Recovery of Copper(II) and Silver(I) from Nitrate Leaching Solution of Industrial Dust via Solvent Extraction with LIX63
Abstract
1. Introduction
2. Materials and Methods
2.1. Leaching Procedure
2.2. Materials
2.3. Solvent Extraction and Stripping
3. Results and Discussions
3.1. Effect of the HNO3 Concentration on the Extraction of Metal Ions
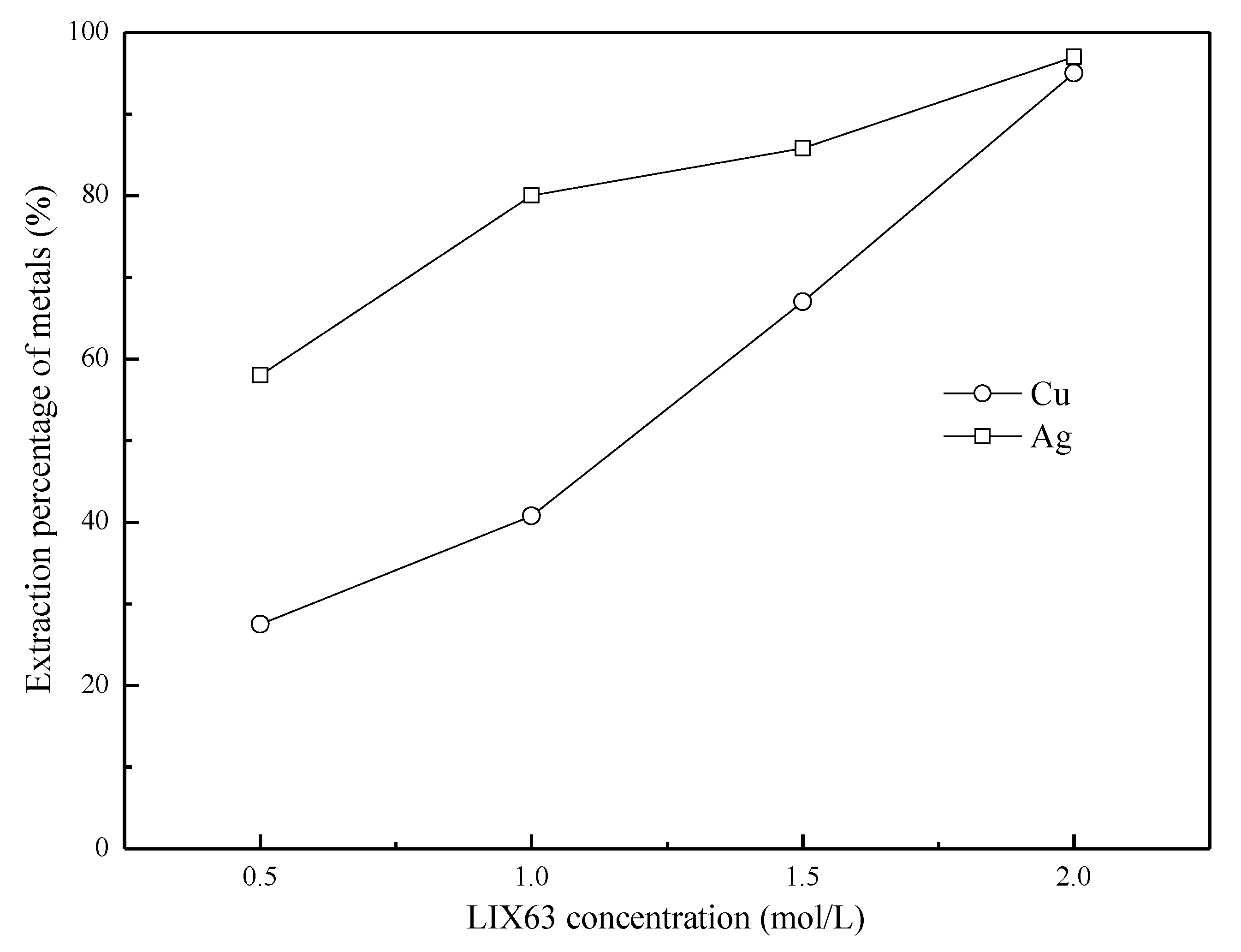
3.2. Effect of the LIX63 Concentration on the Extraction of Cu and Ag
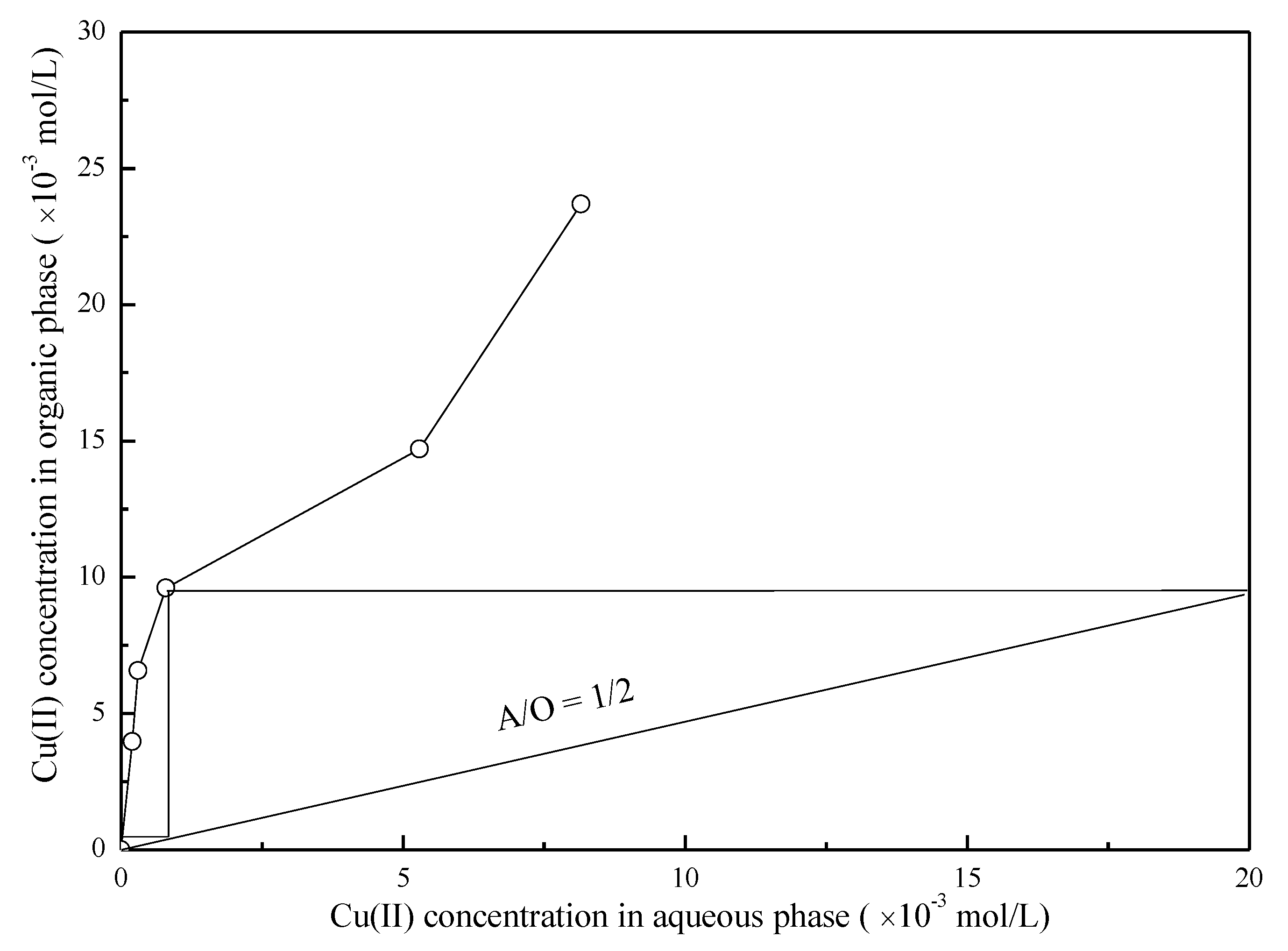
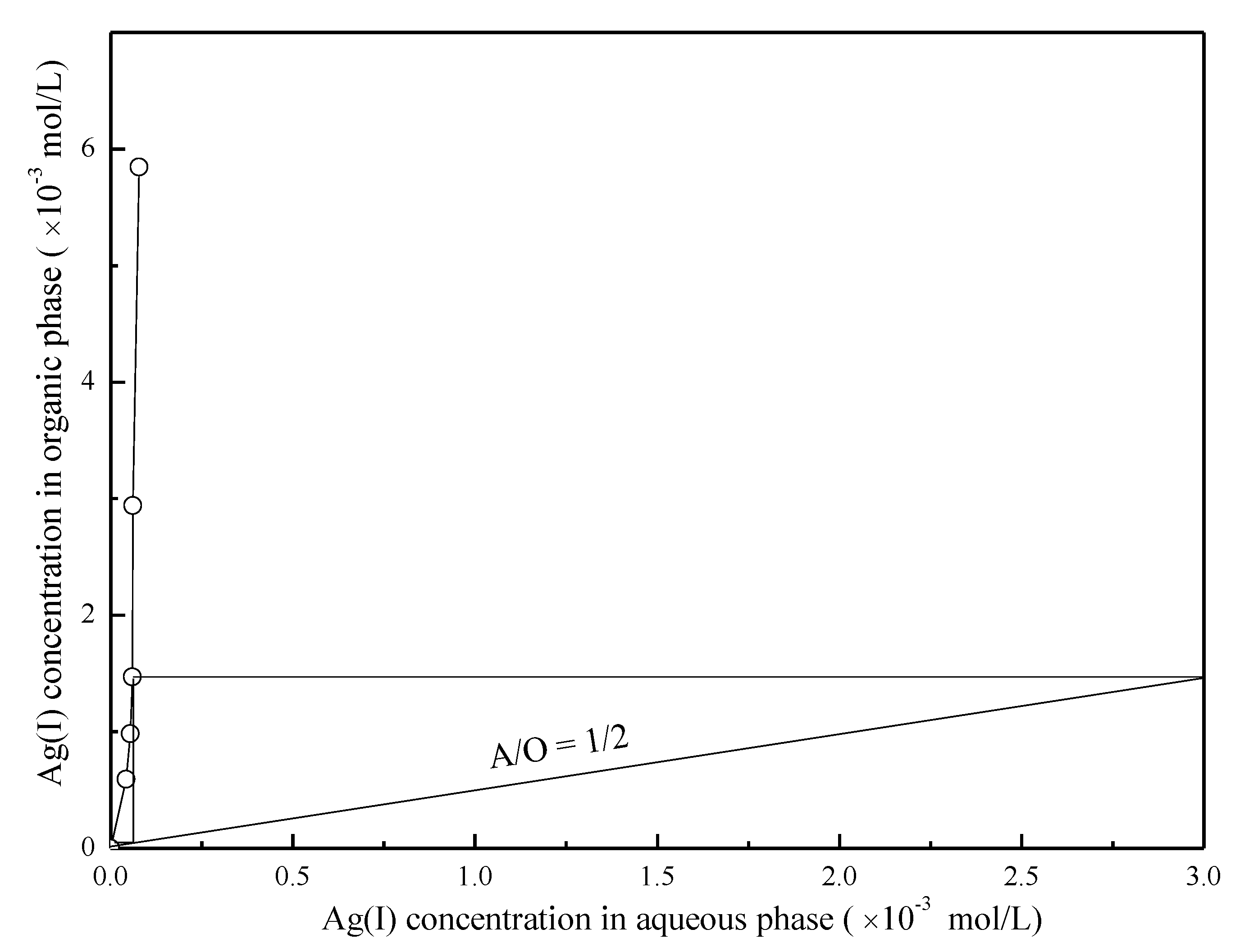
3.3. Effect of the A/O Ratio on the Extraction of Cu(II) and Ag(I)
3.4. Selection of a Strippant for the Stripping of Cu(II) and Ag(I) from the Loaded LIX63
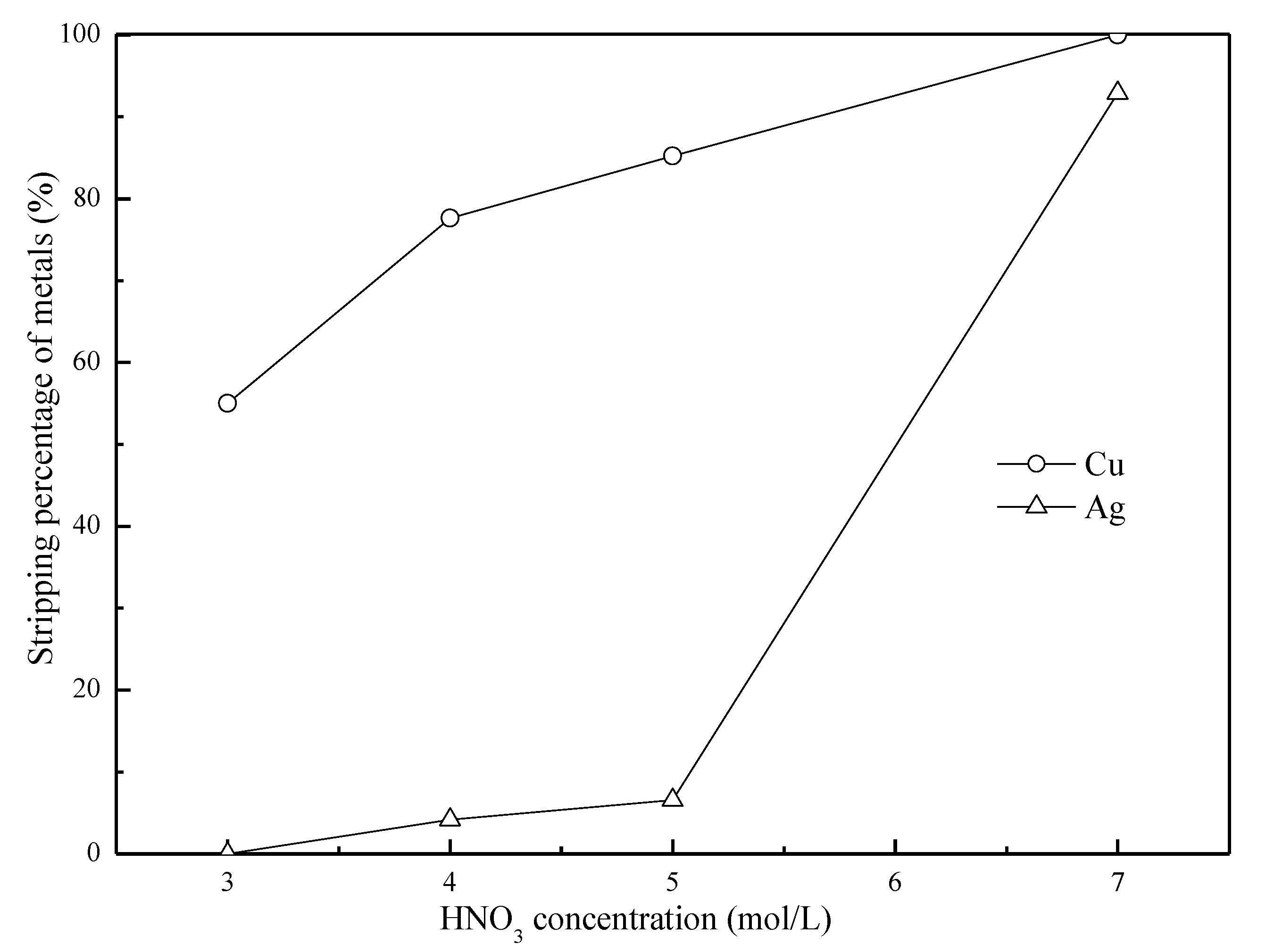
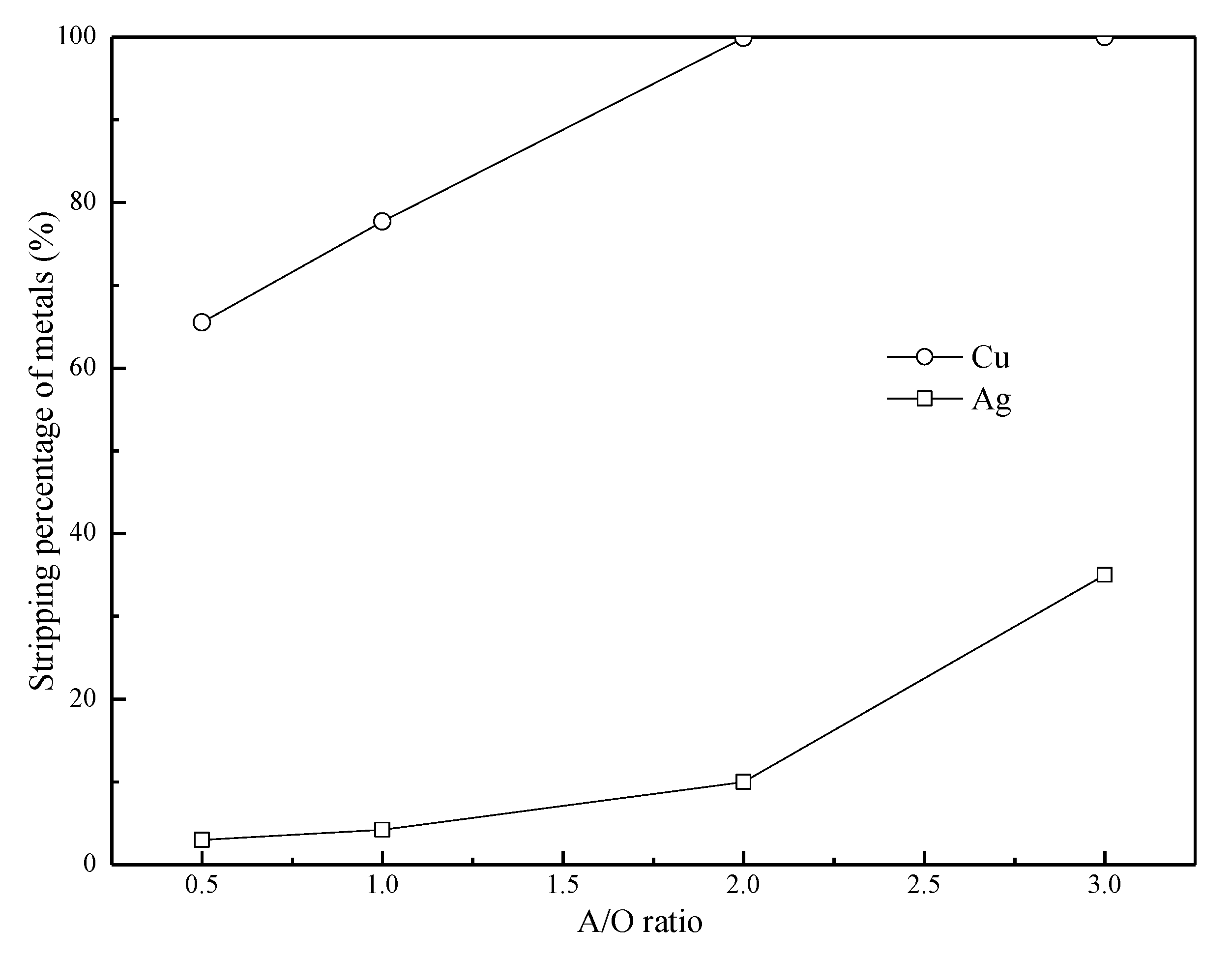
3.5. Effects of the A/O Ratio on the Stripping of Cu(II) and Ag(I)
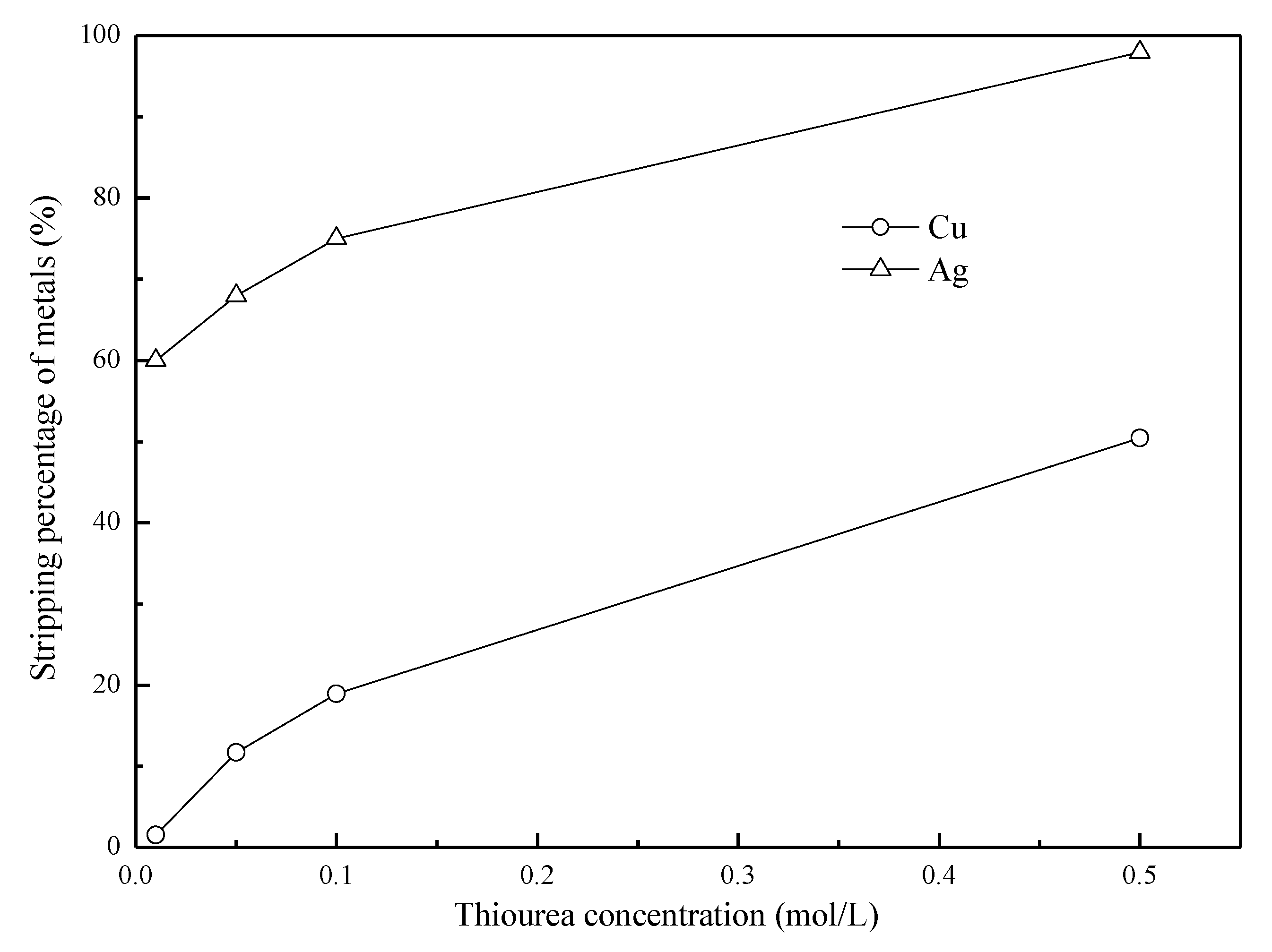
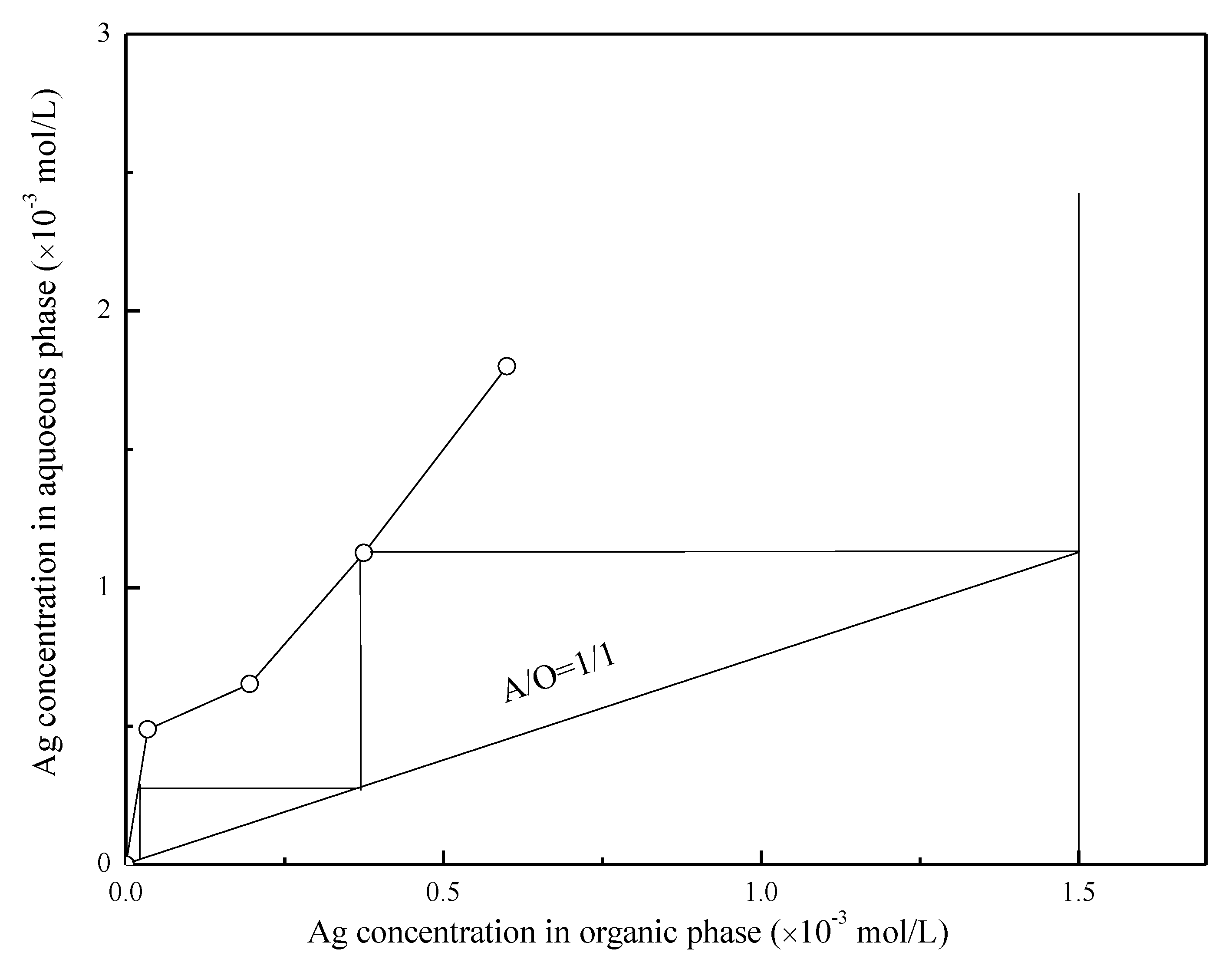
3.6. Effects of the A/O Ratio on the Stripping of Ag(I) with Thiourea
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lemos, L.R.; Santos, I.J.B.; Rodrigues, G.D.; Silva, L.H.M.; Silva, M.C.H. Copper recovery from ore by liquid–liquid extraction using aqueous two-phase system. J. Hazard. Mater. 2012, 237–238, 209–214. [Google Scholar] [CrossRef]
- Meterfi, S.; Meniai, A.H.; Chikhi, M. Elimination of Cu (II) from aqueous solutions by liquid-liquid extraction. Test of sodium diethyldithiocarbamate (SDDT) as an extracting agent. Energy Proc. 2012, 18, 1165–1174. [Google Scholar] [CrossRef][Green Version]
- Kasaie, M.; Bahmanyar, H.; Moosavian, M.A. A kinetic study on solvent extraction of copper from sulfate solution with Cupromex-3302 using Lewis cell. J. Environ. Chem. Eng. 2017, 5, 3044–3050. [Google Scholar] [CrossRef]
- AbdEl-Ghaaffar, M.A.; Abdel-Wahab, Z.H.; Elwakeel, K.Z. Extraction and separation studies of silver (I) and copper (II) from their aqueous solution using chemically modified melamine resins. Hydrometallurgy 2009, 96, 27–34. [Google Scholar] [CrossRef]
- Song, X.; Gunawan, P.; Jiang, R.; Leong, S.S.J.; Wang, K.; Xu, R. Surface activated carbon nanospheres for fast adsorption of silver ions from aqueous solutions. J. Hazard. Mater. 2011, 194, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Fornalczyk, A. Industrial catalysts as a source of valuable metals. J. Achiev. Mater. Manuf. Eng. 2012, 55, 864–869. [Google Scholar]
- Sari, A.; Tüzen, M. Adsorption of silver from aqueous solution onto raw vermiculite and manganese oxide-modified vermiculite. Microporous Mesoporous Mater. 2013, 170, 155–163. [Google Scholar] [CrossRef]
- Pant, D.; Joshi, D.; Upreti, M.K.; Kotnala, R.K. Chemical and biological extraction of metals present in E waste: A hybrid technology. Waste Manag. 2012, 32, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.R.; Park, K.H.; Mohapatra, D. Process development for the separation and recovery of copper from sulfate leach liquors of synthetic Cu–Ni–Co–Fe matte using LIX 84 and LIX 973N. Hydrometallurgy 2007, 87, 51–57. [Google Scholar] [CrossRef]
- Barik, G.; Nathsarma, K.C.; Sarangi, K. Recovery of copper from a waste heat boiler dust leach liquor using LIX 84I and LIX 622N. Solvent. Extr. Ion. Exch. 2013, 31, 198–209. [Google Scholar] [CrossRef]
- Lu, J.; Dreisinger, D. Solvent extraction of copper from chloride solution I: Extraction isotherms. Hydrometallurgy 2013, 137, 13–17. [Google Scholar] [CrossRef]
- Ruiz, M.C.; González, I.; Rodriguez, V.; Padilla, R. Solvent Extraction of copper from sulfate-chloride solutions using LIX 84-IC and LIX 860-IC. Miner. Process. Extr. Metall. Rev. 2021, 24, 1–8. [Google Scholar] [CrossRef]
- Sombhatla, S.S.; Kumar, A.; Mashruwala, S.; Rokkam, K.K.; Shukla, A. Comparative study of organic solvents for extraction of copper from ammoniacal carbonate solution. Hydrometallurgy 2016, 166, 94–97. [Google Scholar] [CrossRef]
- Tang, J.F.; Steenari, B.M. Solvent extraction separation of copper and zinc from MSWI fly ash leachates. Waste Manag. 2015, 44, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Bernard, K.R.; Zhang, W.; Zhu, Z.; Pranolo, Y. Recovery of nickel, cobalt, copper and zinc in sulfate and chloride solutions using synergistic solvent extraction. Chin. J. Chem Eng. 2016, 24, 237–248. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, W.; Meng, H.; Liu, Y.M.; Dai, Y. Extraction equilibria of copper (II) with D2EHPA in kerosene from aqueous solutions in acetate buffer media. J. Chem. Eng. Data 2007, 52, 438–441. [Google Scholar] [CrossRef]
- Fouad, E.A. Separation of copper from aqueous sulfate solutions by mixtures of Cyanex301 and LIX984N. Hazrd. Mater. 2009, 166, 720–727. [Google Scholar] [CrossRef] [PubMed]
- El-Hefny, N.E.; Daoud, J.A. Extraction of copper (II) by CYANEX 302 in kerosene from different aqueous media. Solvent. Extr. Ion. Exch. 2007, 259, 831–843. [Google Scholar] [CrossRef]
- Staszak, K.; Regel-Rosocka, M.; Wieszczycka, K.; Burmistrzak, P. Copper (II) sulfate solutions treatment by solvent extraction with Na-Cyanex272. Sep. Purif. Technol. 2012, 85, 183–192. [Google Scholar] [CrossRef]
- Mishra, S.; Devi, N.B. Extraction of copper (II) from hydrochloric acid solution by Cyanex921. Hydrometallurgy 2011, 107, 29–33. [Google Scholar] [CrossRef]
- Devi, N.B.; Mishra, S. Liqiud-liquid extraction of copper(II) from chloride media by Cyanex 923 in kerosene. J. S. Afr. Inst. Min. Metall. 2012, 112, 859–864. [Google Scholar]
- Feizollahi, S.; Azizi, A. Solvent extraction of copper from an industrial sulfate liquor using Chemorex CP-150. J. Mining Environ. 2018, 9, 905–916. [Google Scholar]
- Stankovic, V.; Outarra, L.; Zonnevijlle, F.; Comninellis, C. Solvent extraction of silver from nitric acid solutions by calix[4]arene amide derivatives. Sep. Purif. Technol. 2008, 61, 366–374. [Google Scholar] [CrossRef]
- Ohto, K.; Murakami, E.; Shinohara, T.; Shiratsuchi, K.; Inoue, K.; Iwasaki, M. Selective extraction of silver over palladium with ketonic derivatives of calixarenes from highly concentrated nitric acid. Anal. Chim. Acta 1997, 341, 275–283. [Google Scholar] [CrossRef]
- Sole, K.C.; Ferguson, T.L.; Hiskey, J.B. Solvent extraction of silver by cyanex272, cyanex 302 and cyanex 301. Solvent Extr. Ion Exch. 1994, 12, 1033–1050. [Google Scholar] [CrossRef]
- Lee, Y.I.; Park, S.H.; Kim, J.S.; Kim, D.W.; Choi, K.Y. Selective extraction of silver(I) ion by a new acyclic diazapolyether compound bearing dicarboxylate functional end groups. Bull. Korean Chem. Soc. 1999, 20, 487–490. [Google Scholar] [CrossRef]
- Löfström-Engdahl, E.; Aneheim, E.; Ekberg, C.; Foreman, M.; Skarnemark, G.S.; Hájková, Z.; Grűner, B. The extraction of silver and the effect of diluent, ligand side group and solvent composition. Proc. Chem. 2012, 7, 239–244. [Google Scholar] [CrossRef][Green Version]
- Cho, S.Y.; Kim, T.Y.; Sun, P.P. Recovery of silver from leachate of silicon solar cells by solvent extraction with TOPO. Sep. Purif. Technol. 2019, 215, 516–520. [Google Scholar] [CrossRef]
- Song, H.I.; Min, B.J.; Cho, S.Y.; Sun, P.P. Solvent extraction of silver from nitric acid solution with 5,8-diethyl-7-hydroxy-dodecan-6-oxime. J. Chem. Eng. Jpn. 2015, 48, 829–833. [Google Scholar] [CrossRef]
- Sun, P.P.; Min, B.J.; Kim, S.T.; Cho, S.Y. Separation of silver (I) and zinc (II) from nitrate solutions by solvent extraction with LIX63. Mater. Trans. 2017, 58, 287–290. [Google Scholar] [CrossRef]
- Xing, W.D.; Lee, M.S.; Senanayake, G. Recovery of metals from chloride leach solutions of anode slimes by solvent extraction. Part II: Recovery of silver and copper with LIX 63 and Alamine 336. Hydrometallurgy 2018, 180, 49–57. [Google Scholar] [CrossRef]

| Extractant | Media | Diluent | Remark | Ref. |
|---|---|---|---|---|
| LIX 84, LIX 973N | H2SO4 | kerosene | 1. The extraction behavior of the two extractants for copper(II) was compared. 2. A copper(II) extraction of 95–99% was achieved via three extraction stages with LIX 84 and two extraction stages with LIX 973N. | [9] |
| LIX 84I, LIX 622N | H2SO4 | kerosene | 1. Cu(II) extractions of 98.64% and 99.95% were obtained using LIX 84I and LIX 622N, respectively. 2. Stripping was conducted by using 2 mol/L H2SO4. | [10] |
| LIX84-I, LIX612N-LV, XI-04003, LIX984N | HCl | kerosene | 1. The copper loading capacity order for four extractants was LIX984N > LIX612N-LV and XI04003 > LIX84-I. 2. At a pH of <0.5, copper extraction efficiency increased quickly with increasing pH, while at a pH value above 0.5, it increased only slightly with increasing pH. | [11] |
| LIX 84-I, LIX 984N, LIX54-100 | Ammonia | Escaid110 | Extraction efficiency was much higher for systems using hydroxy reagents than that using β-diketone oximes. | [12] |
| LIX-54, cinnamate, β diketone | Ammoniacal carbonate | Arol-light | 1. Solvents cinnamate and a mixture of β-diketone were suitable alternatives to LIX 54. 2. About 97.3%, 99.7%, and 99.6% of copper(II) can be extracted using LIX 54, cinnamate, and β-diketone, respectively. 3. Stripping was conducted by using 2 mol/L H2SO4. | [13] |
| LIX860N-I | HCl | kerosene | 1. Fe(III) and Pb(II) were co-extracted with Cu(II) at pH 2. 2. Stripping was conducted using 1.5 mol/L H2SO4. | [14] |
| LIX63 + Versatic10; LIX63 + Versatic10 + TBP | HCl | Shellsol D70 | 1. Using a Versatic 10/LIX63/TBP system, 95% of Cu(II) and 12% of Ni(II), Zn(II), Co(II), and Cl(I) were extracted. 2. At pH 3–3.5, impurities of Ni(II), Zn(II), Co(II), and Cl(I) were stripped, and Cu(II) was transferred to a sulfate solution. 3. Cu(II) was recovered by electrowinning. 4. The Versatic 10/LIX63 system was used to separate Cu(II) from Fe(III) in strong chloride solutions. | [15] |
| D2EHPA | Acetate buffer | kerosene | 1. Acetate ions greatly improved the Cu(II) extraction efficiency. 2. At an initial pH value of 4.44, acetate ion concentration was 0.18 mol/L, and the single stage extraction efficiency was >99.5%. | [16] |
| Cyanex 301, LIX 984N | H2SO4, | kerosene | 1. The extraction equilibria of Cu(II) with Cyanex 301, LIX 984N, and their mixtures were investigated. 2. Stripping efficiency was improved by the addition of LIX 984N to Cyanex 301. | [17] |
| Cyanex 302 | H2SO4/ HCl/ HNO3 | kerosene | 1. Cyanex 302 was a potential extractant for the extraction of copper(II) from aqueous solutions. 2. The endothermic nature of the extraction process for the three studied systems was in the order: chloride > nitrate > sulfate media. | [18] |
| Cyanex 272, Na-Cyanex 272 | H2SO4 | kerosene | 1. The solvent extraction of Cu(II) with Na-Cyanex 272 was more efficient than that with Cyanex 272 | [19] |
| Cyanex 921 | HCl | kerosene | 1. Cu(II) extraction was quantitative using 0.5 mol/L Cyanex 921 and 5 mol/L HCl. 2. By using using 0.2 mol/L H2SO4 or 0.5 mol/L HCl, 99.7% Cu(II) was recovered from the loaded organic phase. | [20] |
| Cyanex 923 | HCl | kerosene | 1. The extraction mechanism was investigated. | [21] |
| Extractant | Media | Diluent | Remark | Ref. |
|---|---|---|---|---|
| Cyanex 272 Cyanex 302 Cyanex 301 | HNO3 | kerosene | 1. Extraction mechanisms were investigated. | [25] |
| ketonic derivatives of calixarenes | HNO3 HCl | / | 1. Tetrameric ketone facilitated selective extraction of Ag(I) over Pd(II) using ~1 mol/L HNO3. 2. It effectively removed traces of Ag(I) from a large excess of Pd(II). | [26] |
| Bis-triazin-bi-pyridine (BTBP) | HNO3 | / | 1. The side group of the ligand affected the distribution of Ag(I). 2. CyMe4-BTBP is a more efficient extractant for Ag(I) compared to C2-BTBP. | [27] |
| Trioctyl Phosphine oxide (TOPO) | HNO3 | kerosene | 1. TOPO selectively extracted Ag(I) over Al using 0.1 mol/L HNO3 solution. 2. The selective stripping of Ag(I) was successful using a 0.1 mol/L thiourea solution. 3. TOPO was regenerated by stripping of co-loaded Al(III) using 3 mol/L HNO3. | [28] |
| LIX63 | HNO3 | kerosene | 1. Ag(I) extraction followed a solvating extraction mechanism, when the concentration of HNO3 was higher than 0.1 mol/L. | [29] |
| LIX63 | HNO3 | kerosene | 1. The selective extraction of Ag(I) over Zn(II) was achieved by using LIX63, when the HNO3 concentration was 0.001–1 mol/L. 2. The stripping of Ag(I) was conducted by using 5 mol/L HNO3. | [30] |
| LIX 63, Alamine 336; Cyanex 301 | HCl | kerosene | 1. Cu(II) was recovered by extraction with LIX 63 and stripping with an HCl solution, while Ag(I) was recovered by extraction with Alamine 336 followed by stripping with NH4SCN solution. 2. Cu(II) and Ag(I) were simultaneously extracted by Cyanex 301. 3. Stripping from Cyanex 301 was achieved using diluted aqua regia. | [31] |
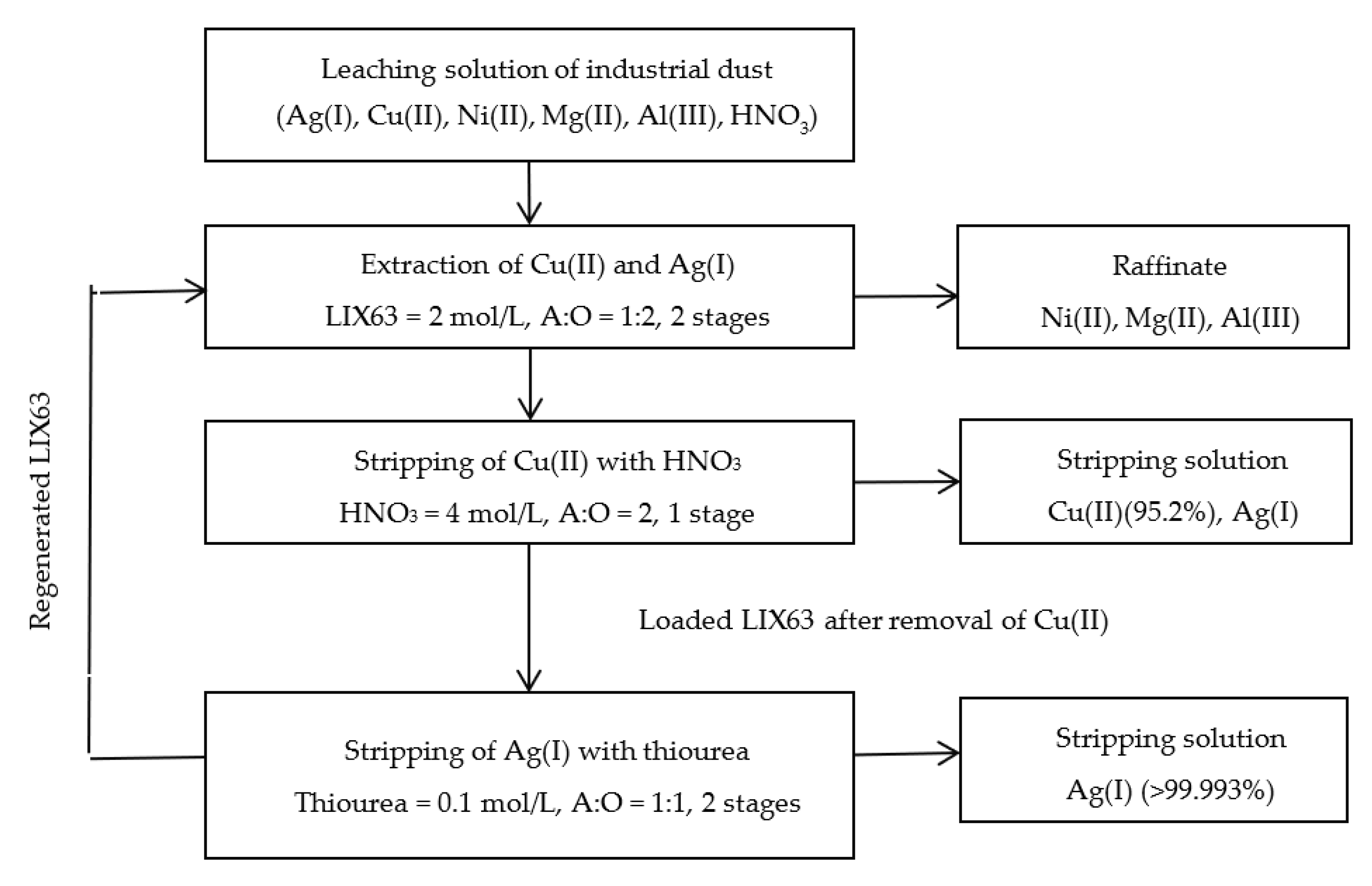
| LIX63 | HNO3 | kerosene | 1. LIX63 exhibited selectivity for the extraction of Cu(II) and Ag(I) over Ni(II), Mg(II), and Al(III) at low concentrations of HNO3. 2. Cu(II) was selectively stripped over Ag(I) from the loaded LIX63 using 4 mol/L HNO3. 3. After the removal of Cu(II), Ag(I) was stripped from the loaded LIX63 using 0.1 mol/L thiourea. | Present study |
| Strippants | Stripping Percentage (%) | |
|---|---|---|
| Cu(II) | Ag(I) | |
| 1 mol/L Na2S2O3 (pH 4.7) | 63.89 | 98.90 |
| 1 mol/L NH4NO3 (pH 4.6) | 0.6 | 0.8 |
| 1 mol/L (NH4)2SO4 (pH 5.1) | 2.1 | 6.7 |
| 0.5 mol/L CH3COONH4 (pH 7.1) | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, P.-P.; Kim, T.-Y.; Seo, H.; Cho, S.-Y. Recovery of Copper(II) and Silver(I) from Nitrate Leaching Solution of Industrial Dust via Solvent Extraction with LIX63. Metals 2021, 11, 1300. https://doi.org/10.3390/met11081300
Sun P-P, Kim T-Y, Seo H, Cho S-Y. Recovery of Copper(II) and Silver(I) from Nitrate Leaching Solution of Industrial Dust via Solvent Extraction with LIX63. Metals. 2021; 11(8):1300. https://doi.org/10.3390/met11081300
Chicago/Turabian StyleSun, Pan-Pan, Tae-Young Kim, Hyeon Seo, and Sung-Yong Cho. 2021. "Recovery of Copper(II) and Silver(I) from Nitrate Leaching Solution of Industrial Dust via Solvent Extraction with LIX63" Metals 11, no. 8: 1300. https://doi.org/10.3390/met11081300
APA StyleSun, P.-P., Kim, T.-Y., Seo, H., & Cho, S.-Y. (2021). Recovery of Copper(II) and Silver(I) from Nitrate Leaching Solution of Industrial Dust via Solvent Extraction with LIX63. Metals, 11(8), 1300. https://doi.org/10.3390/met11081300







