Effect of the Thermomechanical Treatment on the Corrosion of UNSM Processed Inconel 718: An Electrochemical Study
Abstract
:1. Introduction
2. Materials and Methods
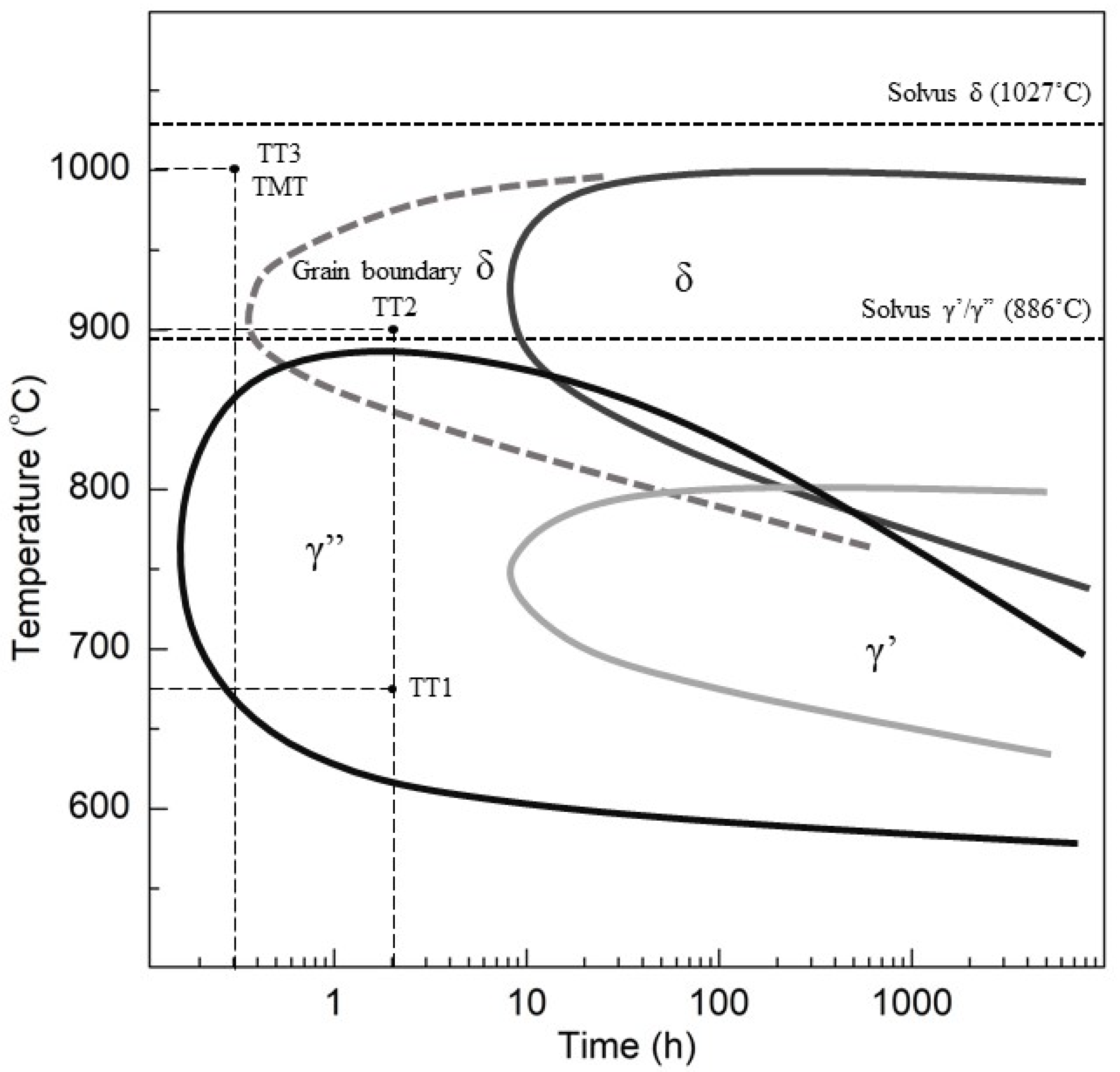
2.1. Materials and Thermomechanical Processing
2.2. Electrochemical Techniques
2.3. Characterization Techniques
3. Results
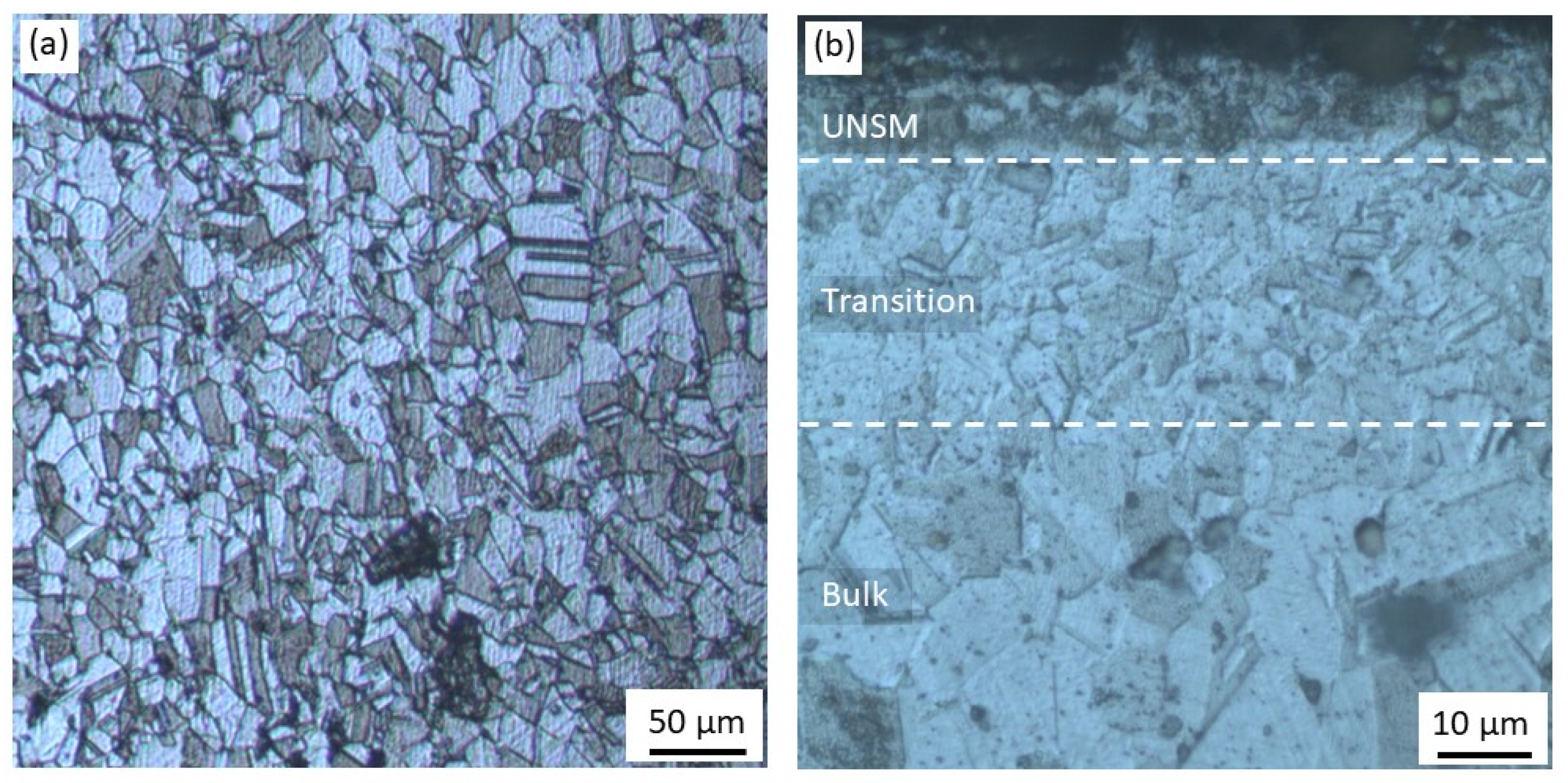
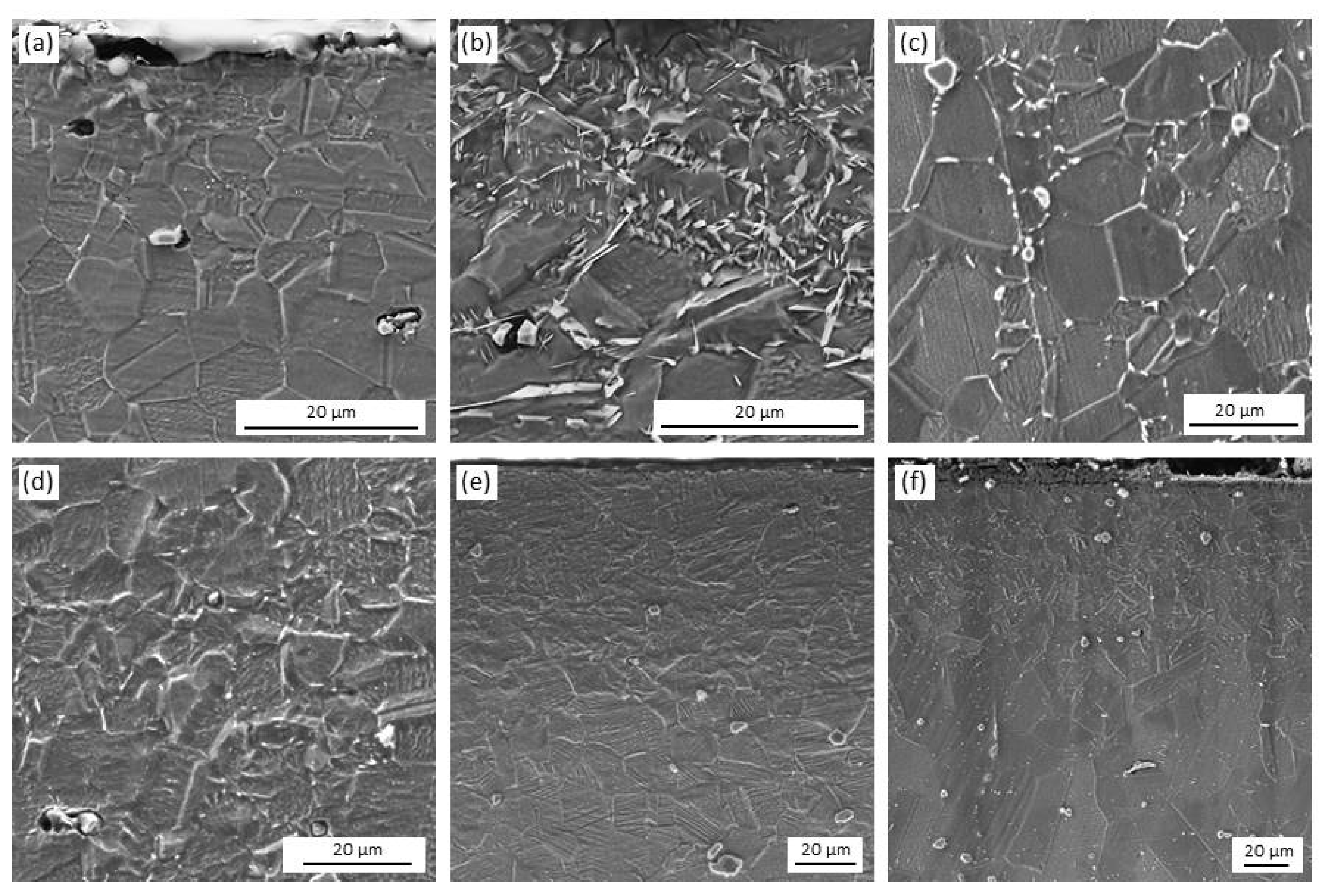
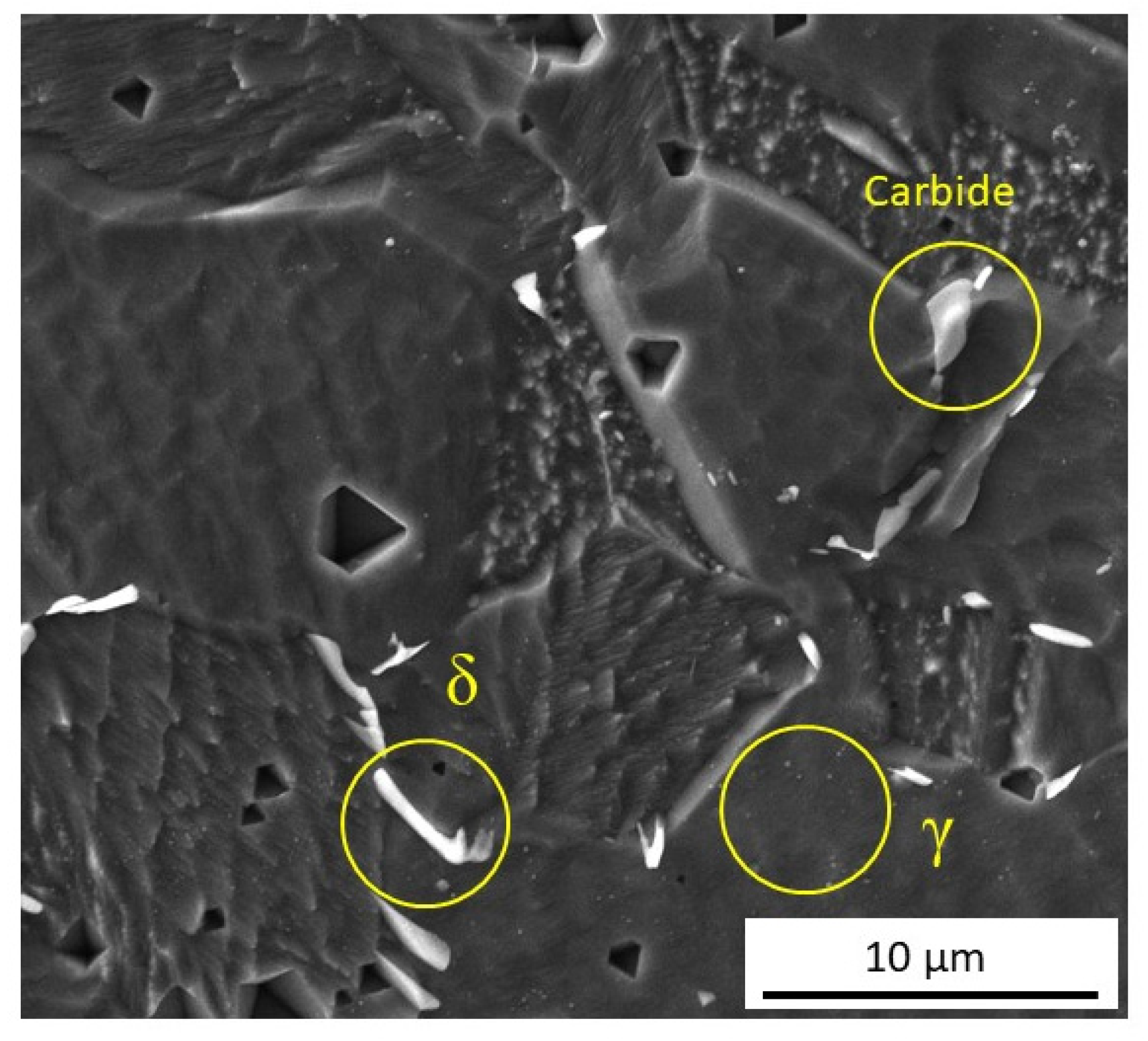
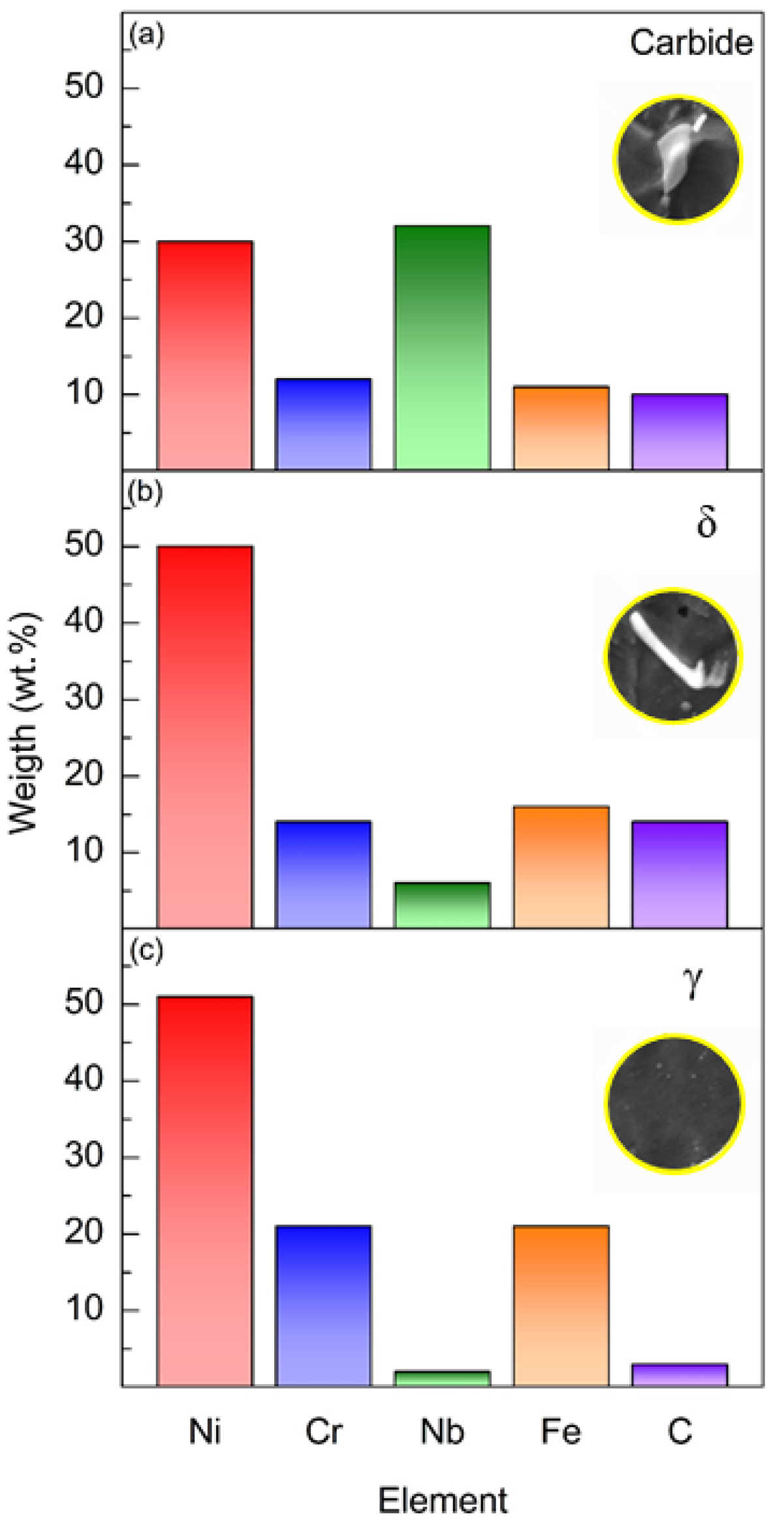
3.1. Microstructure Characterization
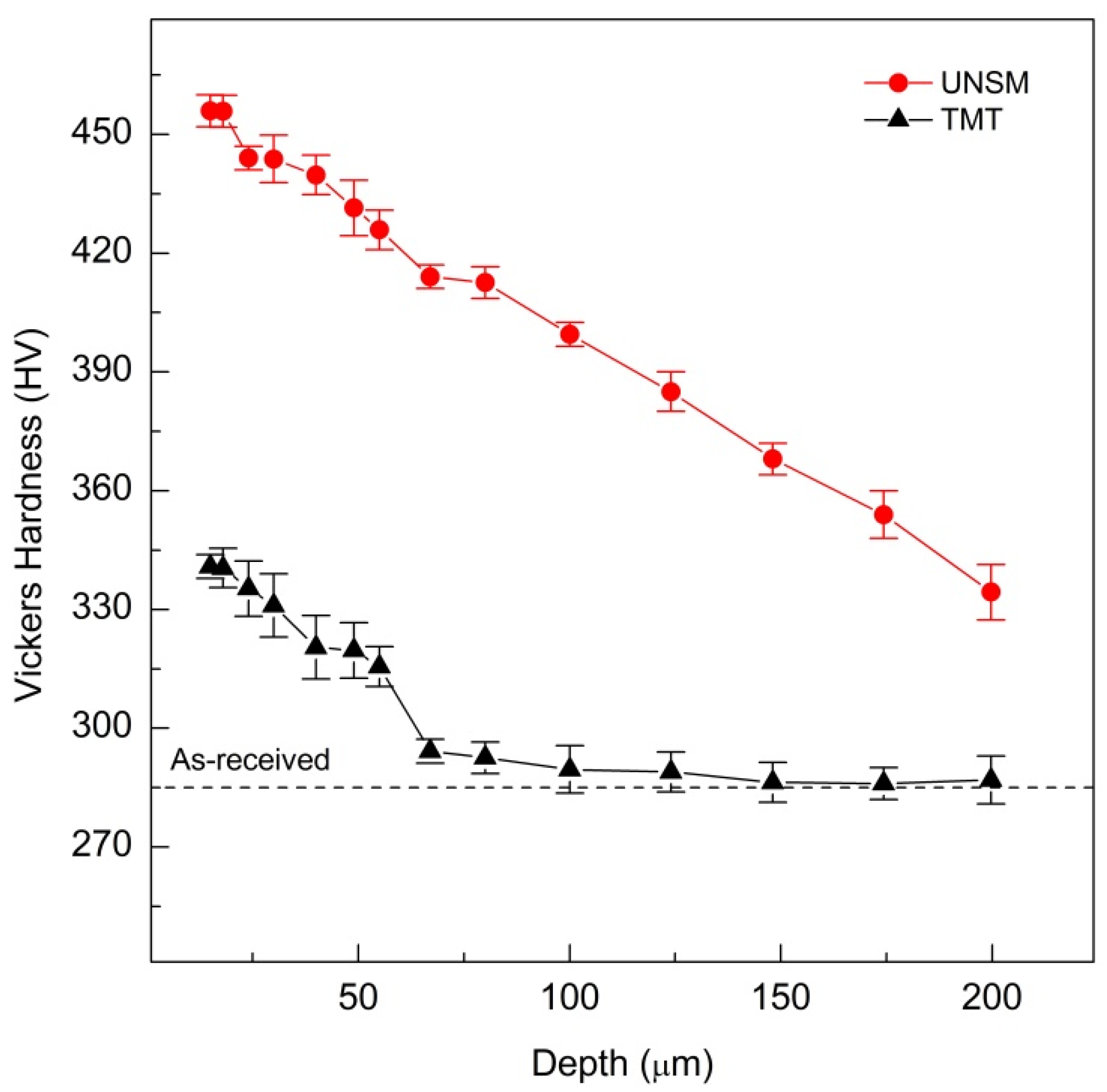
3.2. Vickers Hardness
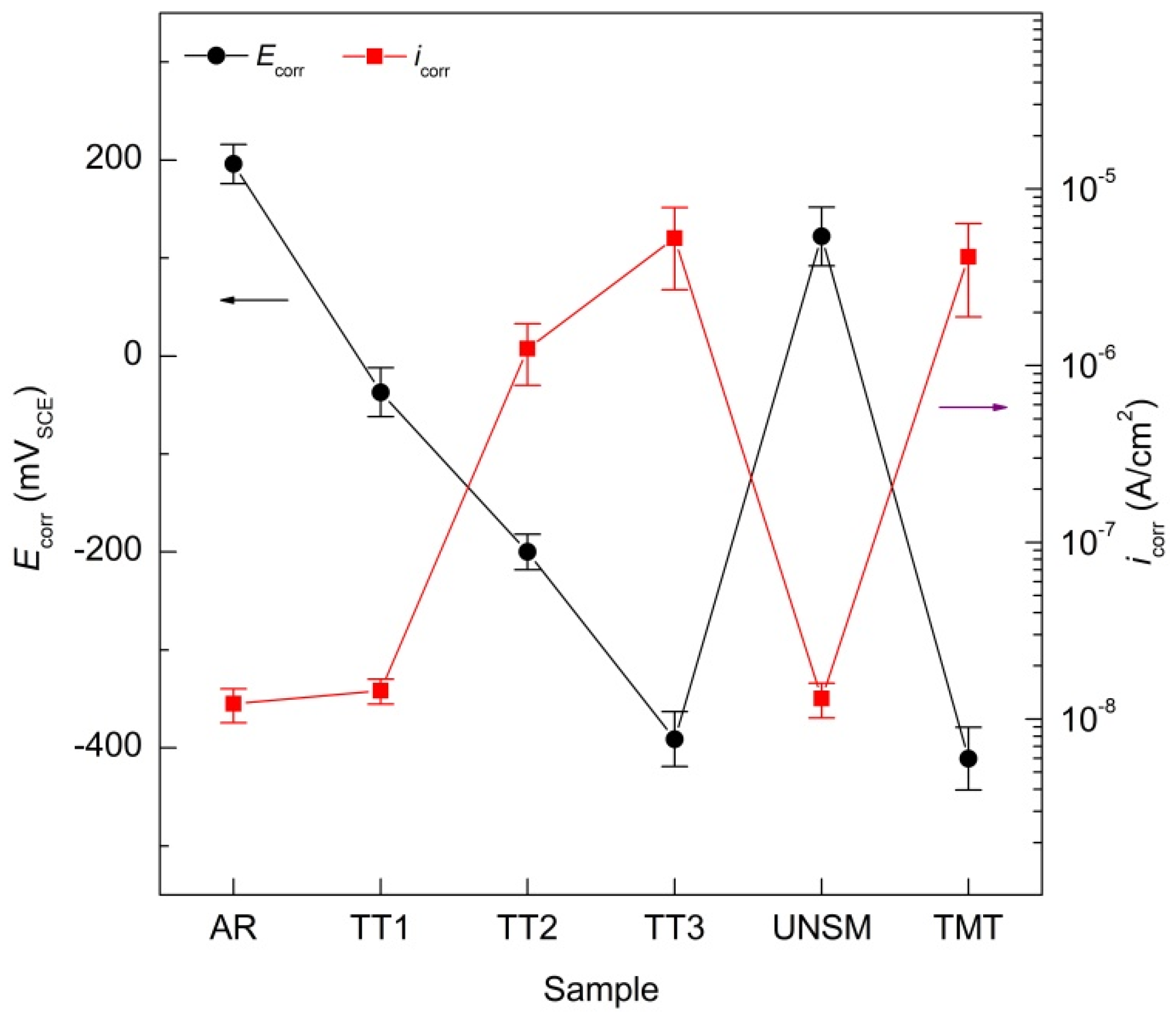
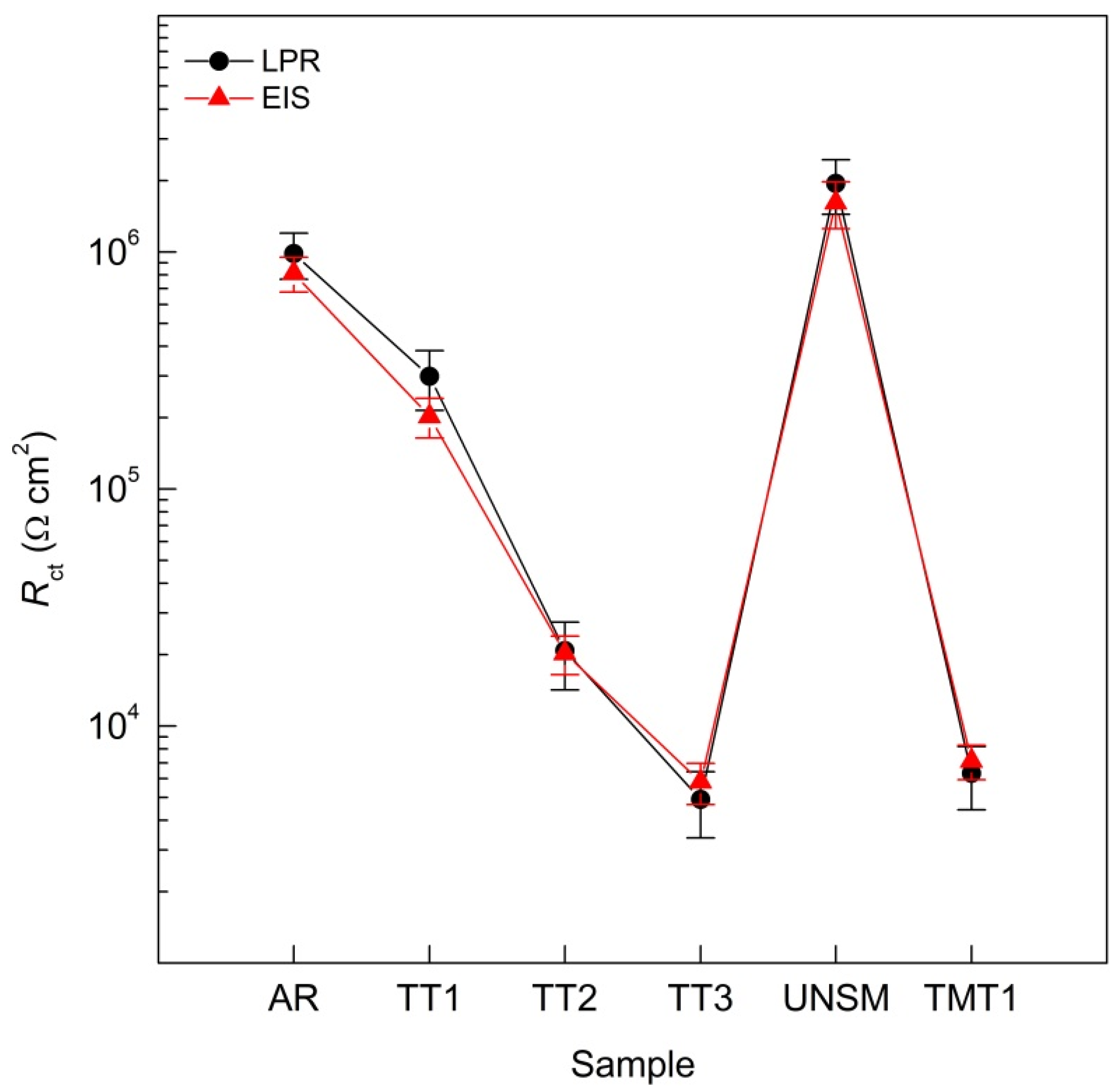
3.3. Linear Polarization Resistance (LPR)
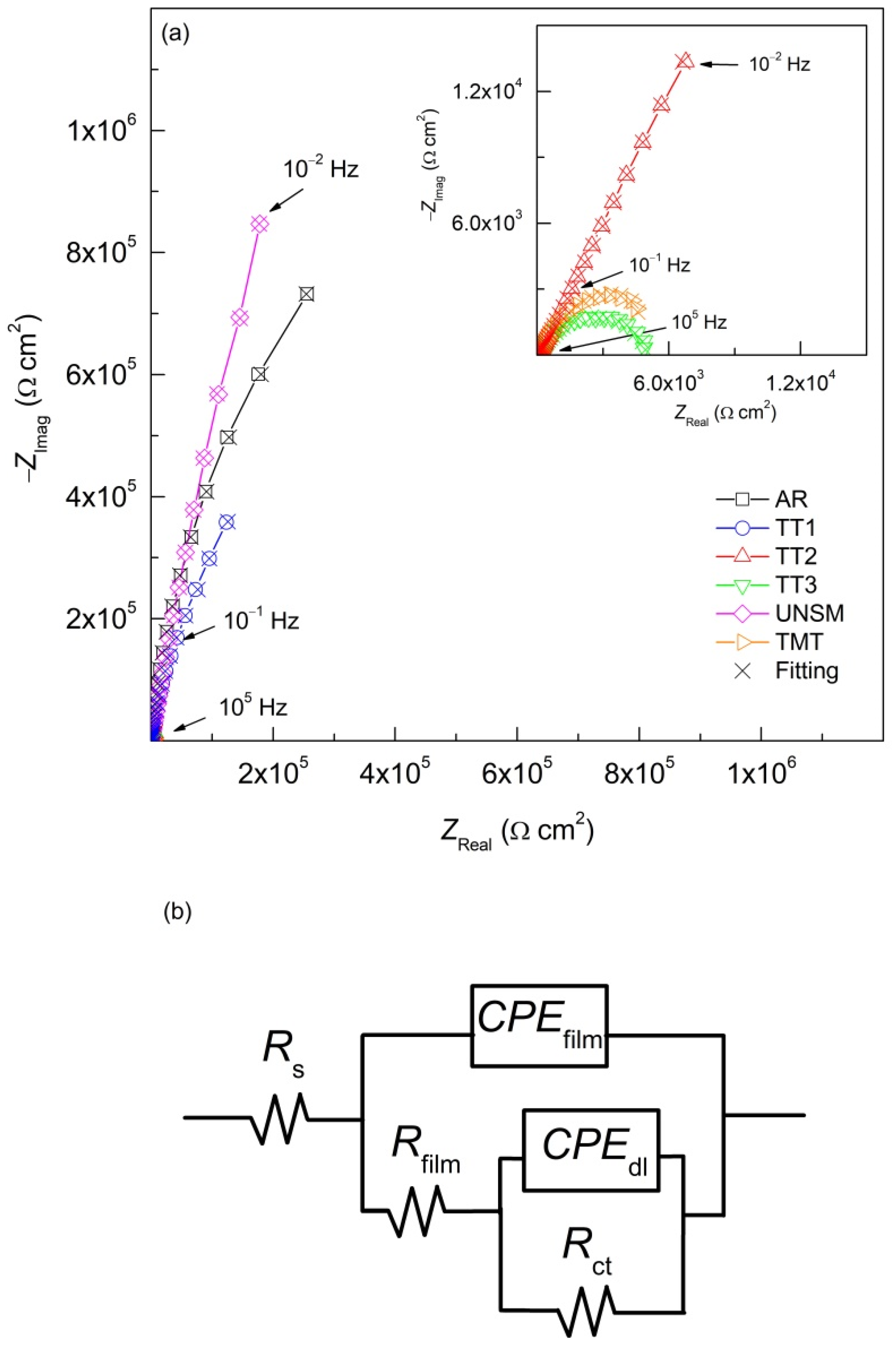
3.4. Electrochemical Impedance Spectroscopy (EIS)
4. Discussion
4.1. Microstructure Characterization
4.2. Vickers Hardness
4.3. Linear Polarization Resistance (LPR)
4.4. Electrochemical Impedance Spectroscopy (EIS)
4.5. Infinite Focus Microscopy (IFM)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azadian, S.; Wei, L.Y.; Warren, R. Delta phase precipitation in Inconel 718. Mater. Charact. 2004, 53, 7–16. [Google Scholar] [CrossRef]
- Hosseini, E.; Popovich, V.A. A review of mechanical properties of additively manufactured Inconel 718. Addit. Manuf. 2019, 30, 100877. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Guo, Q.; Ma, Z.; Huang, Y.; Li, H.; Liu, Y. Hot tensile behavior of cold-rolled Inconel 718 alloy at 650 °C: The role of δ phase. Mater. Sci. Eng. A 2018, 722, 136–146. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yang, S.; Kim, Y.K.; AlMangour, B.; Lee, K.A. Effect of post-treatment on the microstructure and high-temperature oxidation behaviour of additively manufactured Inconel 718 alloy. Corros. Sci. 2019, 158, 108082. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Contla-Pacheco, A.D.; Figueroa-López, U.; Martínez-Trinidad, J.; Garduño-Alva, A.; Ortega-Avilés, M. Sliding wear resistance of nickel boride layers on an Inconel 718 superalloy. Surf. Coat. Technol. 2019, 124862. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Liu, Y.; Guo, Q.; Huang, Y.; Li, H.; Yu, J. Effect of hot deformation on γ″ and δ phase precipitation of Inconel 718 alloy during deformation&isothermal treatment. J. Alloys Compd. 2017, 716, 65–72. [Google Scholar] [CrossRef]
- Jelvani, S.; Shoja Razavi, R.; Barekat, M.; Dehnavi, M.R.; Erfanmanesh, M. Evaluation of solidification and microstructure in laser cladding Inconel 718 superalloy. Opt. Laser Technol. 2019, 120, 105761. [Google Scholar] [CrossRef]
- Kulkarni, A.; Dwivedi, D.K.; Vasudevan, M. Dissimilar metal welding of P91 steel-AISI 316L SS with Incoloy 800 and Inconel 600 interlayers by using activated TIG welding process and its effect on the microstructure and mechanical properties. J. Mater. Process. Technol. 2019, 274, 116280. [Google Scholar] [CrossRef]
- Kañetas, P.P.; Osorio, L.R.; Mata, M.G.; De La Garza, M.; López, V.P. Influence of the delta phase in the microstructure of the Inconel 718 subjected to “Delta-processing” heat treatment and hot deformed. Procedia Mater. Sci. 2015, 8, 1160–1165. [Google Scholar] [CrossRef] [Green Version]
- Rafiei, M.; Mirzadeh, H.; Malekan, M. Micro-mechanisms and precipitation kinetics of delta (δ) phase in Inconel 718 superalloy during aging. J. Alloys Compd. 2019, 795, 207–212. [Google Scholar] [CrossRef]
- Anderson, M.; Thielin, A.L.; Bridier, F.; Bocher, P.; Savoie, J. δ Phase precipitation in Inconel 718 and associated mechanical properties. Mater. Sci. Eng. A 2017, 679, 48–55. [Google Scholar] [CrossRef]
- Luo, S.; Huang, W.; Yang, H.; Yang, J.; Wang, Z.; Zeng, X. Microstructural evolution and corrosion behaviors of Inconel 718 alloy produced by selective laser melting following different heat treatments. Addit. Manuf. 2019, 30, 100875. [Google Scholar] [CrossRef]
- Gill, A.; Telang, A.; Mannava, S.R.; Qian, D.; Pyoun, Y.S.; Soyama, H.; Vasudevan, V.K. Comparison of mechanisms of advanced mechanical surface treatments in nickel-based superalloy. Mater. Sci. Eng. A 2013, 576, 346–355. [Google Scholar] [CrossRef]
- Bazarbayev, Y.; Kattoura, M.; Mao, K.S.; Song, J.; Vasudevan, V.K.; Wharry, J.P. Effects of corrosion-inhibiting surface treatments on irradiated microstructure development in Ni-base alloy 718. J. Nucl. Mater. 2018, 512, 276–287. [Google Scholar] [CrossRef]
- Amanov, A.; Pyun, Y.S. Local heat treatment with and without ultrasonic nanocrystal surface modification of Ti-6Al-4V alloy: Mechanical and tribological properties. Surf. Coat. Technol. 2017, 326, 343–354. [Google Scholar] [CrossRef]
- Kattoura, M.; Mannava, S.R.; Qian, D.; Vasudevan, V.K. Effect of ultrasonic nanocrystal surface modification on elevated temperature residual stress, microstructure, and fatigue behavior of ATI 718Plus alloy. Int. J. Fatigue 2018, 110, 186–196. [Google Scholar] [CrossRef]
- Amanov, A.; Umarov, R. The effects of ultrasonic nanocrystal surface modification temperature on the mechanical properties and fretting wear resistance of Inconel 690 alloy. Appl. Surf. Sci. 2018, 441, 515–529. [Google Scholar] [CrossRef]
- Martin, U.; Ress, J.; Bosch, J.; Bastidas, D.M. Evaluation of the DOS by DL−EPR of UNSM processed Inconel 718. Metals 2020, 10, 204. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Liu, D.; Qin, H.; Zhang, X.; Zhang, H.; Zhang, R.; Ren, Z.; Ma, C.; Amanov, A.; Pyun, Y.-S.; et al. The effect of ultrasonic nanocrystal surface modification on low temperature nitriding of ultra-high strength steel. Surf. Coat. Technol. 2019, 375, 205–214. [Google Scholar] [CrossRef]
- Kim, K.-T.; Lee, J.-H.; Kim, Y.-S. Effect of ultrasonic nano-crystal surface modification (UNSM) on the passivation behavior of aged 316L stainless steel. Materials 2017, 10, 713. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, J.J.; Cao, G.H.; Russell, A.M.; Zhou, Z.J.; Li, C.P.; Chen, G.F. Improved plasticity of Inconel 718 superalloy fabricated by selective laser melting through a novel heat treatment process. Mater. Des. 2019, 180, 107915. [Google Scholar] [CrossRef]
- Chandrasekar, R.; Lo, C.C.H.; Frishman, A.M.; Larson, B.F.; Nakagawa, N. Quantification of precipitates and their effects on the response of nickel-base superalloy to shot peening. AIP Conf. Proc. 2012, 1430, 1437–1444. [Google Scholar]
- ASTM G3-14. Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]
- ASTM E112-13. Standard Test Methods for Determining Average Grain Size; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar] [CrossRef]
- Kattoura, M.; Telang, A.; Mannava, S.R.; Qian, D.; Vasudevan, V.K. Effect of Ultrasonic Nanocrystal Surface Modification on residual stress, microstructure and fatigue behavior of ATI 718Plus alloy. Mater. Sci. Eng. A 2018, 711, 364–377. [Google Scholar] [CrossRef]
- Babu, U.R.; Kondraivendhan, B. Effect of IR drop on reinforced concrete corrosion measurements. IOP Conf. Ser. Mater. Sci. Eng. 2020, 829, 012015. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, L.; Ni, H.; Hao, W.; Man, C.; Chen, S.; Wang, X.; Liu, Z.; Li, X. Influence of temperature on the electrochemical and passivation behavior of 2507 super duplex stainless steel in simulated desulfurized flue gas condensates. Corros. Sci. 2017, 118, 31–48. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Fernández-Jiménez, A.; Palomo, A.; González, J.A. A study on the passive state stability of steel embedded in activated fly ash mortars. Corros. Sci. 2008, 50, 1058–1065. [Google Scholar] [CrossRef]
- Bautista, A.; Paredes, E.C.; Velasco, F.; Alvarez, S.M. Corrugated stainless steels embedded in mortar for 9 years: Corrosion results of non-carbonated, chloride-contaminated samples. Constr. Build. Mater. 2015, 93, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Ohta, K.; Ishida, H. Comparison among several numerical integration methods for Kramers-Kronig transformation. Appl. Spectrosc. 1988, 42, 952–957. [Google Scholar] [CrossRef]
- Ress, J.; Martin, U.; Bosch, J.; Bastidas, D.M. pH-Triggered release of NaNO2 corrosion inhibitors from novel colophony microcapsules in simulated concrete pore solution. ACS Appl. Mater. Interfaces 2020, 12, 46686–46700. [Google Scholar] [CrossRef]
- Nabavi, B.; Goodarzi, M.; Khan, A.K. Metallurgical effects of nitrogen on the microstructure and hot corrosion behavior of alloy 718 weldment. Mater. Charact. 2019, 157, 109916. [Google Scholar] [CrossRef]
- Alizadeh, M.; Bordbar, S. The influence of microstructure on the protective properties of the corrosion product layer generated on the welded API X70 steel in chloride solution. Corros. Sci. 2013, 70, 170–179. [Google Scholar] [CrossRef]
- Pierce, D.T.T.; Jiménez, J.A.A.; Bentley, J.; Raabe, D.; Wittig, J.E.E. The influence of stacking fault energy on the microstructural and strain-hardening evolution of Fe–Mn–Al–Si steels during tensile deformation. Acta Mater. 2015, 100, 178–190. [Google Scholar] [CrossRef]
- Meric de Bellefon, G.; van Duysen, J.C.; Sridharan, K. Composition-dependence of stacking fault energy in austenitic stainless steels through linear regression with random intercepts. J. Nucl. Mater. 2017, 492, 227–230. [Google Scholar] [CrossRef]
- Weng, S.; Huang, Y.H.; Xuan, F.Z.; Luo, L.H. Correlation between microstructure, hardness and corrosion of welded joints of disc rotors. Procedia Eng. 2015, 130, 1761–1769. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Xiu, M.; Tan, Y.T.; Wei, J.; Wang, P. Pitting corrosion of SLM Inconel 718 sample under surface and heat treatments. Appl. Surf. Sci. 2019, 490, 556–567. [Google Scholar] [CrossRef]
- Huang, G.; Liu, G.Q.; Feng, M.; Zhang, M.; Hu, B.; Wang, H. The effect of cooling rates from temperatures above the γ′ solvus on the microstructure of a new nickel-based powder metallurgy superalloy. J. Alloys Compd. 2018, 747, 1062–1072. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Bastidas, D.M. Interpretation of impedance data for porous electrodes and diffusion processes. Corrosion 2007, 63, 515–521. [Google Scholar] [CrossRef]
- Huang, V.M.W.; Vivier, V.; Orazem, M.E.; Pébère, N.; Tribollet, B. The apparent constant-phase-element behavior of a disk electrode with faradaic reactions a global and local impedance analysis. J. Electrochem. Soc. 2007, 154, C99–C107. [Google Scholar] [CrossRef] [Green Version]
- Hakiki, N.B.; Boudin, S.; Rondot, B.; Da Cunha Belo, M. The electronic structure of passive films formed on stainless steels. Corros. Sci. 1995, 37, 1809–1822. [Google Scholar] [CrossRef]
- Fajardo, S.; Bastidas, D.M.; Criado, M.; Bastidas, J.M. Electrochemical study on the corrosion behaviour of a new low-nickel stainless steel in carbonated alkaline solution in the presence of chlorides. Electrochim. Acta 2014, 129, 160–170. [Google Scholar] [CrossRef] [Green Version]


| Element | Al | C | Cr | Fe | Mo | Nb | Ni | Ti |
|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 0.46 | 0.03 | 17.92 | 18.80 | 3.05 | 5.05 | 53.71 | 0.97 |
| Sample | Treatment |
|---|---|
| AR | As-received |
| TT1 | Annealed at 675 °C for 2 h + water quenched |
| TT2 | Annealed at 900 °C for 2 h + water quenched |
| TT3 | (Annealed at 1000 °C 10 min + water quenched) × 3 |
| UNSM | UNSM treated × 3 |
| TMT | (UNSM treated + annealed at 1000 °C for 10 min + water quenched) × 3 |
| Sample | Rs | Rfilm | Yfilm | nfilm | Rct | Ydl | ndl | Ceff,dl | χ2 (*) |
|---|---|---|---|---|---|---|---|---|---|
| Ω cm2 | Ω cm2 | S/cm2 s film | Ω cm2 | S/cm2 sndl | µF/cm2 | ||||
| AR | 3.78 | 7.46 × 105 | 2.47 × 10−5 | 0.78 | 8.12 × 105 | 1.66 × 10−5 | 0.95 | 9.89 | 1.40 × 10−3 |
| TT1 | 4.06 | 1.13 × 105 | 9.94 × 10−6 | 0.78 | 2.02 × 105 | 2.97 × 10−5 | 0.92 | 13.81 | 4.93 × 10−3 |
| TT2 | 3.88 | 1.53 × 103 | 1.94 × 10−5 | 0.74 | 2.15 × 104 | 5.85 × 10−5 | 0.91 | 26.44 | 2.96 × 10−3 |
| TT3 | 4.93 | 4.38 × 102 | 3.66 × 10−5 | 0.78 | 5.81 × 103 | 1.98 × 10−4 | 0.85 | 21.23 | 1.32 × 10−3 |
| UNSM | 3.94 | 4.52 × 105 | 1.85 × 10−6 | 0.79 | 1.61 × 106 | 1.24 × 10−5 | 0.96 | 8.13 | 1.61 × 10−3 |
| TMT | 3.48 | 1.27 × 103 | 1.24 × 10−4 | 0.78 | 7.13 × 103 | 8.10 × 10−5 | 0.83 | 15.12 | 1.14 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, U.; Ress, J.; Bastidas, D.M. Effect of the Thermomechanical Treatment on the Corrosion of UNSM Processed Inconel 718: An Electrochemical Study. Metals 2021, 11, 1488. https://doi.org/10.3390/met11091488
Martin U, Ress J, Bastidas DM. Effect of the Thermomechanical Treatment on the Corrosion of UNSM Processed Inconel 718: An Electrochemical Study. Metals. 2021; 11(9):1488. https://doi.org/10.3390/met11091488
Chicago/Turabian StyleMartin, Ulises, Jacob Ress, and David M. Bastidas. 2021. "Effect of the Thermomechanical Treatment on the Corrosion of UNSM Processed Inconel 718: An Electrochemical Study" Metals 11, no. 9: 1488. https://doi.org/10.3390/met11091488
APA StyleMartin, U., Ress, J., & Bastidas, D. M. (2021). Effect of the Thermomechanical Treatment on the Corrosion of UNSM Processed Inconel 718: An Electrochemical Study. Metals, 11(9), 1488. https://doi.org/10.3390/met11091488







