Degradation Analysis of Thin Mg-xAg Wires Using X-ray Near-Field Holotomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Magnesium Wires
2.2. In Vitro Degradation
2.3. Near-Field Holotomography
2.4. Image Processing and Analysis
3. Results and Discussion
3.1. Material Analysis
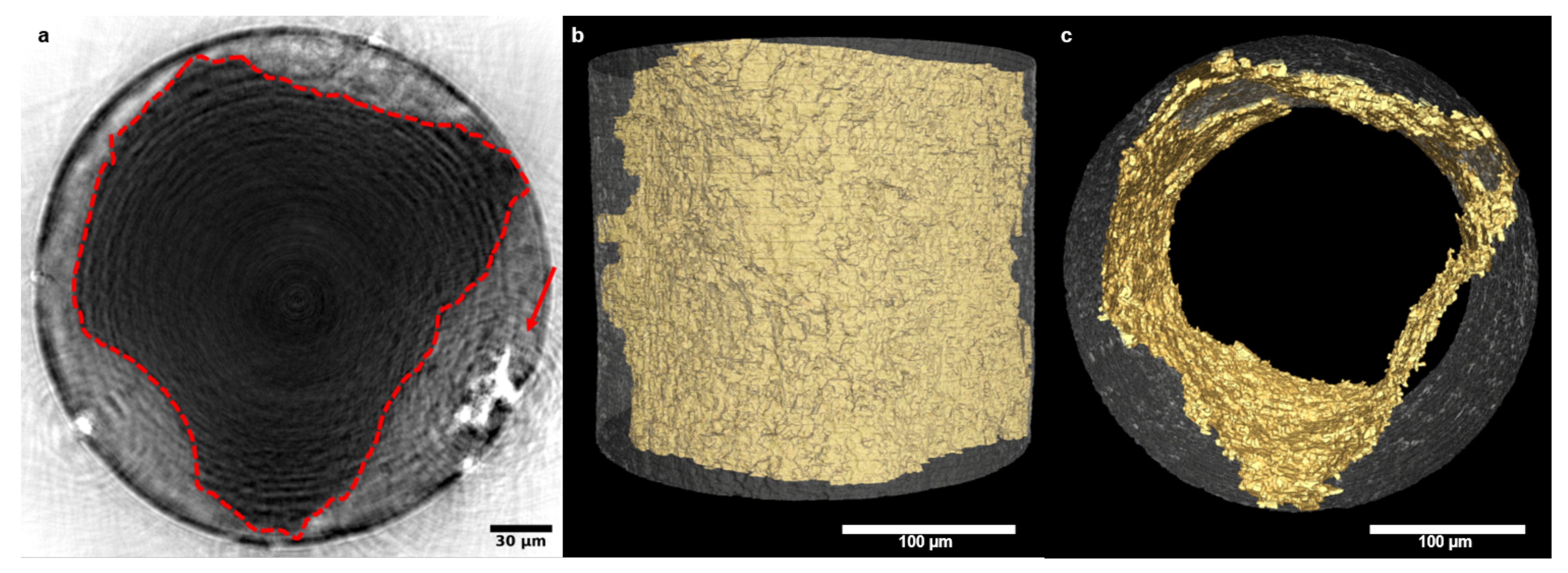
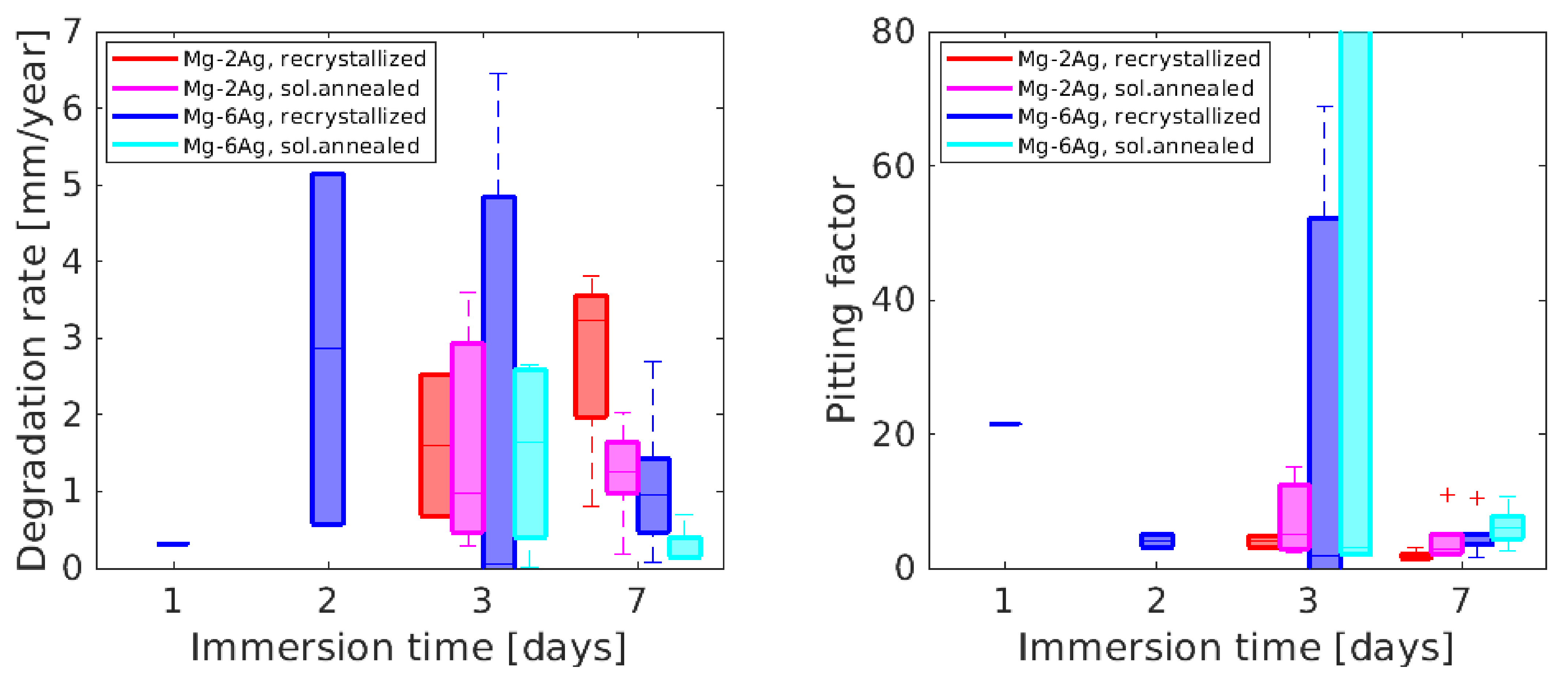
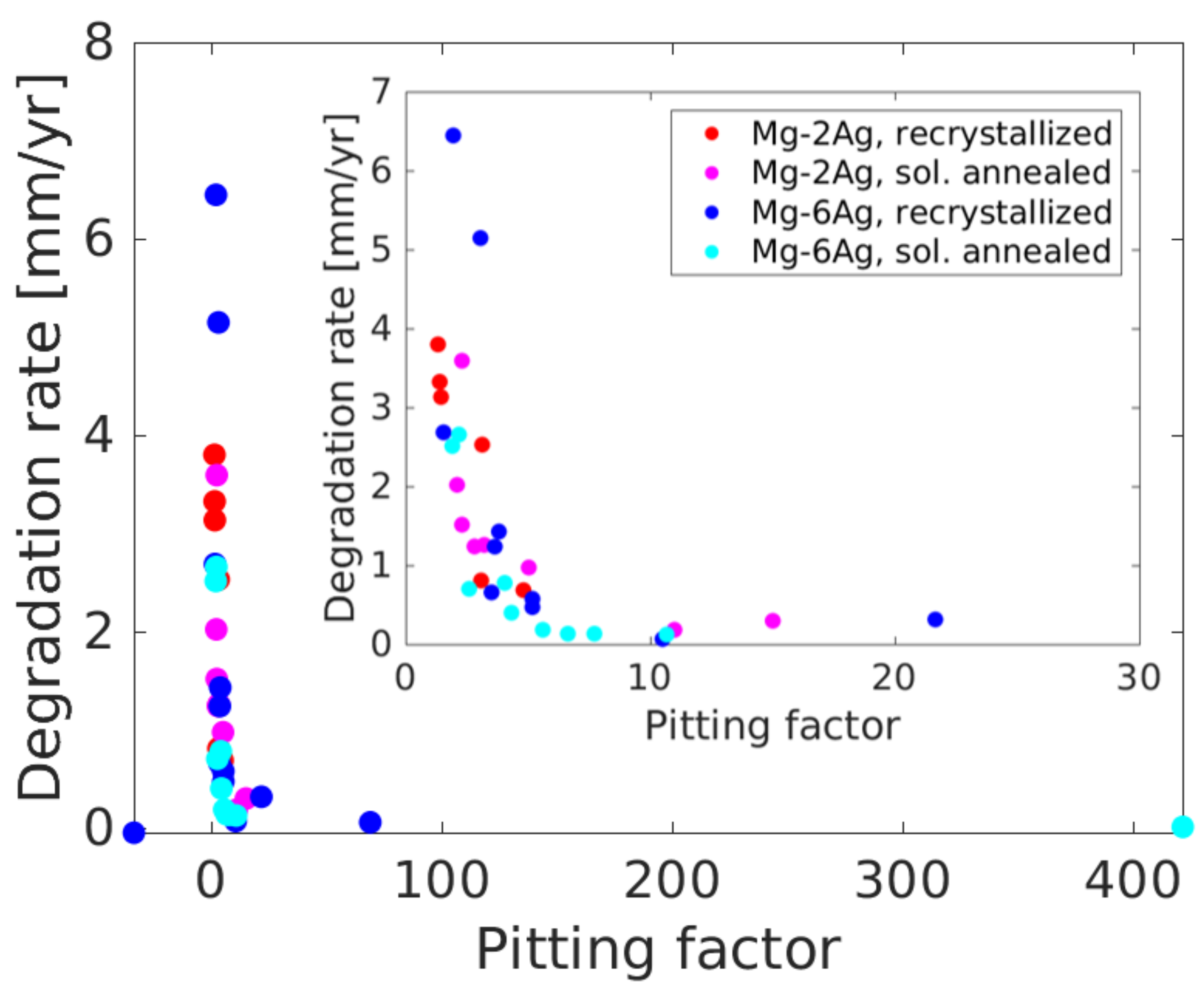
3.2. Degradation Analysis Based on NFHT
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SEM | Scanning electron microscopy |
| BSE | Back-scattering electron |
| TXM | Transmission X-ray microscopy |
| NFHT | Near-field holotomography |
| FOV | Field-of-view |
| OSA | Order sorting aperture |
Appendix A. Supplementary Figures
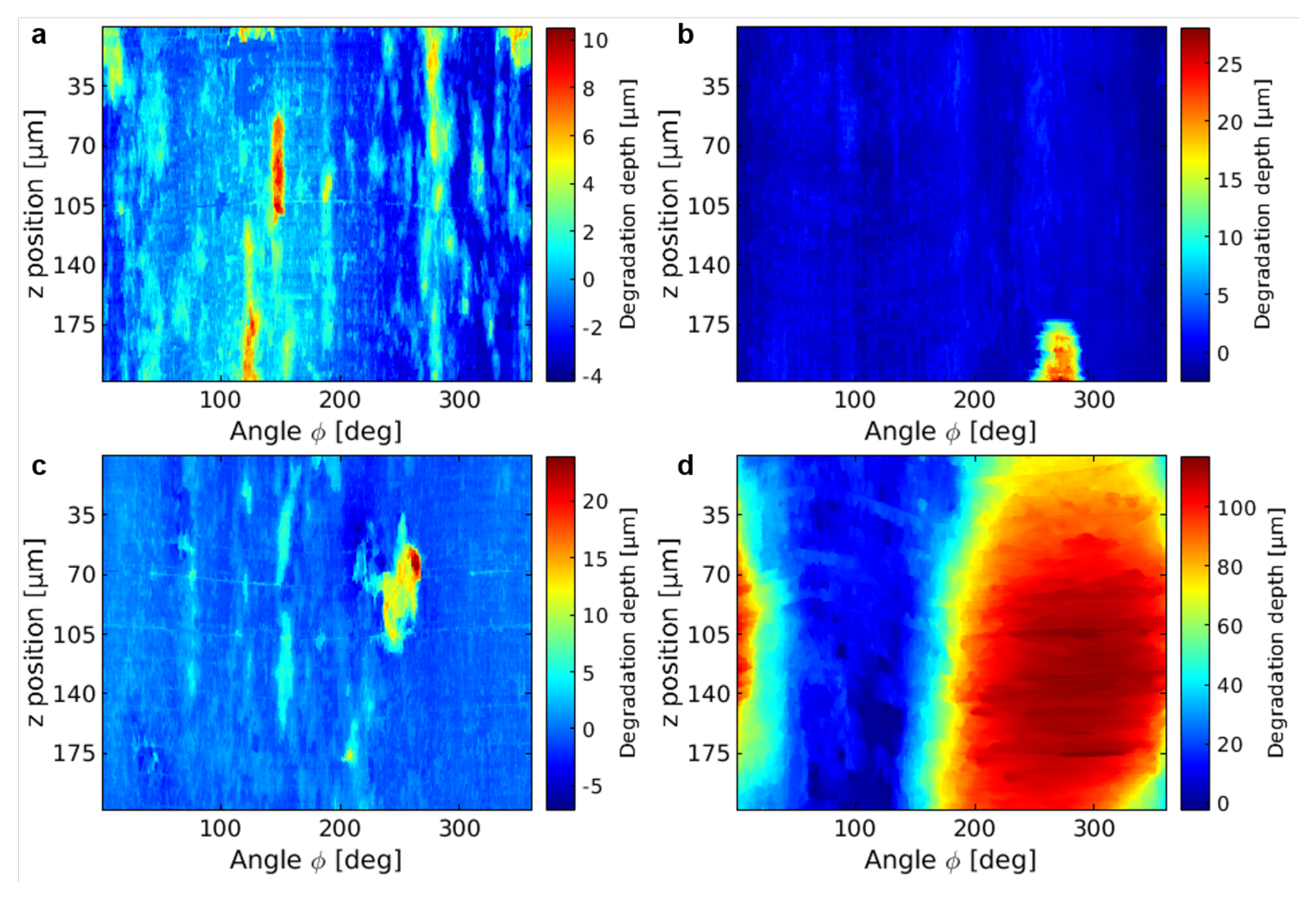
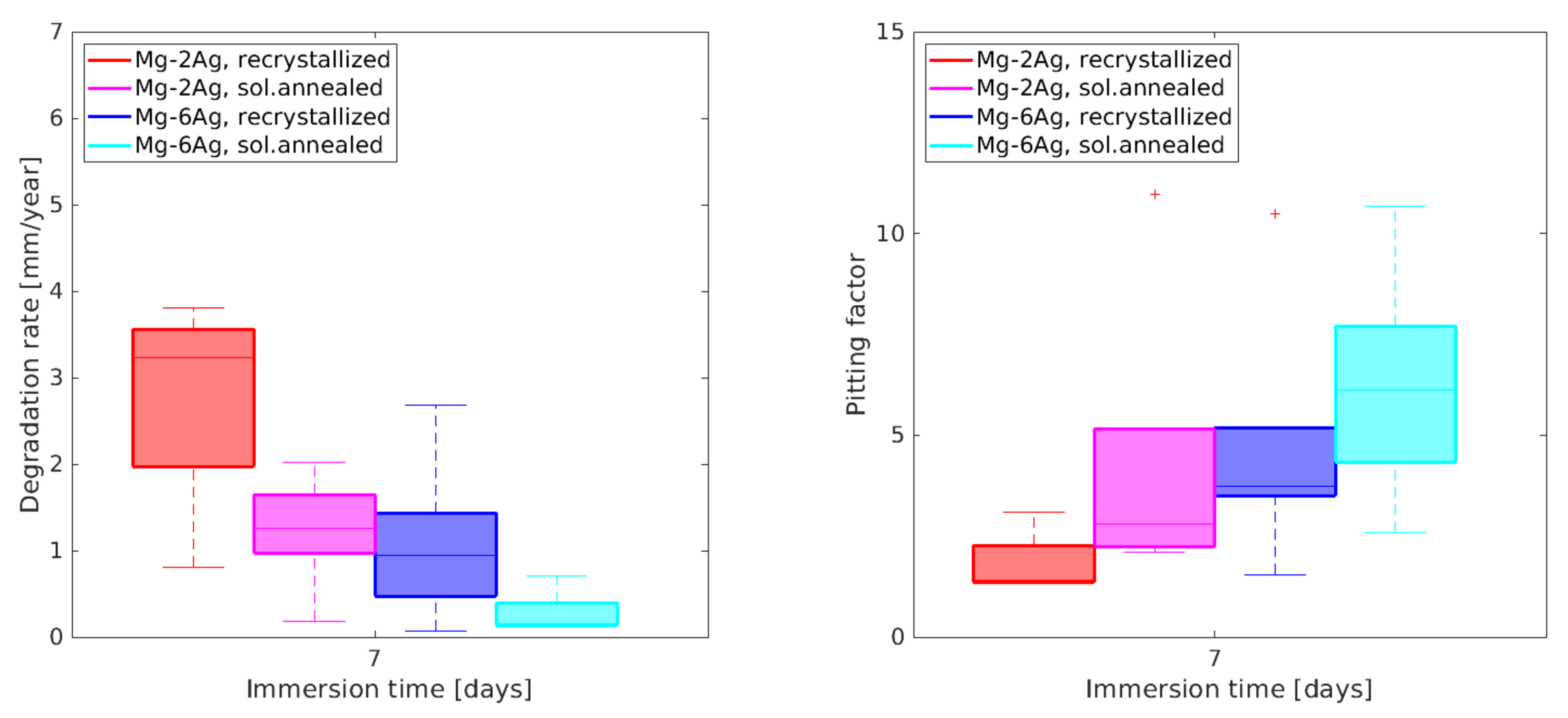
References
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Seitz, J.M.; Lucas, A.; Kirschner, M. Magnesium-Based Compression Screws: A Novelty in the Clinical Use of Implants. JOM 2016, 68, 1177–1182. [Google Scholar] [CrossRef]
- Bennett, J.; Hemptinne, Q.D.; McCutcheon, K. Magmaris resorbable magnesium scaffold for the treatment of coronary heart disease: overview of its safety and efficacy. Expert Rev. Med. Devices 2019, 16, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Huse, E.C. A New Ligature? Chic. Med. J. Exam. 1878, 38, 171–172. [Google Scholar]
- Seitz, J.M.; Utermöhlen, D.; Wulf, E.; Klose, C.; Bach, F.W. The Manufacture of Resorbable Suture Material from Magnesium—Drawing and Stranding of Thin Wires. Adv. Eng. Mater. 2011, 13, 1087–1095. [Google Scholar] [CrossRef]
- Rostock, P. Ist das Magnesium als Naht- und Sehienungsmaterialfiir Knochenoperationen geeignet? Arch. Orthop. Unf.-Chir. 1937, 38, 486–492. [Google Scholar] [CrossRef]
- Alaneme, K.; Okotete, E. Enhancing plastic deformability of Mg and its alloys—A review of traditional and nascent developments. J. Magnes. Alloy. 2017, 5, 460–475. [Google Scholar] [CrossRef]
- Wiese, B.; Willumeit-Römer, R.; Letzig, D.; Bohlen, J. Alloying effect of silver in magnesium on the development of microstructure and mechanical properties by indirect extrusion. J. Magnes. Alloy. 2021, 9, 112–122. [Google Scholar] [CrossRef]
- Liu, Z.; Schade, R.; Luthringer, B.; Hort, N.; Rothe, H.; Müller, S.; Liefeith, K.; Willumeit-Römer, R.; Feyerabend, F. Influence of the Microstructure and Silver Content on Degradation, Cytocompatibility, and Antibacterial Properties of Magnesium-Silver Alloys in Vitro. Oxidative Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nienaber, M.; Yi, S.; Kainer, K.U.; Letzig, D.; Bohlen, J. On the Direct Extrusion of Magnesium Wires from Mg-Al-Zn Series Alloys. Metals 2020, 10, 1208. [Google Scholar] [CrossRef]
- Kustra, P.; Milenin, A.; Byrska-Wójcik, D.; Grydin, O.; Schaper, M. The process of ultra-fine wire drawing for magnesium alloy with the guaranteed restoration of ductility between passes. J. Mater. Process. Technol. 2017, 247, 234–242. [Google Scholar] [CrossRef]
- Tie, D.; Feyerabend, F.; Hort, N.; Hoeche, D.; Kainer, K.; Willumeit, R.; Mueller, W. In vitro mechanical and corrosion properties of biodegradable Mg-Ag alloys. Mater. Corros. 2014, 65, 569–576. [Google Scholar] [CrossRef]
- Liu, Z.; Feyerabend, F.; Bohlen, J.; Willumeit-Römer, R.; Letzig, D. Mechanical properties and degradation behavior of binary magnesium-silver alloy sheets. J. Phys. Chem. Solids 2019, 133, 142–150. [Google Scholar] [CrossRef]
- Tie, D.; Feyerabend, F.; Müller, W.D.; Schade, R.; Liefeith, K.; Kainer, K.; Willumeit, R. Antibacterial biodegradable Mg-Ag alloys. Eur. Cells Mater. 2013, 25, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Estrin, Y.; Martynenko, N.; Anisimova, N.; Temralieva, D.; Kiselevskiy, M.; Serebryany, V.; Raab, G.; Straumal, B.; Wiese, B.; Willumeit-Römer, R.; et al. The effect of equal-channel angular pressing on the microstructure, the mechanical and corrosion properties and the anti-tumor activity of magnesium alloyed with silver. Materials 2019, 12, 3832. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, J.; Hou, R.Q.; Nidadavolu, E.P.S.; Willumeit-Römer, R.; Feyerabend, F. Magnesium degradation under physiological conditions—Best practice. Bioact. Mater. 2018, 3, 174–185. [Google Scholar] [CrossRef]
- van Gaalen, K.; Gremse, F.; Benn, F.; McHugh, P.E.; Kopp, A.; Vaughan, T.J. Automated ex-situ detection of pitting corrosion and its effect on the mechanical integrity of rare earth magnesium alloy—WE43. Bioact. Mater. 2021. [Google Scholar] [CrossRef]
- Zeller-Plumhoff, B.; Tolnai, D.; Wolff, M.; Greving, I.; Hort, N.; Willumeit-Römer, R. Utilizing Synchrotron Radiation for the Characterization of Biodegradable Magnesium Alloys—From Alloy Development to the Application as Implant Material. Adv. Eng. Mater. 2021, 2100197. [Google Scholar] [CrossRef]
- Zeller-Plumhoff, B.; Helmholz, H.; Feyerabend, F.; Dose, T.; Wilde, F.; Hipp, A.; Beckmann, F.; Willumeit-Römer, R.; Hammel, J.U. Quantitative characterization of degradation processes in situ by means of a bioreactor coupled flow chamber under physiological conditions using time-lapse SRµCT. Mater. Corros. 2018, 69, 298–306. [Google Scholar] [CrossRef]
- Zeller-Plumhoff, B.; Laipple, D.; Slominska, H.; Iskhakova, K.; Longo, E.; Hermann, A.; Flenner, S.; Greving, I.; Storm, M.; Willumeit-Römer, R. Evaluating the morphology of the degradation layer of pure magnesium via 3D imaging at resolutions below 40 nm. Bioact. Mater. 2021, 6, 4368–4376. [Google Scholar] [CrossRef]
- Bartels, M.; Krenkel, M.; Haber, J.; Wilke, R.N.; Salditt, T. X-ray holographic imaging of hydrated biological cells in solution. Phys. Rev. Lett. 2015, 114, 48103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cloetens, P.; Ludwig, W.; Baruchel, J.; Van Dyck, D.; Van Landuyt, J.; Guigay, J.P.; Schlenker, M. Holotomography: Quantitative phase tomography with micrometer resolution using hard synchrotron radiation x rays. Appl. Phys. Lett. 1999, 75, 2912–2914. [Google Scholar] [CrossRef] [Green Version]
- Kree, V.; Bohlen, J.; Letzig, D.; Kainer, K. The metallographical examination of magnesium alloys [Metallographische Gefügeuntersuchungen von Magnesiumlegierungen]. Prakt. Metallogr./Pract. Metallogr. 2004, 41, 233–246. [Google Scholar]
- Flenner, S.; Kubec, A.; David, C.; Storm, M.; Schaber, C.F.; Vollrath, F.; Müller, M.; Greving, I.; Hagemann, J. Hard X-ray nano-holotomography with a Fresnel zone plate. Opt. Express 2020, 28, 37514–37525. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, J.; Töpperwien, M.; Salditt, T. Phase retrieval for near-field X-ray imaging beyond linearisation or compact support. Appl. Phys. Lett. 2018, 113, 041109. [Google Scholar] [CrossRef] [Green Version]
- Gürsoy, D.; De Carlo, F.; Xiao, X.; Jacobsen, C. TomoPy: A framework for the analysis of synchrotron tomographic data. Proc. Spie Int. Soc. Opt. Eng. 2014, 9212. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Bian, M.; Huang, X.; Chino, Y. Microstructures and Mechanical Properties of Precipitation-Hardenable Magnesium-Silver-Calcium Alloy Sheets. Metals 2020, 10, 1632. [Google Scholar] [CrossRef]
- Flenner, S.; Storm, M.; Kubec, A.; Longo, E.; Döring, F.; Pelt, D.M.; David, C.; Müller, M.; Greving, I. Pushing the temporal resolution in absorption and Zernike phase contrast nanotomography: enabling fast in situ experiments. J. Synchrotron Radiat. 2020, 27, 1339–1346. [Google Scholar] [CrossRef]
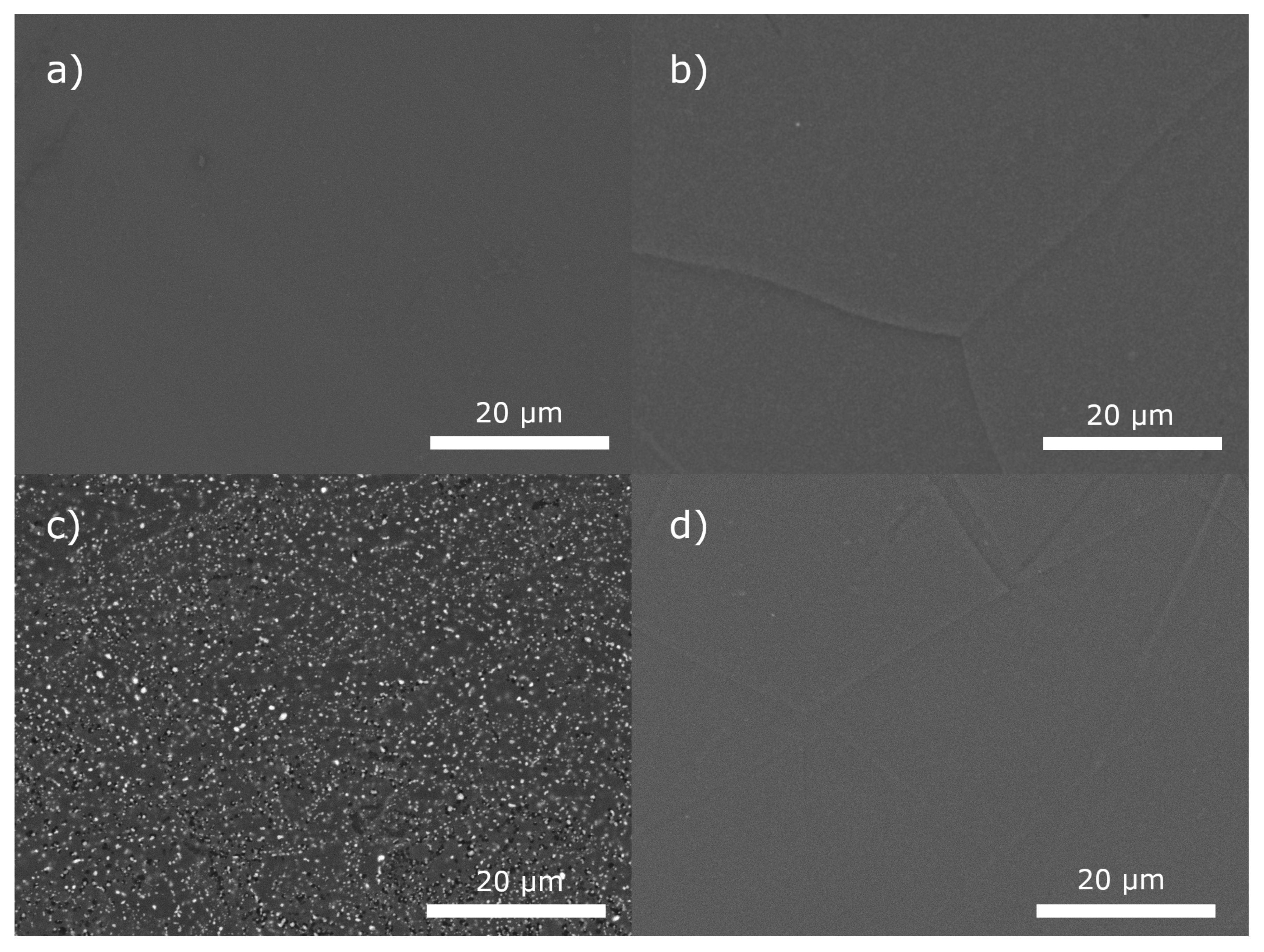
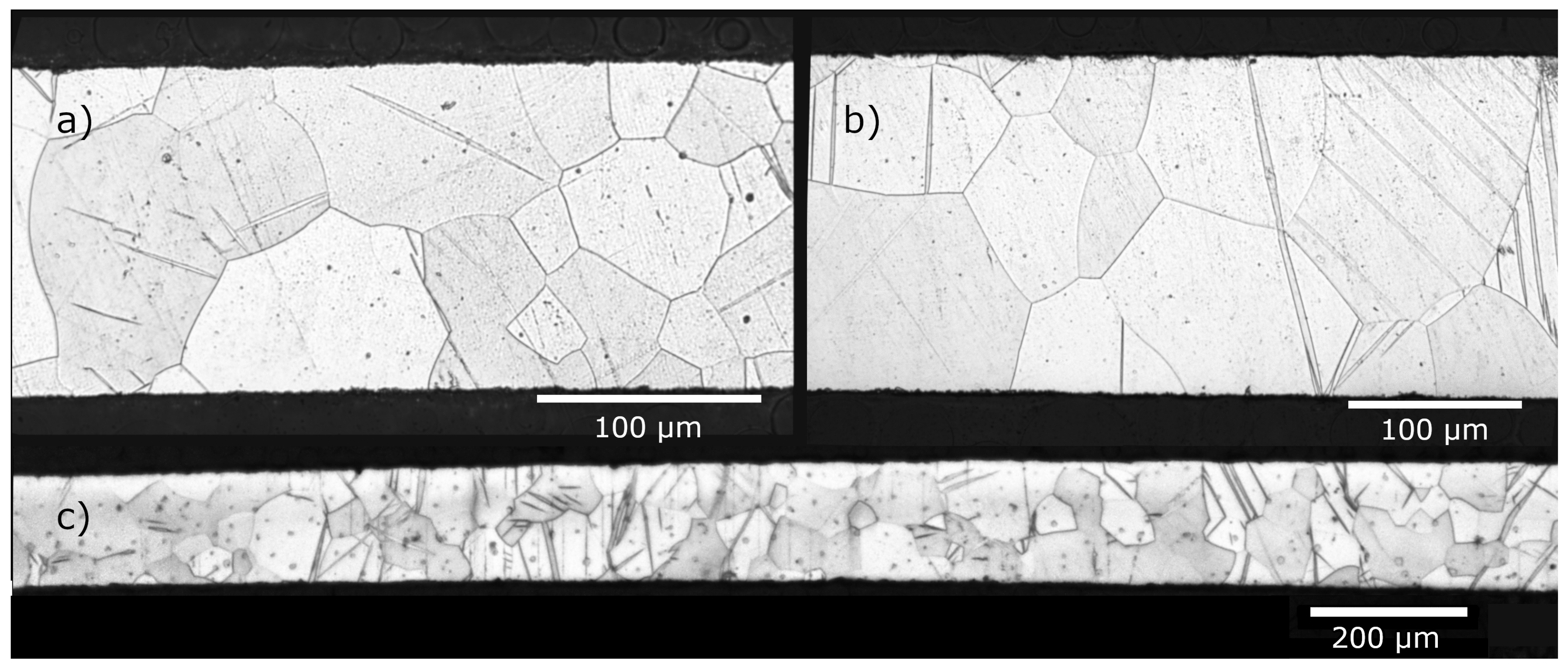
| Alloy | Ag (wt.%) | Fe (wt.%) | Ni (wt.%) | Cu (wt.%) |
|---|---|---|---|---|
| Mg-2Ag | 1.97 (±0.06) | 0.0027 (±0.0006) | 0.004 (±0.002) | 0.0013 (±0.005) |
| Mg-6Ag | 5.99 (±0.20) | 0.0016 (±0.0002) | <0.0002 | 0.0004 (±0.001) |
| Degradation Time (Days) | Mg-2Ag Recrystallized | Mg-2Ag sol. Annealed | Mg-6Ag Recrystallized | Mg-6Ag sol. Annealed |
|---|---|---|---|---|
| 0 | – | 1 | – | 1 |
| 1 | – | – | 1 | – |
| 2 | – | – | 2 | – |
| 3 | 4 | 3 | 3 | 4 |
| 7 | 4 | 5 | 6 | 6 |
| Alloy | Recrystallized | Solution Annealed |
|---|---|---|
| Mg-2Ag | 10 ± 1 m | 40 ± 5 m |
| Mg-6Ag | 11 ± 1 m | 61 ± 10 m |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, S.; Wolf, A.; Sanders, D.; Iskhakova, K.; Ćwieka, H.; Bruns, S.; Flenner, S.; Greving, I.; Hagemann, J.; Willumeit-Römer, R.; et al. Degradation Analysis of Thin Mg-xAg Wires Using X-ray Near-Field Holotomography. Metals 2021, 11, 1422. https://doi.org/10.3390/met11091422
Meyer S, Wolf A, Sanders D, Iskhakova K, Ćwieka H, Bruns S, Flenner S, Greving I, Hagemann J, Willumeit-Römer R, et al. Degradation Analysis of Thin Mg-xAg Wires Using X-ray Near-Field Holotomography. Metals. 2021; 11(9):1422. https://doi.org/10.3390/met11091422
Chicago/Turabian StyleMeyer, Sebastian, Andreas Wolf, Daniela Sanders, Kamila Iskhakova, Hanna Ćwieka, Stefan Bruns, Silja Flenner, Imke Greving, Johannes Hagemann, Regine Willumeit-Römer, and et al. 2021. "Degradation Analysis of Thin Mg-xAg Wires Using X-ray Near-Field Holotomography" Metals 11, no. 9: 1422. https://doi.org/10.3390/met11091422
APA StyleMeyer, S., Wolf, A., Sanders, D., Iskhakova, K., Ćwieka, H., Bruns, S., Flenner, S., Greving, I., Hagemann, J., Willumeit-Römer, R., Wiese, B., & Zeller-Plumhoff, B. (2021). Degradation Analysis of Thin Mg-xAg Wires Using X-ray Near-Field Holotomography. Metals, 11(9), 1422. https://doi.org/10.3390/met11091422







