Spray-Pyrolytic Tunable Structures of Mn Oxides-Based Composites for Electrocatalytic Activity Improvement in Oxygen Reduction
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Material Synthesis and Electrode Preparation
2.2.1. Material Synthesis
2.2.2. Electrode Preparation
2.3. Measurements
2.3.1. Material Characterization
2.3.2. Electrochemical Measurements
3. Results and Discussion
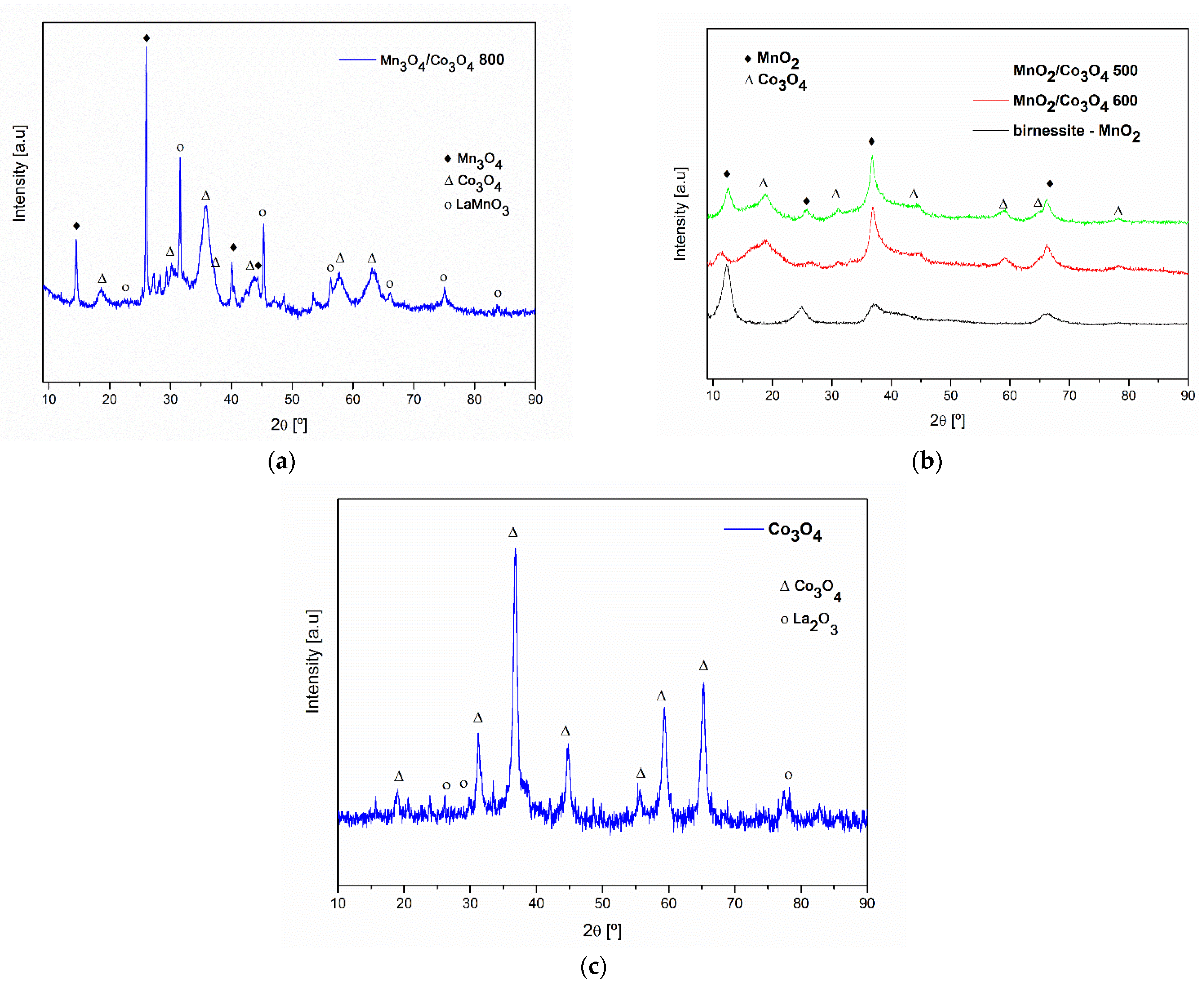
3.1. XRD Analysis
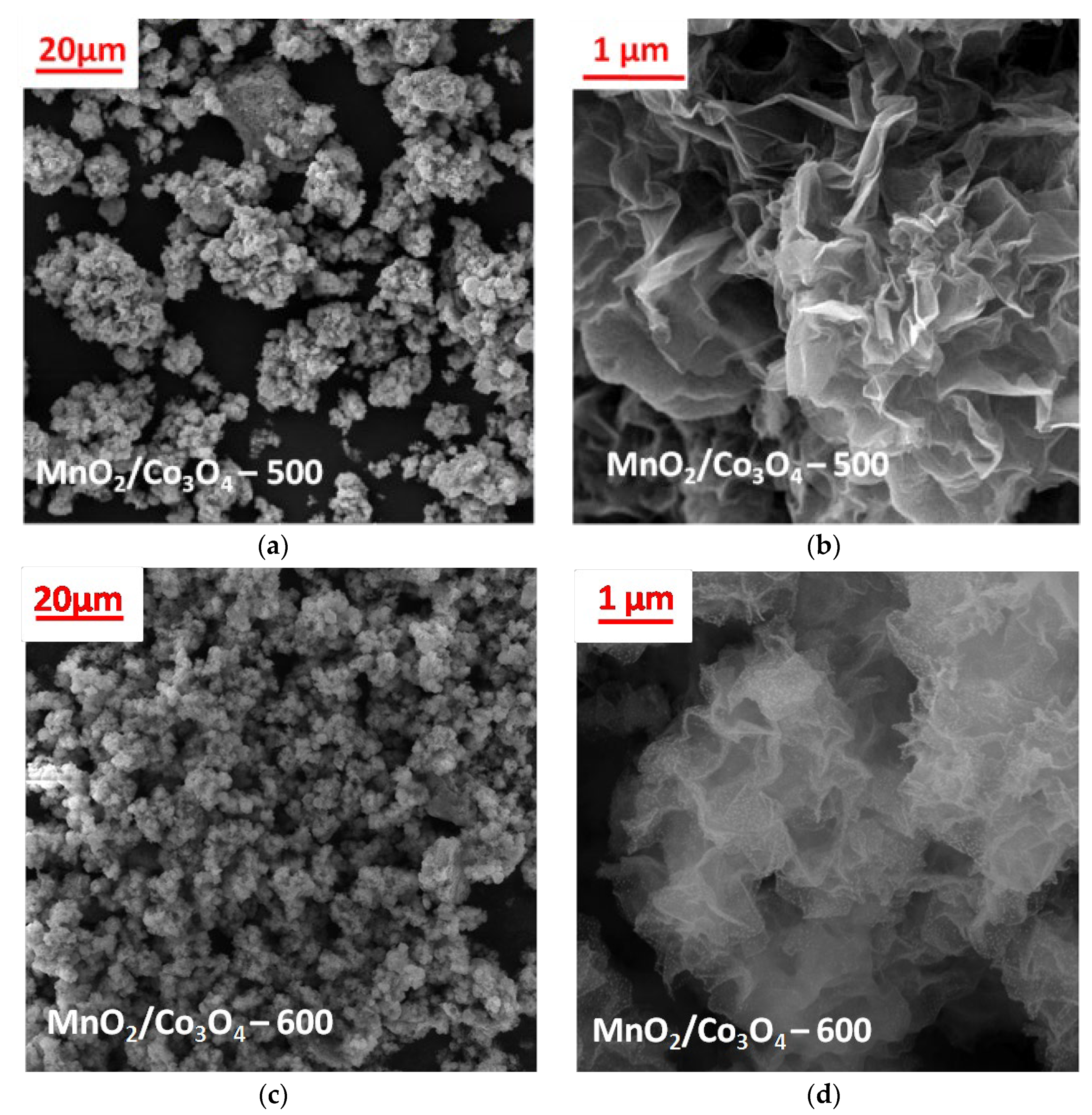
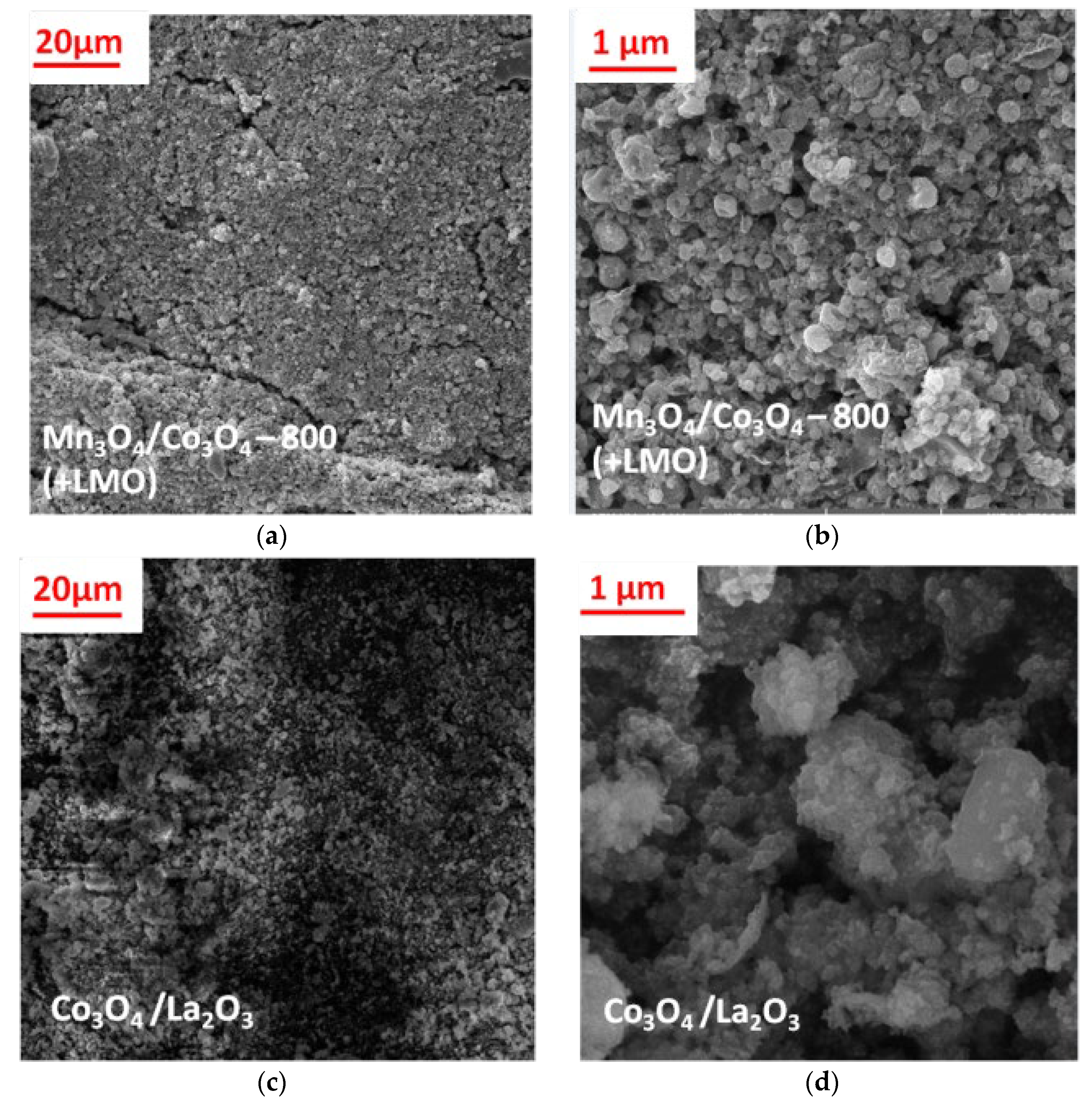
3.2. SEM and EDS Characterization
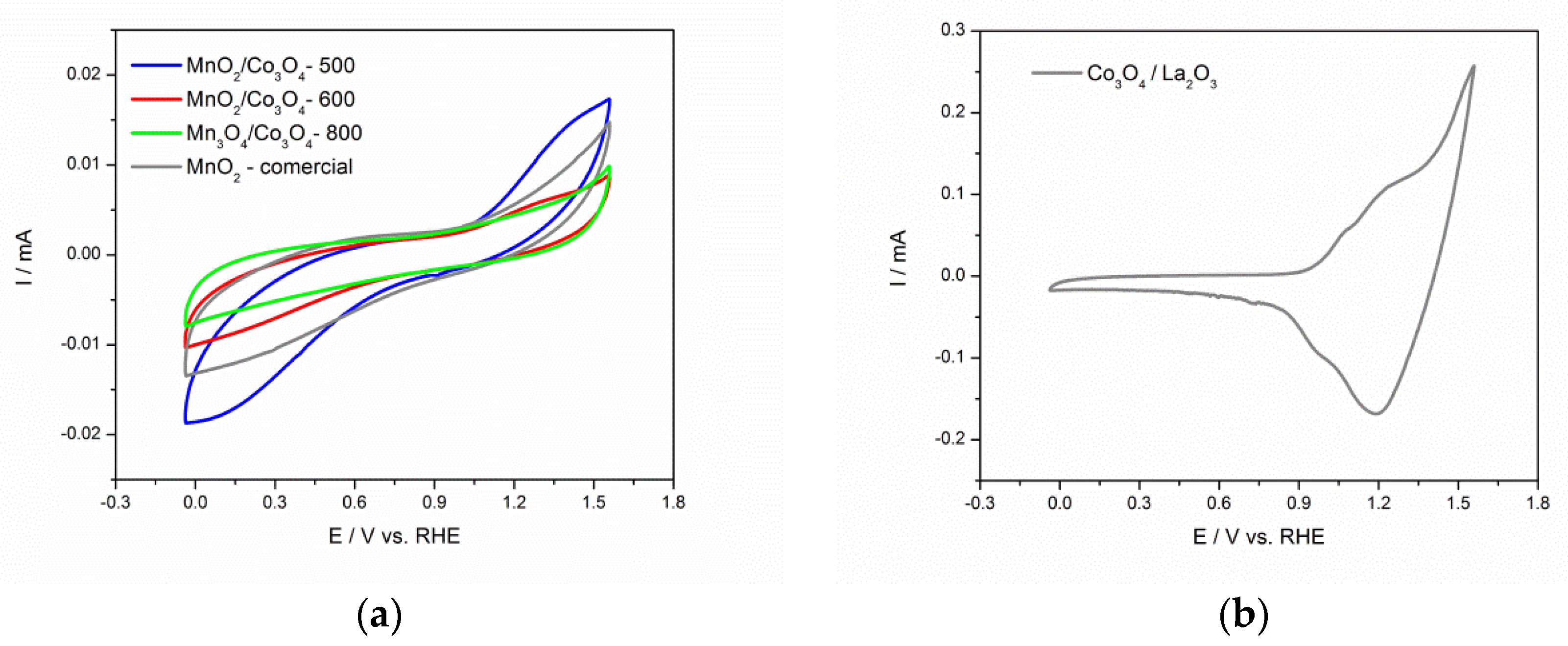
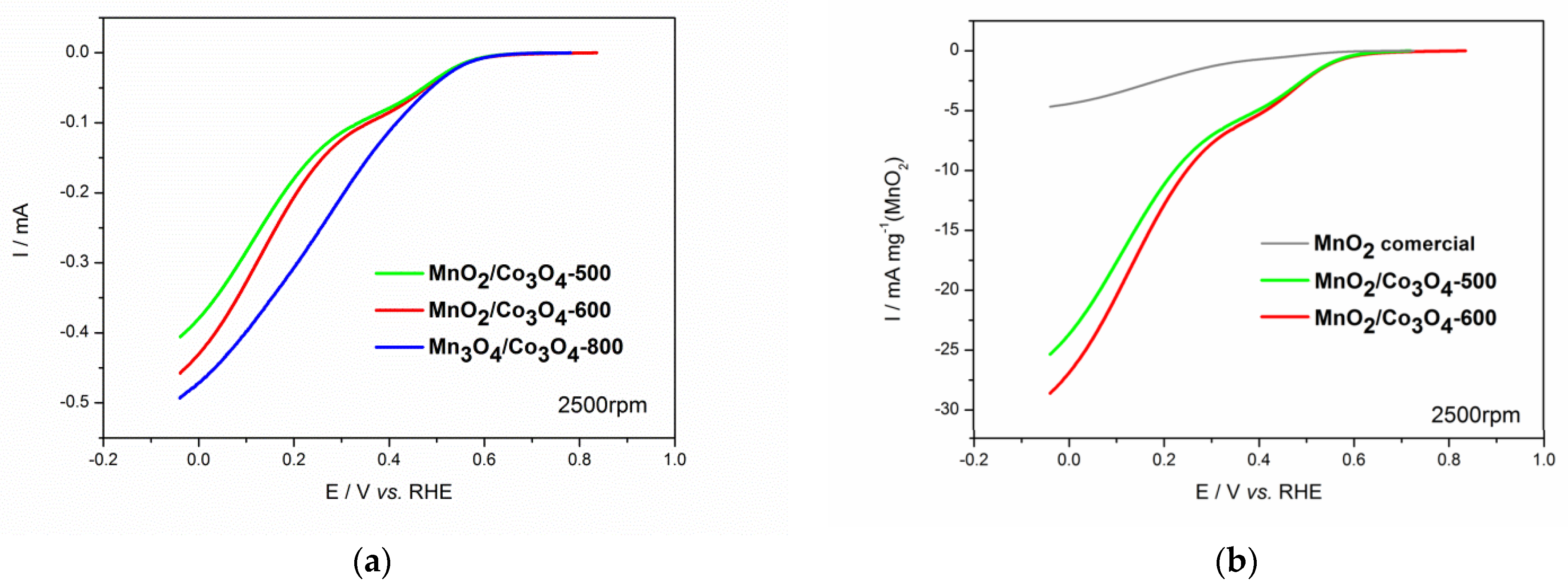
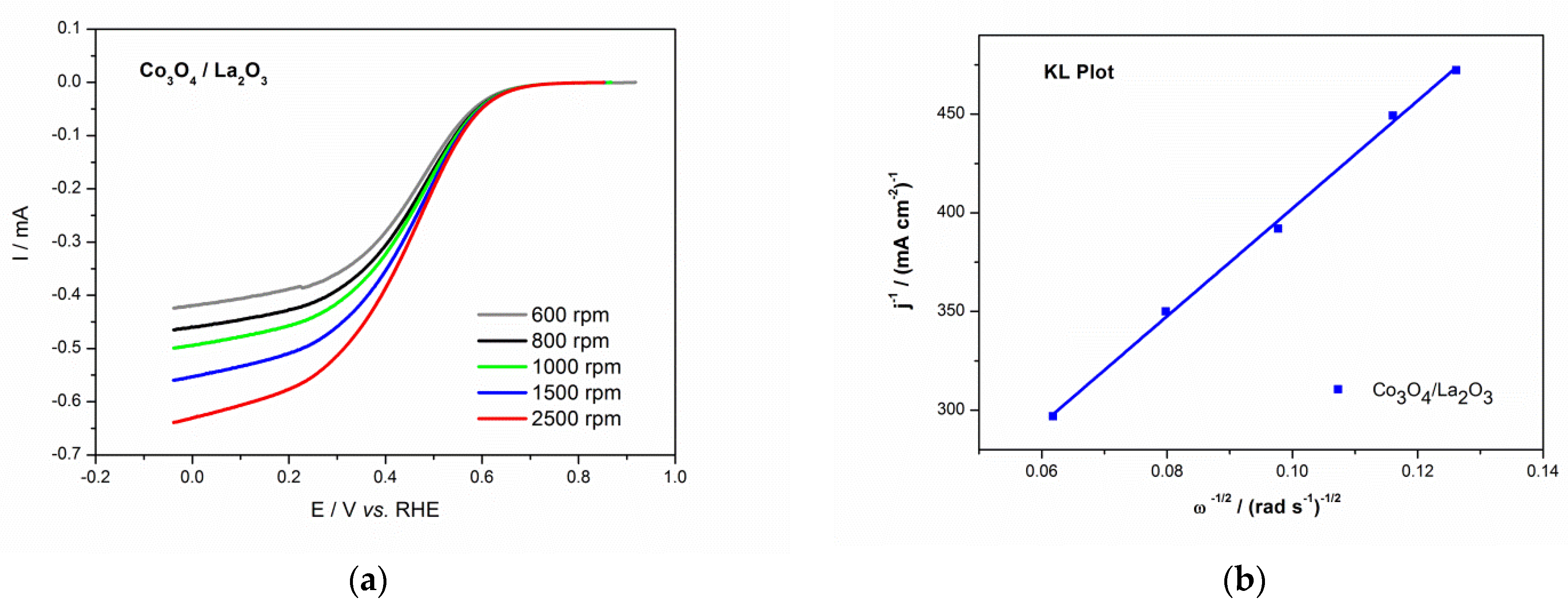
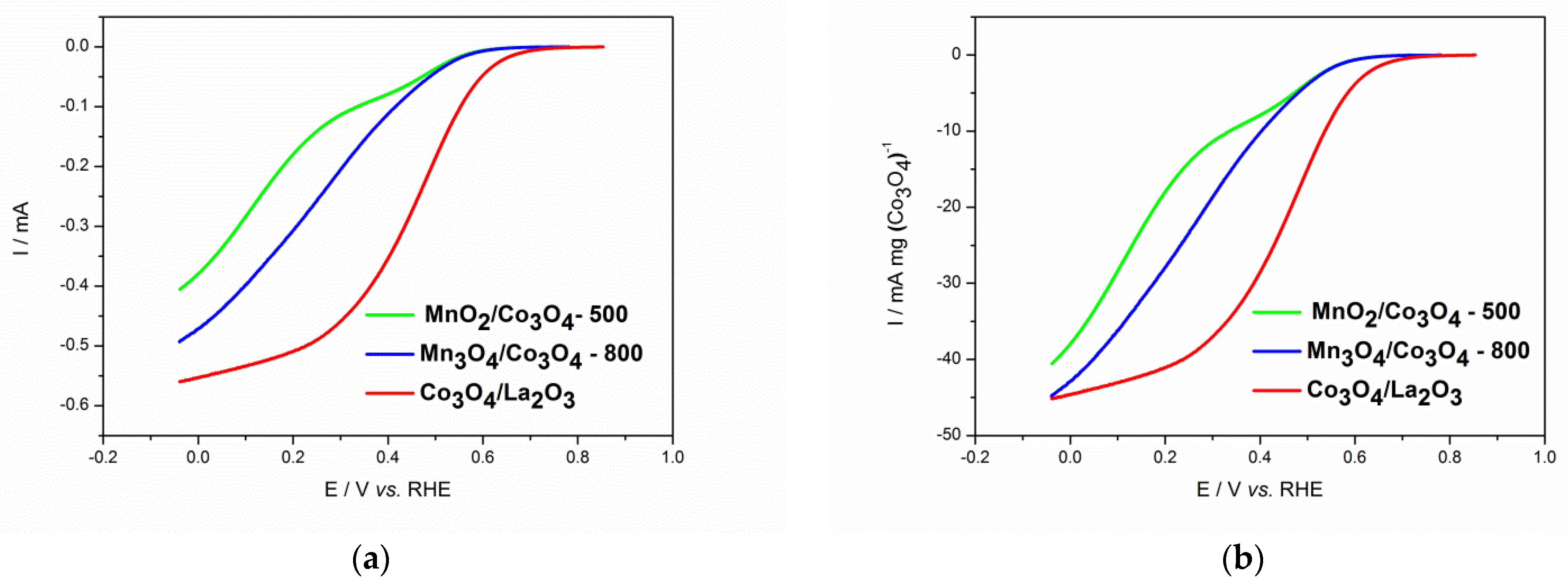
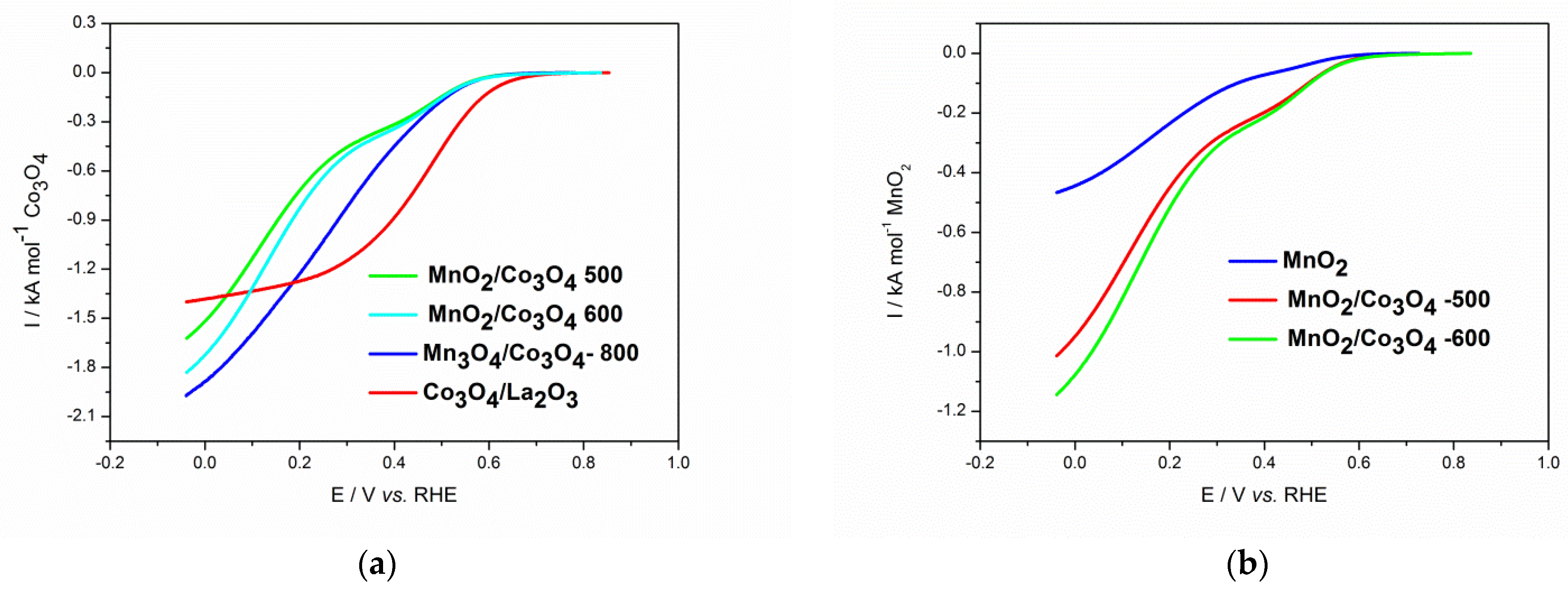
3.3. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Diaz, D.F.R.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Qu, Y.; Yuan, T.; Wang, W.; Wu, Y.; Li, Y. Review of Metal Catalysts for Oxygen Reduction Reaction: From Nanoscale Engineering to Atomic Design. Chem 2019, 5, 1486–1511. [Google Scholar] [CrossRef]
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustain. Energy Fuels 2020, 4, 15–30. [Google Scholar] [CrossRef]
- Xiong, Y.; Xiao, L.; Yang, Y.; DiSalvo, F.J.; Abruña, H.D. High-Loading Intermetallic Pt3Co/C Core–Shell Nanoparticles as Enhanced Activity Electrocatalysts toward the Oxygen Reduction Reaction (ORR). Chem. Mater. 2018, 30, 1532–1539. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Y.-C.; Chen, C.; Yang, X.-D.; Zhou, Z.-Y.; Sun, S.-G. A mesoporous Fe/N/C ORR catalyst for polymer electrolyte membrane fuel cells. Chin. J. Catal. 2016, 37, 1103–1108. [Google Scholar] [CrossRef]
- Gómez-Marín, A.M.; Feliu, J.M. Oxygen Reduction on Platinum Single Crystal Electrodes. In Encyclopedia of Interfacial Chemistry; Wandelt, K.B.T.-E., Ed.; Elsevier: Oxford, UK, 2018; pp. 820–830. ISBN 978-0-12-809894-3. [Google Scholar]
- Yamada, I.; Takamatsu, A.; Asai, K.; Shirakawa, T.; Ohzuku, H.; Seno, A.; Uchimura, T.; Fujii, H.; Kawaguchi, S.; Wada, K.; et al. Systematic Study of Descriptors for Oxygen Evolution Reaction Catalysis in Perovskite Oxides. J. Phys. Chem. C 2018, 122, 27885–27892. [Google Scholar] [CrossRef] [Green Version]
- Kodama, K.; Nagai, T.; Kuwaki, A.; Jinnouchi, R.; Morimoto, Y. Challenges in applying highly active Pt-based nanostructured catalysts for oxygen reduction reactions to fuel cell vehicles. Nat. Nanotechnol. 2021, 16, 140–147. [Google Scholar] [CrossRef]
- Miura, A.; Rosero-Navarro, C.; Masubuchi, Y.; Higuchi, M.; Kikkawa, S.; Tadanaga, K. Nitrogen-Rich Manganese Oxynitrides with Enhanced Catalytic Activity in the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2016, 55, 7963–7967. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Lu, Z.; Wang, W. Improvement of O2 adsorption for α-MnO2 as an oxygen reduction catalyst by Zr4+ doping. RSC Adv. 2018, 8, 2963–2970. [Google Scholar] [CrossRef] [Green Version]
- Menezes, P.W.; Indra, A.; González-Flores, D.; Sahraie, N.R.; Zaharieva, I.; Schwarze, M.; Strasser, P.; Dau, H.; Driess, M. High-Performance Oxygen Redox Catalysis with Multifunctional Cobalt Oxide Nanochains: Morphology-Dependent Activity. ACS Catal. 2015, 5, 2017–2027. [Google Scholar] [CrossRef]
- Kumar, K.; Canaff, C.; Rousseau, J.; Arrii-Clacens, S.; Napporn, T.W.; Habrioux, A.; Kokoh, K.B. Effect of the Oxide–Carbon Heterointerface on the Activity of Co3O4/NRGO Nanocomposites toward ORR and OER. J. Phys. Chem. C 2016, 120, 7949–7958. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Xiao, Z.; Li, M.; Ding, Y.; Qi, M. Electrocatalytic oxygen reduction by a Co/Co3O4@N-doped carbon composite material derived from the pyrolysis of ZIF-67/poplar flowers. RSC Adv. 2021, 11, 2693–2700. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wei, Z. Transition-metal-oxide-based catalysts for the oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 8194–8209. [Google Scholar] [CrossRef]
- Yin, M.; Miao, H.; Hu, R.; Sun, Z.; Li, H. Manganese dioxides for oxygen electrocatalysis in energy conversion and storage systems over full pH range. J. Power Sources 2021, 494, 229779. [Google Scholar] [CrossRef]
- Nikitina, V.A.; Kurilovich, A.A.; Bonnefont, A.; Ryabova, A.S.; Nazmutdinov, R.R.; Savinova, E.R.; Tsirlina, G.A. ORR on Simple Manganese Oxides: Molecular-Level Factors Determining Reaction Mechanisms and Electrocatalytic Activity. J. Electrochem. Soc. 2018, 165, J3199–J3208. [Google Scholar] [CrossRef]
- Poux, T.; Bonnefont, A.; Kéranguéven, G.; Tsirlina, G.A.; Savinova, E.R. Electrocatalytic Oxygen Reduction Reaction on Perovskite Oxides: Series versus Direct Pathway. ChemPhysChem 2014, 15, 2108–2120. [Google Scholar] [CrossRef] [PubMed]
- Ryabova, A.S.; Napolskiy, F.S.; Poux, T.; Istomin, S.Y.; Bonnefont, A.; Antipin, D.M.; Baranchikov, A.Y.; Levin, E.E.; Abakumov, A.M.; Kéranguéven, G.; et al. Rationalizing the Influence of the Mn(IV)/Mn(III) Red-Ox Transition on the Electrocatalytic Activity of Manganese Oxides in the Oxygen Reduction Reaction. Electrochim. Acta 2016, 187, 161–172. [Google Scholar] [CrossRef]
- Zhong, X.; Oubla, M.; Wang, X.; Huang, Y.; Zeng, H.; Wang, S.; Liu, K.; Zhou, J.; He, L.; Zhong, H.; et al. Boosting oxygen reduction activity and enhancing stability through structural transformation of layered lithium manganese oxide. Nat. Commun. 2021, 12, 3136. [Google Scholar] [CrossRef] [PubMed]
- Dessie, Y.; Tadesse, S.; Eswaramoorthy, R.; Abebe, B. Recent developments in manganese oxide based nanomaterials with oxygen reduction reaction functionalities for energy conversion and storage applications: A review. J. Sci. Adv. Mater. Devices 2019, 4, 353–369. [Google Scholar] [CrossRef]
- Speck, F.D.; Santori, P.G.; Jaouen, F.; Cherevko, S. Mechanisms of Manganese Oxide Electrocatalysts Degradation during Oxygen Reduction and Oxygen Evolution Reactions. J. Phys. Chem. C 2019, 123, 25267–25277. [Google Scholar] [CrossRef]
- Lambert, T.N.; Vigil, J.A.; White, S.E.; Delker, C.J.; Davis, D.J.; Kelly, M.; Brumbach, M.T.; Rodriguez, M.A.; Swartzentruber, B.S. Understanding the Effects of Cationic Dopants on α-MnO2 Oxygen Reduction Reaction Electrocatalysis. J. Phys. Chem. C 2017, 121, 2789–2797. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Fang, H.; Huang, J.; Wu, X.; He, X.; Song, J.; Li, Z.; Yan, Y.; Xu, W.; et al. Synthesis of δ–MnO2/Reduced Graphene Oxide Hybrid In Situ and Application in Mg–Air Battery. J. Electrochem. Soc. 2021, 168, 80518. [Google Scholar] [CrossRef]
- Khan, Z.; Park, S.; Hwang, S.M.; Yang, J.; Lee, Y.; Song, H.-K.; Kim, Y.; Ko, H. Hierarchical urchin-shaped α-MnO2 on graphene-coated carbon microfibers: A binder-free electrode for rechargeable aqueous Na–air battery. NPG Asia Mater. 2016, 8, e294. [Google Scholar] [CrossRef]
- Bi, R.; Liu, G.; Zeng, C.; Wang, X.; Zhang, L.; Qiao, S.-Z. 3D Hollow α-MnO(2) Framework as an Efficient Electrocatalyst for Lithium-Oxygen Batteries. Small 2019, 15, e1804958. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.M.; Rameshkumar, P.; Basirun, W.J.; Juan, J.C.; Huang, N.M. Cobalt oxide nanocubes interleaved reduced graphene oxide as an efficient electrocatalyst for oxygen reduction reaction in alkaline medium. Electrochim. Acta 2017, 237, 61–68. [Google Scholar] [CrossRef]
- Shahid, M.M.; Zhan, Y.; Alizadeh, M.; Sagadevan, S.; Paiman, S.; Oh, W.C. A glassy carbon electrode modified with tailored nanostructures of cobalt oxide for oxygen reduction reaction. Int. J. Hydrogen Energy 2020, 45, 18850–18858. [Google Scholar] [CrossRef]
- Tan, P.; Wu, Z.; Chen, B.; Xu, H.; Cai, W.; Ni, M. Exploring oxygen electrocatalytic activity and pseudocapacitive behavior of Co3O4 nanoplates in alkaline solutions. Electrochim. Acta 2019, 310, 86–95. [Google Scholar] [CrossRef]
- Lu, J.; Dey, S.; Temprano, I.; Jin, Y.; Xu, C.; Shao, Y.; Grey, C.P. Co3O4-Catalyzed LiOH Chemistry in Li–O2 Batteries. ACS Energy Lett. 2020, 5, 3681–3691. [Google Scholar] [CrossRef]
- Jin, J.; Fu, X.; Liu, Q.; Zhang, J. A highly active and stable electrocatalyst for the oxygen reduction reaction based on a graphene-supported g-C3N4@cobalt oxide core–shell hybrid in alkaline solution. J. Mater. Chem. A 2013, 1, 10538–10545. [Google Scholar] [CrossRef]
- Al-Hakemy, A.Z.; Nassr, A.B.A.A.; Naggar, A.H.; Elnouby, M.S.; Soliman, H.M.A.E.-F.; Taher, M.A. Electrodeposited cobalt oxide nanoparticles modified carbon nanotubes as a non-precious catalyst electrode for oxygen reduction reaction. J. Appl. Electrochem. 2017, 47, 183–195. [Google Scholar] [CrossRef]
- Yu, J.; Chen, G.; Sunarso, J.; Zhu, Y.; Ran, R.; Zhu, Z.; Zhou, W.; Shao, Z. Cobalt Oxide and Cobalt-Graphitic Carbon Core–Shell Based Catalysts with Remarkably High Oxygen Reduction Reaction Activity. Adv. Sci. 2016, 3, 1600060. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, H.; Diao, P.; Chang, W.; Hong, G.; Li, Y.; Gong, M.; Xie, L.; Zhou, J.; Wang, J.; et al. Oxygen Reduction Electrocatalyst Based on Strongly Coupled Cobalt Oxide Nanocrystals and Carbon Nanotubes. J. Am. Chem. Soc. 2012, 134, 15849–15857. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Kim, H.J.; Kim, S. Embedded cobalt oxide nano particles on carbon could potentially improve oxygen reduction activity of cobalt phthalocyanine and its application in microbial fuel cells. RSC Adv. 2014, 4, 44065–44072. [Google Scholar] [CrossRef]
- Kostuch, A.; Gryboś, J.; Wierzbicki, S.; Sojka, Z.; Kruczała, K. Selectivity of Mixed Iron-Cobalt Spinels Deposited on a N,S-Doped Mesoporous Carbon Support in the Oxygen Reduction Reaction in Alkaline Media. Materials 2021, 14, 820. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, P.; Dai, S. Recent Advances of Lanthanum-Based Perovskite Oxides for Catalysis. ACS Catal. 2015, 5, 6370–6385. [Google Scholar] [CrossRef]
- Xu, N.; Qiao, J.; Zhang, X.; Ma, C.; Jian, S.; Liu, Y.; Pei, P. Morphology controlled La2O3/Co3O4/MnO2–CNTs hybrid nanocomposites with durable bi-functional air electrode in high-performance zinc–air energy storage. Appl. Energy 2016, 175, 536–544. [Google Scholar] [CrossRef]
- Wang, N.; Liu, J.; Gu, W.; Song, Y.; Wang, F. Toward Synergy of Carbon and La2O3 in Their Hybrid as Efficient Catalyst for Oxygen Reduction Reaction. RSC Adv. 2016, 6, 77786–77795. [Google Scholar] [CrossRef]
- Liu, K.; Lei, Y.; Wang, G. Correlation between oxygen adsorption energy and electronic structure of transition metal macrocyclic complexes. J. Chem. Phys. 2013, 139, 204306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, Q.; Zhang, Y.; Jiang, X.; Yang, Z.; Xue, Y.; Yan, Y.-M.; Sun, K. La2O3 Doped Carbonaceous Microspheres: A Novel Bifunctional Electrocatalyst for Oxygen Reduction and Evolution Reactions with Ultrahigh Mass Activity. J. Phys. Chem. C 2014, 118, 20229–20237. [Google Scholar] [CrossRef]
- Sugawara, Y.; Kobayashi, H.; Honma, I.; Yamaguchi, T. Effect of Metal Coordination Fashion on Oxygen Electrocatalysis of Cobalt–Manganese Oxides. ACS Omega 2020, 5, 29388–29397. [Google Scholar] [CrossRef]
- Li, M.; Xiong, Y.; Liu, X.; Bo, X.; Zhang, Y.; Han, C.; Guo, L. Facile synthesis of electrospun MFe2O4 (M = Co, Ni, Cu, Mn) spinel nanofibers with excellent electrocatalytic properties for oxygen evolution and hydrogen peroxide reduction. Nanoscale 2015, 7, 8920–8930. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Liu, X.; Zong, Y.; Hor, T.S.A.; Yu, A.; Liu, Z. Co3O4 nanoparticle-modified MnO2 nanotube bifunctional oxygen cathode catalysts for rechargeable zinc–air batteries. Nanoscale 2013, 5, 4657–4661. [Google Scholar] [CrossRef]
- Yang, X.; Peng, W.; Fu, K.; Mao, L.; Jin, J.; Yang, S.; Li, G. Nanocomposites of honeycomb double-layered MnO2 nanosheets /cobalt doped hollow carbon nanofibers for application in supercapacitor and primary zinc-air battery. Electrochim. Acta 2020, 340, 135989. [Google Scholar] [CrossRef]
- Li, X.; Nengneng, X.; Li, H.; Wang, M.H.; Zhang, L.; Qiao, J. 3D hollow sphere Co3O4/MnO2-CNTs: Its high-performance bi-functional cathode catalysis and application in rechargeable zinc-air battery. Green Energy Environ. 2017, 2, 316–328. [Google Scholar] [CrossRef]
- Che, H.; Lv, Y.; Liu, A.; Mu, J.; Zhang, X.; Bai, Y. Facile synthesis of three dimensional flower-like Co3O4@MnO2 core-shell microspheres as high-performance electrode materials for supercapacitors. Ceram. Int. 2017, 43, 6054–6062. [Google Scholar] [CrossRef]
- Eraković, S.; Pavlović, M.M.; Stopić, S.; Stevanović, J.; Mitrić, M.; Friedrich, B.; Panić, V. Interactive promotion of supercapacitance of rare earth/CoO3-based spray pyrolytic perovskite microspheres hosting the hydrothermal ruthenium oxide. Electrochim. Acta 2019, 321, 134721. [Google Scholar] [CrossRef]
- Pavlović, M.M.; Pantović Pavlović, M.R.; Eraković Pantović, S.G.; Stevanović, J.S.; Stopić, S.R.; Friedrich, B.; Panić, V. V The Roles of Constituting Oxides in Rare-Earth Cobaltite-Based Perovskites on their Pseudocapacitive Behavior. J. Electroanal. Chem. 2021, 897, 115556. [Google Scholar] [CrossRef]
- Zhang, T.; Ge, X.; Zhang, Z.; Tham, N.N.; Liu, Z.; Fisher, A.; Lee, J.Y. Improving the Electrochemical Oxygen Reduction Activity of Manganese Oxide Nanosheets with Sulfurization-Induced Nanopores. ChemCatChem 2018, 10, 422–429. [Google Scholar] [CrossRef]
- Chen, B.; Miao, H.; Hu, R.; Yin, M.; Wu, X.; Sun, S.; Wang, Q.; Li, S.; Yuan, J. Efficiently optimizing the oxygen catalytic properties of the birnessite type manganese dioxide for zinc-air batteries. J. Alloys Compd. 2021, 852, 157012. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, D.; Lou, X.W. Shape-Controlled Synthesis of MnO2 Nanostructures with Enhanced Electrocatalytic Activity for Oxygen Reduction. J. Phys. Chem. C 2010, 114, 1694–1700. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.; Ang, H.-M.; Tadé, M.O.; Wang, S. Manganese oxides at different oxidation states for heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions. Appl. Catal. B Environ. 2013, 142–143, 729–735. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yang, Y.; Relefors, A.; Kong, X.; Siso, G.M.; Wickman, B.; Kiros, Y.; Soroka, I.L. Tuning morphology, composition and oxygen reduction reaction (ORR) catalytic performance of manganese oxide particles fabricated by γ-radiation induced synthesis. J. Colloid Interface Sci. 2021, 583, 71–79. [Google Scholar] [CrossRef] [PubMed]
- BOSE, V.; BIJU, V. Mixed valence nanostructured Mn3O4 for supercapacitor applications. Bull. Mater. Sci. 2015, 38, 865–873. [Google Scholar] [CrossRef] [Green Version]
- Sankar, V.; Kalpana, D.; Kalai Selvan, R. Electrochemical properties of microwave-assisted reflux-synthesized Mn3O4 nanoparticles in different electrolytes for supercapacitor applications. J. Appl. Electrochem. 2012, 42, 463–470. [Google Scholar] [CrossRef]
- Fink, M.; Eckhardt, J.; Khadke, P.; Gerdes, T.; Roth, C. Bifunctional α—MnO 2 and Co 3 O 4 Catalyst for Oxygen Electrocatalysis in Alkaline Solution. ChemElectroChem 2020, 7, 4822–4836. [Google Scholar] [CrossRef]
- Xia, H.; Zhu, D.; Luo, Z.; Yu, Y.; Shi, X.; Yuan, G.; Xie, J. Hierarchically Structured Co3O4@Pt@MnO2 Nanowire Arrays for High-Performance Supercapacitors. Sci. Rep. 2013, 3, 2978. [Google Scholar] [CrossRef]
- Paulraj, A.R.; Kiros, Y. La0.1Ca0.9MnO3/Co3O4 for oxygen reduction and evolution reactions (ORER) in alkaline electrolyte. J. Solid State Electrochem. 2018, 22, 1697–1710. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.; Chen, B.; Jiang, Z.; Niu, X.; Cheng, S.; Zhen, Z.; Jiang, Y.; Rong, H.; Jiang, Z.-J. High catalytic activity of Co3O4 nanoparticles encapsulated in a graphene supported carbon matrix for oxygen reduction reaction. RSC Adv. 2016, 6, 50349–50357. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, L.; Mai, L.; Han, C.; An, Q.; Xu, X.; Liu, X.; Zhang, Q. Hierarchical mesoporous perovskite La0.5Sr0.5CoO2.91 nanowires with ultrahigh capacity for Li-air batteries. Proc. Natl. Acad. Sci. USA 2012, 109, 19569–19574. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.-P.; Sun, H.-H.; Manthiram, A. Design of a sectionalized MnO2-Co3O4 electrode via selective electrodeposition of metal ions in hydrogel for enhanced electrocatalytic activity in metal-air batteries. Nano Energy 2016, 30, 130–137. [Google Scholar] [CrossRef] [Green Version]

| Element | Sample | ||||
|---|---|---|---|---|---|
| MnO2/Co3O4-500 | MnO2/Co3O4-600 | Mn3O4/Co3O4/LaMnO3-800 | Co3O4 (+La2O3) | MnO2 | |
| O | 68.71 | 62.92 | 61.78 | 69.77 | 60.29 |
| La | 2.04 | 3.78 | 3.11 | 2.26 | / |
| Co | 9.88 | 11.24 | 12.98 | 27.96 | / |
| Mn | 19.37 | 22.06 | 22.13 | / | 32.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varničić, M.; Pavlović, M.M.; Eraković Pantović, S.; Mihailović, M.; Pantović Pavlović, M.R.; Stopić, S.; Friedrich, B. Spray-Pyrolytic Tunable Structures of Mn Oxides-Based Composites for Electrocatalytic Activity Improvement in Oxygen Reduction. Metals 2022, 12, 22. https://doi.org/10.3390/met12010022
Varničić M, Pavlović MM, Eraković Pantović S, Mihailović M, Pantović Pavlović MR, Stopić S, Friedrich B. Spray-Pyrolytic Tunable Structures of Mn Oxides-Based Composites for Electrocatalytic Activity Improvement in Oxygen Reduction. Metals. 2022; 12(1):22. https://doi.org/10.3390/met12010022
Chicago/Turabian StyleVarničić, Miroslava, Miroslav M. Pavlović, Sanja Eraković Pantović, Marija Mihailović, Marijana R. Pantović Pavlović, Srećko Stopić, and Bernd Friedrich. 2022. "Spray-Pyrolytic Tunable Structures of Mn Oxides-Based Composites for Electrocatalytic Activity Improvement in Oxygen Reduction" Metals 12, no. 1: 22. https://doi.org/10.3390/met12010022
APA StyleVarničić, M., Pavlović, M. M., Eraković Pantović, S., Mihailović, M., Pantović Pavlović, M. R., Stopić, S., & Friedrich, B. (2022). Spray-Pyrolytic Tunable Structures of Mn Oxides-Based Composites for Electrocatalytic Activity Improvement in Oxygen Reduction. Metals, 12(1), 22. https://doi.org/10.3390/met12010022








