Abstract
A new bauxite-type vanadium ore with a grade of 0.96% V2O5 was found in Hanzhong, China, having quartz, illite, and calcite as the main mineral constituents and vanadium that mainly occurs in the aluminosilicate lattice of illite by replacing Al3+ with V3+ in isomorphism form. In this study, a novel process of low-temperature sulfating roasting–water leaching is presented to extract vanadium from the bauxite-type vanadium ore. Addition of sulfuric acid enhanced the conversion of vanadium to NaVO3 in the sulfating roasting process, and addition of ammonium molybdate improved the leaching efficiency in water leaching. The results showed that a leaching efficiency of 90.33% was obtained under optimal test conditions. The calculation results of standard Gibbs free energy (ΔrGθ) further verified that the formation of NaVO3 is feasible.
1. Introduction
Vanadium is a strategic transition metal that has been extensively utilized in the steelmaking, green chemistry, energy storage, and the aviation industries owing to its excellent hardness, high tensile strength and resistance to corrosion [1,2]. The estimated total reserves of vanadium resources in the world amount to approximately 38 million tons, and China, ranking third in the world, is extremely rich in vanadium resources [3]. At present, the processes for extracting vanadium from its ore include sodium roasting, calcification roasting, non-salt roasting, and direct acid leaching [4].
The basic principle of sodium roasting is that during the roasting process of vanadium ore and sodium salts (Na2CO3, Na2SO4, NaCl, etc.), the structure of the vanadium ore is destroyed by high temperature, and the low-valent vanadium is oxidized to high-valent vanadium to the maximum extent, converted into sodium vanadate, and then leached with water to obtain a vanadium-containing solution; the final vanadium product is obtained from the vanadium-containing solution by precipitation or calcination [5,6,7]. The sodium roasting extraction process was first proposed by Brick in 1912 and has been widely used for vanadium extraction in China since the 1980s. On the one hand, it is a simple process that requires simple equipment, but on the other hand, it emits a large number of corrosive gases (HCl, Cl2, SO2, etc.) during the roasting process, which corrode the equipment and seriously pollute the air [4,8,9]. Li [10] studied the asynchronous extraction of vanadium and chromium from vanadium slag using a stepwise sodium roasting–water leaching process. Under the following optimal conditions, that is, a roasting temperature of 1073 K, V/Na molar ratio of 0.30, roasting time of 180 min, leaching temperature of 298 K, liquid-to-solid ratio of 3:1, and leaching time of 60 min, the leaching efficiency approached 87.9%. Zhao [11] used 6 wt.% NaCl and 10 wt.% Na2SO4 as composite additives for vanadium extraction from stone coal by roasting and explored the mineral phase transition of the roasting process. It was found that vanadium-bearing muscovite and quartz were converted into feldspar group minerals during the roasting process.
To solve the problems of low conversion rate and toxic gas pollution of the sodium roasting process, researchers proposed a calcification roasting process. Zhang [12] studied the formation mechanism of vanadate in the calcification roasting process of vanadium slag and explored the influence of roasting process conditions on efficiency of vanadium oxidation. The maximum recovery rate of vanadium was 93.3% after roasting for 150 min at a roasting temperature of 1123 K, and m (CaO)/m (V2O5) was 0.42. Li [9] studied the selective leaching of vanadium from roasting vanadium slag with (NH4)2CO3 and found that under the following optimal conditions of liquid-to-solid ratio of 20:1, particle size of 45 to 74 μm, leaching temperature of 353 K, (NH4)2CO3 concentration of 600 g/L and leaching time of 70 min, the leaching efficiency of vanadium could reach up to 96.0%. Calcification roasting-acid leaching has the advantages of lower gas emission, less harmful wastewater, and less noxious solid waste pollution. However, owing to the selectivity of calcification roasting to vanadium ores, its application has limitations and cannot be applied to various types of vanadium ore [13].
In view of the disadvantages of calcification roasting, researchers have studied the processing technology of extracting vanadium by non-salt roasting. Non-salt roasting is a vanadium extraction process in which the vanadium ore is directly roasted and oxidized without any additives. Low-valent vanadium is directly converted into high-valent vanadium by atmospheric oxygen at high temperature, and a vanadium-containing solution is obtained by leaching [14]. Li [15] investigated the non-salt roasting process on vanadium slag, and studied the effects of particle size, oxygen concentration, and temperature on the rate of isothermal oxidation of vanadium slag were investigated. Maximum vanadium recovery rate of 92.8% was achieved by roasting the vanadium slag at 1173 K for 150 min in air. He [5] studied the process of non-salt roasting-alkali leaching of vanadium from stone coal. Under the following conditions of roasting temperature of 1123 K, roasting time of 180 min, NaOH concentration of 2 mol/L, leaching temperature of 363 K, leaching time of 120 min and liquid-to-solid ratio of 3:1, the leaching efficiency reached 88.38%. The non-salt roasting–leaching process has the advantages of causing low pollution, having low economic cost, and high leaching efficiency [16]. However, it is only applicable to those ores, in which vanadium exists by adsorption or in independent minerals. For ores, in which most vanadium exists in by isomorphy in aluminosilicate, the oxidation efficiency and acid leaching efficiency are too low because of the lack of oxidation additives [17].
Different from the roasting-leaching process mentioned above, the direct leaching process has its unique advantages. Vanadium extraction by direct acid leaching is a simple process, with low energy consumption, and a good leaching index. Nejad [18] used direct acid leaching to recover vanadium from vanadium-titanium magnetite; leaching at a temperature of 365 K, nitric acid concentration by volume of 8.9%, H2SO4 concentration of 5.4 mol/L, and liquid-to-solid ratio of 7:1 for 240 min resulted in a leaching efficiency of 86%. Zhu [16] carried out a comparative test between direct leaching and post-roasting leaching of the residues of stone coal used to generate electricity. In the direct leaching test, a leaching efficiency of 74.49% was achieved by leaching at a H2SO4 concentration of 5.4% and a leaching temperature of 363 K for 480 min. In the post-roasting leaching test, after roasting at 1223 K for 60 min and then leaching at an H2SO4 concentration of 0.45%, the leaching efficiency obtained was 76.88%, which was only 2.39% higher than that of the direct leaching method. Deng [19] used an oxygen pressure acid leaching method to study a stone coal vanadium mine in Guizhou. The results showed that under the conditions of leaching time of 180–240 min, temperature of 423 K, H2SO4 dosage of 25–30%, liquid-solid ratio of 1.2:1, raw ore size less than 0.074 mm, additive dosage of 3–5%, and oxygen pressure of 1.2 MPa, the leaching efficiency of vanadium can reach over 92%.
Sodium roasting, calcification roasting, non-salt roasting, direct leaching, and other processes pose issues such as environmental pollution, low leaching efficiency, or selectivity toward certain raw ores. Researchers have made a lot of attempts to develop a cleaner and more effective vanadium ore treatment process. In 2008, Wang first proposed a low-temperature sulfating roasting process for vanadium extraction, which has the advantages of strong applicability, high decomposition efficiency, simple operation, and non-polluting gas production [20]. Wang [21] used sulfuric acid curing-column leaching technology to study stone coal vanadium ore. Under the conditions of raw ore particle size of <12 mm, curing sulfuric acid dosage of 20%, temperature of 398 K, curing for 240 min, and column leaching for 1440 min, the final leaching efficiency reached 83.5%. Shi [22] studied the process of extracting vanadium from shale by sulfuric acid roasting and leaching, and found that the leaching efficiency of vanadium approached 90.1% under the optimal roasting conditions of H2SO4 dosage of 52.5 wt.%, roasting temperature of 453–473 K and roasting time of 90 min. Although the sulfating roasting–water leaching process can achieve good technical indicators, the technology is not mature and in depth theoretical studies are still lacking.
Although there are many studies on the technology and mechanism of vanadium extraction from vanadium slag and stone coal, there are still some gaps in the research on the extraction of vanadium from bauxite–type vanadium ore. As a new type of vanadium ore, bauxite-type vanadium ore has extremely high development and utilization value, it is of great significance to effectively exploit and utilize vanadium resources in it to alleviate the growing demand for resources. In this study, a low-temperature sulfating roasting–water leaching vanadium extraction process was proposed to explore the optimal process conditions for efficient separation of vanadium, the mechanism of mineral phase transition and thermodynamic mechanism during the roasting process of vanadium were investigated to provide theoretical support for efficient separation and extraction of vanadium. X–ray diffraction (XRD), X–ray fluorescence spectrometry (XRF), scanning electron microscopy-X–ray energy spectroscopy (SEM–EDS), and other analytical methods were used to analyze the mineral phase transition and mechanism of the roasting process.
2. Materials and Methods
2.1. Sampling
The raw materials used in this study came from a mining area in Shaanxi Province, China. The ore samples obtained by mining with particle size < 10 mm were crushed to <1 mm by a laboratory jaw crusher, roller crusher and disk crusher, and then a laboratory conical ball mill was used to grind the samples to a specific particle size (such as <0.250 mm, <0.150 mm, <0.096 mm, etc.). Then the phase analysis of samples was carried out by XRD and the results are shown in Figure 1. The main chemical components of the samples were examined using XRF, and the results are listed in Table 1. The main phases in the vanadium mineral were quartz (SiO2), illite ((K, H3O)Al2Si3AlO10(OH)2), and a small amount of calcite (CaCO3). Illite is a mica clay mineral with a dioctahedral structure similar to mica, and its chemical properties are stable. In this bauxite-type vanadium ore, vanadium is mainly present in illite by replacing Al3+ in the aluminosilicate mineral crystal lattice with V3+ in isomorphic form. Table 1 shows that the V2O5 grade in this sample was 0.96%, which is a low-grade vanadium ore. The other major chemical groups are silica (SiO2), iron oxide (Fe2O3), alumina (Al2O3), and calcium oxide (CaO), at relative amounts of 51.86%, 9.26%, 18.42%, and 8.66%, respectively. The main chemical reagents used in this test were sulfuric acid (H2SO4) and ammonium molybdate ((NH4)6Mo7O24), which were analytically pure and produced by the Tianjin Institute of Chemical Reagents Co., Ltd. (Tianjin, China).

Figure 1.
X-ray diffraction (XRD) diffractogram of vanadium ore.

Table 1.
Main chemical components of the vanadium ore (%).
2.2. Experimental
Low-temperature sulfating roasting was performed in a 100 mL ceramic crucible. Thirty grams of vanadium ore of different granularity (particle sizes were <0.250 mm, <0.150 mm, <0.096 mm, <0.074 mm and <0.045 mm, respectively) was added into the cup, and then 13 mL of distilled water and calculated amounts of sulfuric acid (98%) was mixed under stirring. The cup was placed in a resistance furnace to roast at a preset temperature (423–623 K) for 60–180 min. After the furnace cooled to room temperature, the roasted material was crushed to particle size < 0.150 mm and leaching for 120 min under the conditions of liquid-solid ratio of 3:1 and leaching temperature of 333 K. The leached solution and the leached residue were obtained via filtration. The amount of vanadium in the vanadium-containing solution was determined by inductively coupled plasma emission spectrometer (ICP, ICAP6500, ThermoFisher Scientific, MA, USA) and the leaching efficiency was calculated. The experiment was repeated thrice to obtain an average value. The leaching efficiency was calculated using Equation (1):
where, η is the leaching efficiency of vanadium, %; V is the volume of leachate, L; C is the concentration of vanadium in leaching solution, g/L (which was determined by ICP); m is the mass of the sample, g; α is the grade of V2O5 in the sample, %.
2.3. Analysis and Characterization
The vanadium concentration in the leaching solution was analyzed by ICP (ICAP6500, ThermoFisher Scientific). The microstructures of the samples were examined using SEM (LE0440, Leica Cambridge Ltd., Cambridge, UK). EDS (GENESIS 60S, EDAX, Pennsylvania, USA) was used to analyze the micro zone composition of the raw ore, roasted ore, and leaching residue. The main chemical components of the samples were analyzed using XRF (Axios, PANalytical B.V., Almelo, The Netherlands). The phase compositions of the samples were analyzed by XRD (D/Max-Ⅲ A, JEOL Ltd., Tokyo, Japan). The X-ray diffraction (XRD) patterns were recorded with Cu Kα X-ray radiation at 35 kV and 20 mA using a scan speed of 10° min−1 in 2θ ranges from 3° to 80°.
3. Results and Discussion
3.1. Thermodynamic Analysis
In bauxite-type vanadium ores, most of the vanadium occurs in the crystal structure of aluminosilicate minerals. Therefore, destroying the crystal structure of these vanadium-bearing minerals is a prerequisite for leaching vanadium [23]. The methods of destroying the lattice structure of aluminosilicate minerals are usually high-temperature calcination or H+ entry into the lattice to break the chemical bonds. However, high-temperature roasting causes high energy consumption and serious environmental pollution, which is undesirable for today’s increasingly strict environmental protection requirements. Sulfating roasting can not only effectively destroy the crystal lattice of aluminosilicate minerals with low energy consumption, but also oxidize V3+ into a valence state that is easy to leach. The main reaction equations for the sulfating roasting process are shown in Equations (2)–(6) [20]. When the temperature is below the boiling point of sulfuric acid (611 K), the sample reacts with liquid sulfuric acid (reaction (2) and (3)), and when the temperature reaches the boiling point of sulfuric acid, the sulfuric acid changes from liquid to gas (reaction (4)), and the sample reacts with gaseous sulfuric acid (reaction (5) and (6)). Figure 2 shows the calculation results of ΔrGθ in the temperature range 273–673 K.
2V2O3(s) + 4H2SO4(l) + O2(g) = 4VOSO4(s) + 4H2O(g)
O2(s) + H2SO4(l) = VOSO4(s) + H2O(g)
H2SO4(l) = H2SO4(g)
2V2O3(s) + 4H2SO4(g) + O2(g) = 4VOSO4(s) + 4H2O(g)
VO2(s) + H2SO4(g) = VOSO4(s) + H2O(g)
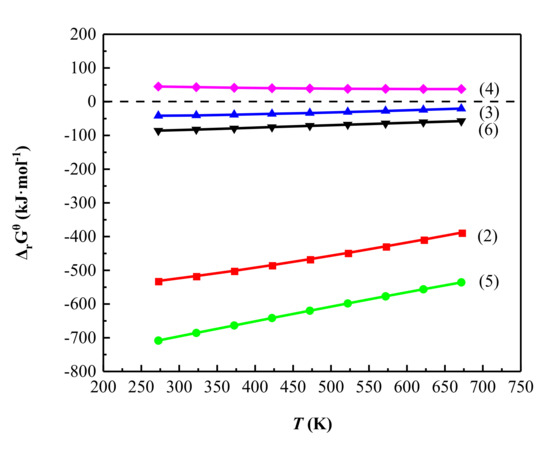
Figure 2.
Correlation of standard Gibbs free energy (ΔrGθ) with temperature for the reactions in Equations (2)–(6).
The ΔrGθ of the reactions is less than 0 in the range of 273–673 K, and the ΔrGθ of the reaction of the sample with liquid sulfuric acid is greater than that with gaseous sulfuric acid at the corresponding temperature. It is seen that the main reactions of the sulfating roasting process are thermodynamically feasible in this temperature range.
3.2. Effects of Roasting Parameters on Vanadium Extraction
3.2.1. Roasting Temperature
When the roasting temperature is low, the permeability of H2SO4 in the vanadium ore increases with increase in the temperature. However, when the temperature is too high, there is a significant increase in energy consumption [24], leading to a secondary reaction of converted vanadium, and massive decomposition of sulfuric acid, which weakens the roasting effect and reduces the conversion rate of the vanadium valence state. To determine the appropriate roasting temperature, the influence of roasting temperature on the roasting effect was investigated under the conditions of a roasting time of 120 min, H2SO4 dosage of 30 wt.%, and particle size of <0.150 mm.
Figure 3a shows that the leaching efficiency of vanadium is greatly affected by the roasting temperature. In the beginning, the leaching efficiency increased with the increase in the roasting temperature and when the roasting temperature reaches 523 K, the maximum leaching efficiency reaches 53.86%. As the roasting temperature continues to rise, the H2SO4 then begins to volatilize and decompose in large quantities, the utilization rate of H2SO4 decreases, and the leaching efficiency begins to decline sharply. Considering the energy consumption and vanadium leaching efficiency, the optimal roasting temperature was determined to be 523 K.
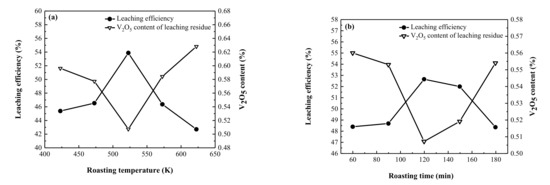
Figure 3.
Effects of (a) roasting temperature and (b) roasting time on vanadium extraction.
3.2.2. Roasting Time
Roasting time is an important factor affecting the roasting process. When the roasting time is too short, the reaction between H2SO4 and vanadium ore is insufficient, and the valence conversion rate of vanadium is very low. However, when the roasting time is too long, the energy consumption increases and the production efficiency decreases [25,26]. The influence of roasting time was investigated at a roasting temperature of 523 K, H2SO4 dosage of 30 wt.%, and particle size of <0.150 mm.
Figure 3b shows that, the leaching efficiency first increased and then decreased with an increase in roasting time. With the roasting time increasing from 60 min to 120 min, the leaching efficiency of vanadium increases from 48.37% to 52.64%, reaching the maximum value. But it was worth noting that when the roasting time rises further, the leaching efficiency decrease. Excessively increasing the roasting time may cause the oxidized vanadium to undergo a secondary reaction to produce a sintering phenomenon, causing the vanadium to be wrapped by silicon and oxygen, which is not conducive to the leaching of vanadium.
3.2.3. Particle Size of Vanadium Ore
When the ore particle size is finer, its lattice structure is more easily destroyed, and vanadium is more easily released. Under the conditions of a roasting temperature of 523 K, roasting time of 120 min, and H2SO4 dosage of 30 wt.%, the influence of particle size of raw ore on roasting was investigated. Figure 4a shows the change in the leaching efficiency with particle size. With a decrease in the raw ore particle size, the leaching efficiency of vanadium initially increases and then decreases. When the particle size was <0.250 mm, the leaching efficiency was 61.27%. When the size of the raw ore was <0.096 mm, the leaching efficiency reached a maximum of 67.15%. Further decrease in the particle size of the raw ore, decreases leaching efficiency. In the roasting process, a smaller particle size results in a larger specific surface area, which is conducive to the reaction of H2SO4 with it and promotes the valence state transformation of vanadium ion. When the particle size is too small, they may aggregate, resulting in a decrease in the specific surface area and hindering the destruction of the aluminosilicate crystal lattice by H2SO4. Therefore, it is considered that the optimal raw ore particle size is <0.096 mm.
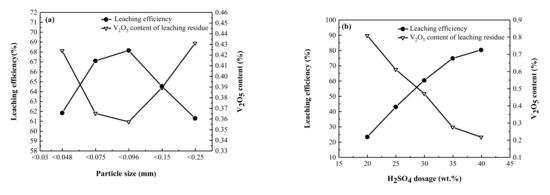
Figure 4.
Effects of (a) particle size of Vanadium Ore and (b) H2SO4 dosage on vanadium extraction.
3.2.4. H2SO4 Dosage
The dosage of H2SO4 is an important factor in the sulfate roasting process. When the dosage of H2SO4 is very low, the reaction between vanadium ore and H2SO4 is insufficient, the crystal lattice of aluminosilicate minerals is not effectively destroyed, and vanadium stored in it does not complete the valence state transformation. When the amount of H2SO4 is high, it aggravates the degree of corrosion on the equipment and increases the production cost, which is not desirable for industrial application. To determine the appropriate dosage of H2SO4, its influence was investigated at a roasting temperature of 523 K, roasting time of 120 min, and particle size of <0.096 mm.
Figure 4b shows that with the increase in H2SO4 dosage, the leaching efficiency shows a trend of continuous improvement. With the increase in H2SO4 dosage, the more the valence conversion rate of vanadium, thereby increasing the leaching efficiency. At H2SO4 dosage of 20 wt.%, the leaching efficiency of vanadium is 23.18%, but when the dosage of H2SO4 is increased to 40 wt.%, the leaching efficiency of vanadium approaches 80.83%. According to the overall relationship between the dosage of H2SO4 and leaching efficiency, it is expected that the increase rate of leaching efficiency will significantly reduce as the dosage of H2SO4 increases. Considering that excessive dosage of H2SO4 leads to serious corrosion of the equipment, increase in production cost, and too many impurities in the leach solution, which are factors not conducive to subsequent operations, the optimal dosage of sulfuric acid determined is 40 wt.%.
3.3. Repeated Tests
The single factor tests have shown that the optimum roasting conditions are a vanadium ore size of <0.096 mm, a roasting temperature of 523 K, a roasting time of 120 min and an H2SO4 dosage of 40 wt.%. However, under the existing conditions, the leaching efficiency is only 80.83%, which cannot achieve the ideal leaching effect. According to existing research, it was found that ammonium molybdate had a promoting effect on the leaching of silicate minerals, and 6 wt.% ammonium molybdate was added as a leaching additive to enhance vanadium extraction [27]. Ammonium molybdate reacts with sulfuric acid to form molybdate and ammonium sulfate in the leaching process, and molybdate reacts with aluminosilicate mineral to form a silica-molybdenum complex, as shown in Equations (7) and (8).
To validate test repeatability, multiple tests were carried out under the conditions of using ammonium molybdate. Table 2 shows that the leaching efficiency of vanadium in the repeated experiments could be increased approximately by 10%, by adding ammonium molybdate. Combining Table 2 and Table 3, it can be seen that the leaching efficiency of vanadium can reach 90.33% through the low-temperature sulfation roasting-water leaching process, and the residual V2O5 in the leaching residue is only 0.11%. In addition, the content of Fe2O3, Al2O3, K2O, etc. in the leaching slag is reduced compared with that of roasted ore. And Table 3 shows that the content of vanadium in the leaching residue was lower and most of the vanadium entered the leaching solution. This shows that low-temperature sulfation roasting can convert the components containing vanadium, iron, aluminum and other elements in the raw ore into a state that is easy to leach.
(NH4)6Mo7O24··4H2O(s) + 3H2SO4(l) = 7H2MoO4(l) + 3(NH4)2SO4(l)
K,H(Al,Mg,Fe)2(Si,Al)4O10(OH)2(H2O)(s) + H2MoO4(l) → K,H(Al,Mg,Fe)2[Si(Mo2O7)6](s) + H2O(l)

Table 2.
Results of the repeated tests of vanadium extraction.

Table 3.
Main chemical composition analysis of leaching residue (%).
3.4. Mechanism Analysis of the Roasting Process
3.4.1. Mineral Phase Transformation Analysis
The low-temperature sulfating roasting–water leaching process was used to treat the bauxite-type vanadium ore, and the leaching efficiency reached 90.33%, which realized the effective separation and enrichment of vanadium from vanadium ore. XRD was used to analyze the raw ore (Figure 1), roasting ore (Figure 5a), and water leaching residue (Figure 5b) to determine the changes in the vanadium-bearing mineral phases in the vanadium ore before and after roasting. Figure 1 shows that the main components of the ore are quartz (SiO2), illite ((K, H3O) Al2Si3AlO10(OH)2), and a small amount of calcite (CaCO3), and vanadium occurs in by isomorphism. Figure 5a shows that the main components of the roasting ore are quartz (SiO2), gypsum (CaSO4), munirite (NaVO3), and misenite (K8(SO4)(SO3OH)6); and quartz (SiO2) and gypsum (CaSO4) are the main components of the water leaching residue in Figure 5b. This indicates that illite reacts with H2SO4 in the process of roasting, and the crystal lattice of aluminosilicate mineral was effectively destroyed, which was separated from illite and reacted to form munirite and misenite, and then through water leaching treatment, aqueous munirite and misenite dissolve, and vanadium ion enters the solution.

Figure 5.
XRD diffractogram of (a) roasted ore and (b) leaching residue.
3.4.2. Analysis of Morphology and Microstructure
SEM and EDS analyses further verified the phase change of vanadium before and after roasting. Figure 6 and Figure 7 show the morphology analysis diagram and micro-area composition analysis of all the samples, respectively.
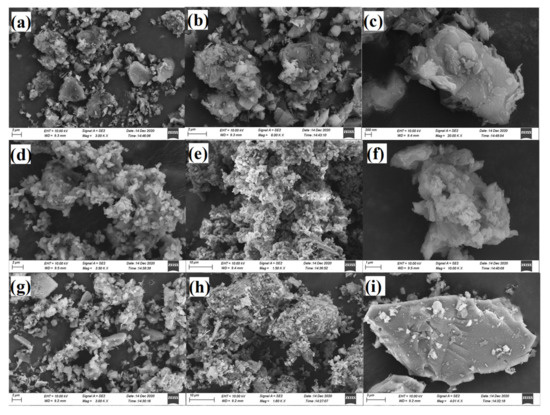
Figure 6.
SEM morphology analysis diagram of raw ore (a–c), roasted ore (d–f), and leaching residue (g–i).
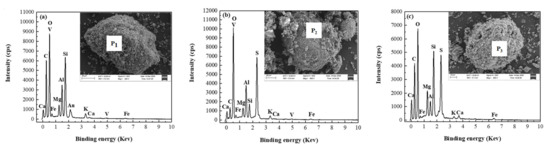
Figure 7.
SEM-EDS analysis of vanadium ore (a), roasted ore (b), and leaching residue (c).
According to SEM analysis, flake minerals exist in the raw ore Figure 6a,c, with large differences in mineral particle size and relatively dispersed distribution among particles without agglomeration. After roasting with H2SO4, there were no obvious flaked minerals in the roasted ore (Figure 6d–f), and the mineral particle size distribution was uniform.
Moreover, sintering caused the mineral particles to gather together and form porous microfine particle aggregates. In the leaching residue (Figure 6g–i), the distribution of mineral particles was uneven, flaky minerals were present, and the surface was relatively smooth. Due to the partial dissolution of mineral particles in the water leaching process, the aggregation among particles was no longer tight.
The EDS analysis results in Figure 7 show that the main chemical elements in the raw ore (Figure 7a) are O, Si, C, Al, V, Mg, Ca, etc.; in the roasting ore (Figure 7b) are O, S, Al, V, Si, Ca, etc.; and in the aqueous leaching residue (Figure 7c) are O, Si, S, C, Mg, Al, Ca, etc., without V. These results indicate that vanadium-bearing minerals dissolved in the water leaching process, and the vanadium entered the aqueous solution, thus achieving the extraction of vanadium.
3.4.3. Thermodynamic Analysis of the Main Chemical Reactions
Based on the results of the analysis, the possible reactions of the bauxite-type vanadium ore in the process of low-temperature sulfating roasting are shown in Equations (9)–(18), and the thermodynamic data of the reactions are calculated. In the process of roasting, V3+ in illite reacts with H2SO4, oxygen, and sodium in the mineral, and V3+ is oxidized to pentavalent vanadium, which is easily leached to produce sodium vanadate. Calcite reacted with H2SO4 to form calcium sulfate. Simultaneously, a small amount of potassium and aluminum in the raw ore reacted with H2SO4 to form potassium sulfate and aluminum sulfate, respectively. As seen in Figure 8, under the test conditions, the ΔrGθ values of the main reactions are all negative. Similarly, before and after the temperature reaches the boiling point of sulfuric acid (611K), the sample will react with liquid sulfuric acid (reactions (9)–(13)) or gaseous sulfuric acid (reactions (14)–(18)). By comparing Figure 8a,b, it can be found that the reactions of the sample with gaseous sulfuric acid are more easily carried out. Thermodynamic analysis of the above reactions proves the rationality and feasibility of the low-temperature sulfating roasting–water leaching process, which provides theoretical support for subsequent research on vanadium extraction from bauxite-type vanadium ore.
Na2O(s) + V2O3(s) + H2SO4(l) + O2(g) = 2NaVO3(s) + H2O(g) + SO3(g)
K2O(s) + H2SO4(l) = K2SO4(s) + H2O(g)
CaO(s) + H2SO4(l) = CaSO4(s) + H2O(g)
Al2O3(s) + 3H2SO4(l) = Al2(SO4)3(s) + 3H2O(g)
Fe2O3(s) + 3H2SO4(l) = Fe2(SO4)3(s) + 3H2O(g)
Na2O(s) + V2O3(s) + H2SO4(g) + O2(g) = 2NaVO3(s) + H2O(g) + SO3(g)
K2O(s) + H2SO4(g) = K2SO4(s) + H2O(g)
CaO(s) + H2SO4(g) = CaSO4(s) + H2O(g)
Al2O3(s) + 3H2SO4(g) = Al2(SO4)3(s) + 3H2O(g)
Fe2O3(s) + 3H2SO4(g) = Fe2(SO4)3(s) + 3H2O(g)
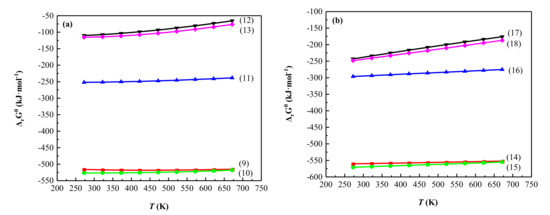
Figure 8.
Correlation of standard Gibbs free energy (ΔrGθ) with temperature for (a) reactions (9)–(13) and (b) reactions (14)–(18).
4. Conclusions
From the results presented in this work the following conclusions may be reached:
- (1)
- The bauxite-type vanadium ore with a lower V2O5 content of 0.96%, is comprised of mica, illite, and calcite. Vanadium occurs in illite aluminosilicate crystals by isomorphism, instead of Al3+, and extracting this vanadium is difficult.
- (2)
- A promising low-temperature sulfating roasting–water leaching process was used to recover vanadium from the vanadium ore. Sulfuric acid was added to promote the release of vanadium-bearing minerals during sulfating roasting and ammonium molybdate was added to enhance vanadium extraction during water leaching. The results of the effects of different roasting process parameters on vanadium extraction showed that an optimal vanadium extraction index with leaching efficiency of 90.33% was obtained under the following conditions: particle size of <0.096 mm, sulfating roasting temperature of 523 K, sulfating roasting time of 120 min, H2SO4 dosage of 40 wt.%, water leaching liquid/solid ratio of 3:1, water leaching temperature of 333 K, water leaching time of 120 min, and ammonium molybdate dosage of 6 wt.%.
- (3)
- The analysis of phase transformation mechanism of sulfating roasting shows that when illite, as the main vanadium-bearing mineral, reacts with H2SO4, the aluminosilicate crystal lattice is destroyed, and Na and V are released with H2SO4 to form NaVO3 and K8(SO4)(SO3OH)6, which are easily leached using water leaching. The thermodynamic calculation theory was in good agreement with the experimental results.
Author Contributions
This is a joint work of the five authors; each author was in charge of their expertise and capability: K.Z. for Writing, review, and conceptualization, J.X. for Software and validation, G.L. for Data curation, W.H. for Methodology, and W.X. for editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Key Laboratory of Guangdong Provincial Key Laboratory of Radioactive and Rare Resource Utilization (Grant No. 2018B030322009), the Sichuan Science and Technology Program (Grant Nos. 2021YJ0057, 2021YFG0268, and 2019FS0452), and the Research Fund Program of Innovation Center of Rare Earth Resources Development and Utilization, China Geological Survey (Grant No. 2021XTZX01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The authors alone are responsible for the content and writing of the article.
References
- Gao, F.; Olayiwola, A.U.; Liu, B.; Wang, S.; Du, H.; Li, J.; Wang, X.; Chen, D.; Zhang, Y. Review of Vanadium Production Part I: Primary Resources. Miner. Process. Extr. Met. Rev. 2021, 4, 1–23. [Google Scholar] [CrossRef]
- Zhang, G.; Luo, D.; Deng, C.; Lv, L.; Liang, B.; Li, C. Simultaneous extraction of vanadium and titanium from vanadium slag using ammonium sulfate roasting-leaching process. J. Alloys Compd. 2018, 742, 504–511. [Google Scholar] [CrossRef]
- USGS. 2010. Mineral Commodity Summaries. Available online: http://minerals.usgs.gov/minerals/pubs/mcs/ (accessed on 20 April 2011).
- Peng, H. A literature review on leaching and recovery of vanadium. J. Environ. Chem. Eng. 2019, 7, 103313. [Google Scholar] [CrossRef]
- He, D.; Feng, Q.; Zhang, G.; Ou, L.; Lu, Y. An environmentally-friendly technology of vanadium extraction from stone coal. Miner. Eng. 2007, 20, 1184–1186. [Google Scholar] [CrossRef]
- Tavakoli, M.; Dreisinger, D. Separation of vanadium from iron by solvent extraction using acidic and neutral organophosporus extractants. Hydrometallurgy 2014, 141, 17–23. [Google Scholar] [CrossRef]
- Van Vuuren, C.; Stander, P. The oxidation of FeV2O4 by oxygen in a sodium carbonate mixture. Miner. Eng. 2001, 14, 803–808. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Bao, S.-X.; Liu, T.; Chen, T.-J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Li, H.-Y.; Wang, K.; Hua, W.-H.; Yang, Z.; Zhou, W.; Xie, B. Selective leaching of vanadium in calcification-roasted vanadium slag by ammonium carbonate. Hydrometallurgy 2016, 160, 18–25. [Google Scholar] [CrossRef]
- Li, H.-Y.; Fang, H.-X.; Wang, K.; Zhou, W.; Yang, Z.; Yan, X.-M.; Ge, W.-S.; Li, Q.-W.; Xie, B. Asynchronous extraction of vanadium and chromium from vanadium slag by stepwise sodium roasting–water leaching. Hydrometallurgy 2015, 156, 124–135. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Zhang, Y.; Song, S.; Bao, S. In-situ investigation on mineral phase transition during roasting of vanadium-bearing stone coal. Adv. Powder Technol. 2017, 28, 1103–1107. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Zhang, L.; Gu, S. Mechanism of vanadium slag roasting with calcium oxide. Int. J. Miner. Process. 2015, 138, 20–29. [Google Scholar] [CrossRef]
- Xiang, J.; Huang, Q.; Lv, X.; Bai, C. Extraction of vanadium from converter slag by two-step sulfuric acid leaching process. J. Clean. Prod. 2018, 170, 1089–1101. [Google Scholar] [CrossRef]
- Li, L.T.; Zhu, P.W.; Shi, Z.L.; Jiang, X.; Zeng, W.Q. Additive-free roasting technique for extracting vanadium from resi-due of mica-type vanadium-bearing stone coal. Chin. J. Rare Met. 2014, 38, 480–486. [Google Scholar] [CrossRef]
- Li, M.; Zheng, S.; Liu, B.; Wang, S.; Dreisinger, D.; Zhang, Y.; Du, H.; Zhang, Y. A clean and efficient method for recovery of vanadium from vanadium slag: Non-salt roasting and ammonium carbonate leaching processes. Miner. Process. Extr. Met. Rev. 2017, 38, 228–237. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Zhang, G.-F.; Feng, Q.-M.; Lu, Y.-P.; Ou, L.-M.; Huang, S.-J. Acid leaching of vanadium from roasted residue of stone coal. Trans. Nonferrous Met. Soc. China 2010, 20, s107–s111. [Google Scholar] [CrossRef]
- Wang, M.; Xiang, X.; Zhang, L.; Xiao, L. Effect of vanadium occurrence state on the choice of extracting vanadium technology from stone coal. Rare Met. 2008, 27, 112–115. [Google Scholar] [CrossRef]
- Nejad, D.G.; Khanchi, A.R.; Taghizadeh, M. Recovery of Vanadium from Magnetite Ore Using Direct Acid Leaching: Optimization of Parameters by Plackett–Burman and Response Surface Methodologies. JOM 2018, 70, 1024–1030. [Google Scholar] [CrossRef]
- Deng, Z.-G.; Wei, C.; Fan, G.; Li, M.-T.; Li, C.-X.; Li, X.-B. Extracting vanadium from stone-coal by oxygen pressure acid leaching and solvent extraction. Trans. Nonferrous Met. Soc. China 2010, 20, s118–s122. [Google Scholar] [CrossRef]
- Liu, W.L.; Wang, X.W.; Wang, M.Y.; Hu, J.; Zhang, L.P. Mineral decomposition process of vanadium recovery from stone coal by low temperature sulphating roasting. Chin. J. Nonferrous Met. 2009, 19, 943–948. (In Chinese) [Google Scholar] [CrossRef]
- Wang, M.; Cheng, Q.; Qi, J.Y.; Li, J.; Ning, X.X.; Jin, J.P. Process of vanadium extraction from stone coal vanadium ore by sulfuric acid low temperature curing and column leaching. Min. Metall. 2020, 29, 62–67. (In Chinese) [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, Y.; Liu, T.; Huang, J. Vanadium Extraction from Shale via Sulfuric Acid Baking and Leaching. JOM 2018, 70, 1972–1976. [Google Scholar] [CrossRef]
- He, D.; Feng, Q.; Zhang, G.; Luo, W.; Ou, L. Study on leaching vanadium from roasted residue of stone coal. Min. Met. Explor. 2008, 25, 181–184. [Google Scholar] [CrossRef]
- Xiao, J.; Xiong, W.; Zou, K.; Chen, T.; Li, H.; Wang, Z. Extraction of Nickel from Magnesia–Nickel Silicate Ore. J. Sustain. Met. 2021, 7, 642–652. [Google Scholar] [CrossRef]
- Xiao, J.; Zou, K.; Chen, T.; Peng, Y.; Ding, W.; Chen, J.; Deng, B.; Li, H.; Wang, Z. Extraction of Sc from Sc-Bearing V–Ti Magnetite Tailings. JOM 2021, 73, 1836–1844. [Google Scholar] [CrossRef]
- Xiao, J.; Zou, K.; Chen, T.; Xiong, W.; Deng, B. Extraction of Manganese and Iron from a Refractory Coarse Manganese Concentrate. Metals 2021, 11, 563. [Google Scholar] [CrossRef]
- Tan, J.F.; Zhang, G.F.; Zhang, Y.; Zhang, Z.H. Aid-leaching reagent of leaching scandium from scandium concentrate in complex silicate. Chin. J. Rare Met. 2012, 2, 304–310. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).