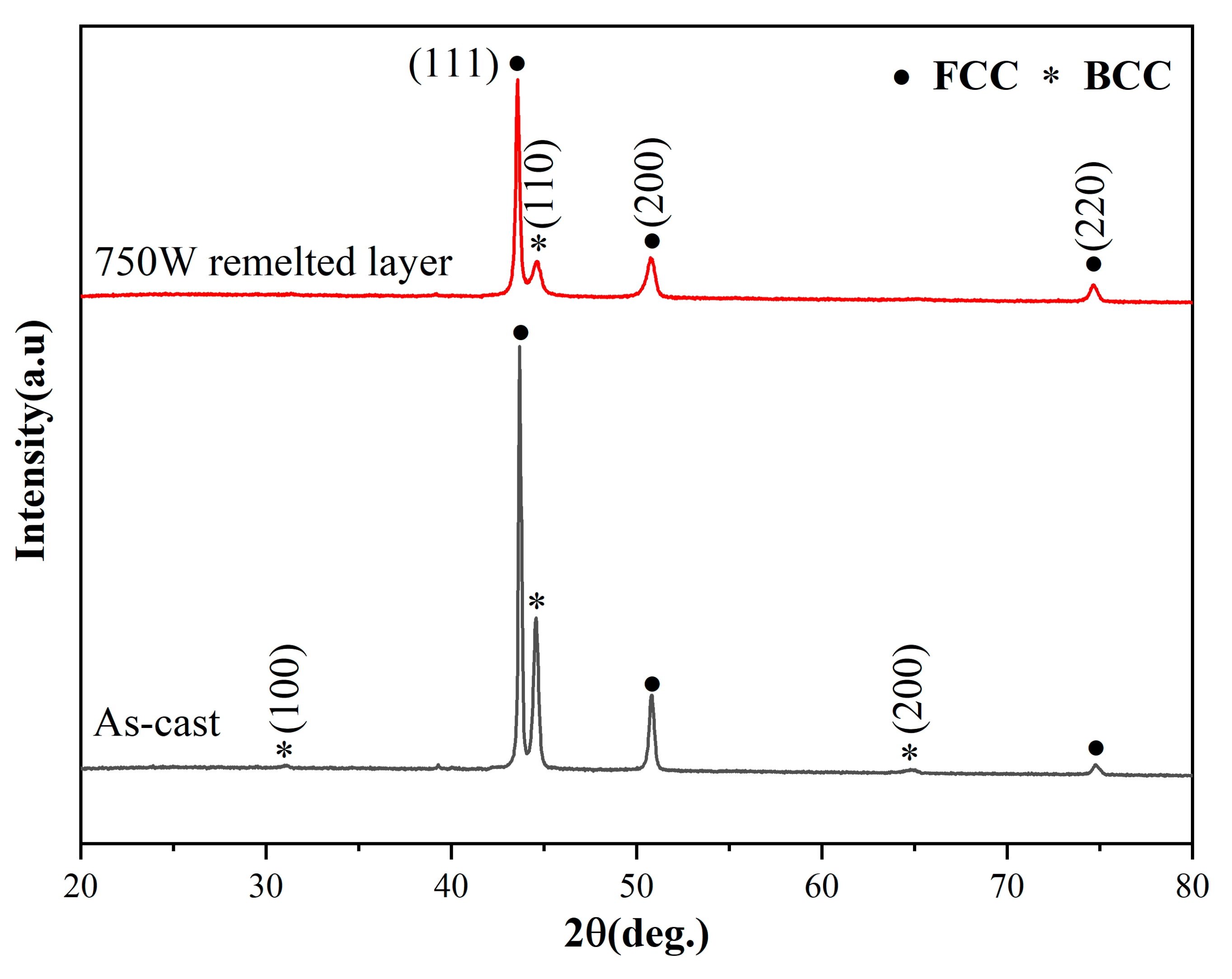
3.1. Phase Analysis
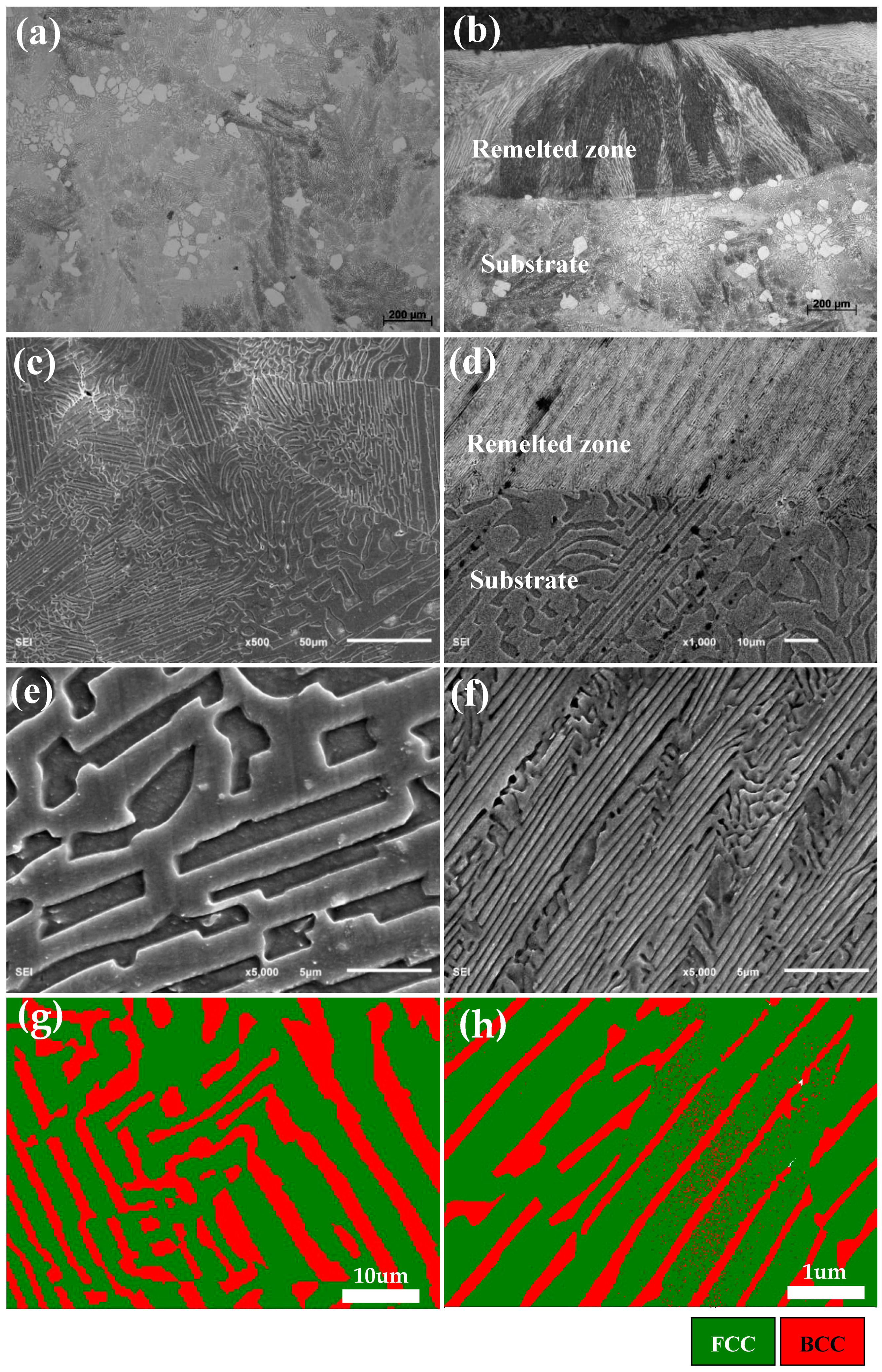
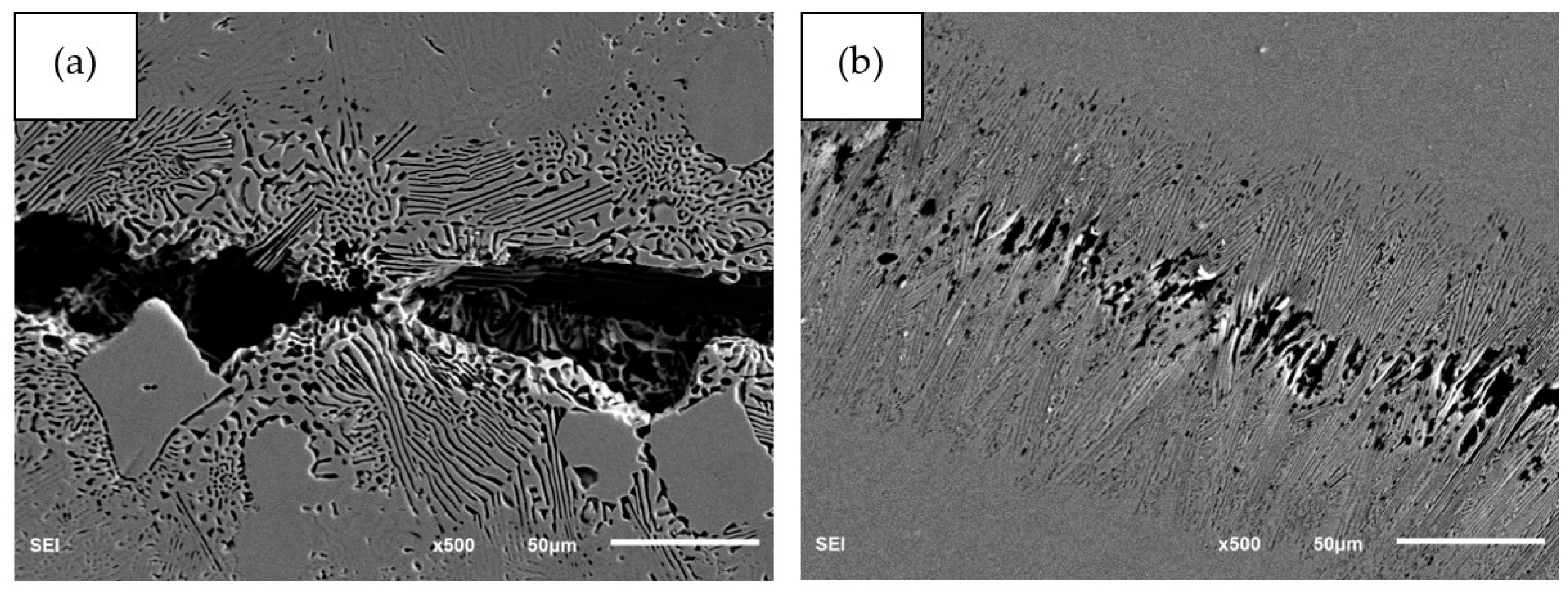
3.2. Microstructure
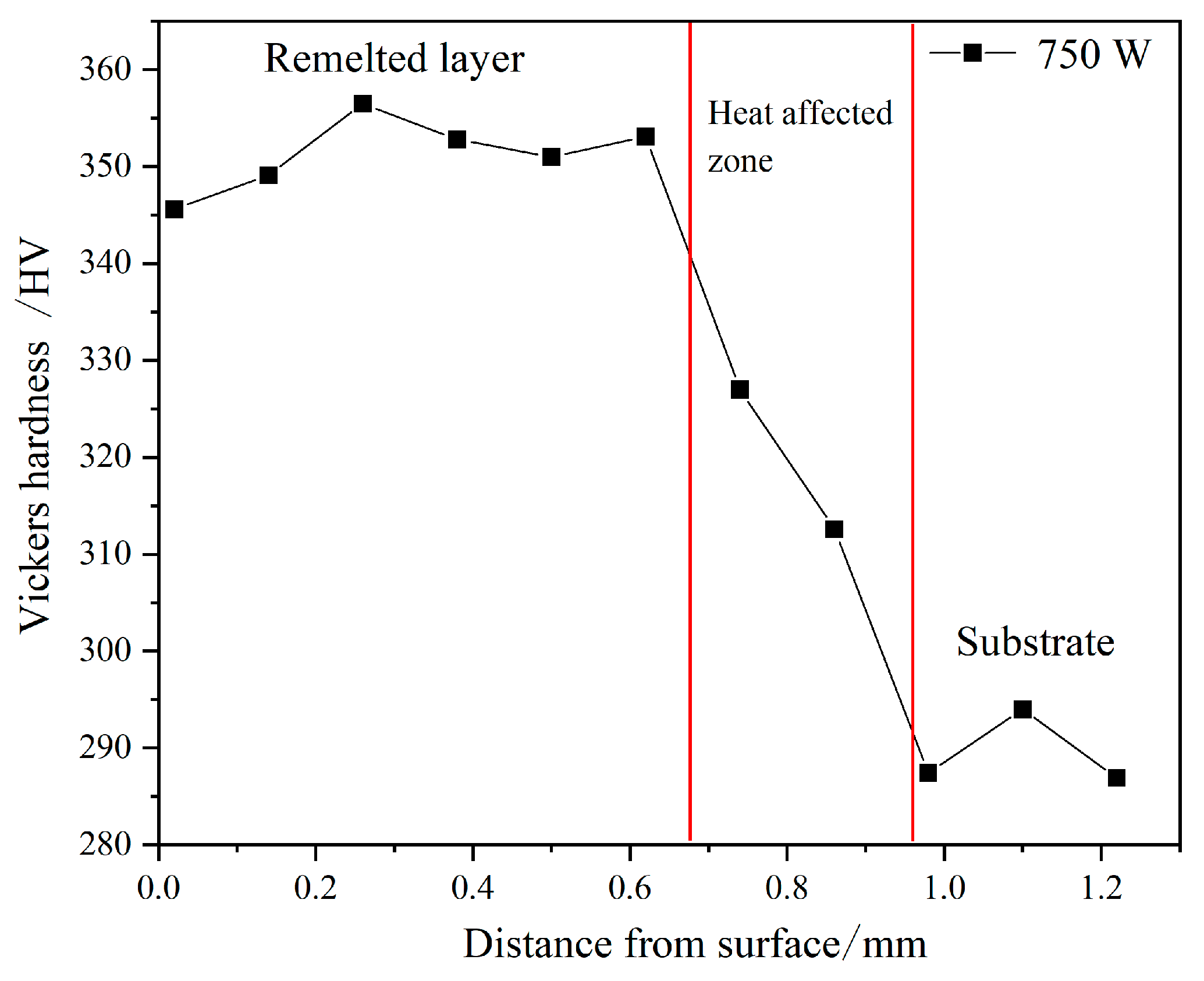
3.3. Hardness Analysis
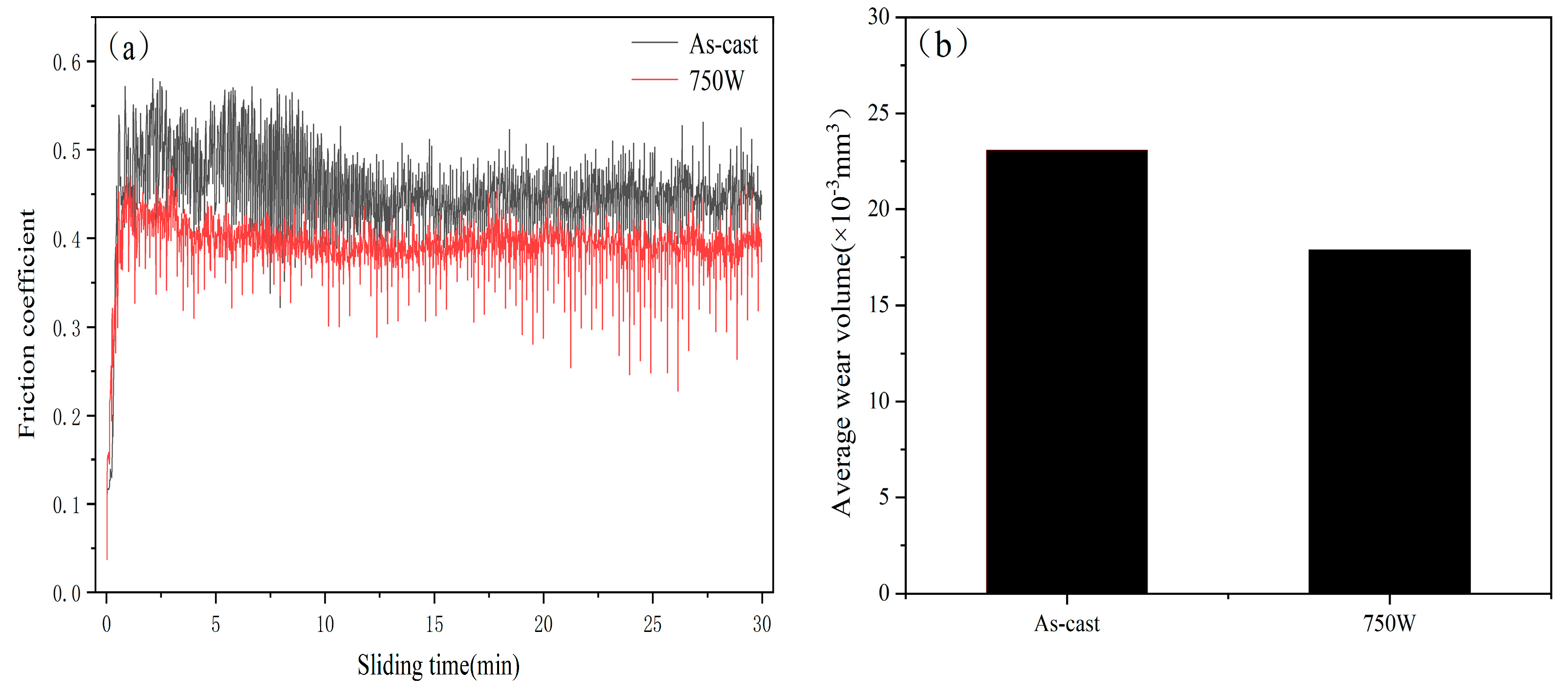
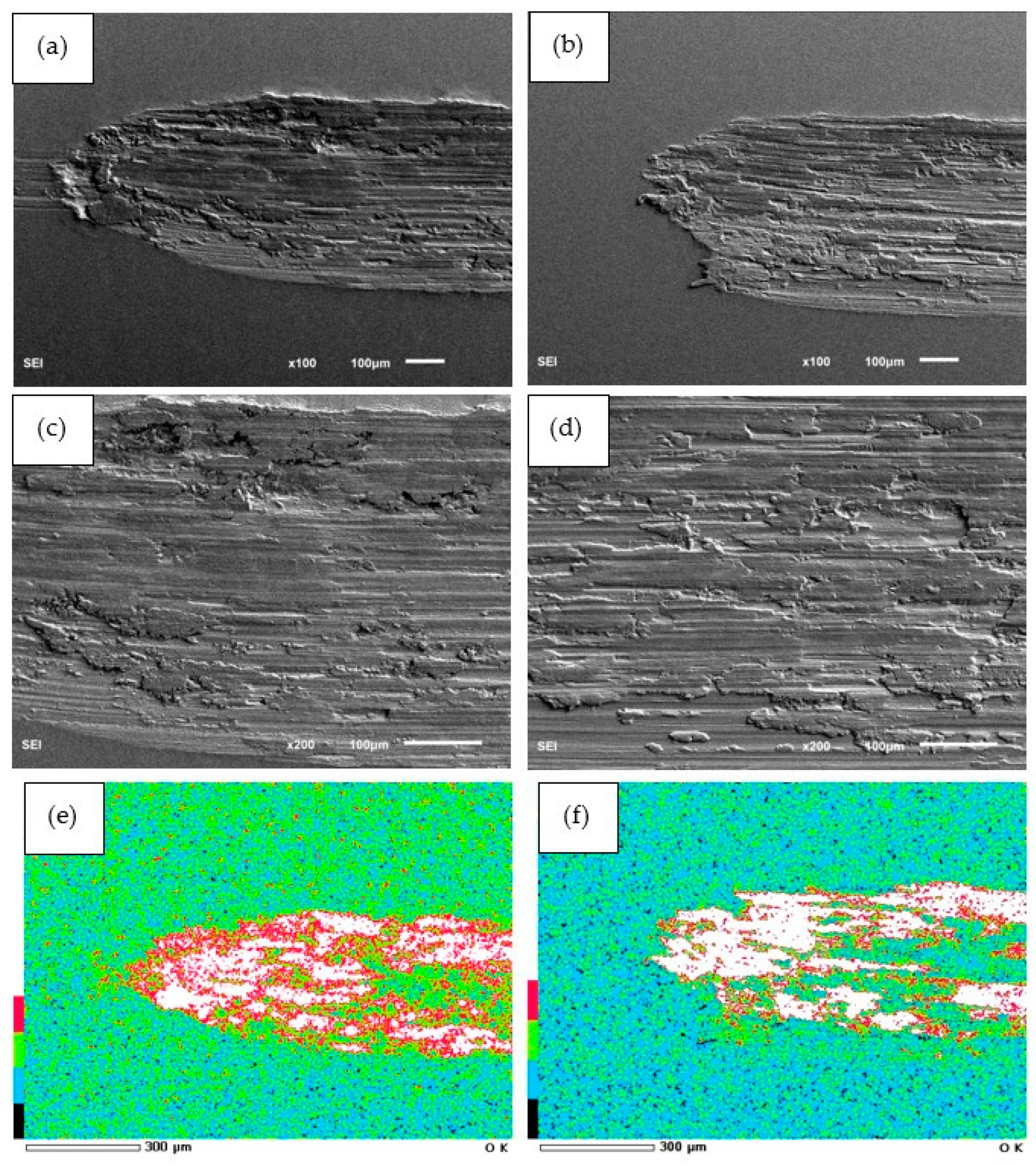
3.4. Friction and Wear Resistance Analysis
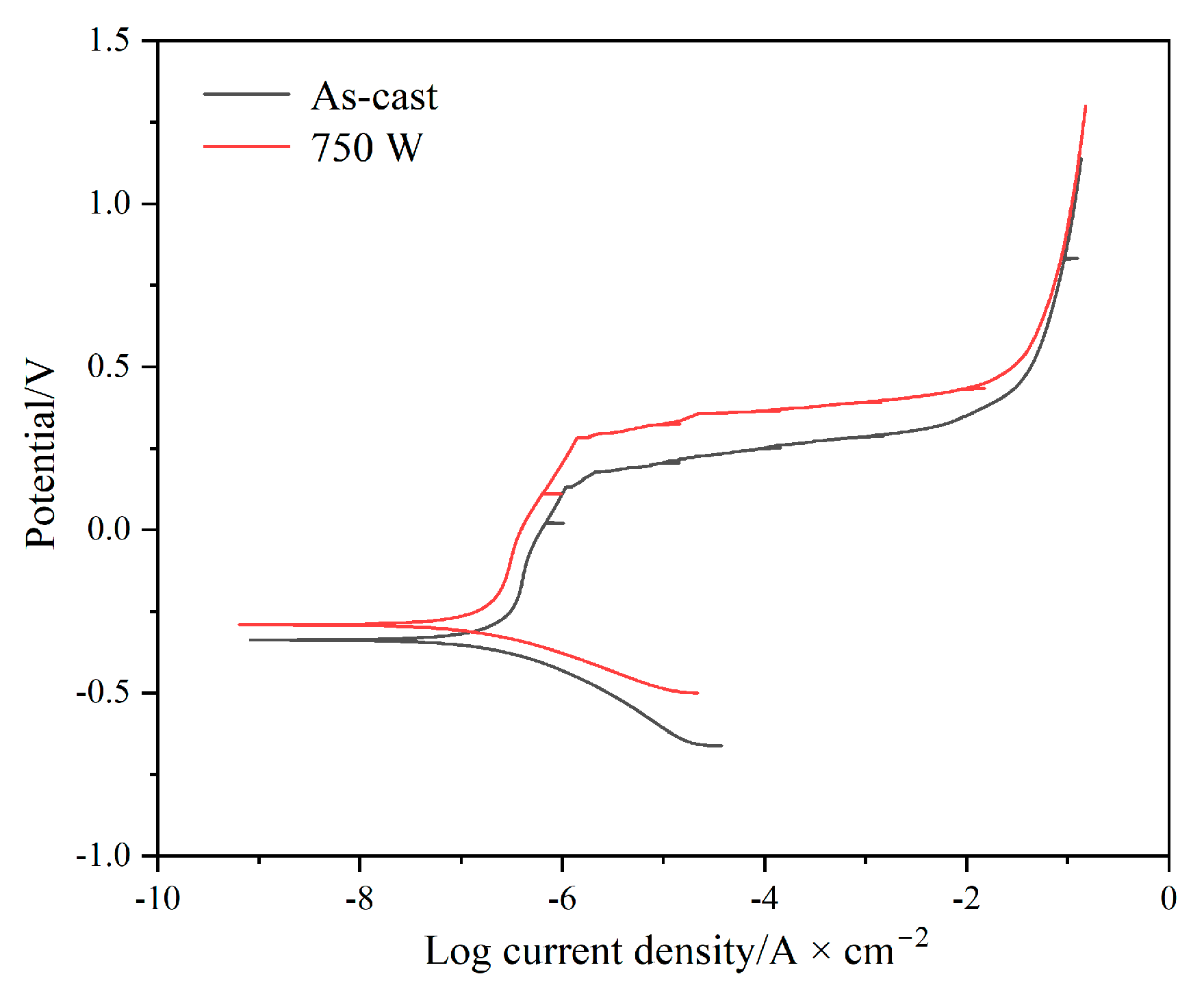
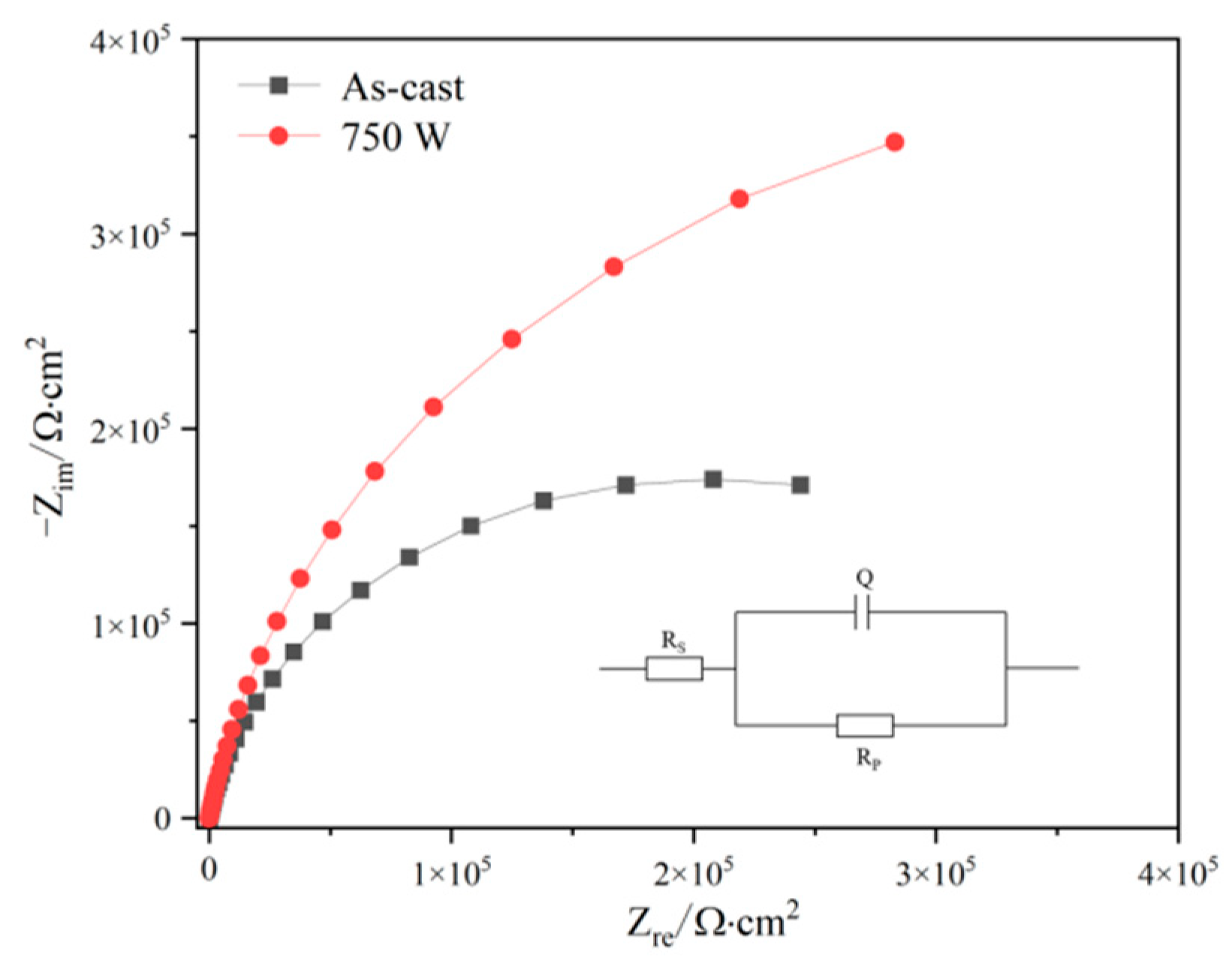
3.5. Electrochemical Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Lu, Y.P.; Dong, Y.; Guo, S.; Yong, D.; Sheng, G.; Li, J.; Kang, H.J.; Wang, T.M.; Wen, B.; Wang, Z.J.; et al. A Promising New Class of High-Temperature Alloys: Eutectic High-Entropy Alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef]
- Bhattacharjee, T.; Zheng, R.X.; Chong, Y.; Sheikh, S.; Guo, S.; Clark, I.T.; Okawa, T.; Wani, I.S.; Bhattacharjee, P.P.; Shibata, A.; et al. Effect of low temperature on tensile properties of AlCoCrFeNi2.1 eutectic high entropy alloy. Mater. Chem. Phys. 2018, 210, 207–212. [Google Scholar] [CrossRef]
- Bhattacharjee, T.; Wani, I.S.; Sheikh, S.; Clark, I.T.; Okawa, T.; Guo, S.; Bhattacharjee, P.P.; Tsuji, N. Simultaneous Strength-Ductility Enhancement of a Nano-Lamellar AlCoCrFeNi2.1 Eutectic High Entropy Alloy by Cryo-Rolling and Annealing. Sci. Rep. 2018, 8, 3276. [Google Scholar] [CrossRef]
- Wani, I.S.; Bhattacharjee, T.; Sheikh, S.; Bhattacharjee, P.P.; Guo, S.; Tsuji, N. Tailoring nanostructures and mechanical properties of AlCoCrFeNi2.1 eutectic high entropy alloy using thermo-mechanical processing. Mater. Sci. Eng. A 2016, 675, 99–109. [Google Scholar] [CrossRef]
- Reddy, S.R.; Sunkari, U.; Lozinko, A.; Saha, R.; Guo, S.; Bhattacharjee, P.P. Microstructural design by severe warm-rolling for tuning mechanical properties of AlCoCrFeNi2.1 eutectic high entropy alloy. Intermetallics 2019, 114, 106601. [Google Scholar] [CrossRef]
- Guo, Y.L.; Jia, L.N.; Kong, B.; Wang, N.; Zhang, H. Single track and single layer formation in selective laser melting of niobium solid solution alloy. Chin. J. Aeronaut. 2018, 31, 860–866. [Google Scholar] [CrossRef]
- Lei, Q.; Ramakrishnan, B.P.; Wang, S.J.; Wang, Y.C.; Mazumder, J.; Misra, A. Structural refinement and nanomechanical response of laser remelted Al-Al2Cu lamellar eutectic. Mater. Sci. Eng. A 2017, 706, 115–125. [Google Scholar] [CrossRef]
- Miao, J.W.; Yao, H.W.; Wang, J.; Lu, Y.P.; Wang, T.M.; Li, T.J. Surface modification for AlCoCrFeNi2.1 eutectic high-entropy alloy via laser remelting technology and subsequent aging heat treatment. J. Alloys Compd. 2022, 894, 162380. [Google Scholar] [CrossRef]
- Hu, J.K.; Liu, Y.; Li, W. Microstructure and corrosion behavior of FeCrNiMnMox high-entropy alloys fabricated by the laser surface remelting. Mater. Corros. 2020, 71, 1747–1754. [Google Scholar] [CrossRef]
- Shuang, S.; Ding, Z.Y.; Chung, D.; Shi, S.Q.; Yang, Y. Corrosion resistant nanostructured eutectic high entropy alloy. Corros. Sci. 2020, 164, 108315. [Google Scholar] [CrossRef]
- Xu, Q.D.; Luo, Y.; Liu, X.D.; Yang, L.; He, S.X.; Wang, X.; Wang, W.Y.; Shi, T.; Li, R.W.; Zhang, P.C. Microstructural evolution and hardness of as-cast Be-Al-Sc-Zr alloy processed by laser surface remelting. Chin. J. Aeronaut. 2021, 34, 131–142. [Google Scholar] [CrossRef]
- Wang, Q.N.; Lu, Y.P.; Yu, Q.; Zhang, Z. The Exceptional Strong Face-centered Cubic Phase and Semi-coherent Phase Boundary in a Eutectic Dual-phase High Entropy Alloy AlCoCrFeNi. Sci. Rep. 2018, 8, 14910. [Google Scholar] [CrossRef]
- Ocelík, V.; Janssen, N.; Smith, S.N.; De Hosson, J.T.M. Additive Manufacturing of High-Entropy Alloys by Laser Processing. JOM 2016, 68, 1810–1818. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Calculations of Mixing Enthalpy and Mismatch Entropy for Ternary Amorphous Alloys. Mater. Trans. JIM 2000, 41, 1372–1378. [Google Scholar] [CrossRef]
- Xiong, T.; Zheng, S.; Pang, J.; Ma, X. High-strength and high-ductility AlCoCrFeNi2.1 eutectic high-entropy alloy achieved via precipitation strengthening in a heterogeneous structure. Scr. Mater. 2020, 186, 336–340. [Google Scholar] [CrossRef]
- Wani, I.S.; Bhattacharjee, T.; Sheikh, S.; Lu, Y.P.; Chatterjee, S.; Bhattacharjee, P.P.; Guo, S.; Tsuji, N. Ultrafine-Grained AlCoCrFeNi2.1 Eutectic High-Entropy Alloy. Mater. Res. Lett. 2016, 4, 174–179. [Google Scholar] [CrossRef]
- Han, T.Y.; Liu, Y.; Liao, M.Q.; Yang, D.N.; Qu, N.; Lai, Z.G.; Zhu, J.C. Refined microstructure and enhanced mechanical properties of AlCrFe2Ni2 medium entropy alloy produced via laser remelting. J. Mater. Sci. Technol. 2022, 99, 18–27. [Google Scholar] [CrossRef]
- Singh, S.; Wanderka, N.; Murty, B.S.; Glatzel, U.; Banhart, J. Decomposition in multi-component AlCoCrCuFeNi high-entropy alloy. Acta Mater. 2011, 59, 182–190. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Nguyen, Q.B.; Ng, F.L.; An, X.H.; Liao, X.Z.; Liaw, P.K.; Nai, S.M.L.; Wei, J. Hierarchical microstructure and strengthening mechanisms of a CoCrFeNiMn high entropy alloy additively manufactured by selective laser melting. Scr. Mater. 2018, 154, 20–24. [Google Scholar] [CrossRef]
- Luo, S.C.; Gao, P.; Yu, H.C.; Yang, J.J.; Wang, Z.M.; Zeng, X.Y. Selective laser melting of an equiatomic AlCrCuFeNi high-entropy alloy: Processability, non-equilibrium microstructure and mechanical behavior. J. Alloys Compd. 2019, 771, 387–397. [Google Scholar] [CrossRef]
- Fan, X.J.; Qu, R.T.; Zhang, R.F. Relation between Strength and Hardness of High-Entropy Alloys. Acta Metall. Sin. (Engl. Lett.) 2021, 34, 1461–1482. [Google Scholar] [CrossRef]
- Ding, Z.Y.; He, Q.F.; Wang, Q.; Yang, Y. Superb strength and high plasticity in laves phase rich eutectic medium-entropy-alloy nanocomposites. Int. J. Plast. 2017, 106, 57–72. [Google Scholar] [CrossRef]
- Hasannaeimi, V.; Ayyagari, A.V.; Muskeri, S.; Salloom, R.; Mukherjee, S. Surface degradation mechanisms in a eutectic high entropy alloy at microstructural length-scales and correlation with phase-specific work function. NPJ Mater. Degrad. 2019, 3, 16. [Google Scholar] [CrossRef]
- Kafexhiu, F.; Podgornik, B.; Feizpour, D. Tribological Behavior of As-Cast and Aged AlCoCrFeNi2.1 CCA. Metals 2020, 10, 208. [Google Scholar] [CrossRef]
- Kang, J.; Li, J.; Zhao, K.Y.; Bai, X.; Yong, Q.L.; Su, J. Passivation Behaviors of Super Martensitic Stainless Steel in Weak Acidic and Weak Alkaline NaCl Solutions. J. Iron Steel Res. Int. 2015, 22, 1156–1163. [Google Scholar] [CrossRef]
- Xiang, Q.; Jiang, B.; Zhang, Y.X.; Chen, X.B.; Song, J.F.; Xu, J.Y.; Fang, L.; Pan, F.S. Effect of rolling-induced microstructure on corrosion behaviour of an as-extruded Mg-5Li-1Al alloy sheet. Corros. Sci. 2017, 119, 14–22. [Google Scholar] [CrossRef]
- Liu, Q.M. Microstructure and Properties of AlCoCrFeNiCux High-Entropy Alloy Fabricated by Laser Additive Manufacturing. Master’s Thesis, Dalian University of Technology, Dalian, China, 2019. (In Chinese). [Google Scholar]
- Svenningsen, G.; Larsen, M.H.; Walmsley, J.C.; Nordlien, J.H.; Nisancioglu, K. Effect of artificial aging on intergranular corrosion of extruded AlMgSi alloy with small Cu content. Corros. Sci. 2006, 48, 1528–1543. [Google Scholar] [CrossRef]
- Okada, T. Pit nucleation originated by coupling of perturbations with local anodic sites on passive metals. Electrochim. Acta 1988, 33, 389–395. [Google Scholar] [CrossRef]
- Liu, T.; Yin, Y.; Chen, S.; Chang, X.; Cheng, S. Super-hydrophobic surfaces improve corrosion resistance of copper in seawater. Electrochim. Acta 2007, 52, 3709–3713. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, S.D.; Sun, W.H.; Gao, Y.; Wang, J.Q. Enhanced corrosion resistance in Fe-based amorphous coatings through eliminating Cr-depleted zones. Corros. Sci. 2018, 136, 161–173. [Google Scholar] [CrossRef]
- Shuang, S.; Yu, Q.; Gao, X.; He, Q.F.; Zhang, J.Y.; Shi, S.Q.; Yang, Y. Tuning the microstructure for superb corrosion resistance in eutectic high entropy alloy. J. Mater. Sci. Technol. 2022, 109, 197–208. [Google Scholar] [CrossRef]
- Quiambao, K.F.; McDonnell, S.J.; Schreiber, D.K.; Gerard, A.Y.; Freedy, K.M.; Lu, P.; Saal, J.E.; Frankel, G.S.; Scully, J.R. Passivation of a corrosion resistant high entropy alloy in non-oxidizing sulfate solutions. Acta Mater. 2019, 164, 362–376. [Google Scholar] [CrossRef]
- Campos, I.; Palomar-Pardavé, M.; Amador, A.; VillaVelázquez, C.; Hadad, J. Corrosion behavior of boride layers evaluated by the EIS technique. Appl. Surf. Sci. 2007, 253, 9061–9066. [Google Scholar] [CrossRef]
- Della Rovere, C.A.; Alano, J.H.; Silva, R.; Nascente, P.A.P.; Otubo, J.; Kuri, S.E. Characterization of passive films on shape memory stainless steels. Corros. Sci. 2012, 57, 154–161. [Google Scholar] [CrossRef]

| Alloy | Atomic Fraction | |||||
|---|---|---|---|---|---|---|
| Al | Co | Cr | Fe | Ni | ||
| Nominal | 16.39 | 16.39 | 16.39 | 16.39 | 34.43 | |
| As-cast | BCC phase | 29.97 | 12.12 | 8.12 | 10.71 | 39.08 |
| FCC phase | 11.37 | 20.84 | 19.62 | 17.24 | 30.93 | |
| 750 W | BCC phase | 17.95 | 15.18 | 16.98 | 15.73 | 34.17 |
| FCC phase | 16.78 | 15.33 | 17.18 | 17.30 | 33.42 | |
| Alloy | Ecorr/mV | Icorr/(A·cm−2) | Epass/mV | Epit/mV | ΔE/mV |
|---|---|---|---|---|---|
| As-cast | −354 | 1.713 × 10−7 | −218 | 132 | 350 |
| 750 W | −308 | 1.362 × 10−7 | −194 | 281 | 475 |
| Sample | Rs/(Ω·cm2) | Rp/(Ω·cm2) | CPE | |
|---|---|---|---|---|
| Y0/(s−n·Ω−1·cm−2) | n | |||
| As-cast | 12.78 | 4.11 × 104 | 2.328 × 10−5 | 0.89 |
| 750 W | 8.74 | 8.72 × 104 | 2.137 × 10−5 | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhang, J.; Li, K.; Zhuang, D.; Zang, Q.; Chen, H.; Lu, Y.; Xu, B.; Zhang, Y. Microstructure and Properties of Laser Surface Remelting AlCoCrFeNi2.1 High-Entropy Alloy. Metals 2022, 12, 1590. https://doi.org/10.3390/met12101590
Chen J, Zhang J, Li K, Zhuang D, Zang Q, Chen H, Lu Y, Xu B, Zhang Y. Microstructure and Properties of Laser Surface Remelting AlCoCrFeNi2.1 High-Entropy Alloy. Metals. 2022; 12(10):1590. https://doi.org/10.3390/met12101590
Chicago/Turabian StyleChen, Jingrun, Jing Zhang, Ke Li, Dongdong Zhuang, Qianhao Zang, Hongmei Chen, Yandi Lu, Bo Xu, and Yan Zhang. 2022. "Microstructure and Properties of Laser Surface Remelting AlCoCrFeNi2.1 High-Entropy Alloy" Metals 12, no. 10: 1590. https://doi.org/10.3390/met12101590
APA StyleChen, J., Zhang, J., Li, K., Zhuang, D., Zang, Q., Chen, H., Lu, Y., Xu, B., & Zhang, Y. (2022). Microstructure and Properties of Laser Surface Remelting AlCoCrFeNi2.1 High-Entropy Alloy. Metals, 12(10), 1590. https://doi.org/10.3390/met12101590






