Kinetic Study of Oxide Growth at High Temperature in Low Carbon Steel
Abstract
:1. Introduction
2. Materials and Methods
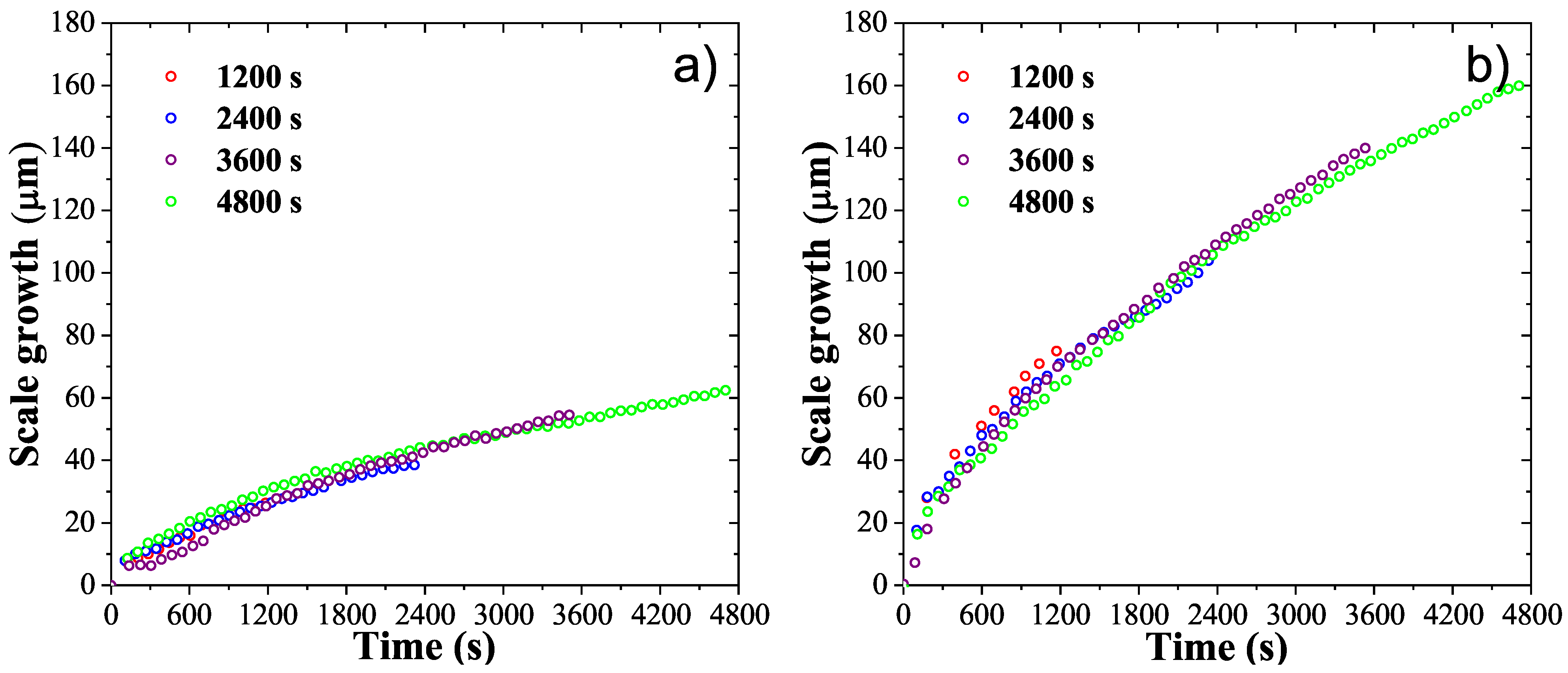
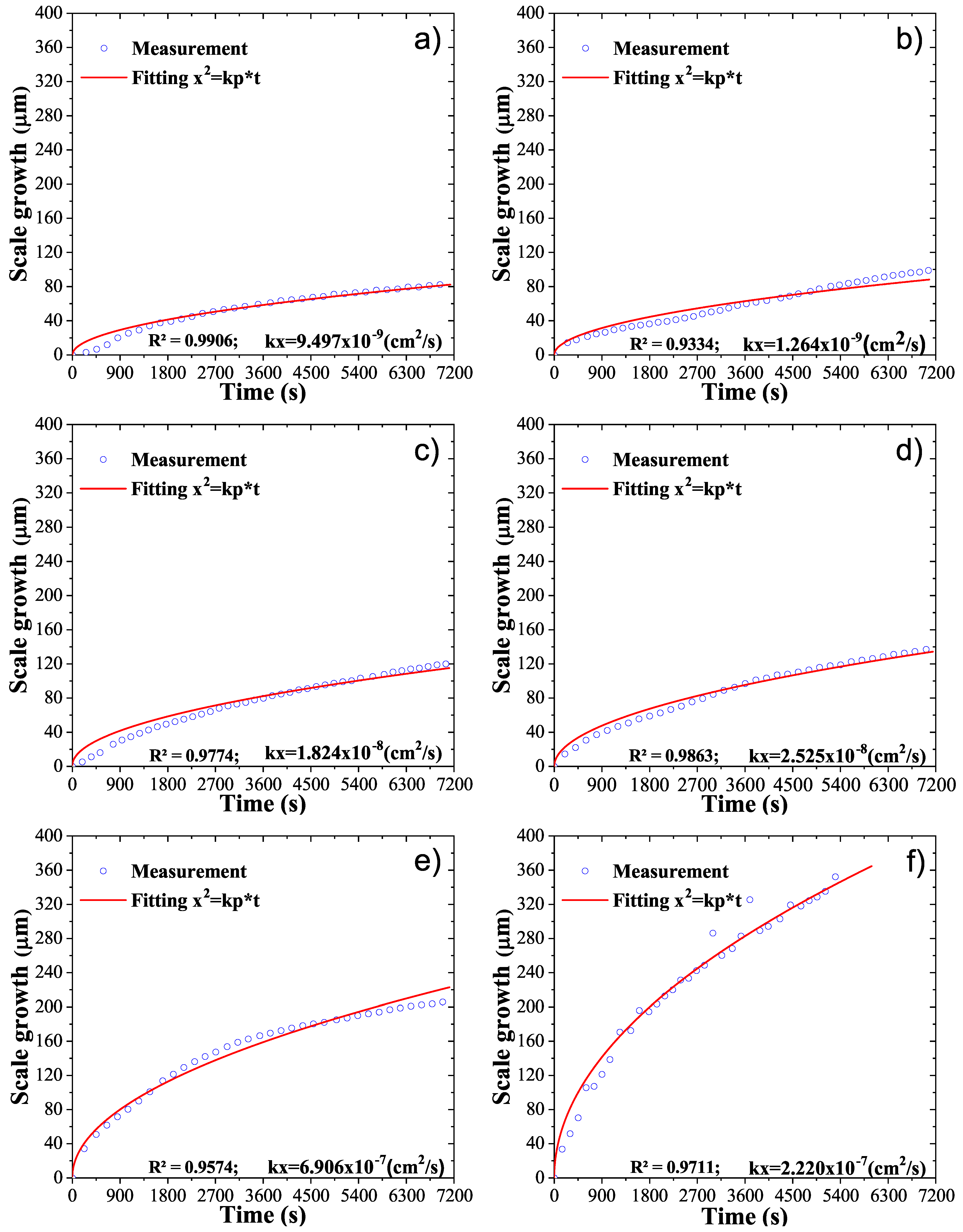
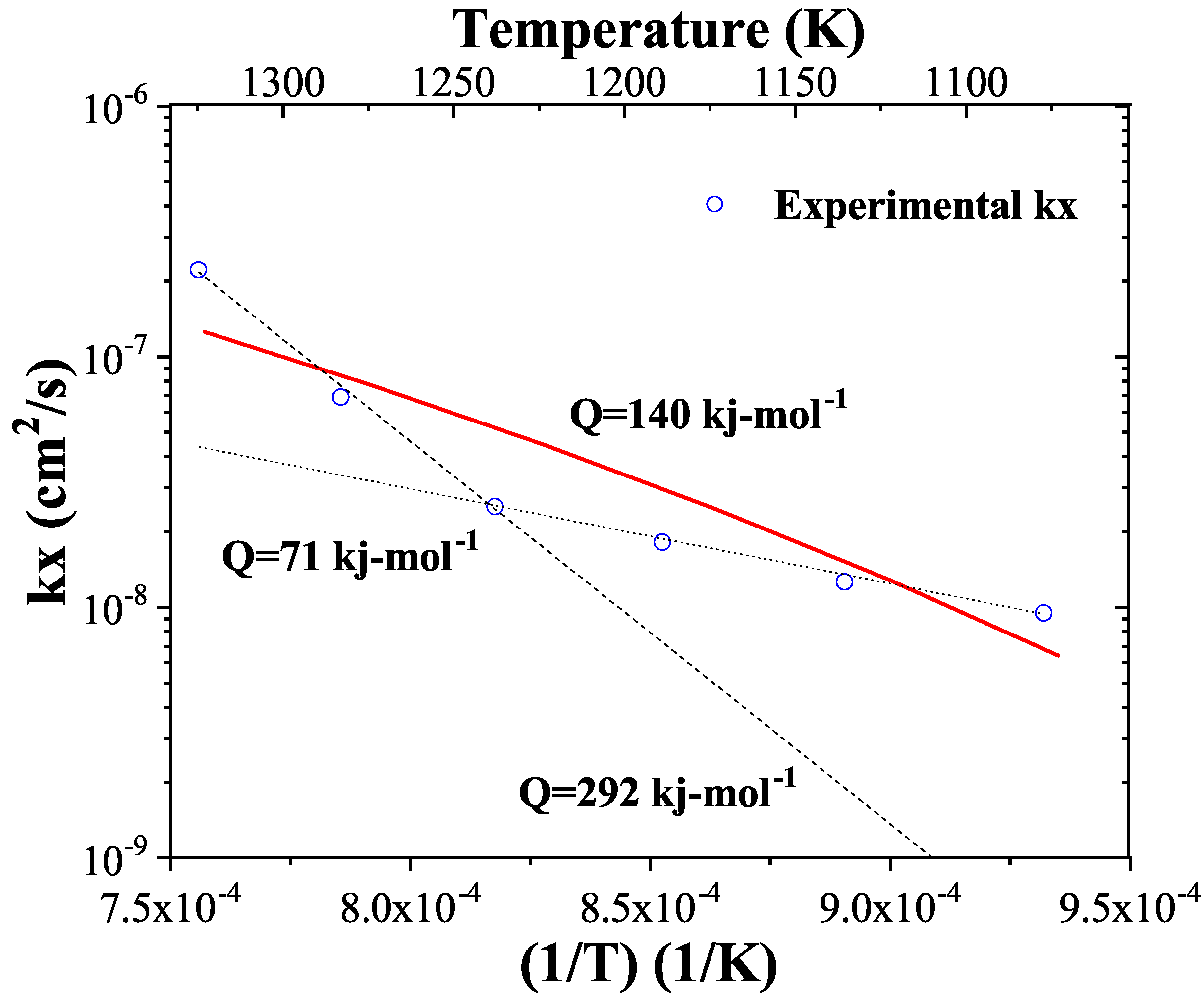
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ormerod IV, R.; Becker, H.; Grandmaison, E.; Pollard, A.; Sobiesiak, A. Effect of process variables on scale formation in steel reheating. J. Chem. Eng 1997, 75, 402–413. [Google Scholar] [CrossRef]
- WOLF, M.M. Scale formation and descaling in continuous casting and hot rolling. Ironmak. Steelmak. 2000, 27, 67–68. [Google Scholar]
- Rdcis, S.; Sail, B. A review on: Efficient energy optimization in reheating furnaces. In Proceedings of the 16th IRF International Conference, Pune, India, 14 December 2014. [Google Scholar]
- Liu, X.; Sun, B.; Wang, J.; Cao, G. Research progress of high temperature iron oxide scale of steel and iron material during hot rolling. Hot Work Technol. 2018, 47, 10–19. [Google Scholar]
- Cheng, L.; Sun, B.; Du, C.; Gao, W.; Cao, G. High-Temperature Oxidation Behavior of Fe–10Cr Steel under Different Atmospheres. Materials 2021, 14, 3453. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Ding, B.; Guan, J. Effect of heating temperature on scale pf W470 high-silicon steel continuous casting billet. Heat. Treat. Met. 2014, 39, 45–48. [Google Scholar]
- Tanei, H.; Kondo, Y. Strain development in oxide scale during phase transformation of FeO. ISIJ Int. 2017, 57, 506–510. [Google Scholar] [CrossRef] [Green Version]
- Shizukawa, Y.; Hayashi, S.; Yoneda, S.; Kondo, Y.; Tanei, H.; Ukai, S. Mechanism of magnetite seam formation and its role for FeO scale transformation. Oxid. Met. 2016, 86, 315–326. [Google Scholar] [CrossRef]
- Chen, R.; Yeun, W. Review of the high-temperature oxidation of iron and carbon steels in air or oxygen. Oxid. Met. 2003, 59, 433–468. [Google Scholar] [CrossRef]
- Blazevic, D.T. Rolled in Scale–the Consistent Problem. In Proceedings of the 4 th International Steel Rolling Conference–the Science and Technology of Flat Rolling, Deauville, France, 1–3 June 1987; Volume 1. [Google Scholar]
- Suarez, L.; Houbaert, Y.; Eynde, X.V.; Colás, R. High temperature deformation of oxide scale. Corros. Sci 2009, 51, 309–315. [Google Scholar] [CrossRef]
- Qiu, H.X.; Hu, X.J.; Chen, S.H.; Fang, F. Study on Growth of Oxide Scale on High Carbon Steel at High Temperature. Appl. Mech. Mater. 2012, 148, 34–37. [Google Scholar] [CrossRef]
- Schwenk, W.; Rahmel, A. Theoretical considerations on phase boundary reactions and mass transfer during the oxidation of iron. Oxid. Met. 1986, 25, 293–303. [Google Scholar] [CrossRef]
- Cramer, S.D.; Covino, B.S., Jr. Corrosion: Fundamentals, Testing, and Protection; ASM International: Novelty, OH, USA, 2003; pp. 122–123. ISBN 978-1-62708-182-5. [Google Scholar]
- Hrabovskỳ, J.; Dobeš, F.; Horskỳ, J. Small Punch Tests at Oxide Scales Surface of Structural Steel and Low Silicon Steel. Oxid. Met. 2014, 82, 297–310. [Google Scholar] [CrossRef]
- Meetham, G. High-temperature materials—A general review. J. Mater. Sci. 1991, 26, 853–860. [Google Scholar] [CrossRef]
- Evans, H.; Hilton, D.; Holm, R.; Webster, S. Influence of silicon additions on the oxidation resistance of a stainless steel. Oxid. Met. 1983, 19, 1–18. [Google Scholar] [CrossRef]
- Liu, X.j.; He, Y.Q.; Cao, G.M.; Jia, T.; Wu, T.Z.; Liu, Z.Y. Effect of Si content and temperature on oxidation resistance of Fe-Si alloys. J. Iron Steel Res. Int. 2015, 22, 238–244. [Google Scholar] [CrossRef]
- Bedworth, R.; Pilling, N. The oxidation of metals at high temperatures. J. Inst. Met. 1923, 29, 529–582. [Google Scholar]
- Tammann, G. Über Anlauffarben von metallen. Z. Anorg. Und Allg. Chem. 1920, 111, 78–89. [Google Scholar] [CrossRef]
- Sheasby, J.; Boggs, W.; Turkdogan, E. Scale growth on steels at 1200 C: Rationale of rate and morphology. Met. Sci. J. 1984, 18, 127–136. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, Z.; Wei, D.; Hao, L.; Zhao, J.; Jiang, L. Oxide scale characterization of ferritic stainless steel and its deformation and friction in hot rolling. Tribol. Int. 2015, 84, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Jiang, Z.; Wei, D.; Zhao, J.; Monaghan, B.J.; Longbottom, R.J.; Jiang, L. Characteristics of oxide scale formed on ferritic stainless steels in simulated reheating atmosphere. Surf. Coat. Technol. 2014, 258, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Abuluwefa, H.; Guthrie, R.; Ajersch, F. The effect of oxygen concentration on the oxidation of low-carbon steel in the temperature range 1000 to 1250 C. Oxid. Met. 1996, 46, 423–440. [Google Scholar] [CrossRef]
- Hu, X.j.; Zhang, B.m.; Chen, S.h.; Fang, F.; Jiang, J.q. Oxide scale growth on high carbon steel at high temperatures. J. Iron Steel Res. Int. 2013, 20, 47–52. [Google Scholar] [CrossRef]
- Krzyzanowski, M.; Beynon, J.H.; Farrugia, D.C. Oxide Scale Behavior in High Temperature Metal Processing; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Torres, M.; Colas, R. A model for heat conduction through the oxide layer of steel during hot rolling. J. Mater. Process. Technol. 2000, 105, 258–263. [Google Scholar] [CrossRef]
- Basabe, V.V.; Szpunar, J.A. Growth rate and phase composition of oxide scales during hot rolling of low carbon steel. ISIJ Int. 2004, 44, 1554–1559. [Google Scholar] [CrossRef] [Green Version]
- Abuluwefa, H.; Guthrie, R.; Ajersch, F. Oxidation of low carbon steel in multicomponent gases: Part I. Reaction mechanisms during isothermal oxidation. Metall. Mater. Trans. 1997, 28, 1633–1641. [Google Scholar] [CrossRef]
- Birks, N.; Nicholson, A. The Journal of the Iron and Steel Institute; Iron and Steel Institute: London, UK, 1970; p. 219. [Google Scholar]
- Browne, K.W.; Dryden, J.; Assefpour, M. Modelling Scaling and Descaling in Hot Strip Mills. Recent advances in heat transfer and micro-structure modelling for metal processing. In Proceedings of the 1995 ASME International Mechanical Engineering Congress and Exposition, San Francisco, CA, USA, 12–17 November 1995; Volume 67, pp. 187–198. [Google Scholar]
- Kubaschewski, O.; Hopkins, B.E. Oxidation of Metals and Alloys; Butterworths: London, UK, 1967. [Google Scholar]
- Paidassi, J. Oxidation of iron in air between 700C and 1250C. Mem. Sci. Rev. Met. 1957, 54, 569585. [Google Scholar]
- Stanley, J.; Vonhoene, J.; Huntoon, R. The oxidation of pure iron. Trans. ASM 1951, 43, 426–453. [Google Scholar]
- Munther, P.A.; Lenard, J.G. The effect of scaling on interfacial friction in hot rolling of steels. J. Mater. Process. Technol. 1999, 88, 105–113. [Google Scholar] [CrossRef]
- Samsonov, G. The Oxide Handbook; Springer Science & Business Media: Cham, Switzerland, 2013. [Google Scholar]
- Suárez, L.; Houbaert, Y.; Eynde, X.V.; Colás, R. Development of an experimental device to study high temperature oxidation. Oxid. Met. 2008, 70, 1–13. [Google Scholar] [CrossRef]
- Suárez, L.; Rodríguez-Calvillo, P.; Houbaert, Y.; Colás, R. Oxidation of ultra low carbon and silicon bearing steels. Corros. Sci. 2010, 52, 2044–2049. [Google Scholar] [CrossRef]
- Yuan, Q.; Xu, G.; Zhou, M.; He, B.; Hu, H. The effect of p on the microstructure and melting temperature of Fe2SiO4 in silicon-containing steels investigated by in situ observation. Metals 2017, 7, 37. [Google Scholar] [CrossRef] [Green Version]
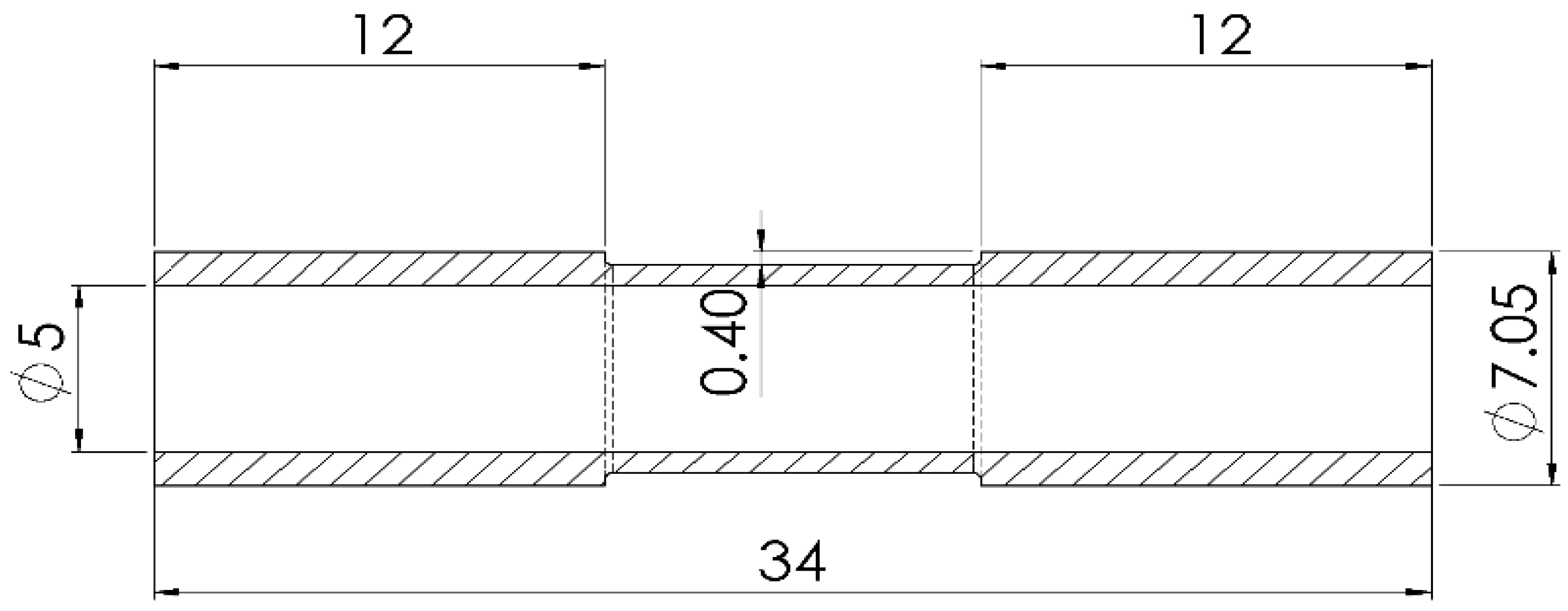


| C | Mn | P | S | Si | Cu | Ni | Cr | Sn | Mo | Al | V |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.07 | 1.43 | 0.25 | 0.25 | 0.83 | 0.08 | 0.08 | 0.08 | 0.01 | 0.01 | 0.01 | 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arreola-Villa, S.A.; Vergara-Hernández, H.J.; Solorio-Diáz, G.; Pérez-Alvarado, A.; Vázquez-Gómez, O.; Chávez-Campos, G.M. Kinetic Study of Oxide Growth at High Temperature in Low Carbon Steel. Metals 2022, 12, 147. https://doi.org/10.3390/met12010147
Arreola-Villa SA, Vergara-Hernández HJ, Solorio-Diáz G, Pérez-Alvarado A, Vázquez-Gómez O, Chávez-Campos GM. Kinetic Study of Oxide Growth at High Temperature in Low Carbon Steel. Metals. 2022; 12(1):147. https://doi.org/10.3390/met12010147
Chicago/Turabian StyleArreola-Villa, Sixtos Antonio, Héctor Javier Vergara-Hernández, Gildardo Solorio-Diáz, Alejandro Pérez-Alvarado, Octavio Vázquez-Gómez, and Gerardo Marx Chávez-Campos. 2022. "Kinetic Study of Oxide Growth at High Temperature in Low Carbon Steel" Metals 12, no. 1: 147. https://doi.org/10.3390/met12010147
APA StyleArreola-Villa, S. A., Vergara-Hernández, H. J., Solorio-Diáz, G., Pérez-Alvarado, A., Vázquez-Gómez, O., & Chávez-Campos, G. M. (2022). Kinetic Study of Oxide Growth at High Temperature in Low Carbon Steel. Metals, 12(1), 147. https://doi.org/10.3390/met12010147









