High-Pressure Torsion for Synthesis of High-Entropy Alloys
Abstract
:1. Introduction
2. Synthesis of High-Entropy Alloys with High Hardness
3. Synthesis of High-Entropy Hydrides for Hydrogen Storage
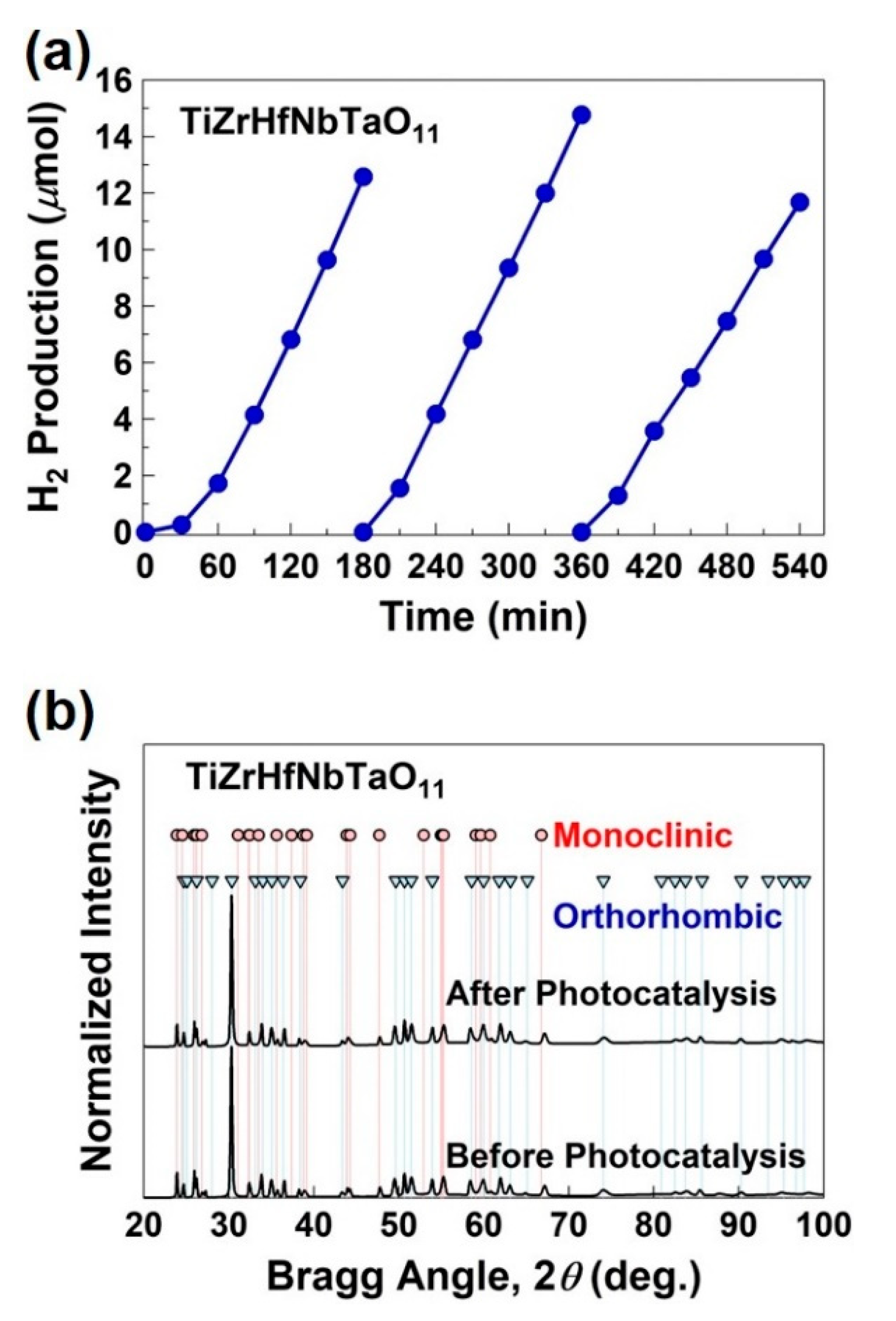
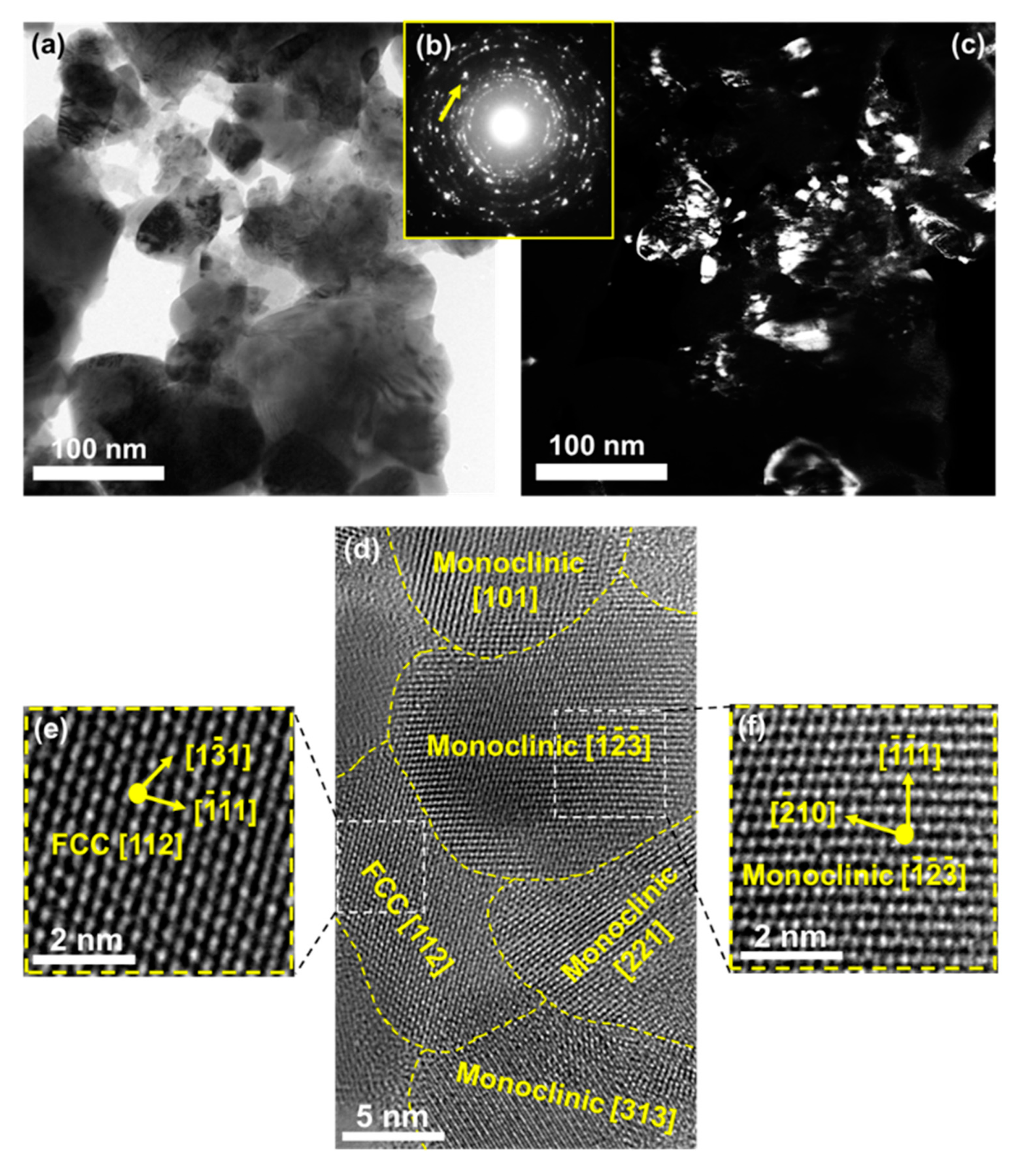
4. Synthesis of High-Entropy Oxides for Photocatalytic Hydrogen Production
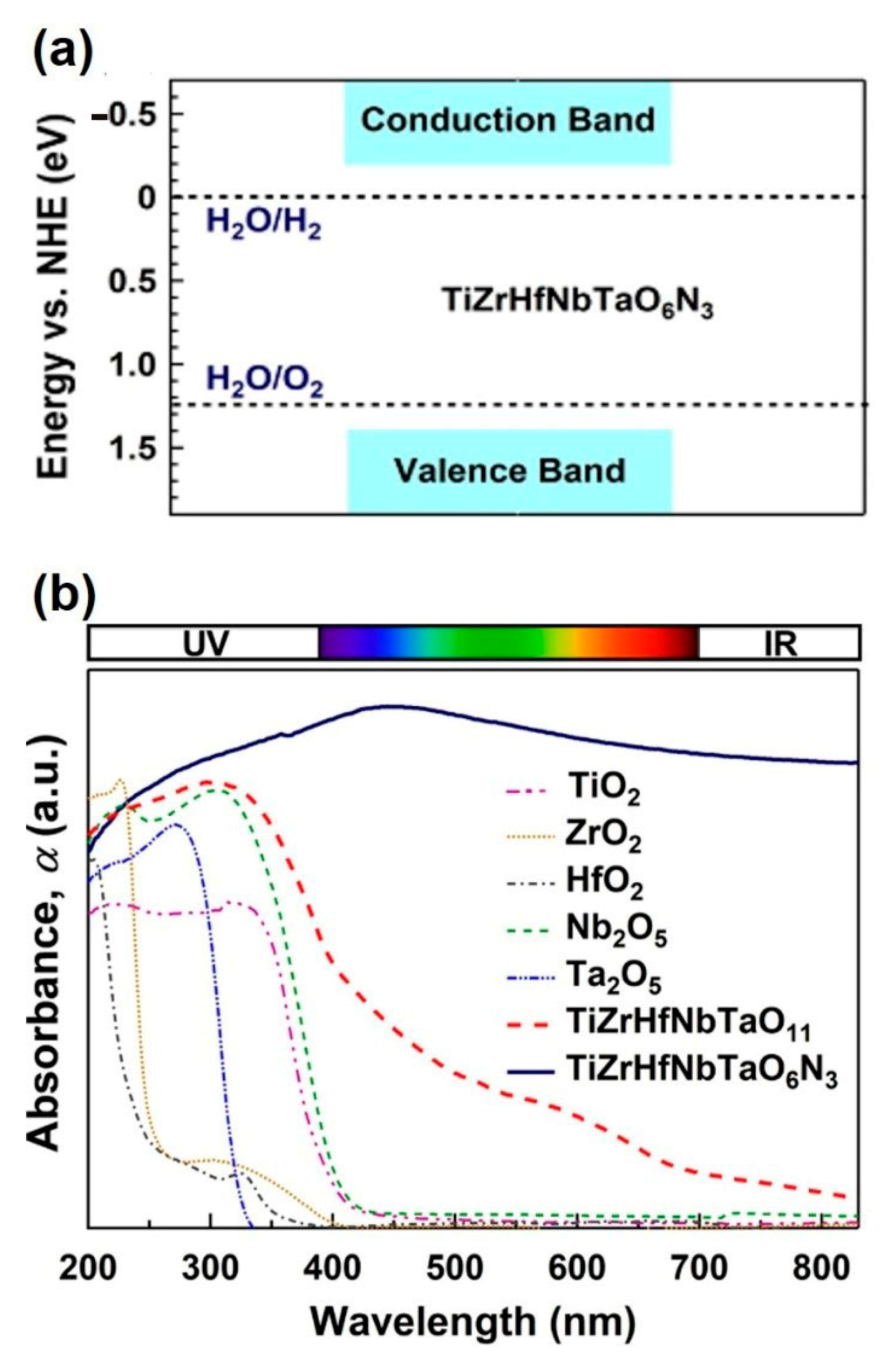
5. Synthesis of High-Entropy Oxynitrides with Large Light Absorbance
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Valiev, R.Z.; Estrin, Y.; Horita, Z.; Langdon, T.G.; Zehetbauer, M.J.; Zhu, Y.T. Producing bulk ultrafine-grained materials by severe plastic deformation. JOM 2006, 58, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Zhilyaev, A.P.; Langdon, T.G. Using high-pressure torsion for metal processing: Fundamentals and applications. Prog. Mater. Sci. 2008, 53, 893–979. [Google Scholar] [CrossRef]
- Bridgman, P.W. Effects of high shearing stress combined with high hydrostatic pressure. Phys. Rev. 1935, 48, 825–847. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z. A review on high-pressure torsion (HPT) from 1935 to 1988. Mater. Sci. Eng. A 2016, 652, 325–352. [Google Scholar] [CrossRef]
- Bryła, K.; Edalati, K. Historical studies by polish scientist on ultrafine-grained materials by severe plastic deformation. Mater. Trans. 2019, 60, 1553–1560. [Google Scholar] [CrossRef] [Green Version]
- Valiev, R.Z.; Kaibyshev, O.A.; Kuznetsov, R.I.; Musalimov, R.S.; Tsenev, N.K. Low-temperature superplasticity of metallic materials. Dokl. Akad. Nauk. SSSR 1988, 301, 864–866. [Google Scholar]
- Valiev, R.Z.; Islamgaliev, R.K.; Alexandrov, I.V. Bulk nanostructured materials from severe plastic deformation. Prog. Mater. Sci. 2000, 45, 103–189. [Google Scholar] [CrossRef]
- Pippan, R.; Scheriau, S.; Taylor, A.; Hafok, M.; Hohenwarter, A.; Bachmaier, A. Saturation of fragmentation during severe plastic deformation. Annu. Rev. Mater. Res. 2010, 40, 319–343. [Google Scholar] [CrossRef]
- Figueiredo, R.B.; Kawasaki, M.; Langdon, T.G. An evaluation of homogeneity and heterogeneity in metals processed by high-pressure torsion. Acta Phys. Pol. A 2012, 122, 425–429. [Google Scholar] [CrossRef]
- Starink, M.J.; Cheng, X.C.; Yang, S. Hardening of pure metals by high-pressure torsion: A physically based model employing volume-averaged defect evolutions. Acta Mater. 2013, 61, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.J.; Yoon, E.Y.; Ahn, D.H.; Park, B.H.; Park, H.W.; Park, L.J.; Estrin, Y.; Kim, H.S. Dislocation density-based finite element analysis of large strain deformation behavior of copper under high-pressure torsion. Acta Mater. 2014, 76, 281–293. [Google Scholar] [CrossRef]
- Bachmaier, A.; Pippan, R. High-pressure torsion deformation induced phase transformations and formations: New material combinations and advanced properties. Mater. Trans. 2019, 60, 1256–1269. [Google Scholar] [CrossRef] [Green Version]
- Levitas, V.I. High-pressure phase transformations under severe plastic deformation by torsion in rotational anvils. Mater. Trans. 2019, 60, 1294–1301. [Google Scholar] [CrossRef] [Green Version]
- Mazilkin, A.; Straumal, B.; Kilmametov, A.; Straumal, P.; Baretzky, B. Phase transformations induced by severe plastic deformation. Mater. Trans. 2019, 60, 1489–1499. [Google Scholar] [CrossRef] [Green Version]
- Alexandrov, I.V.; Zhu, Y.T.; Lowe, T.C.; Islamgaliev, R.K.; Valiev, R.Z. Microstructures and properties of nanocomposites obtained through SPTS consolidation of powders. Metall. Mater. Trans. A 1998, 29, 2253–2260. [Google Scholar] [CrossRef]
- Lee, Z.; Zhou, F.; Valiev, R.Z.; Lavernia, E.J.; Nutt, S.R. Microstructure and microhardness of cryomilled bulk nanocrystalline Al-7.5%Mg alloy consolidated by high pressure torsion. Scr. Mater. 2004, 51, 209–214. [Google Scholar] [CrossRef]
- Borchers, C.; Garve, C.; Tiegel, M.; Deutges, M.; Herz, A.; Edalati, K.; Pippan, R.; Horita, Z.; Kirchheim, R. Nanocrystalline steel obtained by ball milling of iron and graphite subsequently compacted by high-pressure torsion. Acta Mater. 2015, 97, 207–215. [Google Scholar] [CrossRef]
- Ibrahim, N.; Peterlechner, M.; Emeis, F.; Wegner, M.; Divinski, S.V.; Wilde, G. Mechanical alloying via high-pressure torsion of the immiscible Cu50Ta50 system. Mater. Sci. Eng. A 2017, 685, 19–30. [Google Scholar] [CrossRef]
- Han, J.K.; Jang, J.I.; Langdon, T.G.; Kawasaki, M. Bulk-state reactions and improving the mechanical properties of metals through high-pressure torsion. Mater. Trans. 2019, 60, 1131–1138. [Google Scholar] [CrossRef] [Green Version]
- Edalati, K. Metallurgical alchemy by ultra-severe plastic deformation via high-pressure torsion process. Mater. Trans. 2019, 60, 1221–1229. [Google Scholar] [CrossRef] [Green Version]
- Blank, V.D.; Popov, M.Y.; Kulnitskiy, B.A. The effect of severe plastic deformations on phase transitions and structure of solids. Mater. Trans. 2019, 60, 1500–1505. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Ma, Y.; An, Q.; Levitas, V.; Zhang, Y.; Feng, B.; Chaudhuri, J.; Goddard, W.A., III. Shear driven formation of nano-diamonds at sub-gigapascals and 300 K. Carbon 2019, 146, 364–368. [Google Scholar] [CrossRef] [Green Version]
- Ikoma, Y.; Hayano, K.; Edalati, K.; Saito, K.; Guo, Q.; Horita, Z. Phase transformation and nanograin refinement of silicon by processing through high-pressure torsion. Appl. Phys. Lett. 2012, 101, 121908. [Google Scholar] [CrossRef]
- Razavi-Khosroshahi, H.; Edalati, K.; Hirayama, M.; Emami, H.; Arita, M.; Yamauchi, M.; Hagiwara, H.; Ida, S.; Ishihara, T.; Akiba, E.; et al. Visible-light-driven photocatalytic hydrogen generation on nanosized TiO2-II stabilized by high-pressure torsion. ACS Catal. 2016, 6, 5103–5107. [Google Scholar] [CrossRef]
- Edalati, K. Review on recent advancement in severe plastic deformation of oxides by high-pressure torsion (HPT). Adv. Eng. Mater. 2019, 21, 1800272. [Google Scholar] [CrossRef]
- Edalati, K.; Uehiro, R.; Takechi, S.; Wang, Q.; Arita, M.; Watanabe, M.; Ishihara, T.; Horita, Z. Enhanced photocatalytic hydrogen production on GaN-ZnO oxynitride by introduction of strain-induced nitrogen vacancy complexes. Acta Mater. 2020, 185, 149–156. [Google Scholar] [CrossRef]
- Révész, Á.; Kovács, Z. Severe plastic deformation of amorphous alloys. Mater. Trans. 2019, 60, 1283–1293. [Google Scholar] [CrossRef] [Green Version]
- Edalati, K.; Fujita, I.; Sauvage, X.; Arita, M.; Horita, Z. Microstructure and phase transformations of silica glass and vanadium oxide by severe plastic deformation via high-pressure torsion straining. J. Alloys Compd. 2019, 779, 394–398. [Google Scholar] [CrossRef]
- Segal, V.M.; Reznikov, V.I.; Drobyshevskiy, A.E.; Kopylov, V.I. Plastic working of metals by simple shear. Russ. Metall. 1981, 1, 99–105. [Google Scholar]
- Saito, Y.; Utsunomiya, H.; Tsuji, N.; Sakai, T. Novel ultra-high straining process for bulk materials: Development of the accumulative roll-bonding (ARB) process. Acta Mater. 1999, 47, 579–583. [Google Scholar] [CrossRef]
- Beygelzimer, Y.; Varukhin, V.; Synkov, S.; Sapronov, A.; Synkov, V. New techniques for accumulating large plastic deformations using hydroextrusion. Fiz. i Tekhnika Vusokih Davlenii 1999, 9, 109–111. [Google Scholar]
- Edalati, K.; Uehiro, R.; Fujiwara, K.; Ikeda, Y.; Li, H.W.; Sauvage, X.; Valiev, R.Z.; Akiba, E.; Tanaka, I.; Horita, Z. Ultra-severe plastic deformation: Evolution of microstructure, phase transformation and hardness in immiscible magnesium-based systems. Mater. Sci. Eng. A 2017, 701, 158–166. [Google Scholar] [CrossRef]
- Edalati, K.; Emami, H.; Staykov, A.; Smith, D.J.; Akiba, E.; Horita, Z. Formation of metastable phases in magnesium-titanium system by high-pressure torsion and their hydrogen storage performance. Acta Mater. 2015, 99, 150–156. [Google Scholar] [CrossRef]
- Edalati, K.; Emami, H.; Ikeda, Y.; Iwaoka, H.; Tanaka, I.; Akiba, E.; Horita, Z. New nanostructured phases with reversible hydrogen storage capability in immiscible magnesium-zirconium system produced by high-pressure torsion. Acta Mater. 2016, 108, 293–303. [Google Scholar] [CrossRef]
- Mohammadi, A.; Enikeev, N.A.; Murashkin, M.Y.; Arita, M.; Edalati, K. Examination of inverse Hall-Petch relation in nanostructured aluminum alloys by ultra-severe plastic deformation. J. Mater. Sci. Technol. 2021, 91, 78–89. [Google Scholar] [CrossRef]
- Mohammadi, A.; Enikeev, N.A.; Murashkin, M.Y.; Arita, M.; Edalati, K. Developing age-hardenable Al-Zr alloy by ultra-severe plastic deformation: Significance of supersaturation, segregation and precipitation on hardening and electrical conductivity. Acta Mater. 2021, 203, 116503. [Google Scholar] [CrossRef]
- Edalati, K.; Hashiguchi, Y.; Pereira, P.H.R.; Horita, A.; Langdon, T.G. Effect of temperature rise on microstructural evolution during high-pressure torsion. Mater. Sci. Eng. A 2018, 714, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z. Scaling-up of high pressure torsion using ring shape. Mater. Trans. 2009, 50, 92–95. [Google Scholar] [CrossRef] [Green Version]
- Horia, Z.; Tang, Y.; Masuda, T.; Takizawa, Y. Severe plastic deformation under high pressure: Upsizing sample dimensions. Mater. Trans. 2020, 61, 1177–1190. [Google Scholar] [CrossRef]
- Edalati, K.; Lee, S.; Horita, Z. Continuous high-pressure torsion using wires. J. Mater. Sci. 2012, 47, 473–478. [Google Scholar] [CrossRef]
- Ivanisenko, Y.; Kulagin, R.; Fedorov, V.; Mazilkin, A.; Scherer, T.; Baretzky, B.; Hahn, H. High pressure torsion extrusion as a new severe plastic deformation process. Mater. Sci. Eng. A 2016, 664, 247–256. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Hammond, V.H.; Atwater, M.A.; Darling, K.A.; Nguyen, H.Q.; Kecskes, L.J. Equal-channel angular extrusion of a low-density high-entropy alloy produced by high-energy cryogenic mechanical alloying. JOM 2014, 66, 2021–2029. [Google Scholar] [CrossRef]
- Shahmir, H.; Mousavi, T.; He, J.; Lu, Z.; Kawasaki, M.; Langdon, T.G. Microstructure and properties of a CoCrFeNiMn high-entropy alloy processed by equal-channel angular pressing. Mater. Sci. Eng. A 2017, 705, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhao, S.; Ritchie, R.O.; Meyers, M.A. Mechanical properties of high-entropy alloys with emphasis on face-centered cubic alloys. Prog. Mater. Sci. 2019, 102, 296–345. [Google Scholar] [CrossRef]
- George, E.P.; Curtin, W.A.; Tasan, C.C. High entropy alloys: A focused review of mechanical properties and deformation mechanisms. Acta Mater. 2020, 188, 435–474. [Google Scholar] [CrossRef]
- Schuh, B.; Mendez-Martin, F.; Völker, B.; George, E.P.; Clemens, H.; Pippan, R.; Hohenwarter, A. Mechanical properties, microstructure and thermal stability of a nanocrystalline CoCrFeMnNi high-entropy alloy after severe plastic deformation. Acta Mater. 2015, 96, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Shahmir, H.; He, J.; Lu, Z.; Kawasaki, M.; Langdon, T.G. Effect of annealing on mechanical properties of a nanocrystalline CoCrFeNiMn high-entropy alloy processed by high-pressure torsion. Mater. Sci. Eng. A 2016, 676, 294–303. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.H.; Huang, Y.; Huang, Y.Y.; Liao, X.Z.; Langdon, T.G.; Dai, P.Q. Hardening of an Al0.3CoCrFeNi high entropy alloy via high-pressure torsion and thermal annealing. Mater. Lett. 2015, 151, 126–129. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.H.; Huang, Y.; Cheng, H.; Liao, X.Z.; Langdon, T.G.; Dai, P.Q. The effect of grain size on the annealing-induced phase transformation in an Al0.3CoCrFeNi high entropy alloy. Mater. Des. 2016, 105, 381–385. [Google Scholar] [CrossRef] [Green Version]
- Reddy, T.S.; Wani, I.S.; Bhattacharjee, T.; Reddy, S.R.; Saha, R.; Bhattacharjee, P.P. Severe plastic deformation driven nanostructure and phase evolution in a Al0.5CoCrFeMnNi dual phase high entropy alloy. Intermetallics 2017, 91, 150–157. [Google Scholar] [CrossRef]
- Edalati, P.; Mohammadi, A.; Ketabchi, M.; Edalati, K. Ultrahigh hardness in nanostructured dual-phase high-entropy alloy AlCrFeCoNiNb developed by high-pressure torsion. J. Alloys Compd. 2021, 884, 161101. [Google Scholar] [CrossRef]
- Schuh, B.; Völker, B.; Todt, J.; Schell, N.; Perrière, L.; Li, J.; Couzinié, J.P.; Hohenwarter, A. Thermodynamic instability of a nanocrystalline, single-phase TiZrNbHfTa alloy and its impact on the mechanical properties. Acta Mater. 2018, 142, 201–212. [Google Scholar] [CrossRef]
- Shahmir, H.; Tabachnikova, E.; Podolskiy, A.; Tikhonovsky, M.; Langdon, T.G. Effect of carbon content and annealing on structure and hardness of CrFe2NiMnV0.25 high-entropy alloys processed by high-pressure torsion. J. Mater. Sci. 2018, 53, 11813–11822. [Google Scholar] [CrossRef] [Green Version]
- Gubicza, J.; Hung, P.T.; Kawasaki, M.; Han, J.K.; Zhao, Y.; Xue, Y.; Lábár, J.L. Influence of severe plastic deformation on the microstructure and hardness of a CoCrFeNi high-entropy alloy: A comparison with CoCrFeNiMn. Mater. Charact. 2019, 154, 304–314. [Google Scholar] [CrossRef]
- Edalati, P.; Floriano, R.; Mohammadi, A.; Li, Y.; Zepon, G.; Li, H.W.; Edalati, K. Reversible room temperature hydrogen storage in high-entropy alloy TiZrCrMnFeNi. Scr. Mater. 2020, 178, 387–390. [Google Scholar] [CrossRef]
- Edalati, P.; Floriano, R.; Tang, Y.; Mohammadi, A.; Danielle Pereira, K.; Ducati Luchessi, A.; Edalati, K. Ultrahigh hardness and biocompatibility of high-entropy alloy TiAlFeCoNi processed by high-pressure torsion. Mater. Sci. Eng. C 2020, 112, 110908. [Google Scholar] [CrossRef]
- Edalati, P.; Mohammadi, A.; Tang, Y.; Floriano, R.; Fuji, M.; Edalati, K. Phase transformation and microstructure evolution in ultrahard carbon-doped AlTiFeCoNi high-entropy alloy by high-pressure torsion. Mater. Lett. 2021, 302, 130368. [Google Scholar] [CrossRef]
- Lu, Y.; Mazilkin, A.; Boll, T.; Stepanov, N.; Zherebtzov, S.; Salishchev, G.; Ódor, É.; Ungar, T.; Lavernia, E.; Hahn, H.; et al. Influence of carbon on the mechanical behavior and microstructure evolution of CoCrFeMnNi processed by high pressure torsion. Materialia 2021, 16, 101059. [Google Scholar] [CrossRef]
- Kilmametov, A.; Kulagin, R.; Mazilkin, A.; Seils, S.; Boll, T.; Heilmaier, M.; Hahn, H. High-pressure torsion driven mechanical alloying of CoCrFeMnNi high entropy alloy. Scr. Mater. 2019, 158, 29–33. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, A.; Djenadic, R.; Wang, D.; Hein, C.; Kautenburger, R.; Clemens, O.; Hahn, H. Rare earth and transition metal based entropy stabilised perovskite type oxides. J. Eur. Ceram. Soc. 2018, 38, 2318–2327. [Google Scholar] [CrossRef]
- Chen, T.; Shun, T.; Yeh, J.; Wong, M. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coat. Technol. 2004, 188, 193–200. [Google Scholar] [CrossRef]
- Lewin, E. Multi-component and high-entropy nitride coatings–a promising field in need of a novel approach. J. Appl. Phys. 2020, 127, 160901. [Google Scholar] [CrossRef]
- Braic, M.; Braic, V.; Balaceanu, M.; Zoita, C.; Vladescu, A.; Grigore, E. Characteristics of (TiAlCrNbY)C films deposited by reactive magnetron sputtering. Surf. Coat. Technol. 2010, 204, 2010–2014. [Google Scholar] [CrossRef]
- Jiang, S.; Shao, L.; Fan, T.W.; Duan, J.M.; Chen, X.T.; Tang, B.Y. Elastic and thermodynamic properties of high entropy carbide (HfTaZrTi)C and (HfTaZrNb)C from ab initio investigation. Ceram. Int. 2020, 46, 15104–15112. [Google Scholar] [CrossRef]
- Gild, J.; Zhang, Y.; Harrington, T.; Jiang, S.; Hu, T.; Quinn, M.C.; Mellor, V.M.; Zhou, N.; Vecchio, K.; Luo, J. High-entropy metal diborides: A new class of high-entropy materials and a new type of ultrahigh temperature ceramics. Sci. Rep. 2016, 6, 37946. [Google Scholar] [CrossRef]
- Qin, M.; Yan, Q.; Liu, Y.; Luo, J. A new class of high-entropy M3B4 borides. J. Adv. Ceram. 2021, 10, 166–172. [Google Scholar] [CrossRef]
- Wright, A.J.; Luo, J. A step forward from high-entropy ceramics to compositionally complex ceramics: A new perspective. J. Mater. Sci. 2020, 55, 9812–9827. [Google Scholar] [CrossRef] [Green Version]
- Oses, C.; Toher, C.; Curtarolo, S. High-entropy ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- De Marco, M.O.; Li, Y.; Li, H.W.; Edalati, K.; Floriano, R. Mechanical synthesis and hydrogen storage characterization of MgVCr and MgVTiCrFe high-entropy alloy. Adv. Eng. Mater. 2020, 22, 1901079. [Google Scholar] [CrossRef]
- Edalati, P.; Wang, Q.; Razavi-Khosroshahi, H.; Fuji, M.; Ishihara, T.; Edalati, K. Photocatalytic hydrogen evolution on a high-entropy oxide. J. Mater. Chem. A 2020, 8, 3814–3821. [Google Scholar] [CrossRef]
- Edalati, P.; Shen, X.F.; Watanabe, M.; Ishihara, T.; Arita, M.; Fuji, M.; Edalati, K. High-entropy oxyitride as low-bandgap and stable photocatalyst for hydrogen production. J. Mater. Chem. A 2021, 9, 15076–15086. [Google Scholar] [CrossRef]
- González-Masís, J.; Cubero-Sesin, J.M.; Campos-Quirós, A.; Edalati, K. Synthesis of biocompatible high-entropy alloy TiNbZrTaHf by high-pressure torsion. Mater. Sci. Eng. A 2021, 825, 141869. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z. Correlations between hardness and atomic bond parameters of pure metals and semi-metals after processing by high-pressure torsion. Scr. Mater. 2011, 64, 161–164. [Google Scholar] [CrossRef]
- Zuttel, A.; Borgschulte, A.; Schlapbach, L. Hydrogen as a Future Energy Carrier, 1st ed.; Wiley VCH Verlag GmbH & Co, KGaA: Weinheim, Germany, 2008. [Google Scholar]
- von Colbe, J.B.; Ares, J.R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrog. Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; von Colbe, J.B.; Blanchard, D.; Bowman, R.C., Jr.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage–past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Huot, J.; Cuevas, F.; Deledda, S.; Edalati, K.; Filinchuk, Y.; Grosdidier, T.; Hauback, B.C.; Heere, M.; Jensen, T.R.; Latroche, M.; et al. Mechanochemistry of metal hydrides: Recent advances. Materials 2019, 12, 2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Révész, A.; Gajdics, M. High-pressure torsion of non-equilibrium hydrogen storage materials: A review. Energies 2021, 14, 819. [Google Scholar] [CrossRef]
- Edalati, K.; Akiba, E.; Horita, Z. High-pressure torsion for new hydrogen storage materials. Sci. Technol. Adv. Mater. 2018, 19, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, D.; Ek, G.; Cedervall, J.; Zlotea, C.; Møller, K.T.; Hansen, T.C.; Bednarčík, J.; Paskevicius, M.; Sørby, M.H.; Jensen, T.R.; et al. Structure and hydrogenation properties of a HfNbTiVZr high-entropy alloy. Inorg. Chem. 2018, 57, 2103–2110. [Google Scholar] [CrossRef]
- Zlotea, C.; Sow, M.A.; Ek, G.; Couzinié, J.P.; Perrière, L.; Guillot, I.; Bourgon, J.; Møller, K.T.; Jensen, T.R.; Akiba, E.; et al. Hydrogen sorption in TiZrNbHfTa high entropy alloy. J. Alloys Compd. 2019, 775, 667–674. [Google Scholar] [CrossRef]
- Zepon, G.; Leiva, D.R.; Strozi, R.B.; Bedoch, A.; Figueroa, S.J.A.; Ishikawa, T.T.; Botta, W.J. Hydrogen-induced phase transition of MgZrTiFe0.5Co0.5Ni0.5 high entropy alloy. Int. J. Hydrog. Energy 2018, 43, 1702–1708. [Google Scholar] [CrossRef]
- Kunce, I.; Polanski, M.; Bystrzycki, J. Microstructure and hydrogen storage properties of a TiZrNbMoV high entropy alloy synthesized using laser engineered net shaping (LENS). Int. J. Hydrog. Energy 2014, 39, 9904–9910. [Google Scholar] [CrossRef]
- Hu, J.; Shen, H.; Jiang, M.; Gong, H.; Xiao, H.; Liu, Z.; Sun, G.; Zu, X. A DFT Study of Hydrogen storage in high-entropy alloy TiZrHfScMo. Nanomaterials 2019, 9, 461. [Google Scholar] [CrossRef] [Green Version]
- Kao, Y.F.; Chen, S.K.; Sheu, J.H.; Lin, J.T.; Lin, W.E.; Yeh, J.W.; Len, S.J.; Liou, T.H.; Wang, C.W. Hydrogen storage properties of multi-principal-component CoFeMnTixVyZrz alloys. Int. J. Hydrog. Energy 2010, 35, 9046–9059. [Google Scholar] [CrossRef]
- Kunce, I.; Polanski, M.; Bystrzycki, J. Structure and hydrogen storage properties of a high entropy ZrTiVCrFeNi alloy synthesized using laser engineered net shaping (LENS). Int. J. Hydrog. Energy 2013, 38, 12180–12189. [Google Scholar] [CrossRef]
- Yang, S.; Yang, F.; Wu, C.; Chen, Y.; Mao, Y.; Luo, L. Hydrogen storage and cyclic properties of (VFe)60(TiCrCo)40−xZrx (0 ≤ x ≤ 2) alloys. J. Alloys Compd. 2016, 663, 460–465. [Google Scholar] [CrossRef]
- Nygård, M.M.; Ek, G.; Karlsson, D.; Sahlberg, M.; Sørby, M.H.; Hauback, B.C. Hydrogen storage in high-entropy alloys with varying degree of local lattice strain. Int. J. Hydrog. Energy 2019, 44, 29140–29149. [Google Scholar] [CrossRef] [Green Version]
- Dewangan, S.K.; Sharma, V.K.; Sahu, P.; Kumar, V. Synthesis and characterization of hydrogenated novel AlCrFeMnNiW high entropy alloy. Int. J. Hydrog. Energy 2020, 45, 16984–16991. [Google Scholar] [CrossRef]
- Floriano, R.; Zepon, G.; Edalati, K.; Fontana, G.L.B.G.; Mohammadi, A.; Ma, Z.; Li, H.W.; Contieri, R. Hydrogen storage in TiZrNbFeNi high entropy alloys, designed by thermodynamic calculations. Int. J. Hydrog. Energy 2020, 45, 33759–33770. [Google Scholar] [CrossRef]
- Floriano, R.; Zepon, G.; Edalati, K.; Fontana, G.L.B.G.; Mohammadi, A.; Ma, Z.; Li, H.W.; Contieri, R.J. Hydrogen storage properties of new A3B2-type TiZrNbCrFe high-entropy alloy. Int. J. Hydrog. Energy 2021, 46, 23757–23766. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Wang, W.; Tade, M.O.; Shao, Z. Research progress of perovskite materials in photocatalysis- and photovoltaics-related energy conversion and environmental treatment. Chem. Soc. Rev. 2015, 44, 5371–5408. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Domen, K. New non-oxide photocatalysts designed for overall water splitting under visible light. J. Phys. Chem. C 2007, 111, 7851–7861. [Google Scholar] [CrossRef]
- Takata, T.; Pan, C.; Domen, K. Recent progress in oxynitride photocatalysts for visible-light-driven water splitting. Sci. Technol. Adv. Mater. 2015, 16, 033506. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edalati, K.; Li, H.-W.; Kilmametov, A.; Floriano, R.; Borchers, C. High-Pressure Torsion for Synthesis of High-Entropy Alloys. Metals 2021, 11, 1263. https://doi.org/10.3390/met11081263
Edalati K, Li H-W, Kilmametov A, Floriano R, Borchers C. High-Pressure Torsion for Synthesis of High-Entropy Alloys. Metals. 2021; 11(8):1263. https://doi.org/10.3390/met11081263
Chicago/Turabian StyleEdalati, Kaveh, Hai-Wen Li, Askar Kilmametov, Ricardo Floriano, and Christine Borchers. 2021. "High-Pressure Torsion for Synthesis of High-Entropy Alloys" Metals 11, no. 8: 1263. https://doi.org/10.3390/met11081263
APA StyleEdalati, K., Li, H.-W., Kilmametov, A., Floriano, R., & Borchers, C. (2021). High-Pressure Torsion for Synthesis of High-Entropy Alloys. Metals, 11(8), 1263. https://doi.org/10.3390/met11081263









