Thermodynamic Modeling of the Ag-Cu-Sn Ternary System
Abstract
1. Introduction
2. Literature Information
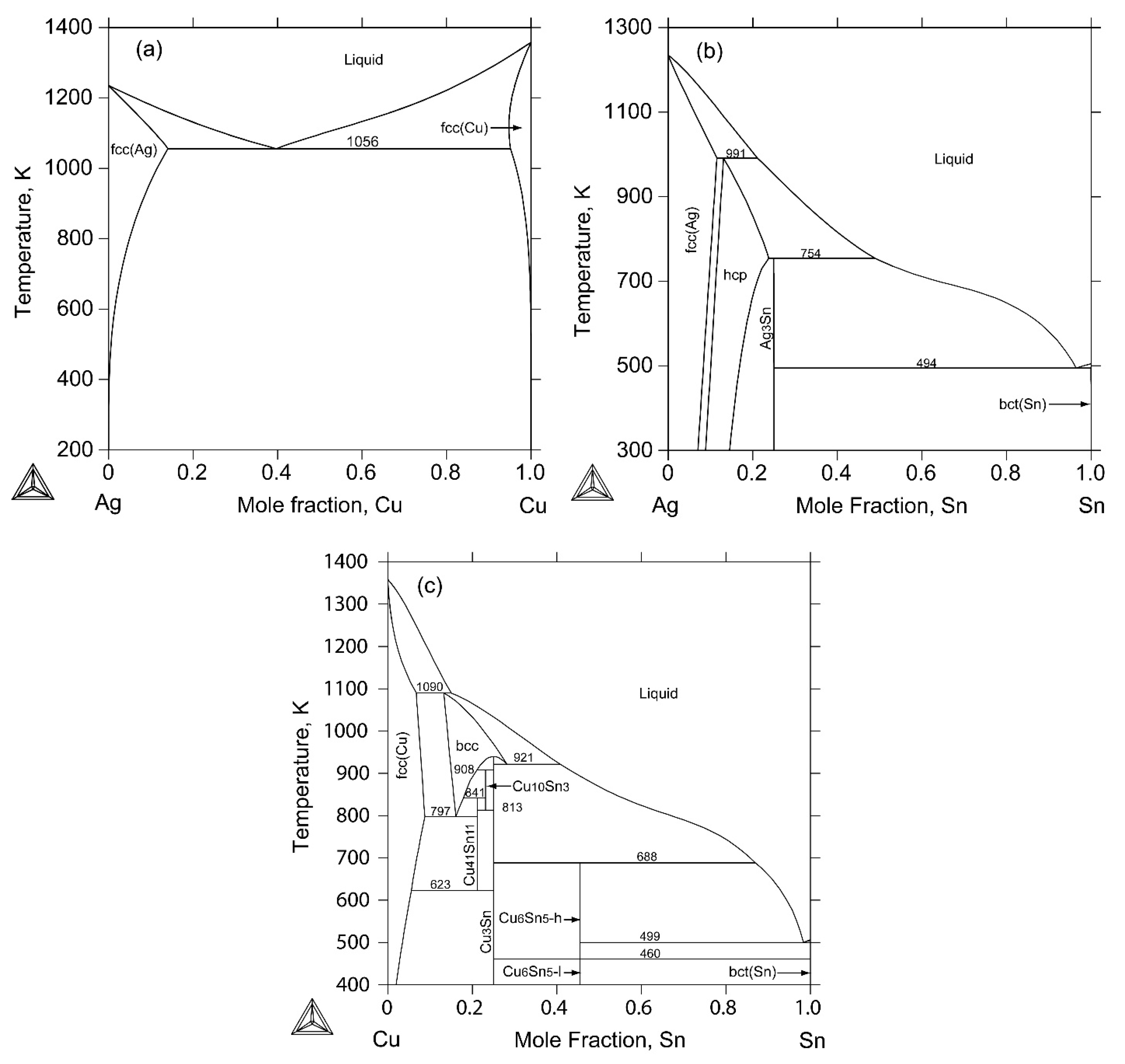
2.1. The Ag-Cu Binary System
2.2. The Ag-Sn Binary System
2.3. The Cu-Sn Binary System
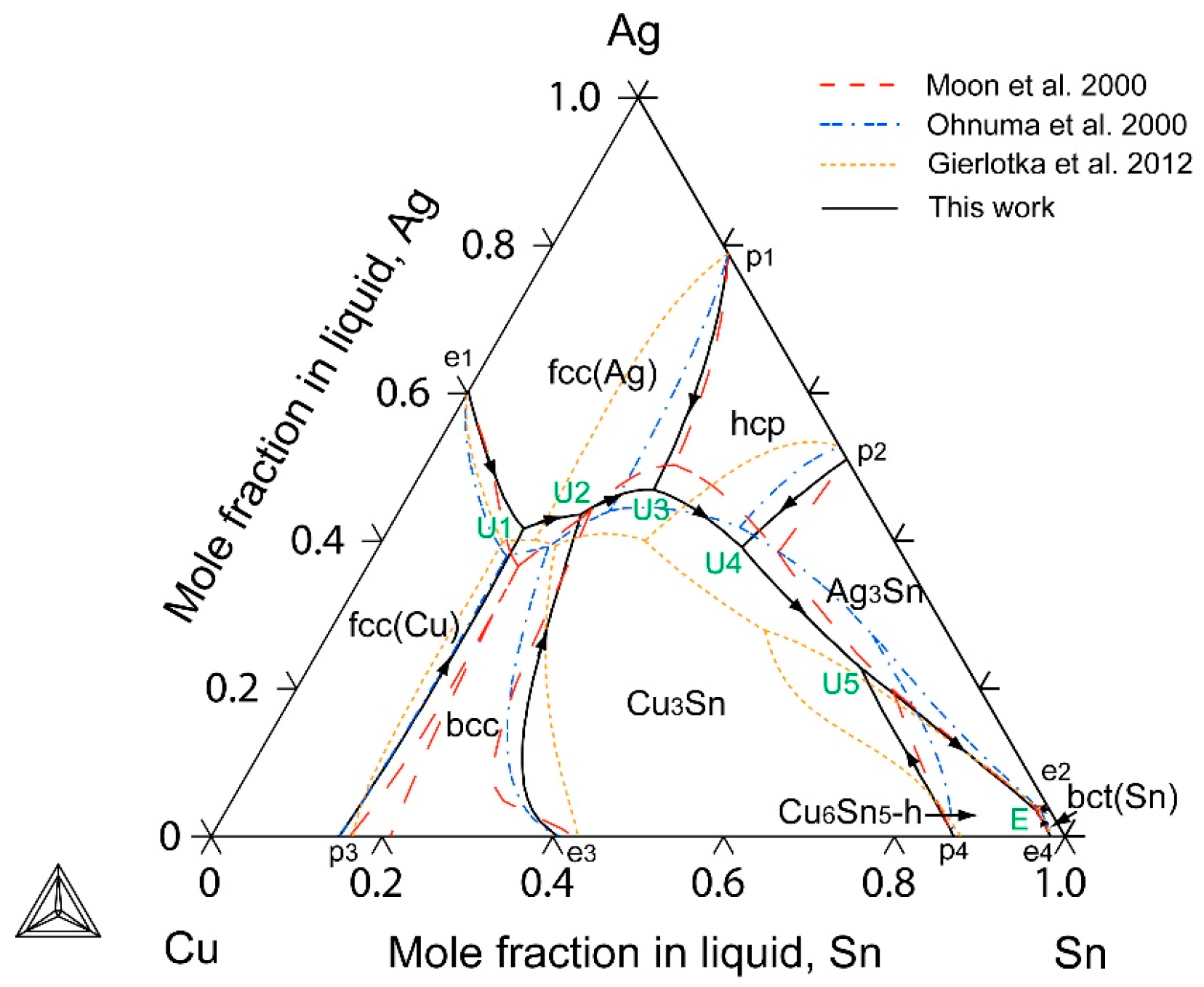
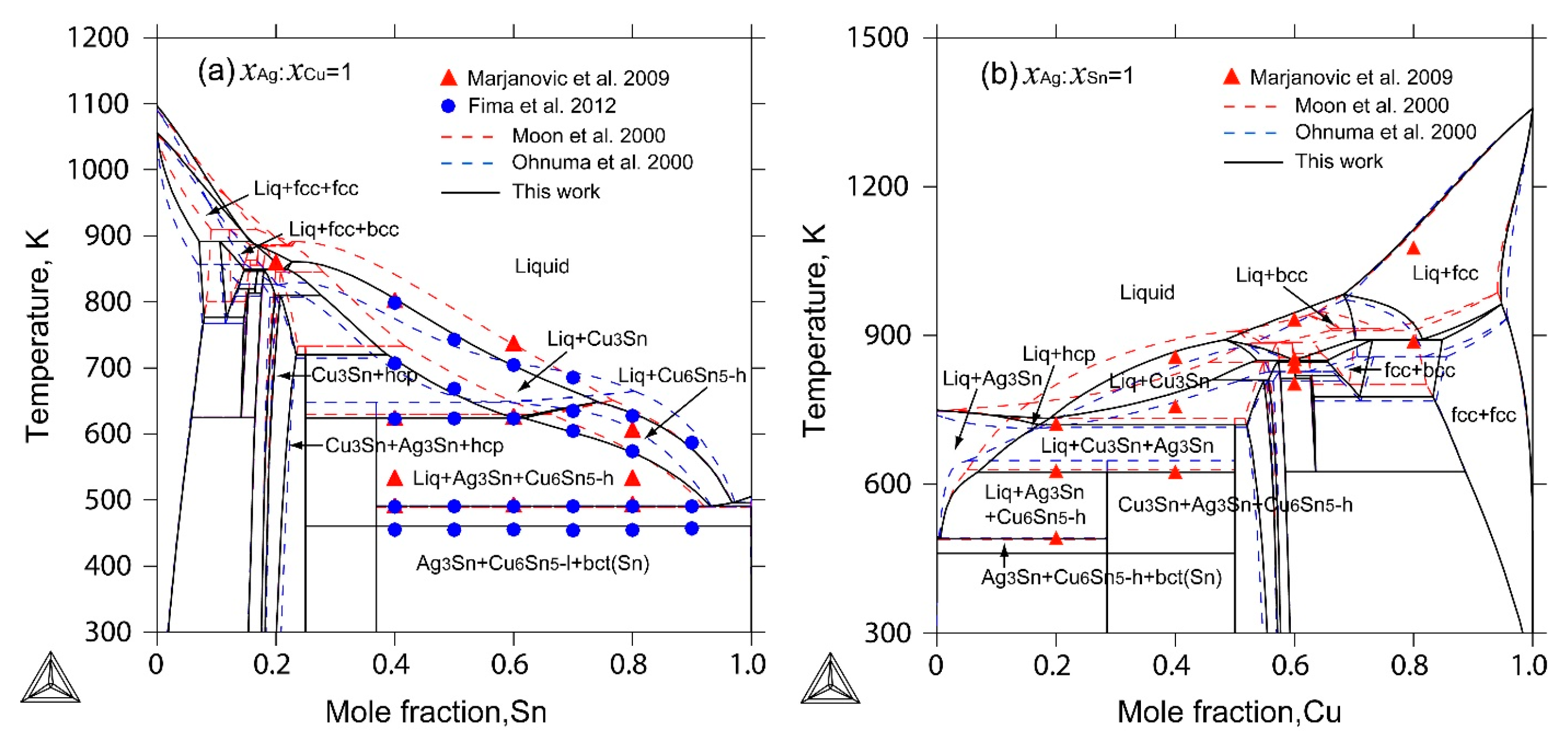
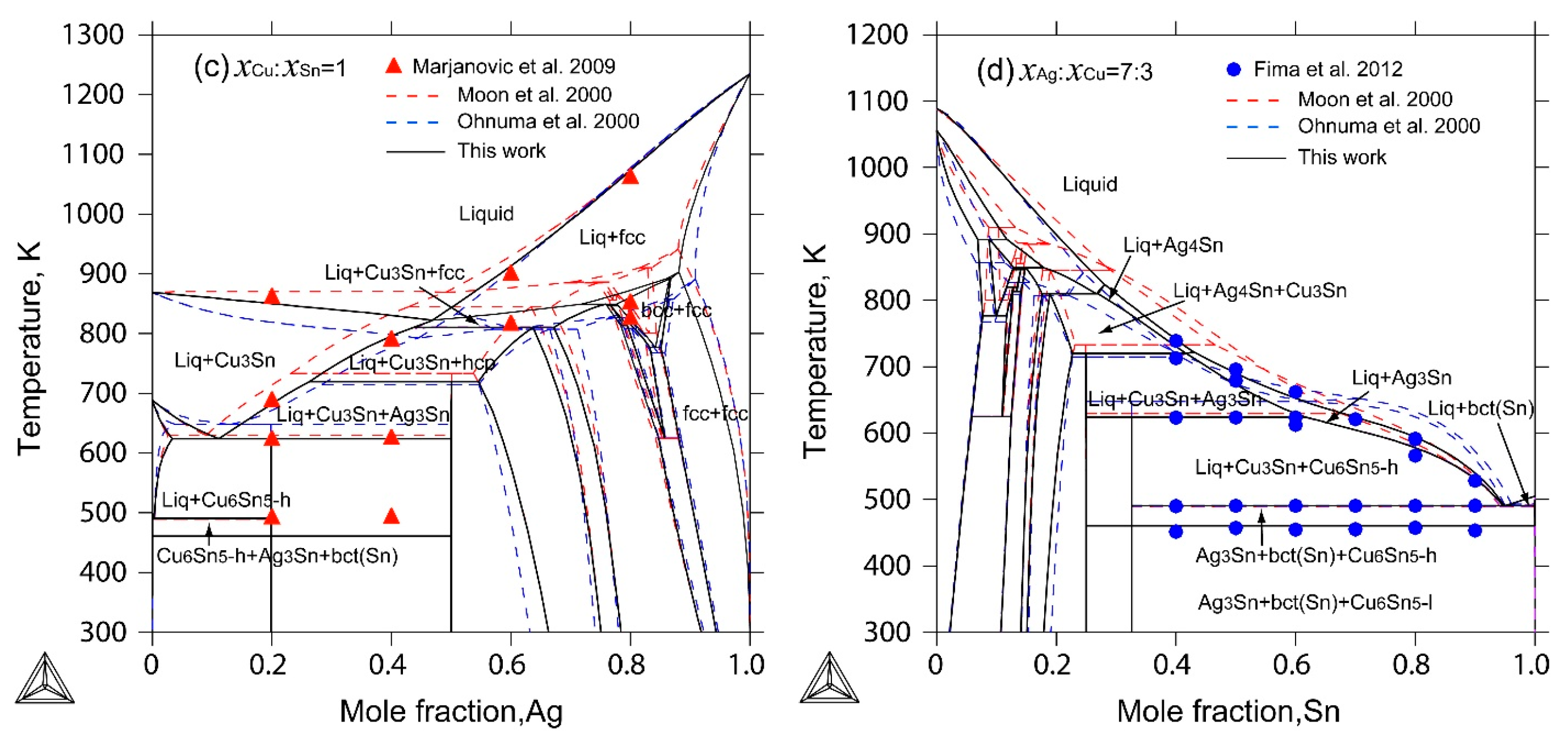
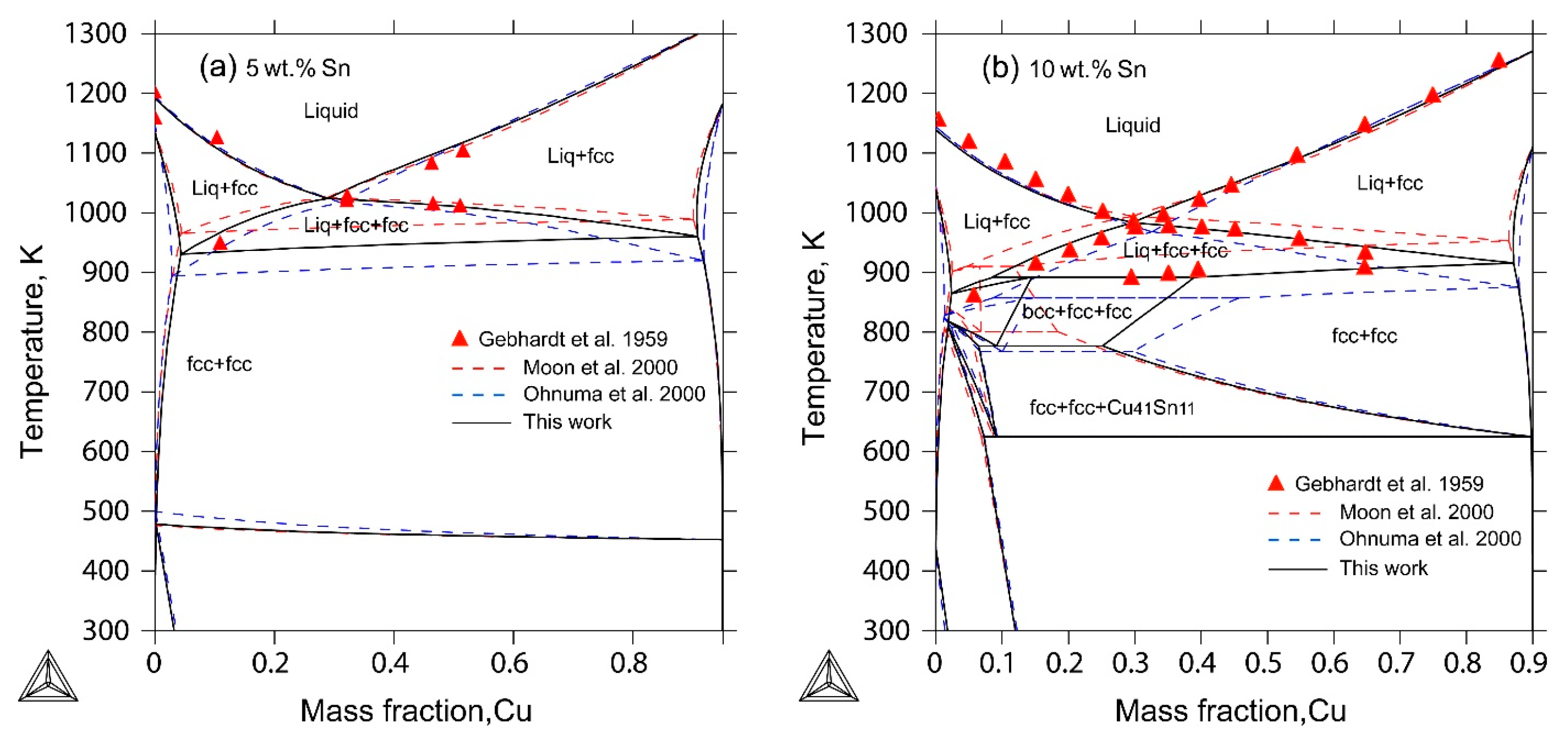
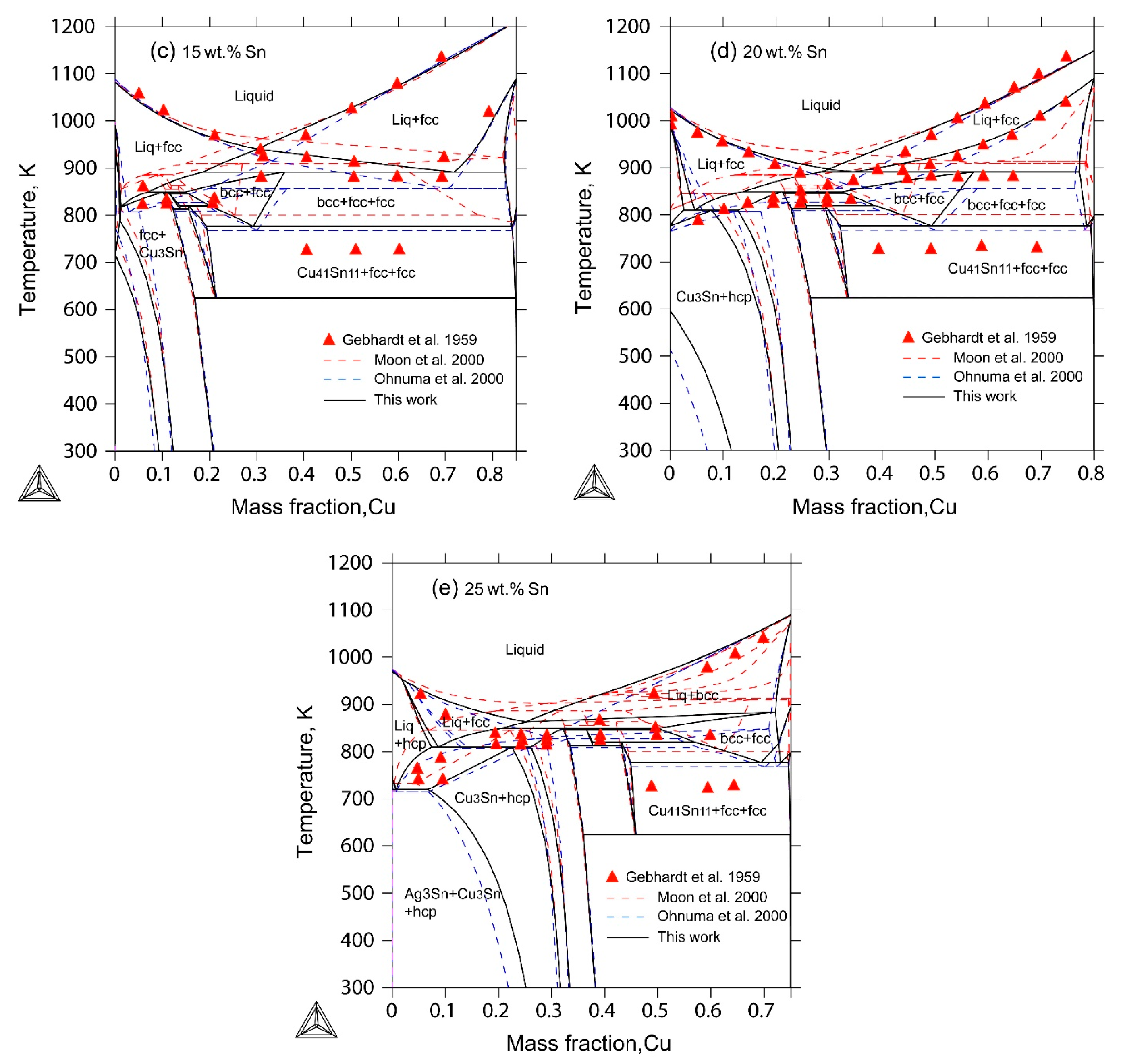
2.4. The Ag-Cu-Sn Ternary System
3. Thermodynamic Models
3.1. Solution Phases
3.2. Intermetallic Compounds
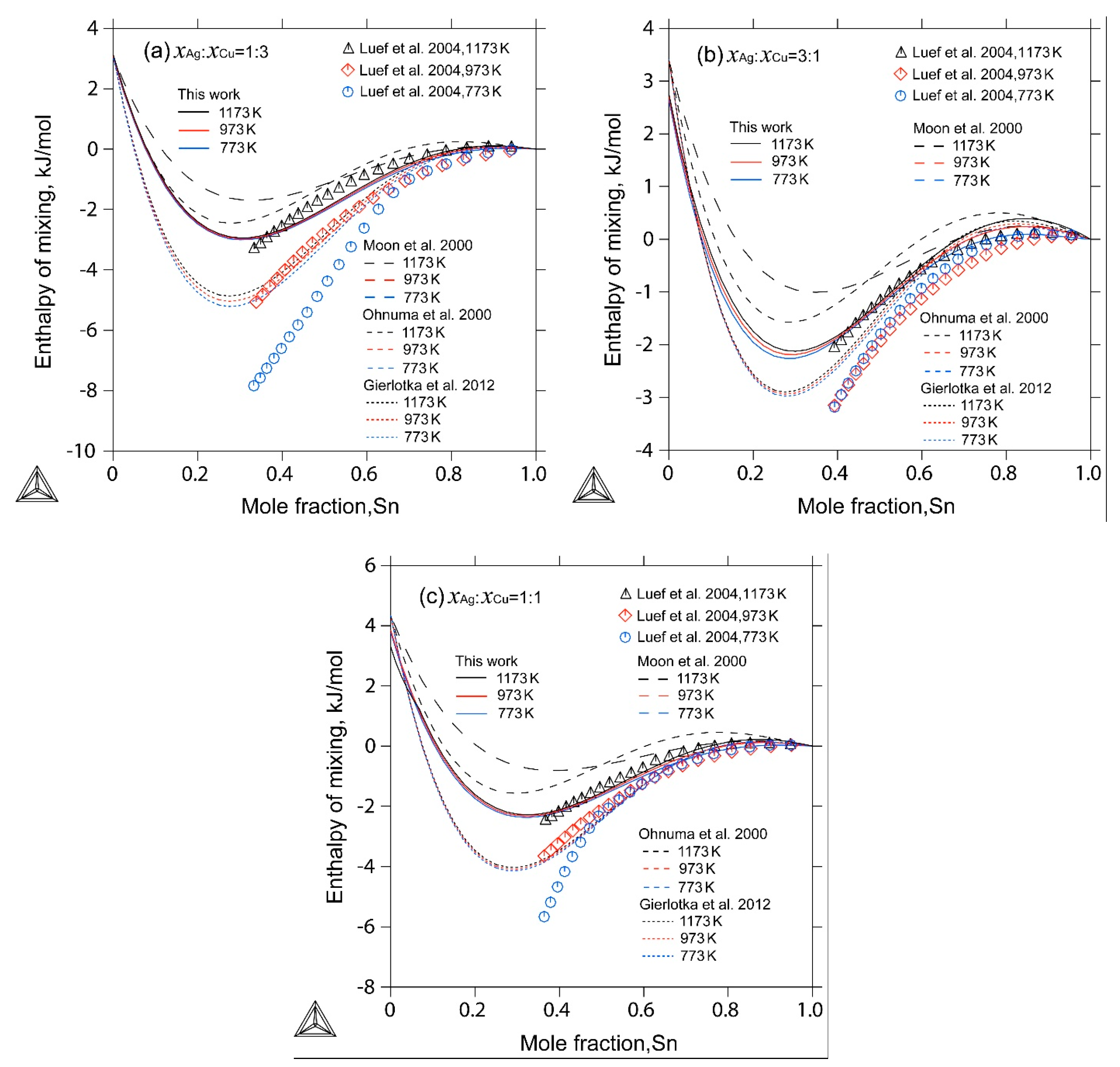
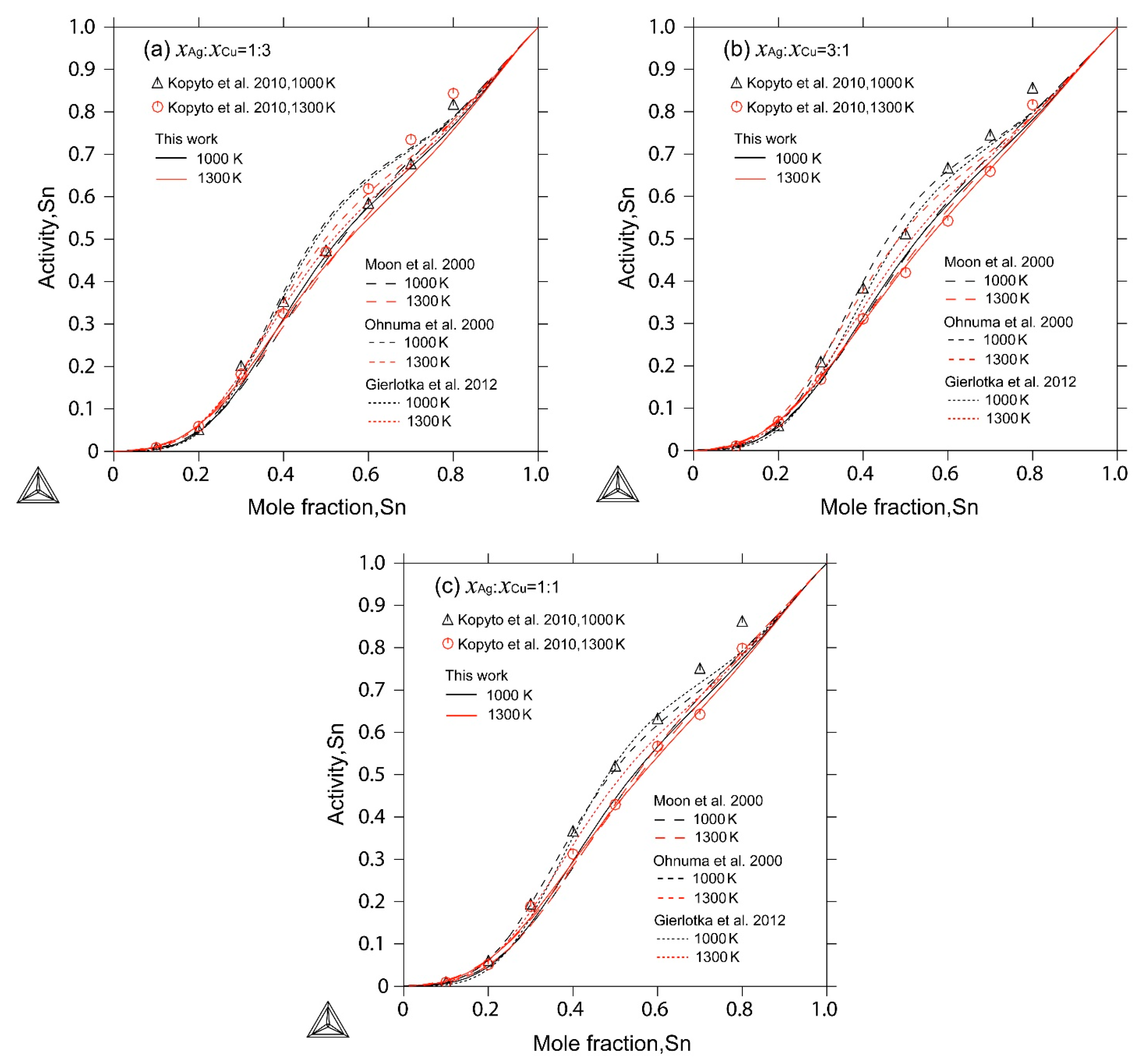
4. Results and Discussion
5. Conclusions
- A set of self-consistent thermodynamic parameters formulating the Gibbs energies of various phases in the Ag-Cu-Sn ternary system were obtained in this work.
- The liquidus projection, vertical sections and isothermal sections were calculated, which are in good agreement with the reported experimental results, assuming that all binary intermetallic compounds have no ternary solubility.
- Thermodynamic parameters of the Ag-Cu-Sn ternary system obtained in this work provide a good basis to develop a compatible thermodynamic database including multicomponent Ag-Cu-Sn-based alloys.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abtew, M.; Selvaduray, G. Lead-free solders in microelectronics. Mater. Sci. Eng. R 2000, 27, 95–141. [Google Scholar] [CrossRef]
- Cheng, S.F.; Huang, C.M.; Pecht, M. A review of lead-free solders for electronics applications. Microelectron. Reliab. 2017, 75, 77–95. [Google Scholar] [CrossRef]
- Anderson, I.E.; Cook, B.A.; Harringa, J.L.; Terpstra, R.L. Sn-Ag-Cu solders and solder joints: Alloy development, microstructure and properties. JOM 2002, 54, 26–29. [Google Scholar] [CrossRef]
- Kroupa, A.; Dinsdale, A.T.; Watson, A.; Vrestal, J.; Zemanova, A. The development of the COST 531 lead-free solders thermodynamic database. JOM 2007, 59, 20–25. [Google Scholar] [CrossRef]
- Wang, X.X.; Peng, J.; Cui, D.T.; Xue, P.; Li, H.; Hu, A.M.; Sun, G.Y. Research and application of silver-based brazing alloys in manufacturing industries: A review. Mater. Rep. 2018, 32, 1477–1485. [Google Scholar] [CrossRef]
- Luo, Q.C.; Xue, S.B.; Wu, J. Influences of Sn on properties of Ag-based and Cu-based brazing filler metals. Crystals 2021, 11, 1403. [Google Scholar] [CrossRef]
- Kanlayasiri, K.; Mongkolwongrojn, M.; Ariga, T. Influence of indium addition on characteristics of Sn-0.3Ag-0.7Cu solder alloy. J. Alloys Compd. 2009, 485, 225–230. [Google Scholar] [CrossRef]
- Leong, Y.M.; Haseeb, A.S.M.A.; Nishikawa, H.; Mokhtari, O. Microstructure and mechanical properties of Sn-1.0Ag-0.5Cu solder with minor Zn additions. J. Mater. Sci. Mater. Electron. 2019, 30, 11914–11922. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, C.Q.; Qi, L.; Chi, C.Y. Kinetics of intermetallic compound layers and shear strength in Bi-bearing SnAgCu/Cu soldering couples. J. Alloys Compd. 2009, 473, 382–388. [Google Scholar] [CrossRef]
- Wang, Y.W.; Lin, Y.W.; Tu, C.T.; Kao, C.R. Effects of minor Fe, Co, and Ni additions on the reaction between SnAgCu solder and Cu. J. Alloys Compd. 2009, 478, 121–127. [Google Scholar] [CrossRef]
- Shi, Y.W.; Tian, J.; Hao, H.; Xia, Z.D.; Lei, Y.P.; Guo, F. Effects of small amount addition of rare earth Er on microstructure and property of SnAgCu solder. J. Alloys Compd. 2008, 453, 180–184. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.F.; Zhang, Z.H.; Wang, H.; Wang, E.Z.; Qiu, T. Microstructure and melting properties of Ag-Cu-In intermediate-temperature brazing alloys. Rare Met. 2015, 5, 324–328. [Google Scholar] [CrossRef]
- Dimitrijevic, S.P.; Manasijevic, D.; Kamberovic, Z.; Dimitrijevic, S.B.; Mitric, M.; Gorgievski, M.; Mladenovic, S. Experimental Investigation of Microstructure and Phase Transitions in Ag-Cu-Zn Brazing Alloys. J. Mater. Eng. Perform. 2018, 27, 1570–1579. [Google Scholar] [CrossRef]
- Chen, S.W.; Hsu, Y.H.; Shih, H.W.; Huang, H.C. Ag-Sb/Cu interfacial reactions and Ag-Cu-Sb phase equilibria. J. Alloys Compd. 2021, 855, 157239. [Google Scholar] [CrossRef]
- Luo, H.T.; Chen, S.W. Phase equilibria of the ternary Ag-Cu-Ni system and the interfacial reactions in the Ag-Cu/Ni couples. J. Mater. Sci. 1996, 31, 5059–5067. [Google Scholar] [CrossRef]
- Wierzbicki, L.J.; Malec, W.; Stobrawa, J.; Cwolek, B.; Juszczyk, B. Studies into new, environmentally friendly Ag-Cu-Zn-Sn brazing alloys of low silver content. Arch. Met. Mater. 2011, 56, 147–158. [Google Scholar] [CrossRef]
- Liu, Z.K. Computational thermodynamics and its applications. Acta Mater. 2020, 200, 745–792. [Google Scholar] [CrossRef]
- Gebhardt, E.; Petzow, G. The constitution of the Ag-Cu-Sn system. Z. Metallkde. 1959, 50, 597–605. [Google Scholar]
- Miller, C.M.; Anderson, I.E.; Smith, J.F. A viable tin-lead solder substitute: Sn-Ag-Cu. J. Electron. Mater. 1994, 23, 595–601. [Google Scholar] [CrossRef]
- Loomans, M.E.; Fine, M.E. Tin-silver-copper eutectic temperature and composition. Met. Mater. Trans A 2000, 31, 1155–1162. [Google Scholar] [CrossRef]
- Moon, K.W.; Boettinger, W.J.; Kattner, U.R.; Biancaniello, F.S.; Handwerker, C.A. Experimental and thermodynamic assessment of Sn-Ag-Cu solder alloys. J. Electron. Mater. 2000, 29, 1122–1136. [Google Scholar] [CrossRef]
- Lewis, D.; Allen, S.; Notis, M.; Scotch, A. Determination of the eutectic structure in the Ag-Cu-Sn system. J. Electron. Mater. 2002, 31, 161–167. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, C.U.; Carper, T.; Puligandla, V. Phase equilibria studies of Sn-Ag-Cu eutectic solder using differential cooling of Sn-3.8Ag-0.7Cu alloys. J. Electron. Mater. 2003, 32, 1297–1302. [Google Scholar] [CrossRef]
- Ohnuma, I.; Miyashita, M.; Anzai, K.; Liu, X.J.; Ohtani, H.; Kainuma, R.; Ishida, K. Phase equilibria and the related properties of Sn-Ag-Cu based Pb-free solder alloys. J. Electron. Mater. 2000, 29, 1137–1144. [Google Scholar] [CrossRef]
- Yen, Y.W.; Chen, S.W. Phase equilibria of the Ag-Sn-Cu ternary system. J. Mater. Res. 2004, 19, 2298–2305. [Google Scholar] [CrossRef]
- Marjanovic, S.; Manasijevic, D.; Minic, D.; Ivkovic, D.; Todorovic, R. Thermal analysis of some alloys in the Ag-Cu-Sn ternary system. J. Optoelectron. Adv. Mater. 2009, 11, 175–179. [Google Scholar]
- Fima, P.; Gazda, A. Thermal analysis of selected Sn-Ag-Cu alloys. J. Therm. Anal. Calorim. 2013, 112, 731–737. [Google Scholar] [CrossRef]
- Gierlotka, W. Thermodynamic description of the quaternary Ag-Cu-In-Sn system. J. Electron. Mater. 2012, 41, 86–108. [Google Scholar] [CrossRef]
- Dinsdale, A.T.; Kroupa, A.; Vizdal, J.; Vrestal, J.; Watson, A.; Zemanova, A. COST 531 Database for Lead-free Solders, ver. 3.0; The European Cooperation in Science and Technology: Brussels, Belgium, 2008; unpublished research. [Google Scholar]
- Luef, C.; Flandorfer, H.; Ipser, H. Lead-free solder materials: Experimental enthalpies of mixing in the Ag-Cu-Sn and Cu-Ni-Sn ternary systems. Z. Metallkde. 2004, 95, 151–163. [Google Scholar] [CrossRef]
- Kopyto, M.; Onderka, B.; Zabdyr, L.A. Thermodynamic properties of the liquid Ag-Cu-Sn lead-free solder alloys. Mater. Chem. Phys. 2010, 122, 480–484. [Google Scholar] [CrossRef]
- He, X.C.; Wang, H.; Liu, H.S.; Jin, Z.P. Thermodynamic description of the Cu-Ag-Zr system. Calphad 2006, 30, 367–374. [Google Scholar] [CrossRef]
- Du, J.Y.; Zemanova, A.; Hutabalian, Y.; Kroupa, A.; Chen, S.W. Phase diagram of Ag-Pb-Sn system. Calphad 2020, 71, 101997. [Google Scholar] [CrossRef]
- Liu, X.J.; Liu, H.S.; Ohnuma, I.; Kainuma, R.; Ishida, K.; Itabashi, S.; Kameda, K.; Yamaguchi, K. Experimental determination and thermodynamic calculation of the phase equilibria in the Cu-In-Sn system. J. Electron. Mater. 2001, 30, 1093–1103. [Google Scholar] [CrossRef]
- Subramanian, P.R.; Perepezko, J.H. The Ag-Cu (Silver-Copper) system. J. Phase Equilib. 1993, 14, 62–75. [Google Scholar] [CrossRef]
- Lim, M.S.S.; Tibballs, J.E.; Rossiter, P.L. An assessment of thermodynamic equilibria in the Ag-Al-Cu-Mg quaternary system in relation to precipitation reactions. Z. Metallkde. 1997, 88, 236–245. [Google Scholar]
- Kusoffsky, A. Thermodynamic evaluation of the ternary Ag-Au-Cu system-including a short range order description. Acta Mater. 2002, 50, 5139–5145. [Google Scholar] [CrossRef]
- Witusiewicz, V.T.; Hecht, U.; Fries, S.G.; Rex, S. The Ag-Al-Cu system: Part I: Reassessment of the constituent binaries on the basis of new experimental data. J. Alloys Compd. 2005, 385, 133–143. [Google Scholar] [CrossRef]
- Oriani, R.A.; Murphy, W.K. Heats of formation of liquid alloys at 1100° by a simple reaction calorimeter. J. Phys. Chem. 1958, 62, 199–202. [Google Scholar] [CrossRef]
- Dobovisek, B.; Paulin, A. Report on calorimetric measurements with DTA at high temperatures. Min. Met. Quart. 1962, 3, 27. [Google Scholar]
- Itagaki, K.; Yazawa, A. Heats of mixing in liquid copper or gold binary alloys. Trans. Jpn. Inst. Met. 1975, 16, 679–686. [Google Scholar] [CrossRef][Green Version]
- Kleppa, O.J.; Watanabe, S. Thermochemistry of alloys of transition metals: Part III. Copper-Silver, -Titanium, Zirconium, and -Hafnium at 1373 K. Met. Trans. B 1982, 13, 391–401. [Google Scholar] [CrossRef]
- Golonka, J.; Botor, J.; Dulat, M. Study of Cu-Ag liquid solutions by combined effusion vaporization and mass spectrometry sensing. Met. Tech. 1979, 6, 267–272. [Google Scholar] [CrossRef]
- Choudary, U.V.; Ghosh, A. Thermodynamics of liquid copper-silver alloys by a solid electrolyte cell. J. Electrochem. Soc. 1970, 117, 1024–1028. [Google Scholar] [CrossRef]
- Fitzner, K.; Guo, Q.; Wang, J.W.; Klepp, O.J. Enthalpies of liquid-liquid mixing in the systems Cu-Ag, Cu-Au and Ag-Au by using an in-situ mixing device in a high temperature single-unit differential calorimeter. J. Alloys Compd. 1999, 291, 190–200. [Google Scholar] [CrossRef]
- Murray, J.L. Calculations of stable and metastable equilibrium diagrams of the Ag-Cu and Cd-Zn systems. Met. Trans. A 1984, 15, 261–268. [Google Scholar] [CrossRef]
- Bienzle, M.; Oishi, T.; Sommer, F.; Ono, K. Thermodynamic Study of the Silver-Rich Ag-Cu Solid Solution. Mater. Trans. JIM 1992, 33, 51–56. [Google Scholar] [CrossRef]
- Karakaya, I.; Thompson, W.T. The Ag-Sn (Silver-Tin) system. J. Phase Equilib. 1987, 8, 340–347. [Google Scholar] [CrossRef]
- Chevalier, P.Y. A thermodynamic evaluation of the Ag-Sn system. Thermochim. Acta 1988, 136, 45–54. [Google Scholar] [CrossRef]
- Kattner, U.R.; Boettinger, W.J. On the Sn-Bi-Ag ternary phase diagram. J. Electron. Mater. 1994, 23, 603–610. [Google Scholar] [CrossRef]
- Oh, C.S.; Shim, J.H.; Lee, B.J.; Lee, D.N. A thermodynamic study on the Ag-Sb-Sn system. J. Alloys Compd. 1996, 238, 155–166. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.S.; Liu, L.B.; Jin, Z.P. Thermodynamic description of the Sn-Ag-Au ternary system. Calphad 2007, 31, 545–552. [Google Scholar] [CrossRef]
- Heycock, C.T.; Neville, F.H. Complete freezing-point curves of binary alloys containing silver or copper together with another metal. Philos. Trans. R. Soc. Lond. A 1897, 189, 25–69. [Google Scholar] [CrossRef]
- Petrenko, G.J. On the alloying of silver with lead and tin. Z. Anorg. Allg. Chem. 1907, 53, 200–211. [Google Scholar]
- Murphy, A.J. The constitution of the alloys of silver and tin. J. Inst. Met. 1926, 35, 107–129. [Google Scholar]
- Dinsdale, A.T. SGTE data for pure elements. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]
- Flandorfer, H.; Luef, C.; Saeed, U. On the temperature dependence of the enthalpies of mixing in liquid binary (Ag, Cu, Ni)-Sn alloys. J. Non Cryst. Solids 2008, 354, 2953–2972. [Google Scholar] [CrossRef]
- Shim, J.H.; Oh, C.S.; Lee, B.J.; Lee, D.N. Thermodynamic assessment of the Cu-Sn system. Z. Metallkde. 1996, 87, 205–212. [Google Scholar] [CrossRef]
- Gierlotka, W.; Chen, S.W.; Lin, S.K. Thermodynamic description of the Cu-Sn system. J. Mater. Res. 2007, 22, 3158–3165. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.L.; Leinenbach, C.; Klotz, U.E.; Uggowitzer, P.J.; Loffler, J.F. Experimental investigation and thermodynamic assessment of the Cu-Sn-Ti ternary system. Calphad 2011, 35, 82–94. [Google Scholar] [CrossRef]
- Miettinen, J. Thermodynamic description of the Cu-Al-Sn system in the copper-rich corner. Met. Mater. Trans. A 2002, 33, 1639–1648. [Google Scholar] [CrossRef]
- Li, M.; Du, Z.M.; Guo, C.P.; Li, C.R. Thermodynamic optimization of the Cu-Sn and Cu-Nb-Sn systems. J. Alloys Compd. 2009, 477, 104–117. [Google Scholar] [CrossRef]
- Liu, X.J.; Wang, C.P.; Ohnuma, I.; Kainuma, R.; Ishida, K. Experimental investigation and thermodynamic calculation of the phase equilibria in the Cu-Sn and Cu-Sn-Mn systems. Met. Mater. Trans. A 2004, 35, 1641–1654. [Google Scholar] [CrossRef]
- Li, D.; Franke, P.; Furtauer, S.; Cupid, D.; Flandorfer, H. The Cu-Sn phase diagram part II: New thermodynamic assessment. Intermetallics 2013, 34, 148–158. [Google Scholar] [CrossRef]
- Redlich, O.; Kister, A.T. Thermodynamics of nonelectrolyte solutions. Ind. Eng. Chem. 1948, 40, 341–345. [Google Scholar] [CrossRef]
- Muggianu, Y.M.; Gambino, M.; Bros, J.P. Enthalpies of formation of liquid alloys bismuth-gallium-tin at 723 K. Choice of an analytical representation of integral and partial excess functions of mixing. J. Chim. Phys. 1975, 75, 83–88. [Google Scholar] [CrossRef]
- Sundman, B.; Jansson, B.; Andersson, J.O. The Thermo-Calc databank system. Calphad 1985, 9, 153–190. [Google Scholar] [CrossRef]

| Reference | Experimental Method * | Experimental Data | Used in Optimization |
|---|---|---|---|
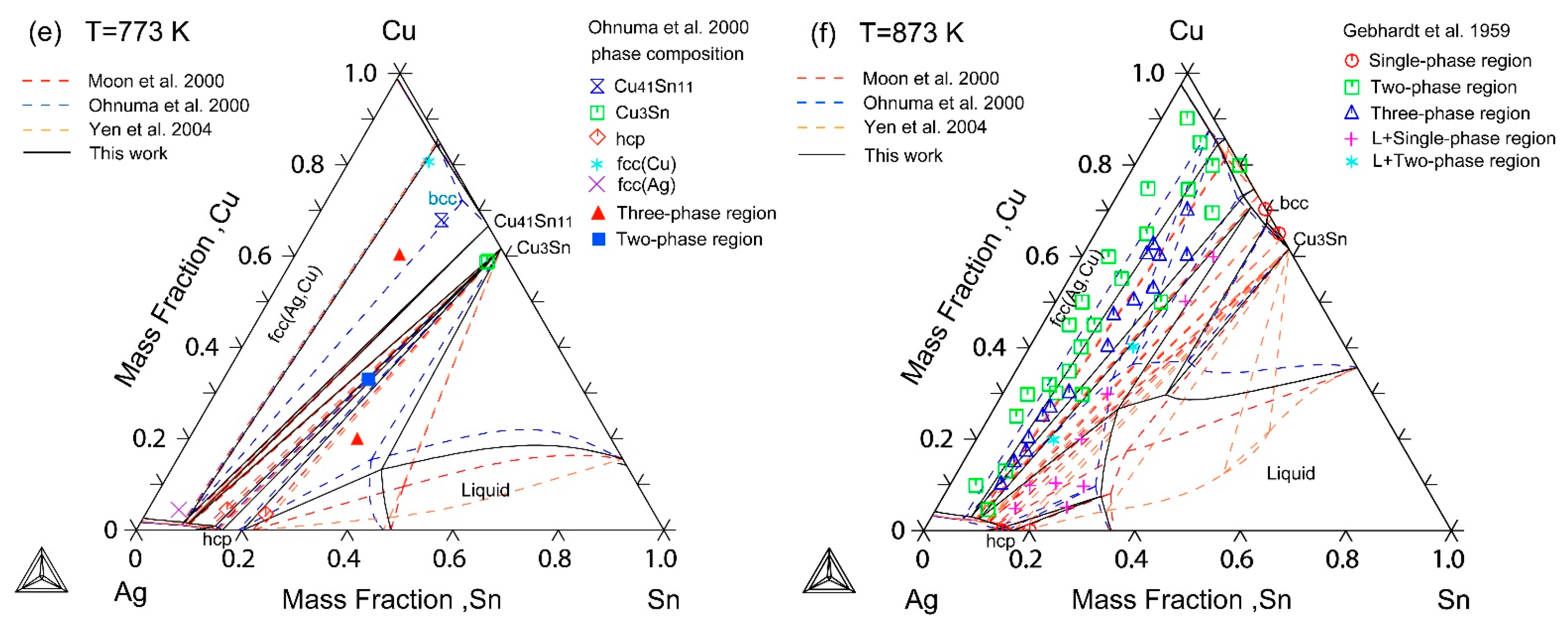
| [18] | DTA, XRD | Liquidus projection Vertical sections: 5 wt%, 10 wt%, 15 wt%, 20 wt%, 25 wt% Sn Isothermal sections: 773 K, 873 K | Yes Yes Yes |
| [19] | DTA, XRD, OM, SEM | Ternary eutectic reaction | Yes |
| [20] | DSC, OM, SEM | Ternary eutectic reaction | Yes |
| [21] | DTA, SEM | Ternary eutectic reaction | Yes |
| [22] | SEM, EPMA | Ternary eutectic reaction | Yes |
| [23] | DSC, SEM | Ternary eutectic reaction | Yes |
| [24] | XRD, SEM | Isothermal sections: 573 K, 673 K, 773 K | Yes |
| [25] | XRD, OM, SEM, EPMA | Isothermal sections: 513 K, 723 K | Yes |
| [26] | DTA | Vertical sections: | Yes |
| [27] | DSC | Vertical sections: | Yes |
| [30] | Calorimetry | Enthalpy of mixing of liquid at 773 K, 973 K, 1173 K for three constant ratios of | Yes |
| [31] | Electromotive force (EMF) | Activity of Sn in liquid 1000 K and 1300 K for three constant ratios of | Yes |
| Phase | Thermodynamic Parameters | Reference |
|---|---|---|
| Liquid | [32] | |
| [32] | ||
| [33] | ||
| [33] | ||
| [33] | ||
| [34] | ||
| [34] | ||
| [34] | ||
| This work | ||
| This work | ||
| This work | ||
| fcc | [32] | |
| [32] | ||
| [33] | ||
| [33] | ||
| [34] | ||
| [34] | ||
| bct | [33] | |
| hcp | [33] | |
| [33] | ||
| bcc | [34] | |
| [34] | ||
| [34] | ||
| This work | ||
| Ag3Sn | [33] | |
| [33] | ||
| Cu3Sn | [34] | |
| Cu10Sn3 | [34] | |
| Cu41Sn11 | [34] | |
| Cu6Sn5-h | [34] | |
| Cu6Sn5-l | [34] |
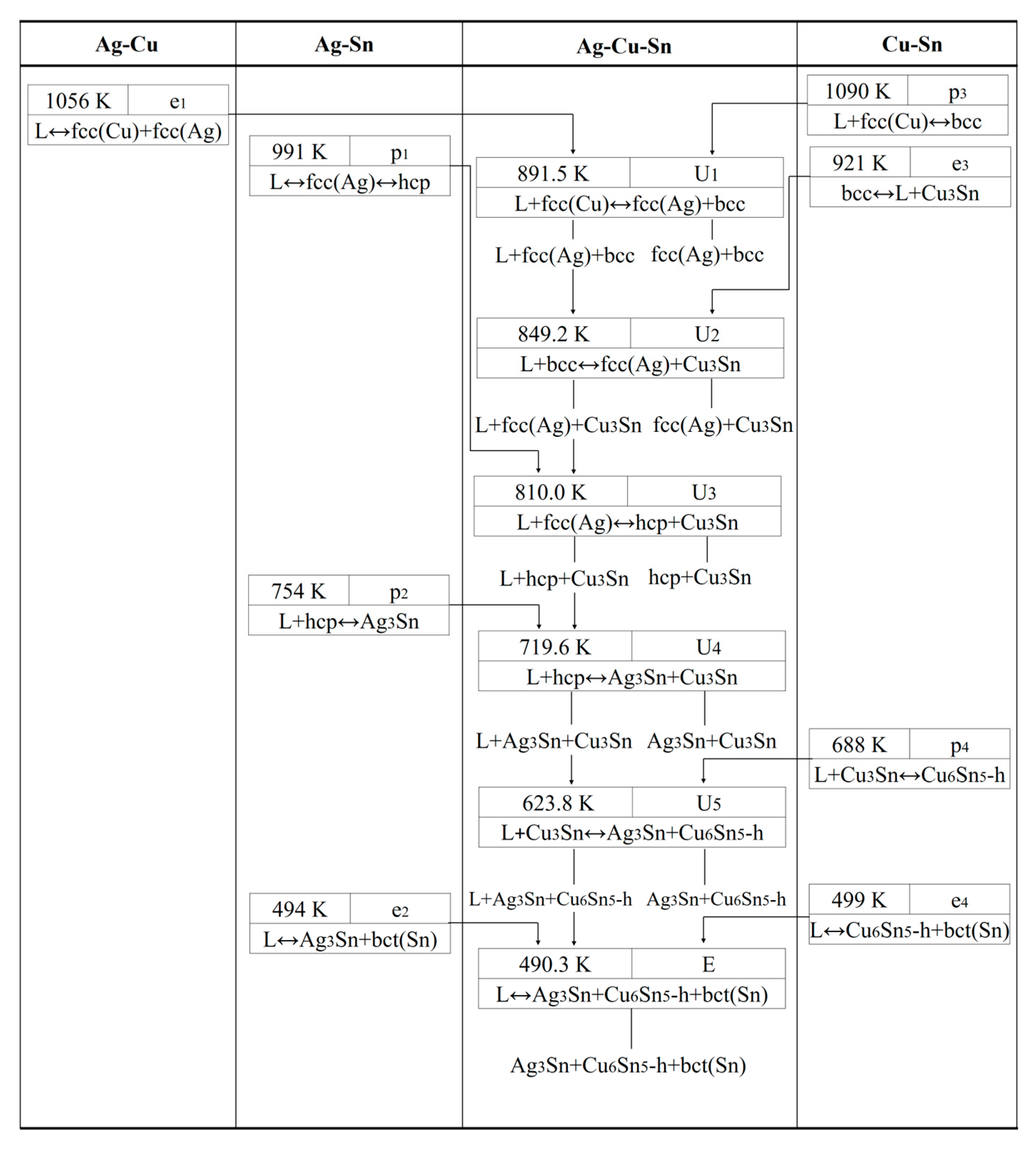
| Invariant Reactions | Type | T(K) | Composition | Reference | |
|---|---|---|---|---|---|
| L + fcc(Cu) ↔ bcc + fcc(Ag) | U1 | 891.5 | 0.417 | 0.425 | This work |
| 878 | 0.545 | 0.224 | Exp. [18] | ||
| 910 | 0.366 | 0.457 | Cal. [21] | ||
| 841 | 0.380 | 0.464 | Cal. [24] | ||
| 857 | 0.401 | 0.460 | Cal. [28] | ||
| L + bcc ↔ fcc(Ag) + Cu3Sn | U2 | 849.2 833 | 0.436 0.492 | 0.345 0.192 | This work Exp. [18] |
| 828 | 0.392 | 0.409 | Cal. [24] | ||
| L + fcc(Ag) ↔ hcp + Cu3Sn | U3 | 810.0 813 | 0.469 0.494 | 0.247 0.152 | This work Exp. [18] |
| 845 | 0.503 | 0.206 | Cal. [21] | ||
| 814 | 0.441 | 0.313 | Cal. [24] | ||
| L + hcp ↔ Ag3Sn + Cu3Sn | U4 | 719.6 713 | 0.391 0.437 | 0.183 0.071 | This work Exp. [18] |
| 733 | 0.385 | 0.144 | Cal. [21] | ||
| 721 | 0.419 | 0.172 | Cal. [24] | ||
| 741 | 0.400 | 0.292 | Cal. [28] | ||
| L + Cu3Sn ↔ Ag3Sn + Cu6Sn5-h | U5 | 623.8 623 | 0.227 0.191 | 0.125 0.034 | This work Exp. [18] |
| 629 | 0.198 | 0.102 | Cal. [21] | ||
| 645 | 0.272 | 0.089 | Cal. [24] | ||
| 632 | 0.281 | 0.215 | Cal. [28] | ||
| L ↔ Ag3Sn + Cu6Sn5-h + bct(Sn) | E | 490.3 498.2 | 0.035 0.036 | 0.016 0.003 | This work Exp. [18] |
| 490.0 | 0.051 | 0.031 | Exp. [19] | ||
| 490.3 490.3 | 0.038 0.038 | 0.016 0.016 | Exp. [20] Exp. [21] | ||
| 490.3 | 0.038 | 0.016 | Exp. [22] | ||
| 490.2 489.5 | 0.039 0.041 | 0.016 0.017 | Exp. [23] Cal. [21] | ||
| 490.7 | 0.035 | 0.011 | Cal. [24] | ||
| 491.9 | 0.039 | 0.003 | Cal. [28] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Q.; Ge, J.; Rong, M.; Li, J.; Jiao, J.; Zhang, L.; Wang, J. Thermodynamic Modeling of the Ag-Cu-Sn Ternary System. Metals 2022, 12, 1557. https://doi.org/10.3390/met12101557
Tong Q, Ge J, Rong M, Li J, Jiao J, Zhang L, Wang J. Thermodynamic Modeling of the Ag-Cu-Sn Ternary System. Metals. 2022; 12(10):1557. https://doi.org/10.3390/met12101557
Chicago/Turabian StyleTong, Qingsong, Jing Ge, Maohua Rong, Jielong Li, Jian Jiao, Lu Zhang, and Jiang Wang. 2022. "Thermodynamic Modeling of the Ag-Cu-Sn Ternary System" Metals 12, no. 10: 1557. https://doi.org/10.3390/met12101557
APA StyleTong, Q., Ge, J., Rong, M., Li, J., Jiao, J., Zhang, L., & Wang, J. (2022). Thermodynamic Modeling of the Ag-Cu-Sn Ternary System. Metals, 12(10), 1557. https://doi.org/10.3390/met12101557






