A Review on Thermophysical Property Assessment of Metal Oxide-Based Nanofluids: Industrial Perspectives
Abstract
:1. Introduction
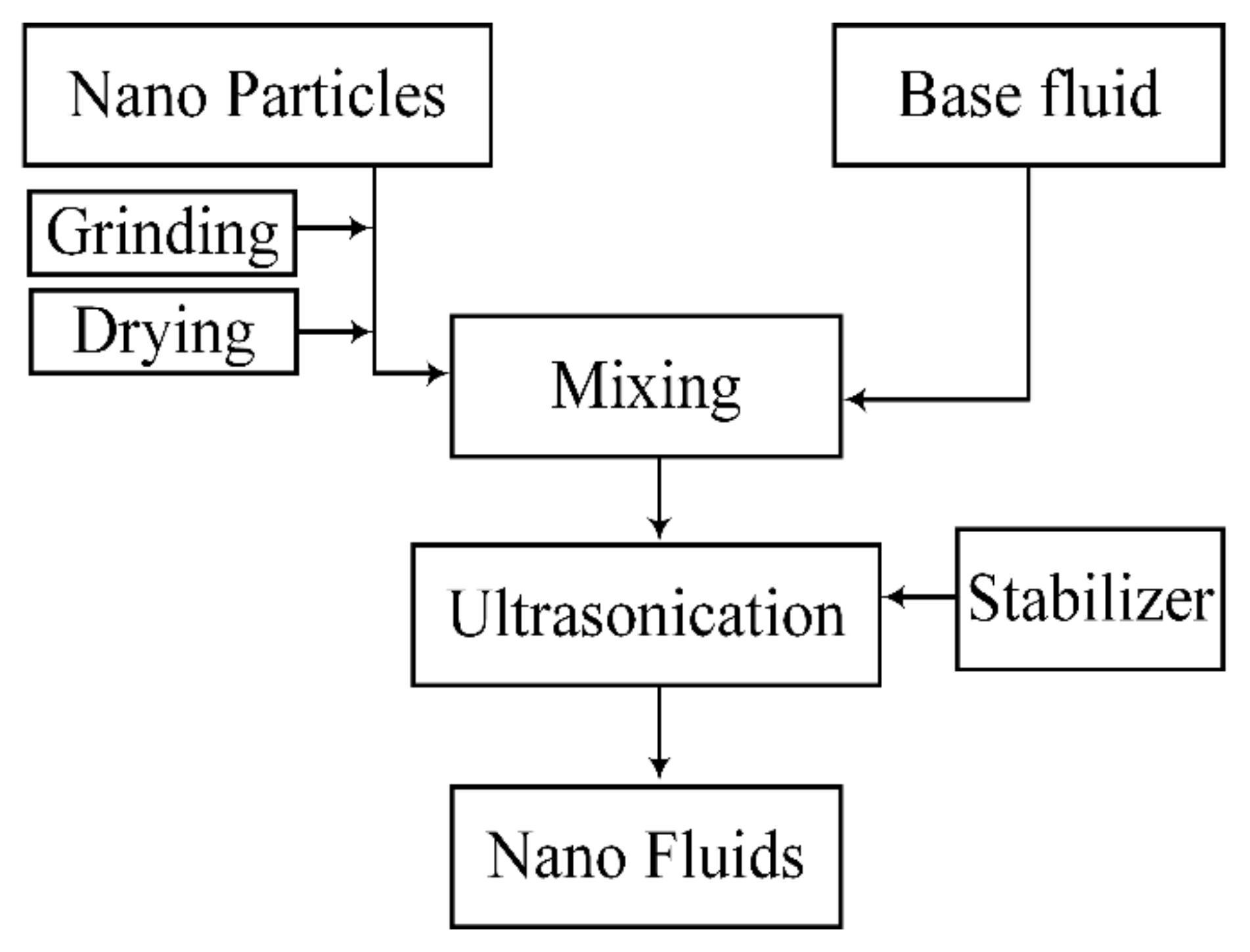
2. Nanofluid Formulation
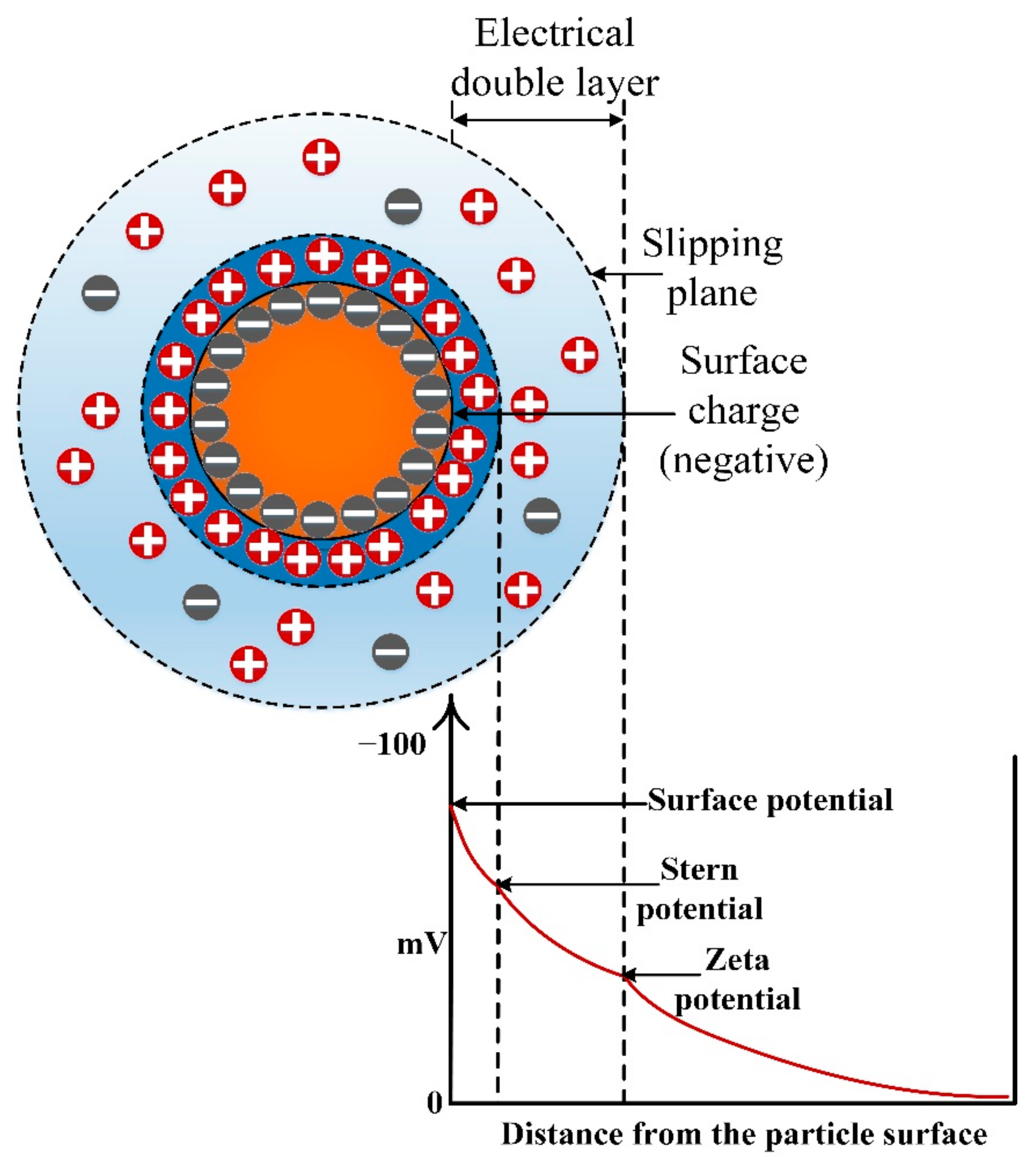
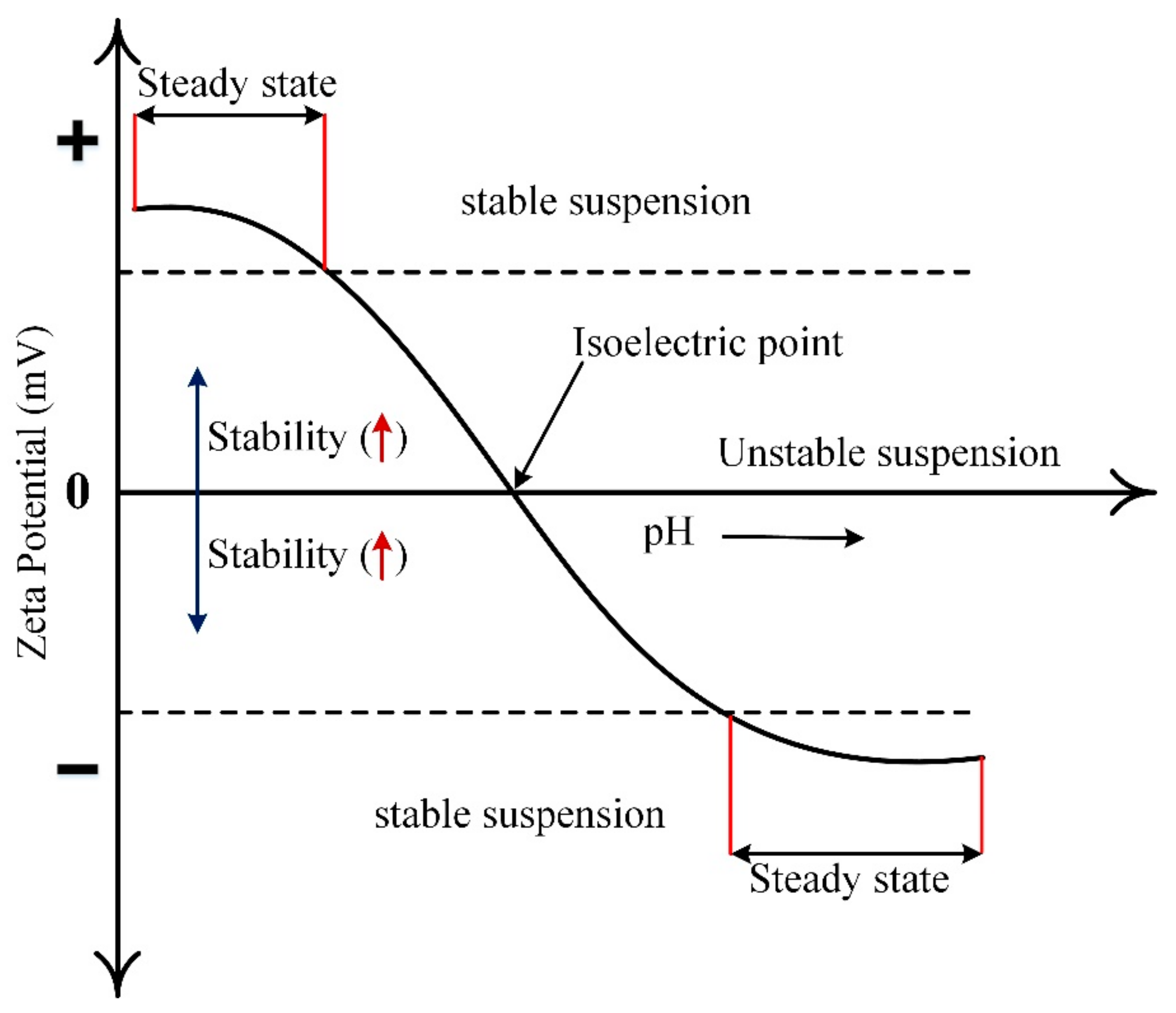
2.1. Stability Assessment
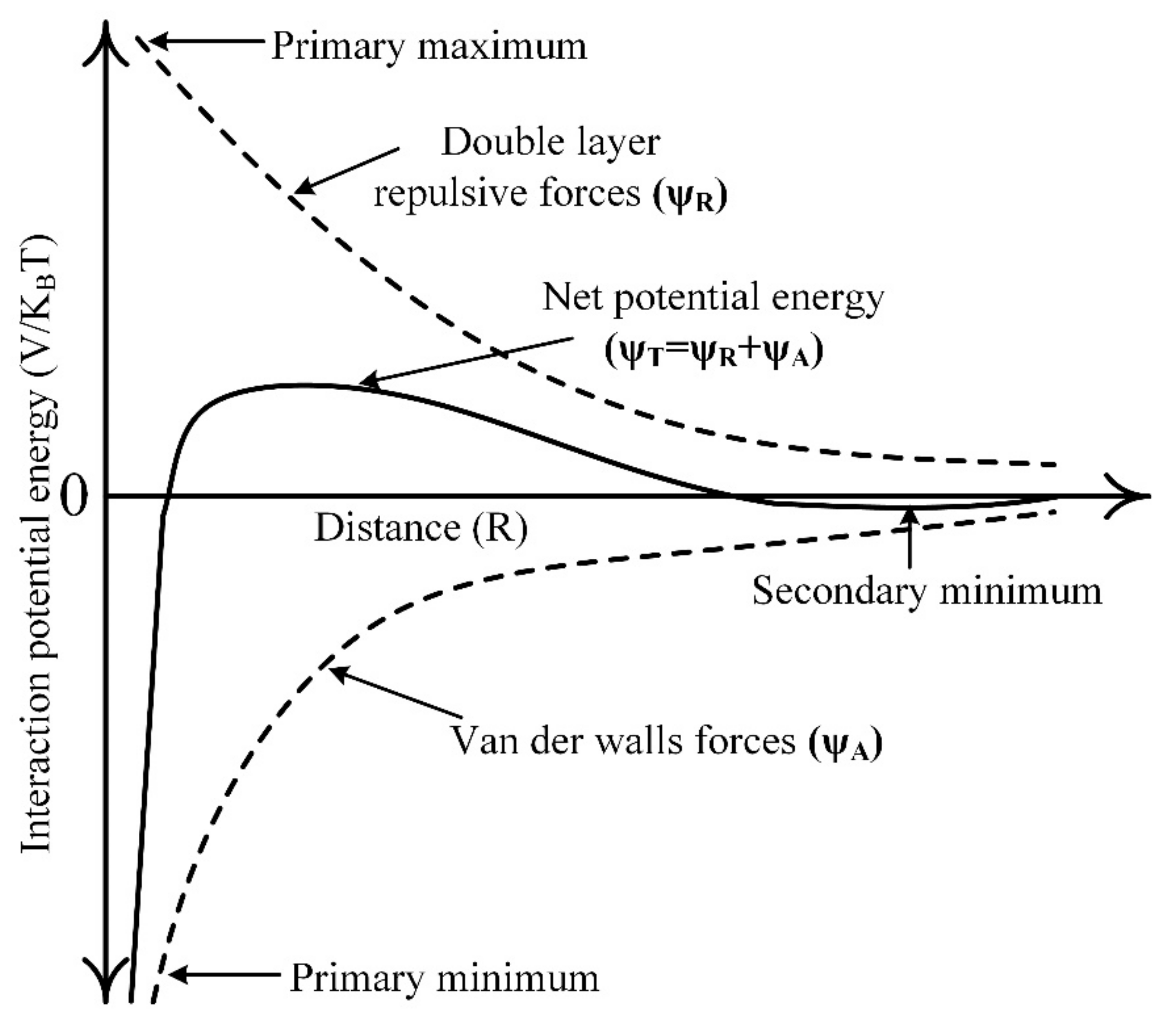
2.2. DLVO Theory and Its Significance
3. Effect of Metal-Oxide Based Nanofluids on Thermophysical Properties
3.1. Specific Heat
3.2. Thermal Conductivity
3.3. Viscosity
3.4. Nucleate Boiling and Wettability
3.5. Effect of Nanoparticle Geometry
3.6. Economic Analysis
3.7. Heat Transfer Merit
4. Future Scope and Challenges for Nanofluid Applications in Various Industrial Sectors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| d | diameter |
| K | thermal conductivity |
| T | temperature |
| Cp | specific heat |
| Kb | Boltzmann constant |
| Re | Reynolds number |
| Pr | Prandtl number |
| m | mass |
| f | friction factor |
| Greek symbols | |
| ρ | density |
| µ | viscosity |
| Φ | solid volume fraction |
| α | thermal diffusivity |
| η | efficiency |
| Subscripts/superscripts | |
| bf | base fluid |
| nf | nanofluid |
| ns | nano structure |
| fr | freezing point |
| p | particle |
References
- Lund, H.; Werner, S.; Wiltshire, R.; Svendsen, S.; Thorsen, J.E.; Hvelplund, F.; Mathiesen, B.V. 4th Generation District Heating (4GDH). Integrating smart thermal grids into future sustainable energy systems. Energy 2014, 68, 1–11. [Google Scholar] [CrossRef]
- Godson, L.; Raja, B.; Lal, D.M.; Wongwises, S. Enhancement of heat transfer using nanofluids—An overview. Renew. Sustain. Energy Rev. 2010, 14, 629–641. [Google Scholar] [CrossRef]
- Bég, O.A.; Espinoza, D.E.S.; Kadir, A.; Shamshuddin, M.; Sohail, A. Experimental study of improved rheology and lubricity of drilling fluids enhanced with nano-particles. Appl. Nanosci. 2018, 8, 1069–1090. [Google Scholar] [CrossRef]
- Sriharan, G.; Harikrishnan, S.; Ali, H.M. Experimental investigation on the effectiveness of MHTHS using different metal oxide-based nanofluids. J. Therm. Anal. 2021, 143, 1251–1260. [Google Scholar] [CrossRef]
- Jamshed, W.; Şirin, C.; Selimefendigil, F.; Shamshuddin, M.D.; Altowairqi, Y.; Eid, M.R. Thermal Characterization of Coolant Maxwell Type Nanofluid Flowing in Parabolic Trough Solar Collector (PTSC) Used Inside Solar Powered Ship Application. Coatings 2021, 11, 1552. [Google Scholar] [CrossRef]
- Chandrasekar, M.; Suresh, S.; Bose, A.C. Experimental studies on heat transfer and friction factor characteristics of Al2O3/water nanofluid in a circular pipe under laminar flow with wire coil inserts. Exp. Therm. Fluid Sci. 2010, 34, 122–130. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Enhanced thermal conductivity of TiO2—Water based nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373. [Google Scholar] [CrossRef]
- Koshy, C.P.; Rajendrakumar, P.K.; Thottackkad, M.V. Evaluation of the tribological and thermo-physical properties of coconut oil added with MoS 2 nanoparticles at elevated temperatures. Wear 2015, 330, 288–308. [Google Scholar] [CrossRef]
- Sujith, S.; Solanki, A.K.; Mulik, R.S. Experimental evaluation on rheological behavior of Al2O3-pure coconut oil nanofluids. J. Mol. Liq. 2019, 286, 110905. [Google Scholar] [CrossRef]
- Esfe, M.H.; Arani, A.A.A.; Esfandeh, S. Experimental study on rheological behavior of monograde heavy-duty engine oil containing CNTs and oxide nanoparticles with focus on viscosity analysis. J. Mol. Liq. 2018, 272, 319–329. [Google Scholar] [CrossRef]
- Nguyen, C.; Desgranges, F.; Roy, G.; Galanis, N.; Maré, T.; Boucher, S.; Mintsa, H.A. Temperature and particle-size dependent viscosity data for water-based nanofluids—Hysteresis phenomenon. Int. J. Heat Fluid Flow 2007, 28, 1492–1506. [Google Scholar] [CrossRef]
- Nadooshan, A.A.; Esfe, M.H.; Afrand, M. Evaluation of rheological behavior of 10W40 lubricant containing hybrid nano-material by measuring dynamic viscosity. Phys. E Low Dimens. Syst. Nanostruct. 2017, 92, 47–54. [Google Scholar] [CrossRef]
- Maxwell, J.C. A Treatise on Electricity and Magnetism; Cambridge University Press: Cambridge, UK, 1873. [Google Scholar] [CrossRef]
- Choi, J.E.S. Enhancing Thermal Conductivity of Fluids with Nanoparticles. 1995. Available online: https://www.osti.gov/biblio/196525-enhancing-thermal-conductivity-fluids-nanoparticles (accessed on 10 December 2021).
- Kim, S.H.; Choi, S.R.; Kim, D. Thermal conductivity of metal-oxide nanofluids: Particle size dependence and effect of laser irradiation. J. Heat Transf. 2007, 129, 298–307. [Google Scholar] [CrossRef]
- Azmi, W.; Sharma, K.V.; Sarma, P.; Mamat, R.; Anuar, S.; Rao, V.D. Experimental determination of turbulent forced convection heat transfer and friction factor with SiO2 nanofluid. Exp. Therm. Fluid Sci. 2013, 51, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Wei, B.; Zou, C.; Li, X. Experimental investigation on stability and thermal conductivity of diathermic oil based TiO2 nanofluids. Int. J. Heat Mass Transf. 2017, 104, 537–543. [Google Scholar] [CrossRef]
- Agarwal, R.; Verma, K.; Agrawal, N.K.; Duchaniya, R.K.; Singh, R. Synthesis, characterization, thermal conductivity and sensitivity of CuO nanofluids. Appl. Therm. Eng. 2016, 102, 1024–1036. [Google Scholar] [CrossRef]
- Wang, X.-J.; Zhu, D.-S.; Yang, S. Investigation of pH and SDBS on enhancement of thermal conductivity in nanofluids. Chem. Phys. Lett. 2009, 470, 107–111. [Google Scholar] [CrossRef]
- Colangelo, G.; Favale, E.; Miglietta, P.; Milanese, M.; De Risi, A. Thermal conductivity, viscosity and stability of Al2O3-diathermic oil nanofluids for solar energy systems. Energy 2016, 95, 124–136. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Liang, X.; Xu, J.; Lee, C.; Liang, Q.; Tao, P.; Deng, T. Dispersion stability of thermal nanofluids. Prog. Nat. Sci. Mater. Int. 2017, 27, 531–542. [Google Scholar] [CrossRef]
- Zheng, T.; Bott, S.; Huo, Q. Techniques for accurate sizing of gold nanoparticles using dynamic light scattering with particular application to chemical and biological sensing based on aggregate formation. ACS Appl. Mater. Interfaces 2016, 8, 21585–21594. [Google Scholar] [CrossRef] [PubMed]
- Mehrali, M.; Sadeghinezhad, E.; Latibari, S.T.; Kazi, S.N.; Mehrali, M.; Zubir, M.N.B.M.; Metselaar, H.S.C. Investigation of thermal conductivity and rheological properties of nanofluids containing graphene nanoplatelets. Nanoscale Res. Lett. 2014, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Malescio, G. Intermolecular potentials—Past, present, future. Nat. Mater. 2003, 2, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental methods in chemical engineering: Zeta potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Ohshima, H. Henry’s function for electrophoresis of a cylindrical colloidal particle. J. Colloid Interface Sci. 1996, 180, 299–301. [Google Scholar] [CrossRef]
- Qi, C.; Luo, T.; Liu, M.; Fan, F.; Yan, Y. Experimental study on the flow and heat transfer characteristics of nanofluids in double-tube heat exchangers based on thermal efficiency assessment. Energy Convers. Manag. 2019, 197, 111877. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q. Investigation on convective heat transfer and flow features of nanofluids. J. Heat Transf. 2003, 125, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K. Specific heat measurement of three nanofluids and development of new correlations. J. Heat Transf. 2009, 131, 071601. [Google Scholar] [CrossRef]
- Zhou, L.-P.; Wang, B.-X.; Peng, X.-F.; Du, X.-Z.; Yang, Y.-P. On the specific heat capacity of CuO nanofluid. Adv. Mech. Eng. 2010, 2010, 172085. [Google Scholar] [CrossRef] [Green Version]
- Sekhar, Y.R.; Sharma, K.V. Study of viscosity and specific heat capacity characteristics of water-based Al2O3 nanofluids at low particle concentrations. J. Exp. Nanosci. 2013, 10, 86–102. [Google Scholar] [CrossRef]
- Shin, D.; Banerjee, D. Specific heat of nanofluids synthesized by dispersing alumina nanoparticles in alkali salt eutectic. Int. J. Heat Mass Transf. 2014, 74, 210–214. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Gracia-Fernández, C.; Legido, J.; Lugo, L. Specific heat of metal oxide nanofluids at high concentrations for heat transfer. Int. J. Heat Mass Transf. 2015, 88, 872–879. [Google Scholar] [CrossRef]
- Barbés, B.; Páramo, R.; Blanco, E.; Casanova, C. Thermal conductivity and specific heat capacity measurements of CuO nanofluids. J. Therm. Anal. 2014, 115, 1883–1891. [Google Scholar] [CrossRef]
- Murshed, S.S. Determination of effective specific heat of nanofluids. J. Exp. Nanosci. 2011, 6, 539–546. [Google Scholar] [CrossRef]
- Shin, D.; Tiznobaik, H.; Banerjee, D. Specific heat mechanism of molten salt nanofluids. Appl. Phys. Lett. 2014, 104, 121914. [Google Scholar] [CrossRef]
- Tiznobaik, H.; Banerjee, D.; Shin, D. Effect of formation of “long range” secondary dendritic nanostructures in molten salt nanofluids on the values of specific heat capacity. Int. J. Heat Mass Transf. 2015, 91, 342–346. [Google Scholar] [CrossRef]
- Hassan, M.A.; Banerjee, D. A soft computing approach for estimating the specific heat capacity of molten salt-based nanofluids. J. Mol. Liq. 2019, 281, 365–375. [Google Scholar] [CrossRef]
- Ercole, D.; Manca, O.; Vafai, K. An investigation of thermal characteristics of eutectic molten salt-based nanofluids. Int. Commun. Heat Mass Transf. 2017, 87, 98–104. [Google Scholar] [CrossRef]
- Sang, L.; Liu, T. The enhanced specific heat capacity of ternary carbonates nanofluids with different nanoparticles. Sol. Energy Mater. Sol. Cells 2017, 169, 297–303. [Google Scholar] [CrossRef]
- Qiao, G.; Lasfargues, M.; Alexiadis, A.; Ding, Y. Simulation and experimental study of the specific heat capacity of molten salt based nanofluids. Appl. Therm. Eng. 2017, 111, 1517–1522. [Google Scholar] [CrossRef]
- Hamilton, R.L.; Crosser, O.K. Thermal Conductivity of Heterogeneous Two-Component Systems. Ind. Eng. Chem. Fundam. 1962, 1, 187–191. [Google Scholar] [CrossRef]
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer konstanten von heterogenen substanzen. I. dielektrizitätskonstanten und leitfähigkeiten der mischkörper aus isotropen Substanzen. Ann. Phys. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- Akita, O.; Yamada, E. Effective thermal conductivity of dispersed materials. Heat Mass Transf. 1980, 13, 27–37. [Google Scholar]
- Sujith, S.V.; Solanki, A.K.; Mulik, R.S. Experimental evaluation in thermal conductivity enhancement and heat transfer optimization of eco-friendly Al2O3–pure coconut oil based nano fluids. J. Therm. Sci. Eng. Appl. 2021, 13, 1–13. [Google Scholar] [CrossRef]
- Yu, W.; Choi, S.U.S. The role of interfacial layers in the enhanced thermal conductivity of nanofluids: A renovated Maxwell model. J. Nanopart. Res. 2003, 5, 167–171. [Google Scholar] [CrossRef]
- Yoo, D.-H.; Hong, K.; Yang, H.-S. Study of thermal conductivity of nanofluids for the application of heat transfer fluids. Thermochim. Acta 2007, 455, 66–69. [Google Scholar] [CrossRef]
- Esfe, M.H.; Amiri, M.K.; Alirezaie, A. Thermal conductivity of a hybrid nanofluid. J. Therm. Anal. Calorim. 2018, 134, 1113–1122. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Routbort, J.L.; Singh, D. Particle shape effects on thermophysical properties of alumina nanofluids. J. Appl. Phys. 2009, 106, 014304. [Google Scholar] [CrossRef]
- Koo, J.; Kleinstreuer, C. A new thermal conductivity model for nanofluids. J. Nanopart. Res. 2004, 6, 577–588. [Google Scholar] [CrossRef]
- Khdher, A.M.; Sidik, N.A.C.; Hamzah, W.A.W.; Mamat, R. An experimental determination of thermal conductivity and electrical conductivity of bio glycol based Al2O3 nanofluids and development of new correlation. Int. Commun. Heat Mass Transf. 2016, 73, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Lin, H. Effective conductivity of composites containing aligned spheroidal inclusions of finite conductivity. J. Appl. Phys. 1996, 79, 6761–6769. [Google Scholar] [CrossRef]
- Krieger, I.M.; Dougherty, T.J. A mechanism for non-newtonian flow in suspensions of rigid spheres. Trans. Soc. Rheol. 1959, 3, 137–152. [Google Scholar] [CrossRef]
- Chhabra, R.P. Non-newtonian fluids: An introduction. In Rheolology Complex Fluids; Springer: New York, NY, USA, 2010; pp. 3–34. [Google Scholar] [CrossRef]
- Esfe, M.H.; Rostamian, H. Non-Newtonian power-law behavior of TiO2 /SAE 50 nano-lubricant: An experimental report and new correlation. J. Mol. Liq. 2017, 232, 219–225. [Google Scholar] [CrossRef]
- Sharma, A.K.; Tiwari, A.K.; Dixit, A.R. Rheological behaviour of nanofluids: A review. Renew. Sustain. Energy Rev. 2015, 53, 779–791. [Google Scholar] [CrossRef]
- Hu, X.; Yin, D.; Chen, X.; Xiang, G. Experimental investigation and mechanism analysis: Effect of nanoparticle size on viscosity of nanofluids. J. Mol. Liq. 2020, 314, 113604. [Google Scholar] [CrossRef]
- Mostafizur, R.; Aziz, A.A.; Saidur, R.; Bhuiyan, M.; Mahbubul, I. Effect of temperature and volume fraction on rheology of methanol based nanofluids. Int. J. Heat Mass Transf. 2014, 77, 765–769. [Google Scholar] [CrossRef]
- Chiam, H.; Azmi, W.; Usri, N.; Mamat, R.; Adam, N. Thermal conductivity and viscosity of Al2O3 nanofluids for different based ratio of water and ethylene glycol mixture. Exp. Therm. Fluid Sci. 2017, 81, 420–429. [Google Scholar] [CrossRef] [Green Version]
- Fedele, L.; Colla, L.; Bobbo, S. Viscosity and thermal conductivity measurements of water-based nanofluids containing titanium oxide nanoparticles. Int. J. Refrig. 2012, 35, 1359–1366. [Google Scholar] [CrossRef]
- Moldoveanu, G.M.; Ibanescu, C.; Danu, M.; Minea, A.A. Viscosity estimation of Al2O3, SiO2 nanofluids and their hybrid: An experimental study. J. Mol. Liq. 2018, 253, 188–196. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Narahari, M.; Theng, J.T.Y.; Pendyala, R. Experimental evaluation of dispersion behavior, rheology and thermal analysis of functionalized zinc oxide-paraffin oil nanofluids. J. Mol. Liq. 2019, 294, 111613. [Google Scholar] [CrossRef]
- Yan, S.-R.; Kalbasi, R.; Nguyen, Q.; Karimipour, A. Rheological behavior of hybrid MWCNTs-TiO2/EG nanofluid: A comprehensive modeling and experimental study. J. Mol. Liq. 2020, 308, 113058. [Google Scholar] [CrossRef]
- Kole, M.; Dey, T. Effect of aggregation on the viscosity of copper oxide—gear oil nanofluids. Int. J. Therm. Sci. 2011, 50, 1741–1747. [Google Scholar] [CrossRef]
- Çolak, A.B. A novel comparative analysis between the experimental and numeric methods on viscosity of zirconium oxide nanofluid: Developing optimal artificial neural network and new mathematical model. Powder Technol. 2021, 381, 338–351. [Google Scholar] [CrossRef]
- Sonawane, S.S.; Juwar, V. Optimization of conditions for an enhancement of thermal conductivity and minimization of viscosity of ethylene glycol based Fe3O4 nanofluid. Appl. Therm. Eng. 2016, 109, 121–129. [Google Scholar] [CrossRef]
- Einstein, A. Eine neue bestimmung der moleküldimensionen. Ann. Phys. 1906, 324, 289–306. [Google Scholar] [CrossRef] [Green Version]
- Brinkman, H.C. The viscosity of concentrated suspensions and solutions. J. Chem. Phys. 1952, 20, 571. [Google Scholar] [CrossRef]
- Lundgren, T.S. Slow flow through stationary random beds and suspensions of spheres. J. Fluid Mech. 1972, 51, 273–299. [Google Scholar] [CrossRef]
- Batchelor, G.K. The effect of Brownian motion on the bulk stress in a suspension of spherical particles. J. Fluid Mech. 1977, 83, 97–117. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Choi, S.U.S. Thermal conductivity of nanoparticle—Fluid mixture. J. Thermophys. Heat Transf. 1999, 13, 474–480. [Google Scholar] [CrossRef]
- Corcione, M. Empirical correlating equations for predicting the effective thermal conductivity and dynamic viscosity of nanofluids. Energy Convers. Manag. 2011, 52, 789–793. [Google Scholar] [CrossRef]
- Namburu, P.K.; Kulkarni, D.P.; Misra, D.; Das, D.K. Viscosity of copper oxide nanoparticles dispersed in ethylene glycol and water mixture. Exp. Therm. Fluid Sci. 2007, 32, 397–402. [Google Scholar] [CrossRef]
- Masoumi, N.; Sohrabi, N.; Behzadmehr, A. A new model for calculating the effective viscosity of nanofluids. J. Phys. D Appl. Phys. 2009, 42, 055501. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.U.; Muthukumar, M. Three-body hydrodynamic effects on viscosity of suspensions of spheres. J. Chem. Phys. 1991, 94, 5180–5189. [Google Scholar] [CrossRef]
- Graham, A.L. On the viscosity of suspensions of solid spheres. Appl. Sci. Res. 1981, 37, 275–286. [Google Scholar] [CrossRef]
- Kim, S.J.; Bang, I.C.; Buongiorno, J.; Hu, L.W. Effects of nanoparticle deposition on surface wettability influencing boiling heat transfer in nanofluids. Appl. Phys. Lett. 2006, 89, 153107. [Google Scholar] [CrossRef] [Green Version]
- Sarafraz, M.; Hormozi, F. Pool boiling heat transfer to dilute copper oxide aqueous nanofluids. Int. J. Therm. Sci. 2015, 90, 224–237. [Google Scholar] [CrossRef]
- Zhang, F.; Jacobi, A.M. Aluminum surface wettability changes by pool boiling of nanofluids. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 506, 438–444. [Google Scholar] [CrossRef]
- Li, S.-Y.; Ji, W.-T.; Zhao, C.-Y.; Zhang, H.; Tao, W.-Q. Effects of magnetic field on the pool boiling heat transfer of water-based α-Fe2O3 and γ-Fe2O3 nanofluids. Int. J. Heat Mass Transf. 2019, 128, 762–772. [Google Scholar] [CrossRef]
- Rohsenow, V.M. A Method of Correlating Heat Transfer Data for Surface Boiling of Liquids; MIT Div. Ind. Cooporation: Cambridge, MA, USA, 1951; p. 31. Available online: https://dspace.mit.edu/handle/1721.1/61431 (accessed on 10 December 2021).
- Pioro, I. Experimental evaluation of constants for the Rohsenow pool boiling correlation. Int. J. Heat Mass Transf. 1998, 42, 2003–2013. [Google Scholar] [CrossRef]
- Kim, S.; Bang, I.; Buongiorno, J.; Hu, L. Surface wettability change during pool boiling of nanofluids and its effect on critical heat flux. Int. J. Heat Mass Transf. 2007, 50, 4105–4116. [Google Scholar] [CrossRef]
- Ciloglu, D.; Bolukbasi, A. A comprehensive review on pool boiling of nanofluids. Appl. Therm. Eng. 2015, 84, 45–63. [Google Scholar] [CrossRef]
- Wen, D.; Wang, B. Effects of surface wettability on nucleate pool boiling heat transfer for surfactant solutions. Int. J. Heat Mass Transf. 2002, 45, 1739–1747. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, P. Effects of nanoparticles’ wettability on vapor bubble coalescence in saturated pool boiling of nanofluids: A lattice Boltzmann simulation. Int. J. Heat Mass Transf. 2020, 154, 119669. [Google Scholar] [CrossRef]
- Ji, W.-T.; Zhao, P.-F.; Zhao, C.-Y.; Ding, J.; Tao, W.-Q. Pool boiling heat transfer of water and nanofluid outside the surface with higher roughness and different wettability. Nanoscale Microscale Thermophys. Eng. 2018, 22, 296–323. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Bahiraei, M.; Monavari, A.; Naseri, M.; Moayedi, H. Irreversibility characteristics of a modified microchannel heat sink operated with nanofluid considering different shapes of nanoparticles. Int. J. Heat Mass Transf. 2020, 151, 119359. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Bhatti, M.M. Forced convection of nanofluid in presence of constant magnetic field considering shape effects of nanoparticles. Int. J. Heat Mass Transf. 2017, 111, 1039–1049. [Google Scholar] [CrossRef]
- Abbasi, M.; Heyhat, M.; Rajabpour, A. Study of the effects of particle shape and base fluid type on density of nanofluids using ternary mixture formula: A molecular dynamics simulation. J. Mol. Liq. 2020, 305, 112831. [Google Scholar] [CrossRef]
- Maheshwary, P.; Handa, C.; Nemade, K. A comprehensive study of effect of concentration, particle size and particle shape on thermal conductivity of titania/water based nanofluid. Appl. Therm. Eng. 2017, 119, 79–88. [Google Scholar] [CrossRef]
- Zahmatkesh, I.; Sheremet, M.; Yang, L.; Heris, S.Z.; Sharifpur, M.; Meyer, J.P.; Ghalambaz, M.; Wongwises, S.; Jing, D.; Mahian, O. Effect of nanoparticle shape on the performance of thermal systems utilizing nanofluids: A critical review. J. Mol. Liq. 2021, 321, 114430. [Google Scholar] [CrossRef]
- Faizal, M.; Saidur, R.; Mekhilef, S.; Alim, M. Energy, economic and environmental analysis of metal oxides nanofluid for flat-plate solar collector. Energy Convers. Manag. 2013, 76, 162–168. [Google Scholar] [CrossRef]
- Alirezaie, A.; Hajmohammad, M.H.; Alipour, A.; Salari, M. Do nanofluids affect the future of heat transfer? “A benchmark study on the efficiency of nanofluids”. Energy 2018, 157, 979–989. [Google Scholar] [CrossRef]
- Esfe, M.H.; Hajmohammad, H.; Moradi, R.; Arani, A.A.A. Multi-objective optimization of cost and thermal performance of double walled carbon nanotubes/water nanofluids by NSGA-II using response surface method. Appl. Therm. Eng. 2017, 112, 1648–1657. [Google Scholar] [CrossRef]
- Abadeh, A.; Rejeb, O.; Sardarabadi, M.; Menezo, C.; Passandideh-Fard, M.; Jemni, A. Economic and environmental analysis of using metal-oxides/water nanofluid in photovoltaic thermal systems (PVTs). Energy 2018, 159, 1234–1243. [Google Scholar] [CrossRef]
- Alirezaie, A.; Hajmohammad, M.H.; Ahangar, M.R.H.; Esfe, M.H. Price-performance evaluation of thermal conductivity enhancement of nanofluids with different particle sizes. Appl. Therm. Eng. 2018, 128, 373–380. [Google Scholar] [CrossRef]
- Rios, M.S.B.-D.L.; Rivera-Solorio, C.I.; Nigam, K. An overview of sustainability of heat exchangers and solar thermal applications with nanofluids: A review. Renew. Sustain. Energy Rev. 2021, 142, 110855. [Google Scholar] [CrossRef]
- Prasher, R.; Song, D.; Wang, J.; Phelan, P. Measurements of nanofluid viscosity and its implications for thermal applications. Appl. Phys. Lett. 2006, 89, 133108. [Google Scholar] [CrossRef]
- Mouromtseff, I.E. Water and forced-air cooling of vacuum tubes nonelectronic problems in electronic tubes. Proc. IRE 1942, 30, 190–205. [Google Scholar] [CrossRef]
- Yang, X.F.; Liu, Z.-H.; Zhao, J. Heat transfer performance of a horizontal micro-grooved heat pipe using CuO nanofluid. J. Micromech. Microeng. 2008, 18, 035038. [Google Scholar] [CrossRef]
- Khandekar, S.; Joshi, Y.; Mehta, B. Thermal performance of closed two-phase thermosyphon using nanofluids. Int. J. Therm. Sci. 2008, 47, 659–667. [Google Scholar] [CrossRef]
- Bahiraei, M.; Heshmatian, S. Electronics cooling with nanofluids: A critical review. Energy Convers. Manag. 2018, 172, 438–456. [Google Scholar] [CrossRef]
- Nazari, M.; Karami, M.; Ashouri, M. Comparing the thermal performance of water, Ethylene Glycol, Alumina and CNT nanofluids in CPU cooling: Experimental study. Exp. Therm. Fluid Sci. 2014, 57, 371–377. [Google Scholar] [CrossRef]
- Guo, W.; Li, G.; Zheng, Y.; Dong, C. Numerical study of nanofluids thermal and hydraulic characteristics considering Brownian motion effect in micro fin heat sink. J. Mol. Liq. 2018, 264, 38–47. [Google Scholar] [CrossRef]
- Kahani, M.; Heris, S.Z.; Mousavi, S.M. Comparative study between metal oxide nanopowders on thermal characteristics of nanofluid flow through helical coils. Powder Technol. 2013, 246, 82–92. [Google Scholar] [CrossRef]
- Vasu, V.; Kumar, K.M. Analysis of nanofluids as cutting fluid in grinding EN-31 steel. Nano-Micro Lett. 2011, 3, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Najiha, M.S.; Rahman, M.; Yusoff, A.R. Flank Wear Characterization in Aluminum Alloy (6061 T6) With Nanofluid Minimum Quantity Lubrication Environment Using an Uncoated Carbide Tool. J. Manuf. Sci. Eng. 2015, 137, 061004. [Google Scholar] [CrossRef] [Green Version]
- Sujith, S.V.; Mulik, R.S. Surface Integrity and Flank Wear Response Under Pure Coconut Oil-Al2O3 Nano Minimum Quantity Lubrication Turning of Al-7079/7 wt%-TiC In Situ Metal Matrix Composites. J. Tribol. 2022, 144, 1–15. [Google Scholar] [CrossRef]
- Sarkar, J.; Ghosh, P.; Adil, A. A review on hybrid nanofluids: Recent research, development and applications. Renew. Sustain. Energy Rev. 2015, 43, 164–177. [Google Scholar] [CrossRef]
- Asadi, A.; Asadi, M.; Rezaniakolaei, A.; Rosendahl, L.; Afrand, M.; Wongwises, S. Heat transfer efficiency of Al2O3-MWCNT/thermal oil hybrid nanofluid as a cooling fluid in thermal and energy management applications: An experimental and theoretical investigation. Int. J. Heat Mass Transf. 2018, 117, 474–486. [Google Scholar] [CrossRef]
- Boubaker, R.; Harmand, S.; Platel, V. Numerical analysis of the impact of nanofluids and vapor grooves design on the performance of capillary evaporators. Transp. Porous Media 2018, 122, 401–419. [Google Scholar] [CrossRef]
- Sarafraz, M.; Pourmehran, O.; Yang, B.; Arjomandi, M. Assessment of the thermal performance of a thermosyphon heat pipe using zirconia-acetone nanofluids. Renew. Energy 2019, 136, 884–895. [Google Scholar] [CrossRef]
- Mebarek-Oudina, F. Convective heat transfer of Titania nanofluids of different base fluids in cylindrical annulus with discrete heat source. Heat Transf. Asian Res. 2019, 48, 135–147. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.; Dureja, J.S.; Dogra, M.; Bhatti, M.S. Environment friendly machining of inconel 625 under nano-fluid minimum quantity lubrication (NMQL). Int. J. Precis. Eng. Manuf. 2018, 19, 1689–1697. [Google Scholar] [CrossRef]
- Behera, B.C.; Chetan; Setti, D.; Ghosh, S.; Rao, P.V. Spreadability studies of metal working fluids on tool surface and its impact on minimum amount cooling and lubrication turning. J. Mater. Process. Technol. 2017, 244, 1–16. [Google Scholar] [CrossRef]
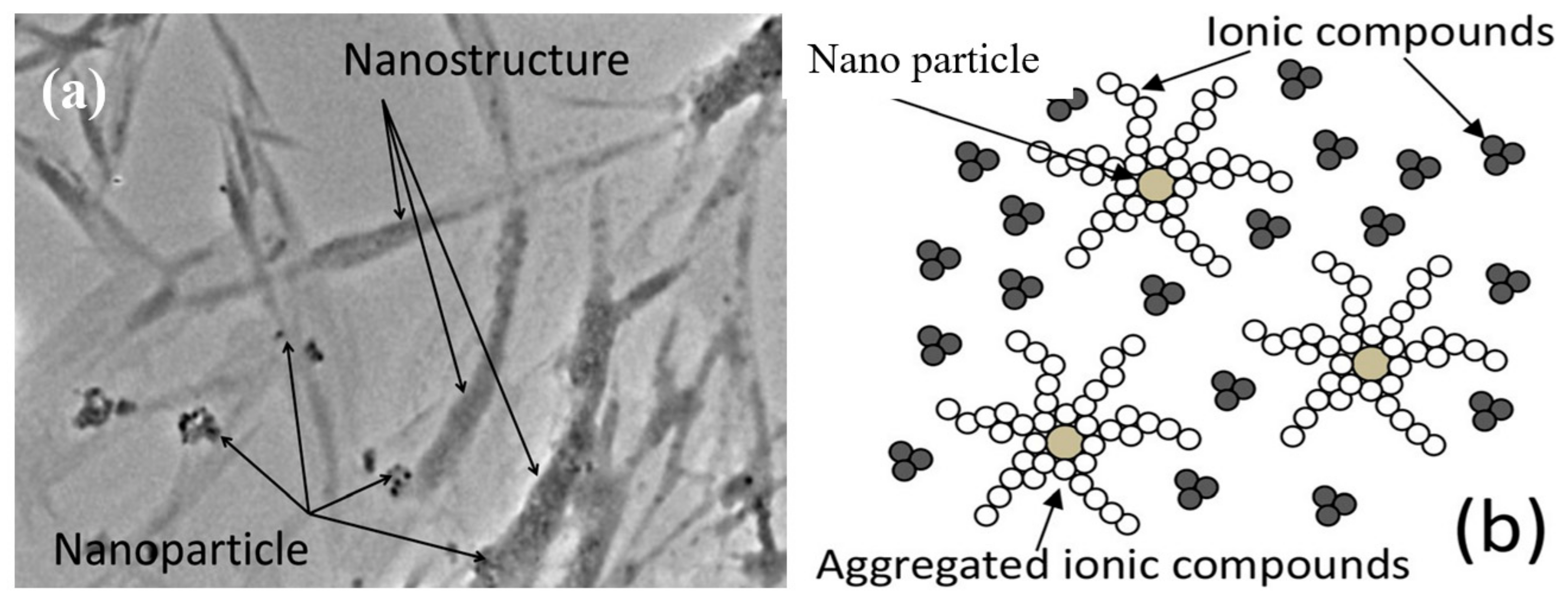
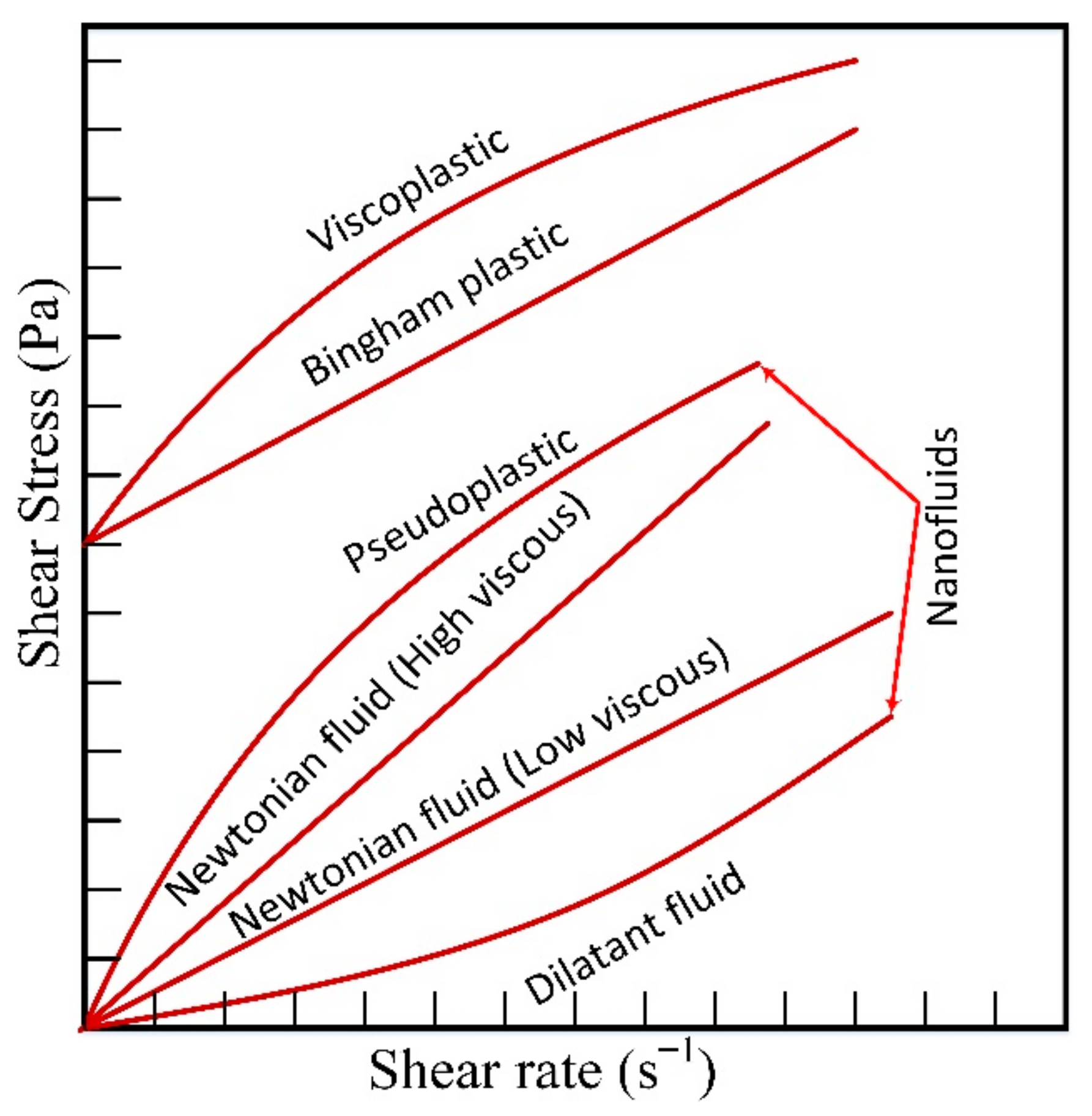
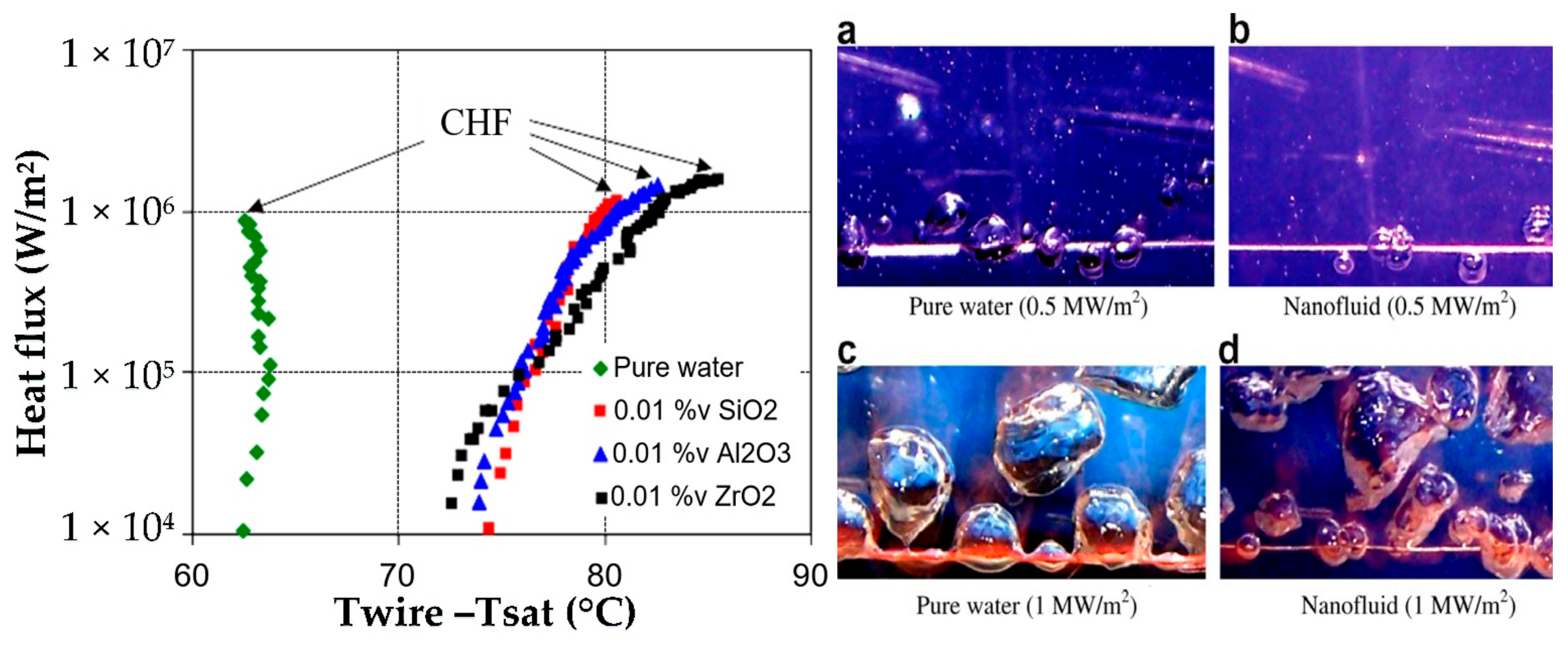
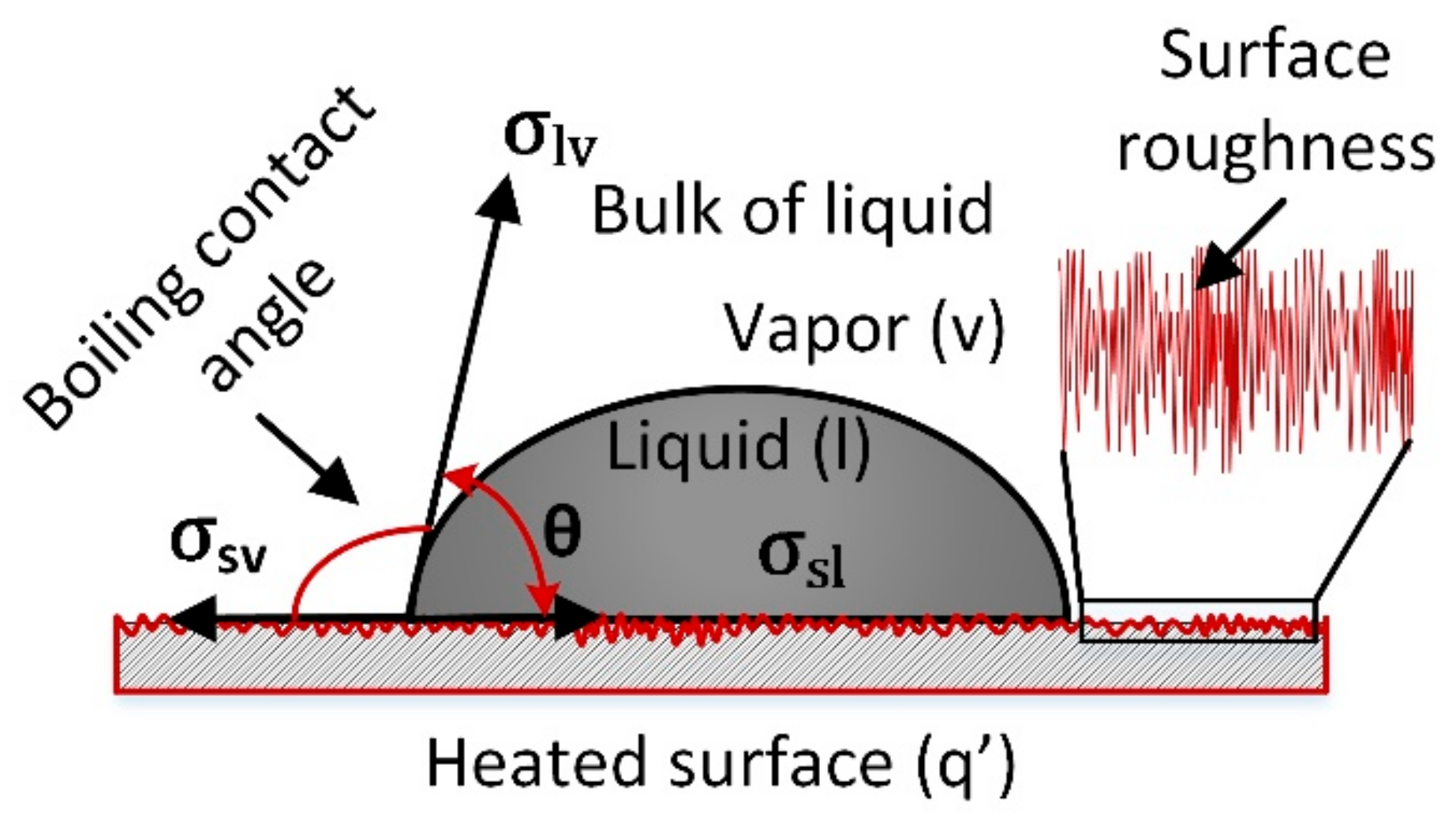
| Models/Correlations | Author |
|---|---|
| Pak and Cho [29] | |
A, B and C are correlation coefficients | Vajjha and Das [30] |
| Zhou et al. [31] | |
| Shekar and Sharma [32] | |
| Donghyun and Debjyoti [33] |
| Models/Correlations | Author |
|---|---|
| Maxwell [13] | |
| Burggeman [44] | |
| Timoofeva [50] | |
| Sujith et al. [46] | |
for cylindrical particles, for spherical particles | You and Choi [47] |
| Hamilton-crosser [43] | |
| Koo and Kleinstreuer [51] | |
| Sadik et al. [52] | |
| Liu and Lin [53] |
| Author | Nanoparticles | Base Fluid | Temperature-Range (°C) | Relative Viscosity (Maximum) |
|---|---|---|---|---|
| Xichen et al. [58] | Al2O3 | Engine oil (SN 5W−40 | Ambient | 1.12 |
| Mostafizur et al. [59] | TiO2 | Methanol | 1–20 | 1.65 |
| Chiam et al. [60] | Al2O3 | 60:40 (W:EG) | 30–70 | 1.67 |
| Fedele et al. [61] | TiO2 | Bidistilled water | 10–70 | 2.8 |
| Sujith et al. [9] | Al2O3 | Coconut oil | 30–140 | 2.5 |
| Georgiana et al. [62] | Al2O3/SiO2 | Distilled water | Ambient | 2.7 |
| Suhaib et al. [63] | ZnO | Paraffin oil | 25–55 | 1.62 |
| Yan et al. [64] | TiO2/MWCNT | Ethylene glycol | 25–55 | 1.94 |
| Kole et al. [65] | CuO | Gearoil | 10–80 | 2.8 |
| Andac et al. [66] | ZrO2 | Water | 10–70 | 1.8 |
| Sonawane [67] | Fe3O4 | Ethylene glycol | 20–80 | 2.18 |
| Models/Correlations | Author |
|---|---|
| Einstein [68] | |
| Brinkman [69] | |
| Batchelor [71] | |
| Wang [72] | |
η = intrinsic viscosity = 2.5 ϕm = 0.605 | Krieger and Dougherty [54] |
df = equivalent diameter of the base fluid molecule | Massimo [73] |
A = 1.8375ϕ2 − 29.643ϕ + 165.56 B = 4 × 10−6ϕ2 − 0.001ϕ + 0.0186 | Namburu et al. [74] |
| Masoumi et al. [75] | |
| Christiana and Kumar [76] | |
h is the inter particle distance | Graham [77] |
| Nanoparticle | Price ($/10 g) |
|---|---|
| TiO2 | 96.81 |
| MgO | 170.5 |
| ZnO | 79 |
| CuO | 68 |
| Fe2O3 | 31.4 |
| Al2O3 | 7.8 |
| Fe3O4 | 16.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sujith, S.V.; Kim, H.; Lee, J. A Review on Thermophysical Property Assessment of Metal Oxide-Based Nanofluids: Industrial Perspectives. Metals 2022, 12, 165. https://doi.org/10.3390/met12010165
Sujith SV, Kim H, Lee J. A Review on Thermophysical Property Assessment of Metal Oxide-Based Nanofluids: Industrial Perspectives. Metals. 2022; 12(1):165. https://doi.org/10.3390/met12010165
Chicago/Turabian StyleSujith, Surendran V., Hansoo Kim, and Joonho Lee. 2022. "A Review on Thermophysical Property Assessment of Metal Oxide-Based Nanofluids: Industrial Perspectives" Metals 12, no. 1: 165. https://doi.org/10.3390/met12010165
APA StyleSujith, S. V., Kim, H., & Lee, J. (2022). A Review on Thermophysical Property Assessment of Metal Oxide-Based Nanofluids: Industrial Perspectives. Metals, 12(1), 165. https://doi.org/10.3390/met12010165







