Corrosion Behavior of AlFeCrCoNiZrx High-Entropy Alloys in 0.5 M Sulfuric Acid Solution
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
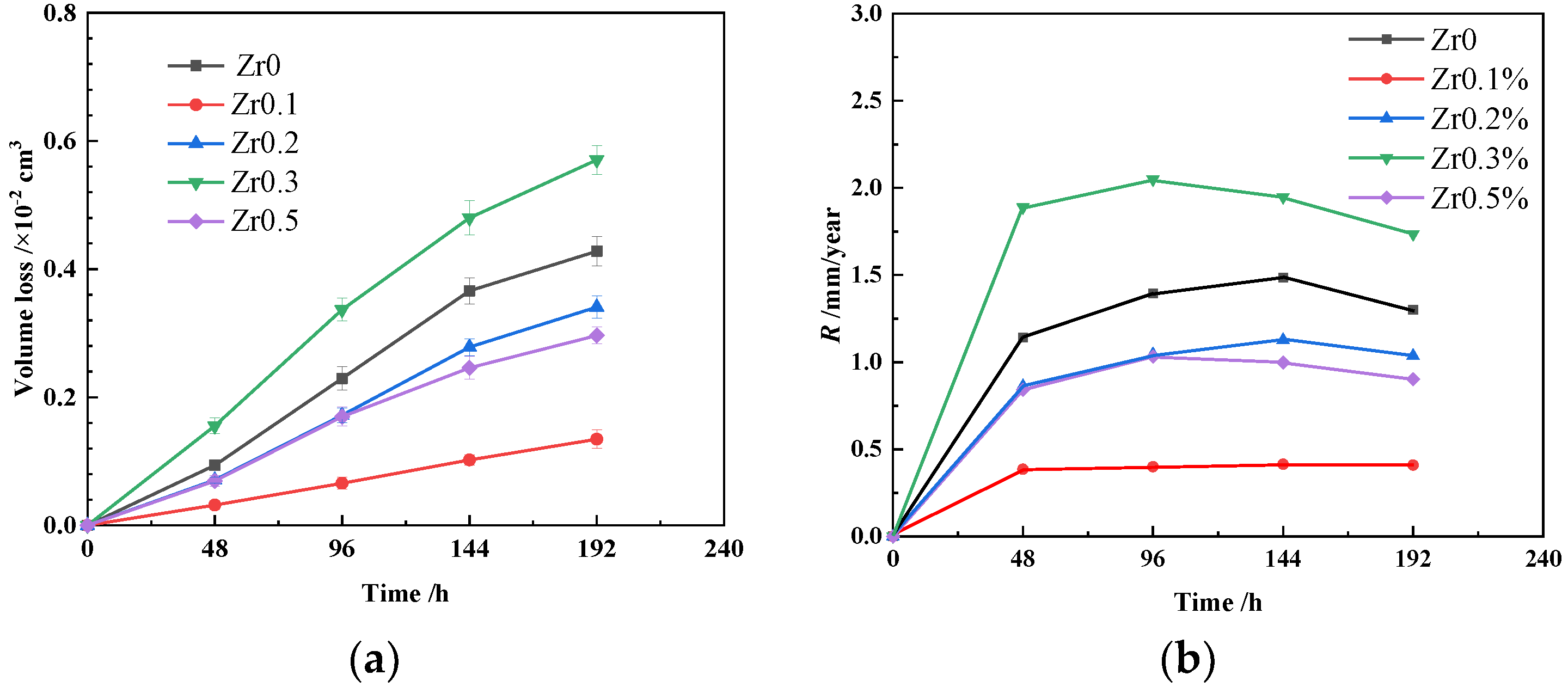
3.1. Immersion Measurements
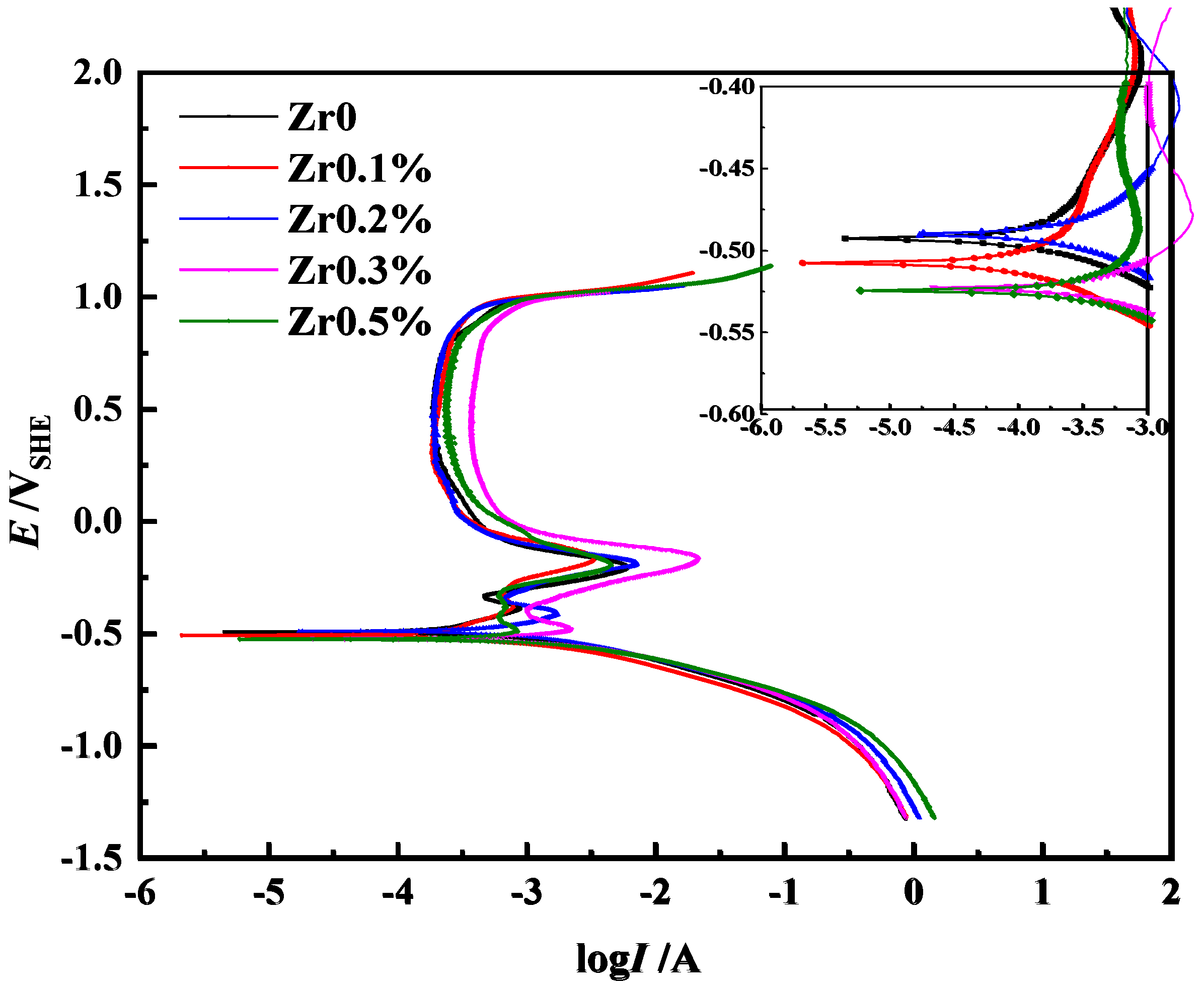
3.2. Potentiodynamic Polarization Measurements
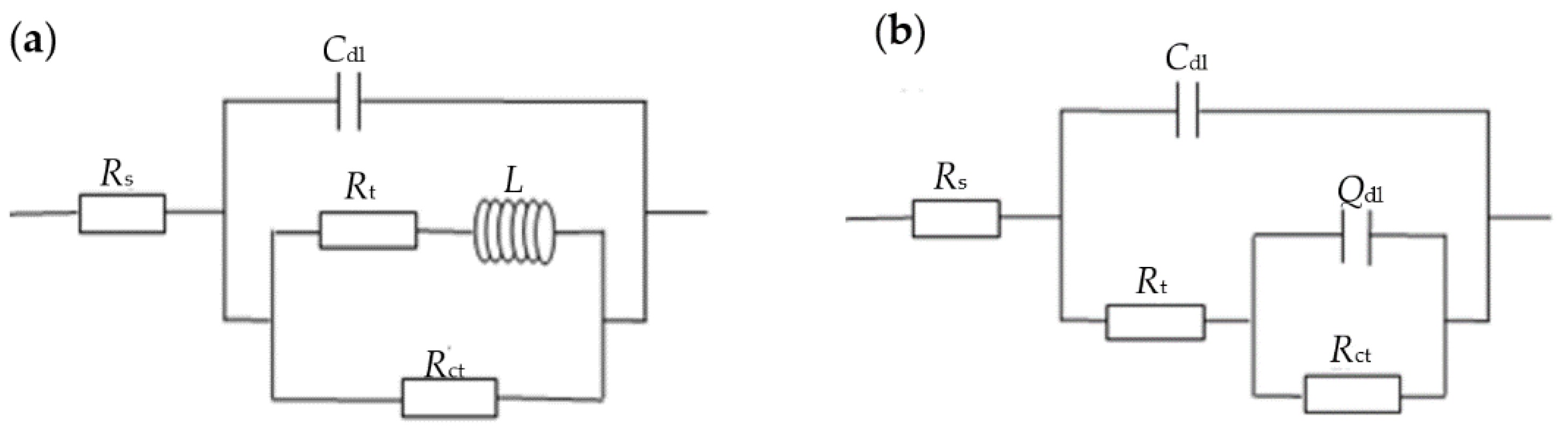
3.3. Electrochemical Impedance Spectroscopy Measurements
4. Conclusions
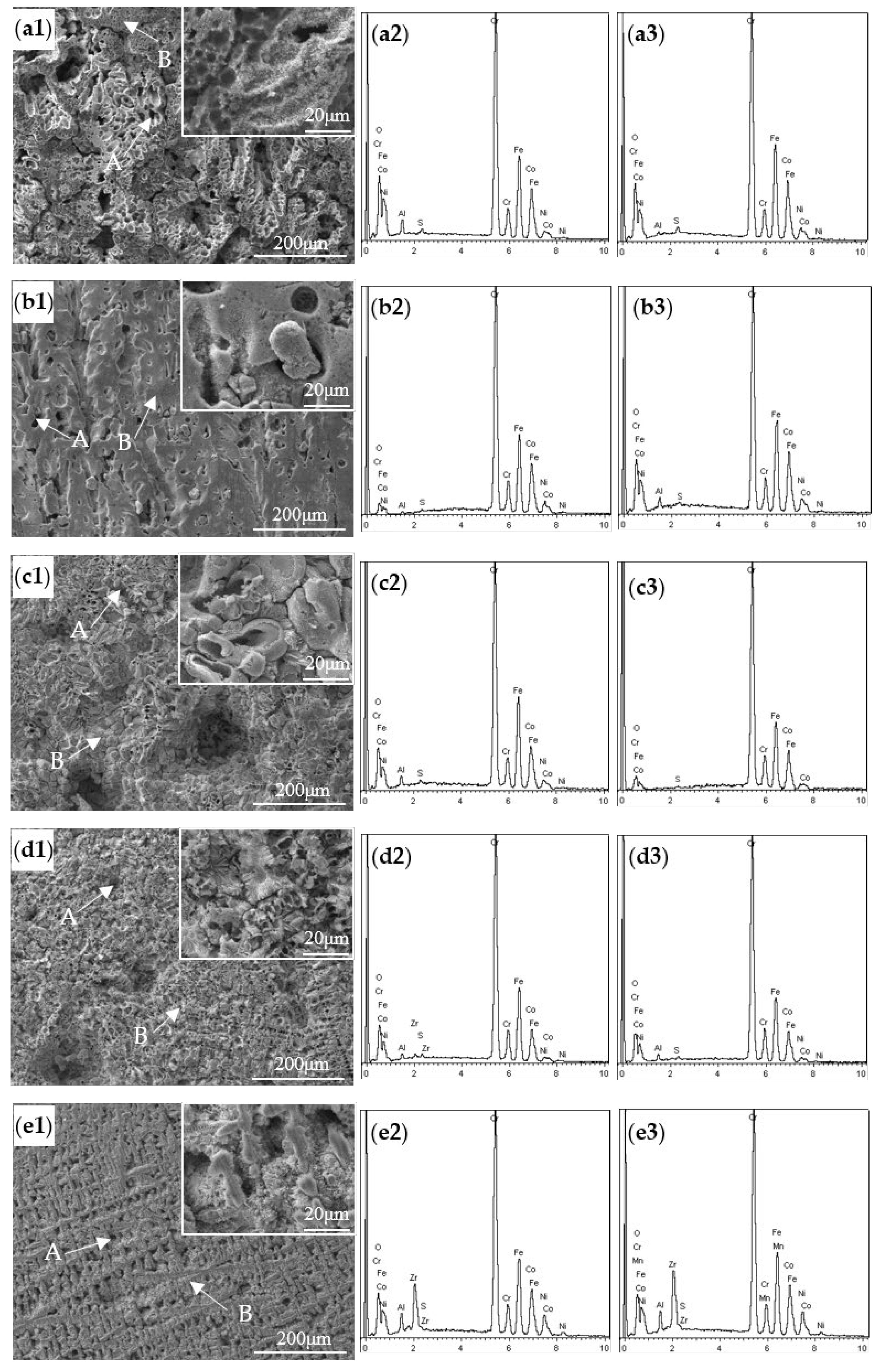
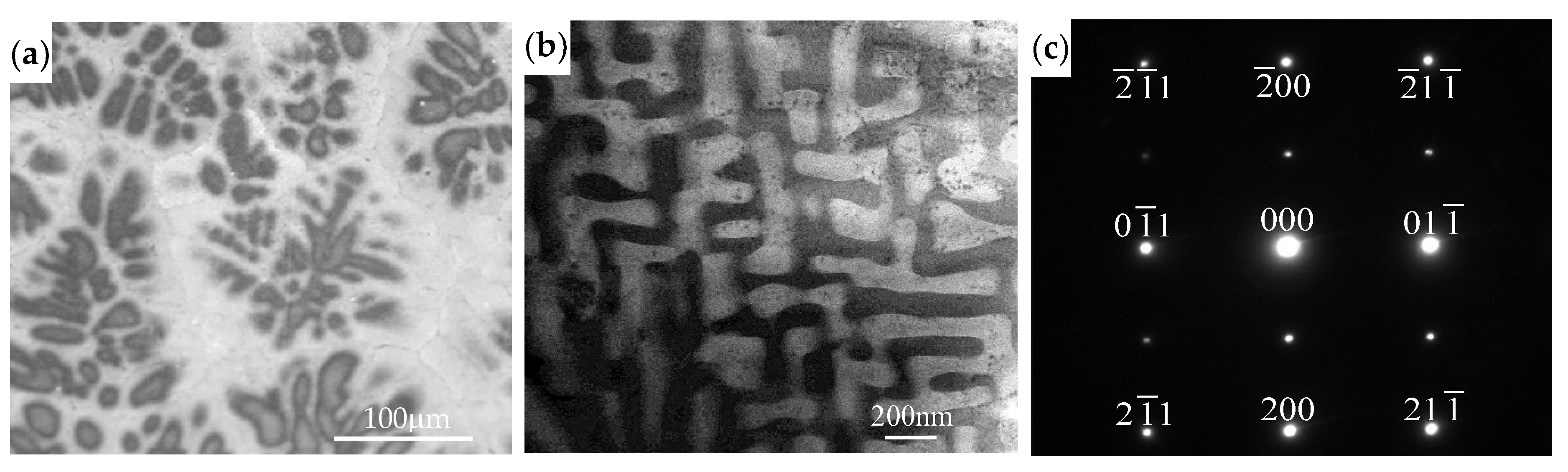
- During the immersion and electrochemical tests of the AlCoCrFeNiZrx alloys in 0.5 M H2SO4 solution, galvanic corrosion occurred both in the dendritic zone for an Al- and Ni-rich, Cr-depleted B2 phase as the anode coupled with the Fe–Cr-rich BCC phase, and in the interdendritic zone for an Al- and Ni-rich, Cr-depleted B2 phase as the anode coupled with the Zr-rich Laves phase.
- For both the immersion tests and the electrochemical measurements in 0.5 M H2SO4 solution, the corrosion properties of the AlCoCrFeNiZrx alloys first increased and then decreased with the increase in the Zr content.
- Zr addition at 0.1, 0.3, and 0.5 mol can be advantageous for improving the corrosion resistance of AlCoCrFeNi alloys. The Zr0.3 alloys presented the worst corrosion resistance to 0.5 M H2SO4 solution due to the serious galvanic corrosion attack on the Al- and Ni-rich, Cr-depleted B2 phases in both the dendrite and interdendrite, whereas the Zr0.1 alloys had the best corrosion resistance due to the segregation of the Zr-rich Laves phase along the grain boundary.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Pouraliakbar, H.; Shim, S.H.; Kim, Y.K.; Rizi, M.S.; Noh, H.; Hong, S.I. Microstructure evolution and mechanical properties of (CoCrNi)90(AlTiZr)5(CuFeMo)5 multicomponent alloy: A pathway through multicomponent alloys toward new superalloys. J. Alloys Compd. 2021, 860, 158412. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shim, S.H.; Lee, B.J.; Hong, S.I. Correlation between mechanical properties and thermodynamic parameters of dual-fcc-phase CoCrFeCuxNi (x = 1, 1.71) and CoCu1.71FeMnNi. Mater. Lett. 2020, 272, 127866. [Google Scholar] [CrossRef]
- Song, J.S.; Lee, B.J.; Moon, W.J.; Hong, S.I. Mechanical performance and microstructural evolution of (NiCo)75Cr17Fe8Cx (x = 0–0.83) medium entropy alloys at room and cryogenic temperatures. Metals 2020, 10, 1646. [Google Scholar] [CrossRef]
- Kozelj, P.; Vrtnik, S.; Jelen, A.; Jazbec, S.; Jaglicic, Z.; Maiti, S.; Feuerbacher, M.; Steurer, W.; Dolinsek, J. Discovery of a superconducting high-entropy alloy. Phys. Rev. Lett. 2014, 113, 107001–107005. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [Green Version]
- Salishchev, G.A.; Tikhonovsky, M.A.; Shaysultanov, D.G.; Stepanov, N.D.; Kuznetsov, A.V.; Kolodiy, I.V.; Tortika, A.S.; Senkov, O.N. Effect of Mn and V on structure and mechanical properties of high-entropy alloys based on CoCrFeNi system. Alloy. Compd. 2014, 591, 11–21. [Google Scholar] [CrossRef]
- Lu, C.L.; Lu, S.Y.; Yeh, J.W.; Hsu, W.K. Thermal expansion and enhanced heat transfer in high-entropy alloys. J. Appl. Crystallogr. 2013, 46, 736–739. [Google Scholar] [CrossRef]
- Xia, S.Q.; Wang, Z.; Yang, T.F.; Zhang, Y. Irradiation behavior in high entropy alloys. J. Iron Steel Res. Int. 2015, 22, 879–884. [Google Scholar] [CrossRef]
- Qiu, Y.; Thomas, S.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion of high entropy alloys. NPJ Mater. Degrad. 2017, 1, 15. [Google Scholar] [CrossRef]
- Kao, Y.F.; Lee, T.D.; Chen, S.K.; Chang, Y.S. Electrochemical passive properties of AlxCoCrFeNi (x = 0, 0.25, 0.50, 1.00) alloys in sulfuric acids. Corros. Sci. 2010, 52, 1026–1034. [Google Scholar] [CrossRef]
- Lee, C.P.; Chang, C.C.; Chen, Y.Y.; Yeh, J.W.; Shih, H.C. Effect of the aluminum content of AlxCrFe1.5MnNi0.5 high-entropy alloys on the corrosion behaviour in aqueous environments. Corros. Sci. 2008, 50, 2053–2060. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Yang, B.; Xie, X.; Brechtl, J.; Dahmen, K.A.; Liaw, P.K. Corrosion of AlxCoCrFeNi high-entropy alloys: Al-content and potential scan-rate dependent pitting behavior. Corros. Sci. 2017, 119, 33–45. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, M.L.; Cui, H.Z.; Zhao, Y.Q.; Song, X.J.; Zeng, Y.; Gao, X.H.; Lu, F.; Wang, C.M.; Song, Q. Effects of Ti-to-Al ratios on the phases, microstructures, mechanical properties, and corrosion resistance of Al2-xCoCrFeNiTix high-entropy alloys. J. Alloys Compd. 2019, 805, 585–596. [Google Scholar] [CrossRef]
- Qiu, Y.; Thomas, S.; Fabijanic, D.; Barlow, A.J.; Fraser, H.L.; Birbilis, N. Microstructural evolution, electrochemical and corrosion properties of AlxCoCrFeNiTiy high entropy alloys. Mater. Des. 2019, 170, 107698. [Google Scholar] [CrossRef]
- Yang, J.; Wu, J.; Zhang, C.Y.; Zhang, S.D.; Yang, B.J.; Emori, W.; Wang, J.Q. Effects of Mn on the electrochemical corrosion and passivation behavior of CoFeNiMnCr high-entropy alloy system in H2SO4 solution. J. Alloys Compd. 2020, 819, 152943. [Google Scholar] [CrossRef]
- Rodriguez, A.; Tylczak, J.H.; Ziomek-Moroz, M. Corrosion behavior of CoCrFeMnNi high-entropy alloys (HEAs) under aqueous acidic conditions. ECS Trans. 2017, 77, 741–752. [Google Scholar] [CrossRef]
- Luo, H.; Li, Z.M.; Mingers, A.M.; Raabe, D. Corrosion behavior of an equiatomic CoCrFeMnNi high-entropy alloy compared with 304 stainless steel in sulfuric acid solution. Corros. Sci. 2018, 191, 131–139. [Google Scholar] [CrossRef]
- Pao, L.; Muto, I.; Sugawara, Y. Pitting at inclusions of the equiatomic CoCrFeMnNi alloy and improving corrosion resistance by potentiodynamic polarization in H2SO4. Corros. Sci. 2021, 191, 109748. [Google Scholar] [CrossRef]
- Hsu, K.M.; Chen, S.H.; Lin, C.S. Microstructure and corrosion behavior of FeCrNiCoMnx (x = 1.0, 0.6, 0.3, 0) high entropy alloys in 0.5 M H2SO4. Corros. Sci. 2021, 190, 109694. [Google Scholar] [CrossRef]
- Chou, Y.L.; Yeh, J.W.; Shih, H.C. The effect of molybdenum on the corrosion behaviour of the high-entropy alloys Co1.5CrFeNi1.5Ti0.5Mox in aqueous environments. Corros. Sci. 2010, 52, 2571–2581. [Google Scholar] [CrossRef]
- Cui, H.B.; Zheng, L.F.; Wang, J.Y. Microstructure evolution and corrosion behavior of directionally solidified FeCoNiCrCu high entropy alloy. Appl. Mech. Mater. 2011, 66–68, 146–149. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, S.; Zhang, C.H.; Zhang, H.; Dong, S.Y. Phase evolution and cavitation erosion-corrosion behavior of FeCoCrAlNiTix high entropy alloy coatings on 304 stainless steel by laser surface alloying. J. Alloys Compd. 2017, 698, 761–770. [Google Scholar] [CrossRef]
- Xiang, C.; Zhang, Z.M.; Fu, H.M.; Han, E.H.; Zhang, H.F.; Wan, J.Q. Microstructure and corrosion behavior of AlCoCrFeNiSi0.1 high-entropy alloy. Intermetallics 2019, 114, 106599. [Google Scholar] [CrossRef]
- Niu, Z.Z.; Xu, J.; Wang, T.; Wang, N.R.; Han, Z.H.; Wang, Y. Microstructure, mechanical properties and corrosion resistance of CoCrFeNiWx (x = 0, 0.2, 0.5) high entropy alloys. Intermetallics 2019, 112, 106550. [Google Scholar] [CrossRef]
- Qiu, X.W. Corrosion behavior of Al2CrFeCoxCuNiTi high-entropy alloy coating in alkaline solution and salt solution. Results Phys. 2019, 12, 1737–1741. [Google Scholar] [CrossRef]
- Wen, D.; Jiang, B.; Wang, Q.; Yu, F.; Li, X.; Tang, R.; Zhang, R.; Chen, G.; Dong, C. Influences of Mo/Zr minor-alloying on the phase precipitation behavior in modified 310S austenitic stainless steels at high temperatures. Mater. Des. 2017, 128, 34–46. [Google Scholar] [CrossRef]
- Yurchenko, N.Y.; Stepanov, N.D.; Gridneva, A.O.; Mishunin, M.V.; Salishchev, G.A. Effect of Cr and Zr on phase stability of refractory Al-Cr-Nb-Ti-V-Zr high-entropy alloys. J. Alloys Compd. 2018, 757, 403–414. [Google Scholar] [CrossRef]
- Chen, J.; Niu, P.Y.; Liu, Y.Z.; Lu, Y.K.; Wang, X.H.; Peng, Y.L.; Liu, J.N. Effect of Zr content on microstructure and mechanical properties of AlCoCrFeNi high entropy alloy. Mater. Des. 2016, 94, 39–44. [Google Scholar] [CrossRef]
- American Society for Testing of Materials. ASTM G31-72 (Reapproved 2004) Standard Practice for Laboratory Immersion Corrosion Testing of Metals; ASTM International: West Conshohocken, PA, USA, 2004; pp. 1–8. [Google Scholar]
- American Society for Testing of Materials. ASTM G5-14 Standard Reference Test Method for Making Potentiodynamic Anodic Polarization Measurements; ASTM International: West Conshohocken, PA, USA, 2004; pp. 1–9. [Google Scholar]
- American Society for Testing of Materials. ASTM G59-97 (Reapproved 2003) Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements; ASTM International: West Conshohocken, PA, USA, 2003; pp. 1–4. [Google Scholar]
- American Society for Testing of Materials. ASTM G102-89(2015)e1 Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements; ASTM International: West Conshohocken, PA, USA, 2015; pp. 1–7. [Google Scholar]
- Tong, C.J.; Chen, M.R.; Chen, S.K.; Yeh, J.W.; Shun, T.T.; Lin, S.J.; Chang, S.Y. Mechanical performance of the AlxCoCrCuFeNi high-entropy alloy system with multi-principal elements. Metall. Mater. Trans. A 2005, 36A, 1263–1271. [Google Scholar] [CrossRef]
- Moazzen, P.; Toroghinejad, M.R.; Karimzadeh, F.; Vleugels, J.; Ravash, H.; Cavaliere, P. Influence of zirconium addition on the microstructure, thermodynamic stability, thermal stability and mechanical properties of mechanical alloyed spark plasma sintered (MA-SPS) FeCoCrNi high entropy alloy. Powder Metall. 2018, 61, 405–416. [Google Scholar] [CrossRef]
- Tang, Z.; Senkov, O.N.; Parish, C.M.; Zhang, C.; Zhang, F.; Santodonato, L.J.; Wang, G.; Zhao, G.; Yang, F.; Liaw, P.K. Tensile ductility of an AlCoCrFeNi multi-phase highentropy alloy through hot isostatic pressing (HIP) and homogenization. Mater. Sci. Eng. A 2015, 647, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.F.; Xia, S.Q.; Liu, S.; Wang, C.X.; Liu, S.S.; Zhang, Y.; Xue, J.M.; Yan, S.; Wang, Y.G. Effects of AL addition on microstructure and mechanical properties of AlxCoCrFeNi High-entropy alloy. Mater. Sci. Eng. A 2010, 648, 15–22. [Google Scholar] [CrossRef]
- Wang, W.R.; Wang, W.L.; Wang, S.C.; Tsai, Y.C.; Lai, C.H.; Yeh, J.W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Mondal, K.; Murty, B.S.; Chatterjee, U.K. Electrochemical behavior of multicomponent amorphous and nanocrystalline Zr-based alloys in different environments. Corros. Sci. 2006, 48, 2212–2225. [Google Scholar] [CrossRef]
- Yen, C.C.; Lu, H.N.; Tsai, M.H.; Wo, B.W.; Lo, Y.C.; Wang, C.C.; Chang, S.Y.; Yen, S.K. Corrosion mechanism of annealed equiatomic AlCoCrFeNi tri-phase high entropy alloy in 0.5 M H2SO4 aerated aqueous solution. Corros. Sci. 2019, 157, 462–471. [Google Scholar] [CrossRef]
- Yamanaka, K.; Shiratori, H.; Mori, M.; Omura, K.; Fujieda, T.; Kuwabara, K.; Chiba, A. Corrosion mechanism of an equimolar AlCoCrFeNi high-entropy alloy additively manufactured by electron beam melting. NPJ Mater. Degrad. 2020, 24, 24. [Google Scholar] [CrossRef]
- Chen, S.K. Electrochemical passive properties of AlxCoCrFeNi (x = 0, 0.25, 0.50, 1.00) high-entropy alloys in sulfuric acids. In Corrosion Resistance; Shih, H., Ed.; IntechOpen: Taiwan, China, 2012; pp. 133–156. [Google Scholar]
- Lins, V.; Reis, G.; Araujo, C.; Matencio, T. Electrochemical impedance spectroscopy and linear polarization applied to evaluation of porosity of phosphate conversion coatings on electrogalvanized steels. Appl. Surf. Sci. 2006, 253, 2875–2884. [Google Scholar] [CrossRef]
- Guo, W.; Dmowski, W.; Noh, J.Y.; Rack, P.; Liaw, P.K.; Egami, T. Local atomic structure of a high-entropy alloy: An-ray and neutron scattering study. Metall. Mater. Trans. A 2012, 44, 1994–1997. [Google Scholar] [CrossRef]
- Hurtado, M.; Sumodjo, P.; Benedetti, A.V. Electrochemical studies with a Cu-5 wt% Ni alloy in 0.5 M H2SO4. Electrochim. Acta 2003, 48, 2791–2798. [Google Scholar] [CrossRef]
- Metikos-Hukovic, M.; Babic, R.; Brinic, S. EIS-in situ characterization of anodic films on antimony and lead-antimony alloys. J. Power Sources 2006, 157, 563–570. [Google Scholar] [CrossRef]
- Trueman, A.R. Determining the probability of stable pit initiation on aluminium alloys using potentiostatic electrochemical measurements. Corros. Sci. 2005, 47, 2240–2256. [Google Scholar] [CrossRef]


| Alloy (wt %) | D | EW | ||||||
|---|---|---|---|---|---|---|---|---|
| Al (26.96) | Fe (55.85) | Cr (52.00) | Co (58.93) | Ni (58.69) | Zr (91.22) | |||
| Zr0 | 10.688 | 22.122 | 20.596 | 23.344 | 23.249 | 0 | 6.718 | 21.038 |
| Zr0.1 | 10.316 | 21.353 | 19.881 | 22.52 | 22.441 | 3.488 | 6.710 | 21.094 |
| Zr0.2 | 9.967 | 20.630 | 19.207 | 21.775 | 21.681 | 6.740 | 6.704 | 21.148 |
| Zr0.3 | 9.644 | 19.961 | 18.584 | 21.053 | 20.978 | 9.780 | 6.697 | 21.198 |
| Zr0.5 | 9.051 | 18.734 | 17.443 | 19.780 | 19.689 | 15.303 | 6.685 | 21.291 |
| Alloy | Regions | Al | Fe | Cr | Co | Ni | Zr |
|---|---|---|---|---|---|---|---|
| Zr0 | interdendritic | 8.71 | 24.29 | 23.06 | 23.08 | 20.86 | - |
| dendritic | 12.34 | 21.96 | 18.52 | 23.19 | 23.99 | - | |
| Zr0.1 | interdendritic | 1.18 | 2.6 | 2.59 | 3.3 | 4.02 | 86.31 |
| dendritic | 10.45 | 21.03 | 22.24 | 23.52 | 22.76 | - | |
| Zr0.2 | interdendritic | 4.77 | 11.47 | 6.68 | 21.09 | 23.43 | 32.56 |
| dendritic | 13.05 | 18.51 | 21.67 | 23.63 | 23.14 | - | |
| Zr0.3 | interdendritic | 4.84 | 12.49 | 6.83 | 20.54 | 22.74 | 32.56 |
| dendritic | 15.52 | 17.69 | 15.98 | 23.14 | 27.67 | - | |
| Zr0.5 | interdendritic | 5.16 | 12.88 | 7.13 | 20.26 | 21.8 | 32.77 |
| dendritic | 7.47 | 23.81 | 37.91 | 18.72 | 12.09 | - |
| Alloys | Regions | Elements (wt %) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Al | Co | Cr | Fe | Ni | Zr | S | O | ||
| Zr0 | A | 0.86 | 18.73 | 44.33 | 26.16 | 3.50 | - | 0.39 | 6.03 |
| B | 0.31 | 20.30 | 41.43 | 27.86 | 4.43 | - | 0.52 | 5.15 | |
| Zr0.1 | A | 0.27 | 17.74 | 47.06 | 25.60 | 5.11 | - | 0.34 | 3.88 |
| B | 5.93 | 18.19 | 38.34 | 25.31 | 8.60 | - | 0.27 | 3.36 | |
| Zr0.2 | A | 1.00 | 16.17 | 44.58 | 29.36 | 3.71 | - | 0.36 | 4.82 |
| B | 0.84 | 16.26 | 43.86 | 29.03 | 5.40 | - | 0.30 | 4.31 | |
| Zr0.3 | A | 0.63 | 12.79 | 49.65 | 27.21 | 2.22 | 1.71 | 0.35 | 5.44 |
| B | 0.79 | 13.94 | 48.83 | 25.77 | 4.51 | - | 0.29 | 5.87 | |
| Zr0.5 | A | 1.76 | 15.27 | 40.52 | 22.16 | 8.17 | 8.80 | 0.44 | 2.88 |
| B | 1.77 | 15.86 | 37.67 | 21.89 | 8.53 | 10.58 | 0.31 | 3.39 | |
| Alloy | Ecorr (VSHE) | Icorr (A·cm−2) | Ip (A·cm−2) | Ep (VSHE) | I’p (A·cm−2) | Epit (VSHE) |
|---|---|---|---|---|---|---|
| Zr0 | −0.491 | 2.30 × 10−4 | 6.00 × 10−3 | −0.207 | 1.89 × 10−4 | 0.988 |
| Zr0.1 | −0.510 | 1.02 × 10−4 | 3.32 × 10−3 | −0.169 | 1.84 × 10−4 | 1.008 |
| Zr0.2 | −0.524 | 2.81 × 10−4 | 7.13 × 10−3 | −0.186 | 1.93 × 10−4 | 1.001 |
| Zr0.3 | −0.525 | 4.06 × 10−4 | 3.58 × 10−2 | −0.184 | 3.70 × 10−4 | 1.010 |
| Zr0.5 | −0.489 | 1.36 × 10−4 | 2.30 × 10−3 | −0.195 | 1.23 × 10−4 | 1.005 |
| Alloy | Icorr (A·cm−2) | EW | KR (mm/year) | CR (mm/year) |
|---|---|---|---|---|
| Zr0 | 2.30 × 10−4 | 21.038 | 2.3552 | 1.301 |
| Zr0.1 | 1.02 × 10−4 | 21.094 | 1.0485 | 0.40915 |
| Zr0.2 | 2.81 × 10−4 | 21.148 | 2.8986 | 1.03614 |
| Zr0.3 | 4.06 × 10−4 | 21.198 | 4.2023 | 1.73494 |
| Zr0.5 | 1.36 × 10−4 | 21.291 | 1.4164 | 0.90121 |
| Alloy | Rs (Ω·cm2) | Cdl (F/cm2) | Rt (Ω·cm2) | Rct (Ω·cm2) | L (H) | Qdl (F/cm2) |
|---|---|---|---|---|---|---|
| Zr0 | 0.6796 ± 0.0097 | 1.03 ± 0.09 × 10−4 | 19.37 ± 1.69 | 47.0 ± 3.01 | 20.93 ± 1.79 | - |
| Zr0.1 | 0.3751 ± 0.0150 | 5.42 ± 0.319 × 10−5 | 41.81 ± 3.01 | 25.73 ± 1.96 | 35.18 ± 3.20 | - |
| Zr0.2 | 0.5057 ± 0.0131 | 2.587 ± 0.141 × 10−5 | 11.22 ± 0.44 | 50.12 ± 4.06 | - | 5.467 ± 0.0217 × 10−5 |
| Zr0.3 | 0.3933 ± 0.0130 | 1.359 ± 0.083 × 10−4 | 316.2 ± 25.04 | 67.94 ± 2.85 | 13.14 ± 1.08 | - |
| Zr0.5 | 0.1577 ± 0.0126 | 3.635 ± 0.222 × 10−5 | 97.92 ± 8.91 | 66.29 ± 5.17 | 32.42 ± 2.77 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Jin, Y.; Gao, W.; Liang, X.; Chen, J.; Zhu, S. Corrosion Behavior of AlFeCrCoNiZrx High-Entropy Alloys in 0.5 M Sulfuric Acid Solution. Metals 2021, 11, 1471. https://doi.org/10.3390/met11091471
Yao Y, Jin Y, Gao W, Liang X, Chen J, Zhu S. Corrosion Behavior of AlFeCrCoNiZrx High-Entropy Alloys in 0.5 M Sulfuric Acid Solution. Metals. 2021; 11(9):1471. https://doi.org/10.3390/met11091471
Chicago/Turabian StyleYao, Yuhong, Yaohua Jin, Wei Gao, Xiaoyu Liang, Jian Chen, and Shidong Zhu. 2021. "Corrosion Behavior of AlFeCrCoNiZrx High-Entropy Alloys in 0.5 M Sulfuric Acid Solution" Metals 11, no. 9: 1471. https://doi.org/10.3390/met11091471
APA StyleYao, Y., Jin, Y., Gao, W., Liang, X., Chen, J., & Zhu, S. (2021). Corrosion Behavior of AlFeCrCoNiZrx High-Entropy Alloys in 0.5 M Sulfuric Acid Solution. Metals, 11(9), 1471. https://doi.org/10.3390/met11091471





