Selective Leaching Trace Elements from Bauxite Residue (Red Mud) without and with Adding Solid NH4Cl Using Microwave Heating
Abstract
:1. Introduction
2. Materials and Methods
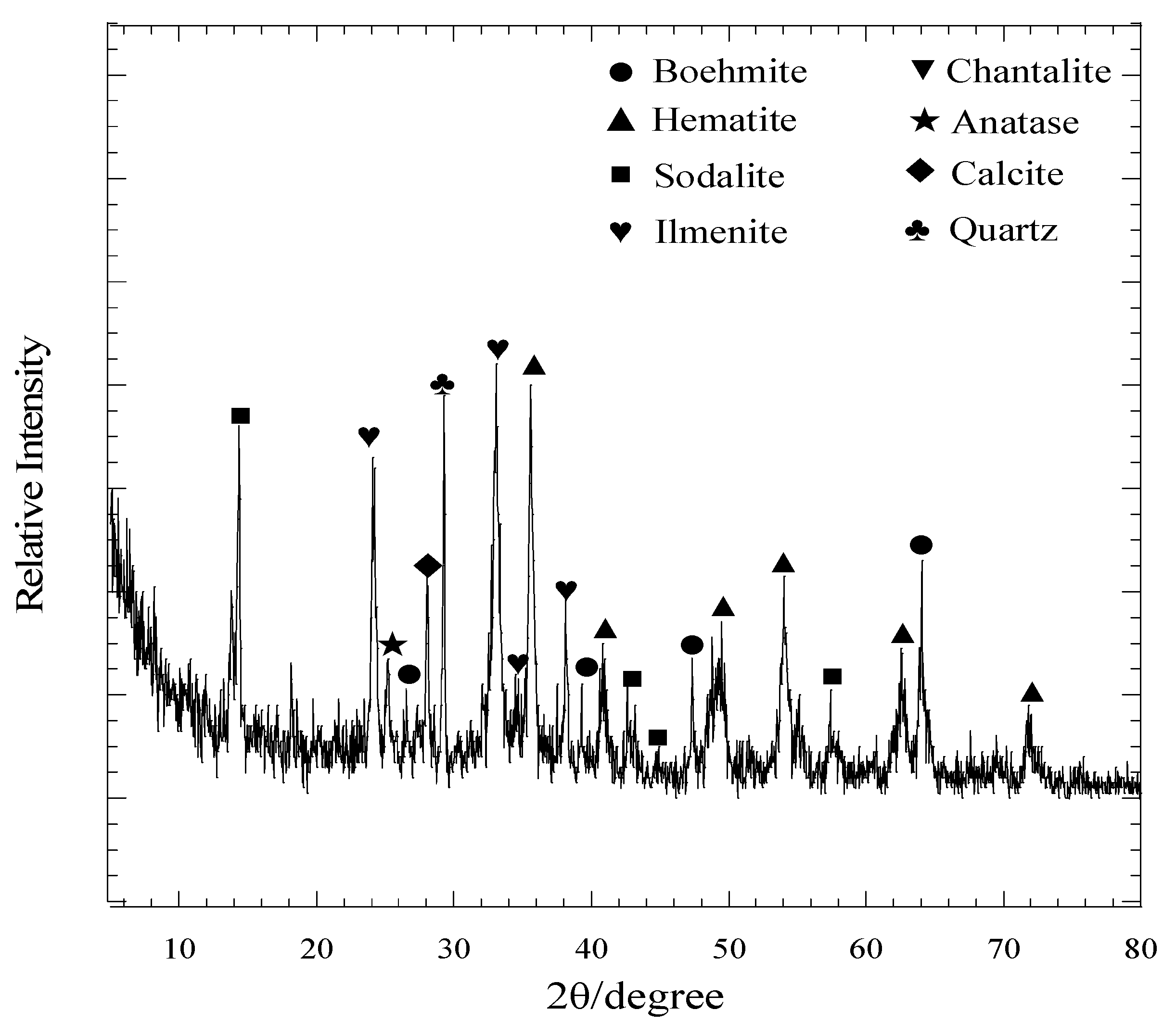
2.1. Material
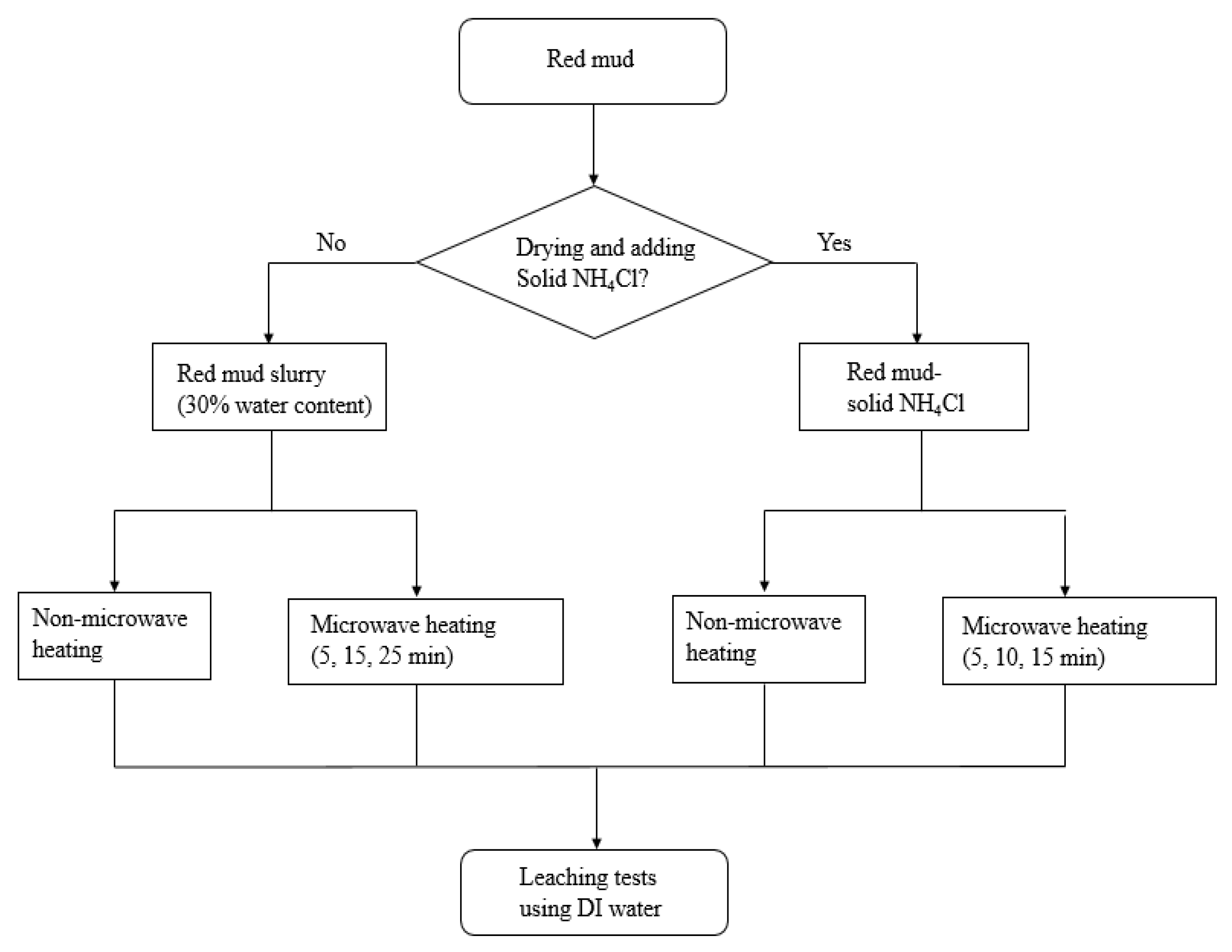
2.2. Methods
3. Results and Discussion
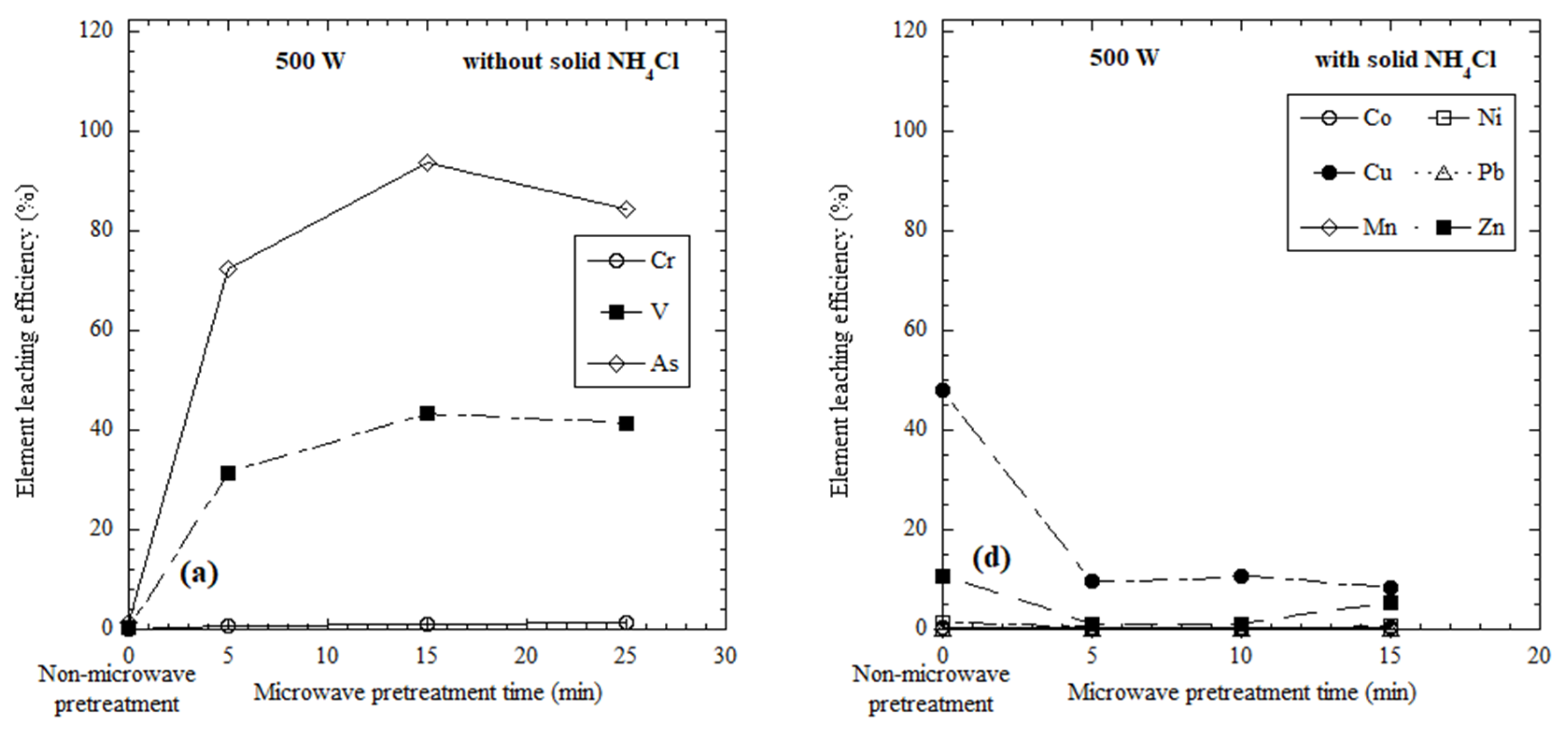
3.1. Elements Leached from Microwave-Heated Red Mud Slurry without the Addition of Solid NH4Cl
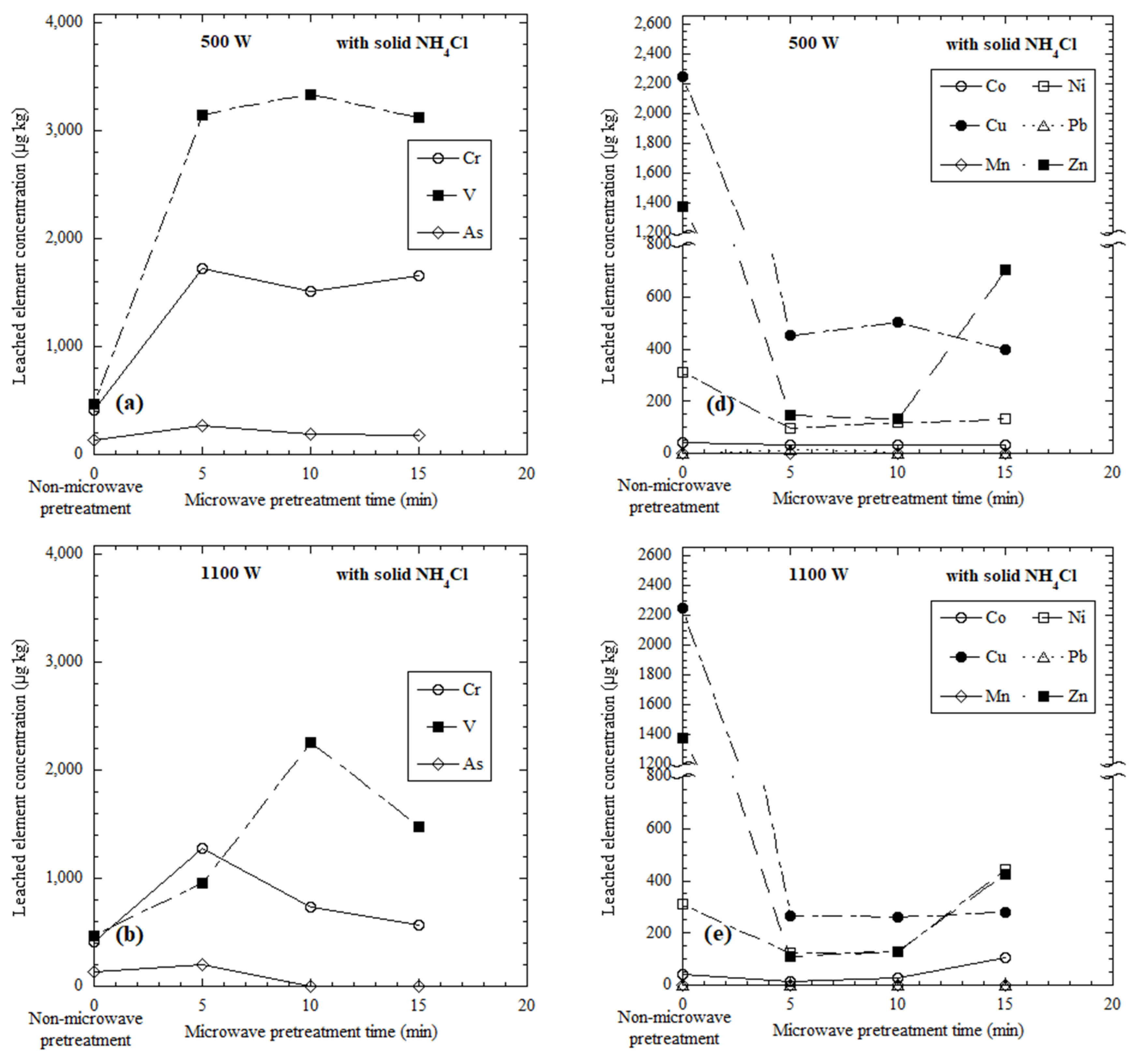
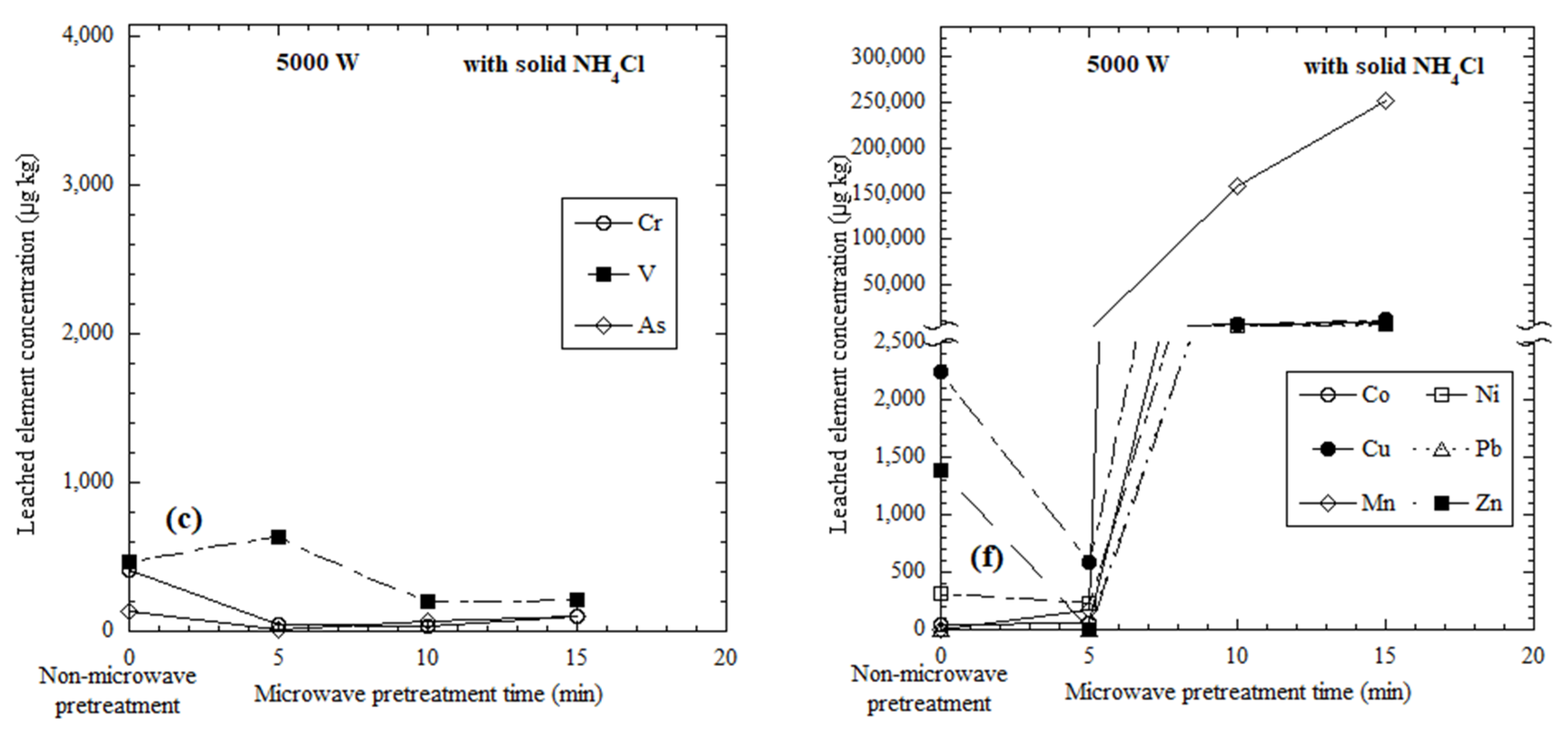
3.2. Elements Leached from Microwave-Heated Dried Red Mud with the Addition of Solid NH4Cl
3.3. Discussion of Selective Leaching from Microwave-Heated Red Mud
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Samouhos, M.; Taxiarchou, M.; Tsakiridis, P.E.; Potiriadis, K. Greek “red mud” residue: A study of microwave reductive roasting followed by magnetic separation for a metallic iron recovery process. J. Hazard. Mater. 2013, 254–255, 193–205. [Google Scholar] [CrossRef]
- Klauber, C.; Gräfe, M.; Power, G. Bauxite residue issues: II. options for residue utilization. Hydrometallurgy 2011, 108, 11–32. [Google Scholar] [CrossRef]
- Liu, W.; Sun, S.; Zhang, L.; Jahanshahi, S.; Yang, J. Experimental and simulative study on phase transformation in Bayer red mud soda-lime roasting system and recovery of Al, Na and Fe. Miner. Eng. 2012, 39, 213–218. [Google Scholar] [CrossRef]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Li, H. Metallurgical process for valuable elements recovery from red mud—A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Reid, S.; Tam, J.; Yang, M.; Azimi, G. Technospheric Mining of Rare Earth Elements from Bauxite Residue (Red Mud): Process Optimization, Kinetic Investigation, and Microwave Pretreatment. Sci. Rep. 2017, 7, 15252. [Google Scholar] [CrossRef] [Green Version]
- Vachon, P.; Tyagi, R.D.; Auclair, J.C.; Wilkinson, K.J. Chemical and biological leaching of aluminum from red mud. Environ. Sci. Technol. 1994, 28, 26–30. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropulu, M.; Lyberopulu, T.; Parissakis, G. Selective separation and determination of scandium from yttrium and lanthanides in red mud by a combined ion exchange/solvent extraction method. Anal. Chim. Acta 1995, 315, 231–237. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropulu, M.; Lyberopulu, T.; Ochsenkühn, K.; Parissakis, G. Recovery of lanthanides and yttrium from red mud by selective leaching. Anal. Chim. Acta 1996, 319, 249–254. [Google Scholar] [CrossRef]
- Smirnov, D.; Molchanova, T. The investigation of sulphuric acid sorption recovery of scandium and uranium from the red mud of alumina production. Hydrometallurgy 1997, 45, 249–259. [Google Scholar] [CrossRef]
- Deep, A.; Malik, P.; Gupta, B. Extraction and separation of Ti(IV) using thiophosphinic acids and its recovery from ilmenite and red mud. Sep. Sci. Technol. 2001, 36, 671–685. [Google Scholar] [CrossRef]
- Qu, Y.; Li, H.; Tian, W.; Wang, X.; Wang, X.; Jia, X.; Shi, B.; Song, G.; Tang, Y. Leaching of valuable metals from red mud via batch and continuous processes by using fungi. Miner. Eng. 2015, 81, 1–4. [Google Scholar] [CrossRef]
- Borra, C.R.; Mermans, J.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner. Eng. 2016, 92, 151–159. [Google Scholar] [CrossRef]
- Ujaczki, E.; Zimmermann, Y.; Gasser, C.; Molnár, M.; Feigl, V.; Lenz, M. Red mud as secondary source for critical raw materials—Purification of rare earth elements by liquid/liquid extraction. J. Chem. Technol. Biotechnol. 2017, 92, 2683–2690. [Google Scholar] [CrossRef]
- Ujaczki, E.; Feigl, V.; Molnár, M.; Cusack, P.; Curtin, T.; Courtney, R.; O’Donoghue, L.; Davris, P.; Hugi, C.; Evangelou, M.W.; et al. Re-using bauxite residues: Benefits beyond (critical raw) material recovery. J. Chem. Technol. Biotechnol. 2018, 93, 2498–2510. [Google Scholar] [CrossRef] [Green Version]
- Hoover, M.; Han, K.; Fuerstenau, D. Segregation roasting of nickel, copper and cobalt from deep-sea manganese nodules. Int. J. Miner. Process. 1975, 2, 173–185. [Google Scholar] [CrossRef]
- Erçag, E.; Apak, R. Furnace smelting and extractive metallurgy of red mud: Recovery of TiO2, Al2O3 and pig iron. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 1997, 70, 241–246. [Google Scholar]
- Kumar, R.; Srivastava, J. Premchand. Utilization of Iron Values of Red Mud for Metallurgical Applications. Natl. Metal. Lab. 1998, 108–119. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropoulou, M.T.; Hatzilyberis, K.S.; Mendrinos, L.N.; Salmas, C.E. Pilot-Plant Investigation of the Leaching Process for the Recovery of Scandium from Red Mud. Ind. Eng. Chem. Res. 2002, 41, 5794–5801. [Google Scholar] [CrossRef]
- Uzun, D.; Gülfen, M. Dissolution kinetics of iron and aluminium from red mud in sulphuric acid solution. Indian J. Chem. Technol. 2007, 14, 263–268. [Google Scholar]
- Agatzini-Leonardou, S.; Oustadakis, P.; Tsakiridis, P.; Markopoulos, C. Titanium leaching from red mud by diluted sulfuric acid at atmospheric pressure. J. Hazard. Mater. 2008, 157, 579–586. [Google Scholar] [CrossRef]
- LI, X.B.; Xiao, W.; Liu, W.; Liu, H.G.; Peng, Z.H.; Zhou, Q.S.; Qi, T.G. Recovery of alumina and ferric oxide from Bayer red mud rich in iron by reduction sintering. Trans. Nonferrous Met. Soc. China 2009, 19, 1342–1347. [Google Scholar] [CrossRef]
- Liu, W.; Yang, J.; Xiao, B. Review on treatment and utilization of bauxite residues in China. Int. J. Miner. Process. 2009, 93, 220–231. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, S.; Ma, S.; Zhang, Y. Recovery of alumina and alkali in Bayer red mud by the formation of andradite-grossular hydrogarnet in hydrothermal process. J. Hazard. Mater. 2011, 189, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA. Sep. Purif. Technol. 2013, 108, 96–102. [Google Scholar] [CrossRef]
- Abhilash; Sinha, S.; Sinha, M.K.; Pandey, B.D. Extraction of lanthanum and cerium from Indian red mud. Int. J. Miner. Process. 2014, 127, 70–73. [Google Scholar] [CrossRef]
- Xia, D.; Picklesi, C. Microwave caustic leaching of electric arc furnace dust. Miner. Eng. 2000, 13, 79–94. [Google Scholar] [CrossRef]
- Krishnan, K.H.; Mohanty, D.; Sharma, K. The effect of microwave irradiations on the leaching of zinc from bulk sulphide concentrates produced from Rampura–Agucha tailings. Hydrometallurgy 2007, 89, 332–336. [Google Scholar] [CrossRef]
- Wu, C.; Kuo, C.; Lo, S.L. Recovery of heavy metals from industrial sludge using various acid extraction approaches. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2009, 59, 289–293. [Google Scholar] [CrossRef]
- Kalidoss, J.; Ray, P.; Chaubey, A.; Padhi, A.; Satapathy, B.; Mukherjee, P. Production of pig iron from red mud waste fines using thermal plasma technology. Int. J. Miner. Metall. Mater. 2012, 19, 679–684. [Google Scholar] [CrossRef]
- Pinto, I.S.; Soares, H.M. Selective leaching of molybdenum from spent hydrodesulphurisation catalysts using ultrasound and microwave methods. Hydrometallurgy 2012, 129–130, 19–25. [Google Scholar] [CrossRef]
- Zhu, D.; Chun, T.; Pan, J.; He, Z. Recovery of Iron From High-Iron Red Mud by Reduction Roasting With Adding Sodium Salt. J. Iron Steel Res. Int. 2012, 19, 1–5. [Google Scholar] [CrossRef]
- Guo, Y.H.; Gao, J.J.; Xu, H.J.; Zhao, K.; Shi, X.F. Nuggets Production by Direct Reduction of High Iron Red Mud. J. Iron Steel Res. Int. 2013, 20, 24–27. [Google Scholar] [CrossRef]
- Peng, Z.; Hwang, J.Y. Microwave-assisted metallurgy. Int. Mater. Rev. 2015, 60, 30–63. [Google Scholar] [CrossRef]
- Kruesi, P.R.; Frahm, V.H. Process for the Recovery of Nickel, Cobalt and Manganese from Their Oxides and Silicates. U.S. Patent AU535773B2, 5 April 1984. [Google Scholar]
- Worner, H.K. Microwave Irradiation of Composites. U.S. Patent AU0001623, 6 March 1990. [Google Scholar]
- Zhang, X.; Ma, G.; Tong, Z.; Xue, Z. Microwave-assisted selective leaching behavior of calcium from Basic Oxygen Furnace (BOF) slag with ammonium chloride solution. J. Min. Metall. Sect. B Metall. 2017, 53, 139–146. [Google Scholar] [CrossRef]
- Kim, J.S.; Jo, H.Y.; Choi, N.C. Calcium and sodium recovery from microwave-pretreated red mud with added solid ammonium chloride. J. Chem. Technol. Biotechnol. 2019, 94, 3960–3969. [Google Scholar] [CrossRef]
- Jo, H.J.; Jo, H.Y.; Rha, S.; Lee, P.K. Direct aqueous mineral carbonation of waste slate using ammonium salt solutions. Metals 2015, 5, 2413. [Google Scholar] [CrossRef]
- Haque, K.E. Microwave energy for mineral treatment processes—A brief review. Int. J. Miner. Process. 1999, 57, 1–24. [Google Scholar] [CrossRef]
- Khattak, H.; Bianucci, P.; Slepkov, A. Linking plasma formation in grapes to microwave resonances of aqueous dimers. Proc. Natl. Acad. Sci. USA 2019, 116, 4000–4005. [Google Scholar] [CrossRef] [Green Version]
- Haynes, W. CRC Handbook of Chemistry and Physics: A Ready-reference Book of Chemical and Physical Data. In CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]



| Material | Elemental Composition (mg/kg) | |||||
|---|---|---|---|---|---|---|
| Al | Fe | Ca | Na | Mg | K | |
| As-received red mud | 58,124 | 99,045 | 17,191 | 47,083 | 267 | 209 |
| Si | As | Ga | V | Cr | Pb | |
| 80 | 7.5 | 16.9 | 229.9 | 176.3 | 16.7 | |
| Mn | Zn | Ni | Cu | Co | Ba | |
| 145.8 | 12.9 | 23.3 | 4.7 | 17.1 | 8.2 | |
| Sample | Solvents | Solid-to-Liquid Ratio (g/L) | Output Power (W) | Pretreatment Time (min) | Concentration (μg/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr | V | As | Co | Cu | Mn | Ni | Pb | Zn | |||||
| Red mud slurry | DI water | 100 | - | 0 | 380 | 530 | 110 | 30 | 420 | 40 | 260 | <a 1 | 440 |
| Red mud- solid NH4Cl | DI water | 100 | - | 0 | 410 | 470 | 130 | 40 | 2250 | <1 | 310 | <1 | 1380 |
| Red mud slurry | DI water | 100 | 500 | 5 | 1640 | 72,300 | 5440 | 40 | 123 | <1 | 82 | <1 | 1320 |
| 15 | 2420 | 99,480 | 7030 | 19 | 186 | <1 | 25 | <1 | 470 | ||||
| 25 | 3400 | 95,100 | 6340 | 19 | 213 | <1 | 16 | <1 | <1 | ||||
| 1100 | 5 | 2160 | 61,600 | 3830 | 17 | 110 | <1 | 19 | <1 | 44 | |||
| 15 | 8850 | 71,700 | 4760 | 16 | 176 | <1 | 27 | <1 | 285 | ||||
| 25 | 24,500 | 74,800 | 4030 | 0 | 123 | <1 | 13 | <1 | <1 | ||||
| 5000 | 5 | 5050 | 128,000 | 9550 | 30 | 332 | <1 | 33 | <1 | <1 | |||
| 15 | 50,300 | 143,000 | 2430 | 0 | 29 | <1 | 0 | <1 | <1 | ||||
| 25 | 37,600 | 158,000 | 2750 | 0 | 108 | <1 | 0 | <1 | <1 | ||||
| Red mud- solid NH4Cl | DI water | 100 | 500 | 5 | 1720 | 3150 | 262 | 33 | 455 | <1 | 94 | 12 | 147 |
| 10 | 1520 | 3330 | 194 | 31 | 503 | <1 | 121 | <1 | 134 | ||||
| 15 | 1660 | 3130 | 176 | 31 | 398 | <1 | 135 | <1 | 704 | ||||
| 1100 | 5 | 1280 | 952 | 204 | 16 | 267 | <1 | 124 | <1 | 110 | |||
| 10 | 731 | 2260 | <1 | 29 | 263 | <1 | 130 | <1 | 127 | ||||
| 15 | 566 | 1480 | <1 | 105 | 280 | <1 | 445 | <1 | 426 | ||||
| 5000 | 5 | 50 | 632 | 17 | 60 | 591 | 168 | 223 | <1 | <1 | |||
| 10 | 30 | 204 | 66 | 5170 | 6590 | 157,000 | 4470 | 3660 | 3650 | ||||
| 15 | 98 | 215 | 97 | 7990 | 10,900 | 251,000 | 5990 | 3070 | 7350 | ||||
| Element | Optimal Conditions | Leaching Efficiency (%) | ||
|---|---|---|---|---|
| Solid NH4Cl | Output Power (W) | Pretreatment Time (min) | ||
| V | No | 5000 | 25 | 69.0 |
| Cr | No | 5000 | 15 | 18.2 |
| As | No | 5000 | 5 | 100 |
| Mn | Yes | 5000 | 15 | 100 |
| Cu | Yes | 5000 | 15 | 100 |
| Co | Yes | 5000 | 15 | 46.7 |
| Zn | Yes | 5000 | 15 | 57.0 |
| Ni | Yes | 5000 | 15 | 25.7 |
| Pb | Yes | 5000 | 15 | 18.4 |
| Fe | Yes | 5000 | 15 | 9.4 |
| Mineral | Chemical Formula | As Received Red Mud | Red Mud-Solid NH4Cl | ||
|---|---|---|---|---|---|
| wt.% | Microwave Heating Time at 5000 W | ||||
| wt.% | wt.% | wt.% | |||
| Sodium aluminum silicates | Na4Al3Si3O14.35 | 21 | 5 | a n.d. | <1 |
| Sodalite | ClNa4Al3Si3O12 | 7 | 8 | <1 | n.d |
| Hematite | Fe2O3 | 22 | 26 | 7 | 10 |
| Quartz | SiO2 | 9 | 7 | 6 | 2 |
| Chantalite | CaAl2SiO8H4 | <1 | n.d. | n.d. | n.d. |
| Calcite | CaCO3 | 6 | 3 | <1 | <1 |
| Boehmite | AlOOH | 16 | 14 | <1 | <1 |
| Anatase | TiO2 | 4 | 5 | 1 | <1 |
| Nosean | CNa8Al6Si6O27 | 3 | <1 | <1 | n.d. |
| Ilmenite | FeTiO3 | 11 | n.d. | n.d. | n.d. |
| Halite | NaCl | n.d. | 19 | 30 | 25 |
| Labradorite | Na0.35Ca0.65Al1.65Si2.35O8 | n.d. | n.d. | 20 | 24 |
| Spinel | FeAl2O4 | n.d. | 2 | 7 | 12 |
| Corundum | Al2O3 | n.d. | <1 | 11 | 6 |
| Anorthite | CaAl2Si2O8 | n.d. | 1 | 3 | 6 |
| Maghemite | γFe2O3 | n.d. | <1 | <1 | 2 |
| Cristobalite | SiO2 | n.d. | 2 | 2 | 3 |
| Albite | NaAlSi3O8 | n.d. | n.d. | 4 | 4 |
| Rutile | TiO2 | n.d. | 3 | 5 | 4 |
| Magnetite | Fe3O4 | n.d. | 1 | <1 | <1 |
| Total | - | 99 | 96 | 95 | 98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-S.; Choi, N.-C.; Jo, H.Y. Selective Leaching Trace Elements from Bauxite Residue (Red Mud) without and with Adding Solid NH4Cl Using Microwave Heating. Metals 2021, 11, 1281. https://doi.org/10.3390/met11081281
Kim J-S, Choi N-C, Jo HY. Selective Leaching Trace Elements from Bauxite Residue (Red Mud) without and with Adding Solid NH4Cl Using Microwave Heating. Metals. 2021; 11(8):1281. https://doi.org/10.3390/met11081281
Chicago/Turabian StyleKim, Jin-Seok, Nag-Choul Choi, and Ho Young Jo. 2021. "Selective Leaching Trace Elements from Bauxite Residue (Red Mud) without and with Adding Solid NH4Cl Using Microwave Heating" Metals 11, no. 8: 1281. https://doi.org/10.3390/met11081281
APA StyleKim, J.-S., Choi, N.-C., & Jo, H. Y. (2021). Selective Leaching Trace Elements from Bauxite Residue (Red Mud) without and with Adding Solid NH4Cl Using Microwave Heating. Metals, 11(8), 1281. https://doi.org/10.3390/met11081281






