The Effect of Solidification Rate on the Microstructure and Mechanical Properties of Pure Magnesium
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
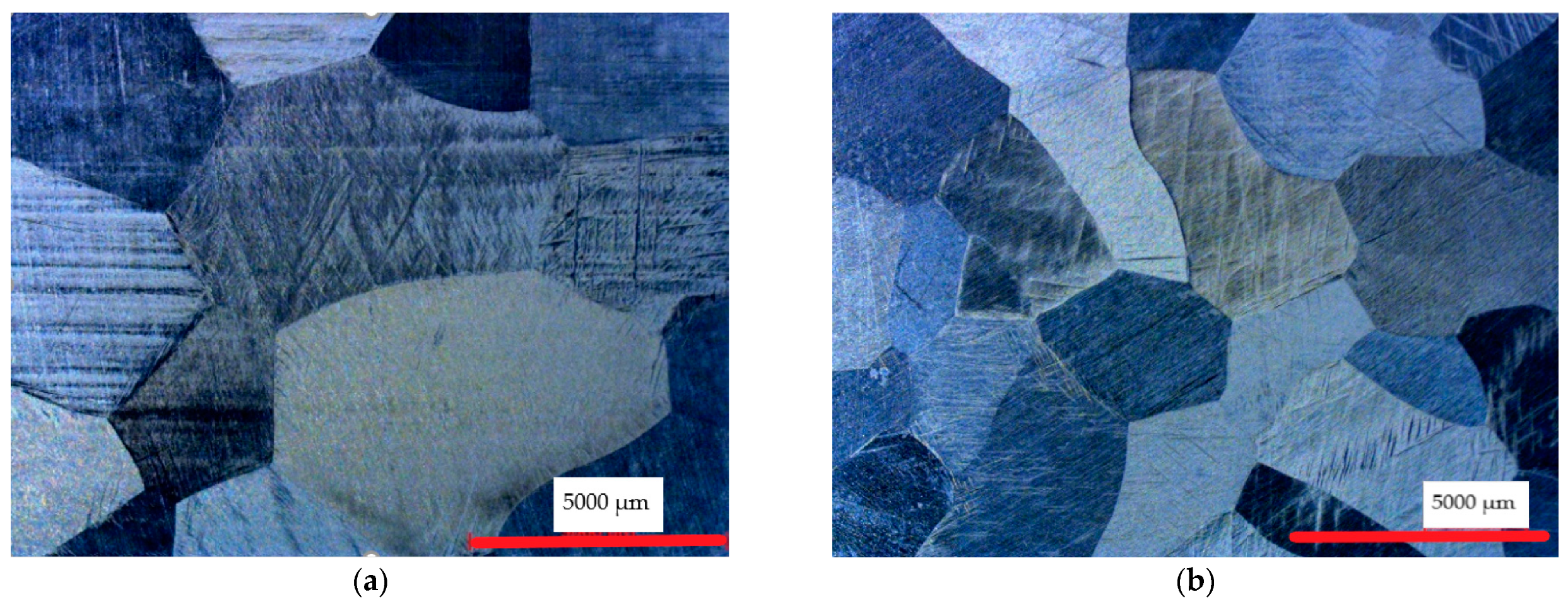
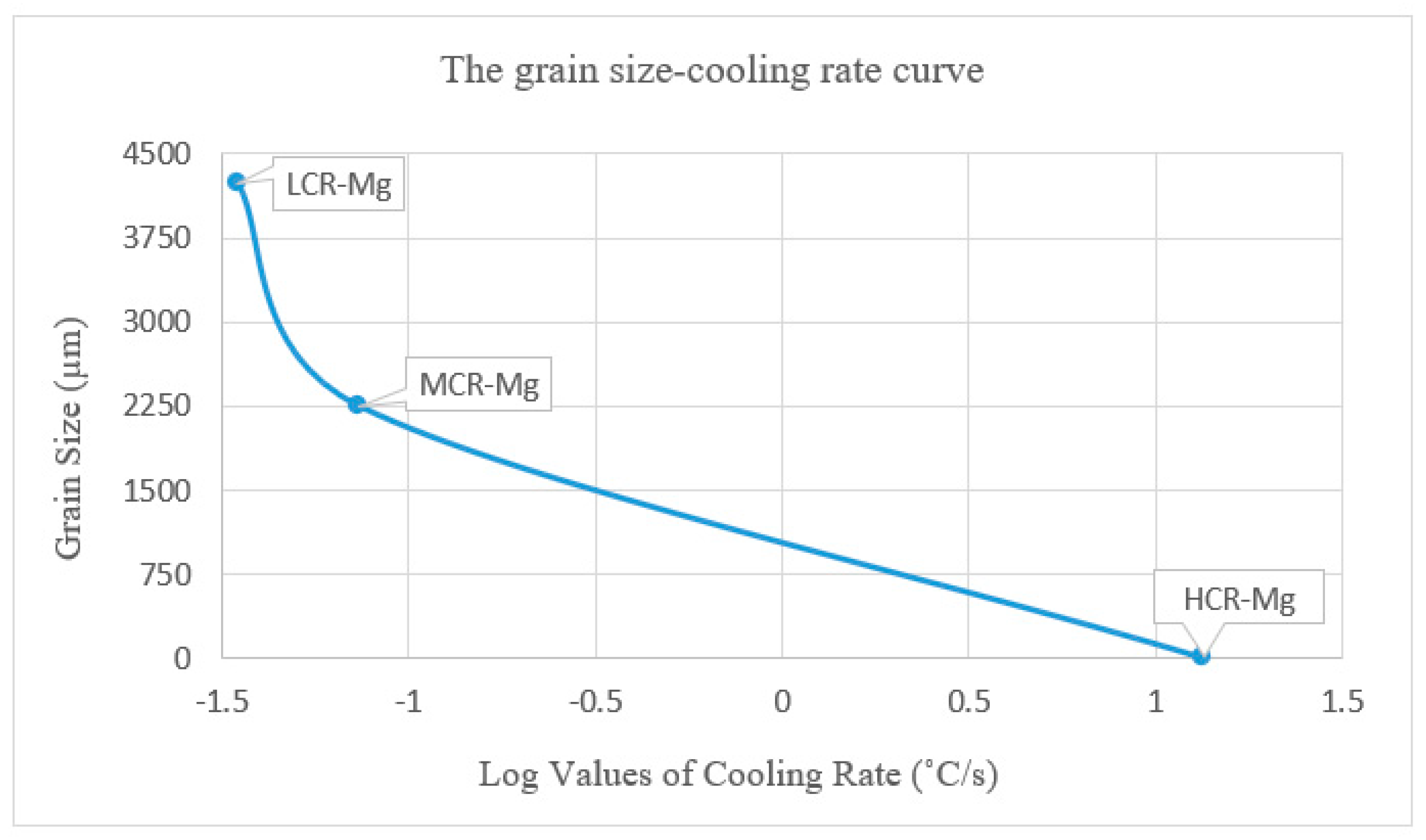
3.1. Microstructure
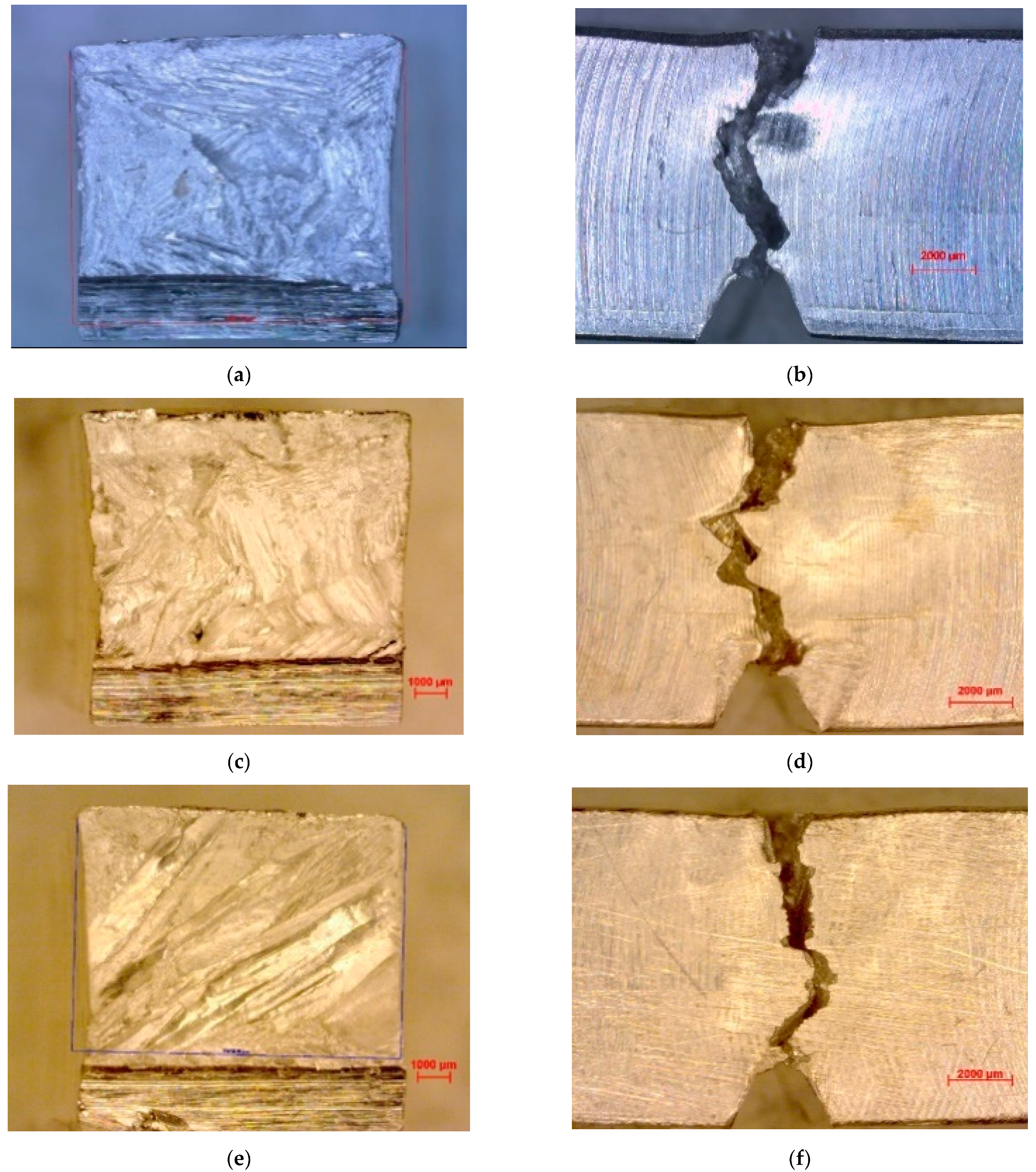
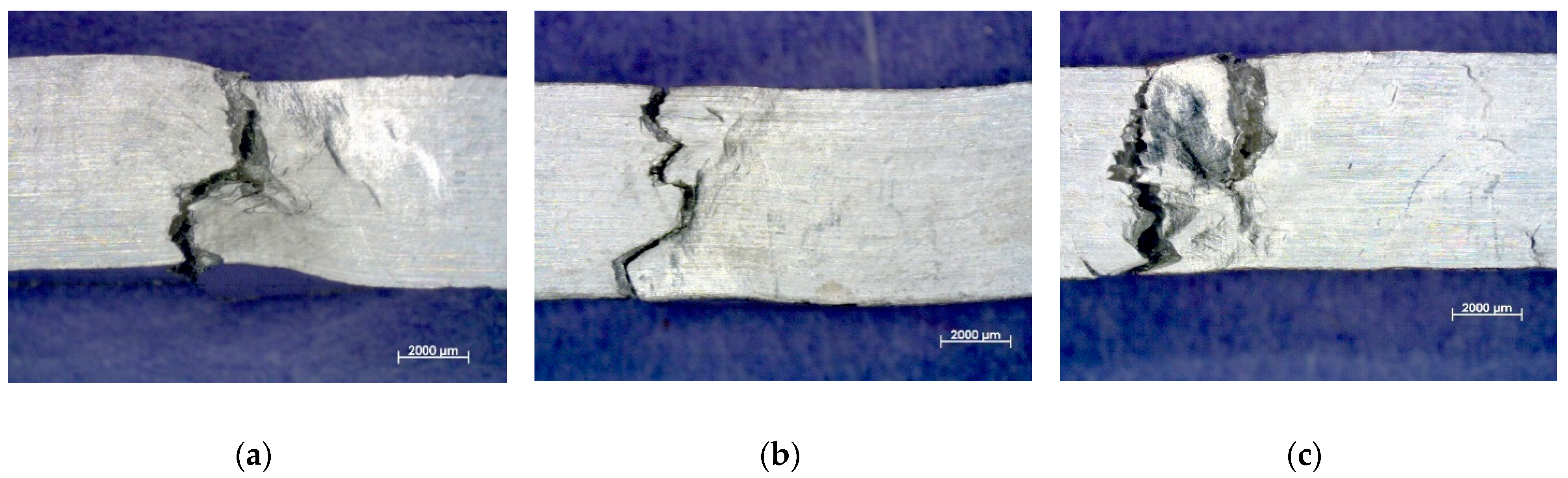
3.2. Impact and Fracture Mechanism
3.3. Hardness
3.4. Tensile and Fracture Mechanism
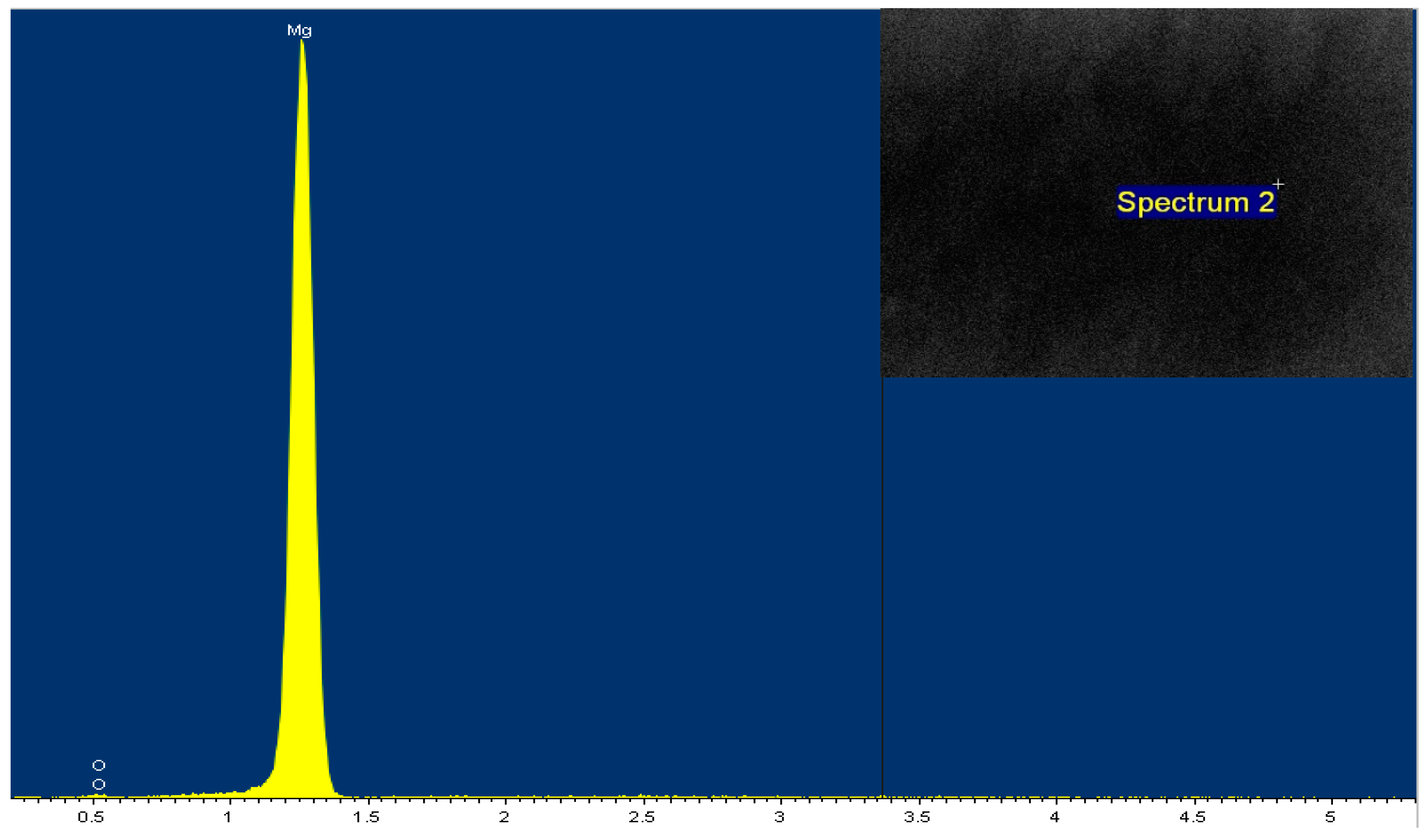
3.5. SEM and EDS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brooks, E.K.; Ehrensberger, M.T. Bio-Corrosion of Magnesium Alloys for Orthopaedic Applications. J. Funct. Biomater. 2017, 8, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Sun, J.; Qiu, K.; Yang, Y.; Pu, Z.; Li, L.; Zheng, Y. Effects of alloying elements (Ca and Sr) on microstructure, mechanical property and in vitro corrosion behavior of biodegradable Zn–1.5Mg alloy. J. Alloys Compd. 2016, 664, 444–452. [Google Scholar] [CrossRef]
- International Magnesium Association. International Magnesium Association. Available online: https://www.intlmag.org/ (accessed on 1 January 2019).
- Abdessameud, S.; Mezbahul-Islam, M.; Medraj, M.; Mikuli, E. Thermodynamic Modeling of Hydrogen Storage Capacity in Mg-Na Alloys. Sci. World J. 2014, 2014, 16. [Google Scholar] [CrossRef] [Green Version]
- Pogorielov, M.; Husak, E.; Solodivnik, A.; Zhdanov, S. Magnesium-based biodegradable alloys: Degradation, application, and alloying elements. (Report). Interv. Med. Appl. Sci. 2017, 9, 27–38. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Li, N.; Zheng, Y. Novel Magnesium Alloys Developed for Biomedical Application. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- ASM Charles Moosbrugger. In Engineering Properties of Magnesium Alloys; ASM International: Almere, The Netherlands, 2017; Chapter 1, 3.
- Bakhsheshi-Rad, H.; Idris, M.; Abdul-Kadir, M.; Ourdjini, A.; Medraj, M.; Daroonparvar, M.; Hamzah, E. Mechanical and bio-corrosion properties of quaternary Mg-Ca-Mn-Zn alloys compared with binary Mg-Ca alloys. Mater. Des. 2014, 53, 283–292. [Google Scholar] [CrossRef]
- Guo, K.K.W. A Review of Magnesium/Magnesium Alloys Corrosion and its Protection. Recent Pat. Corros. Sci. 2010, 2, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Radha, R.; Sreekanth, D. Insight of magnesium alloys and composites for orthopedic implant applications—A review. J. Magnes. Alloys 2017, 5, 286–312. [Google Scholar] [CrossRef]
- Stulikova, I.; Smola, B. Mechanical properties and phase composition of potential biodegradable Mg–Zn–Mn–base alloys with addition of rare earth elements. Mater. Charact. 2010, 61, 952–958. [Google Scholar] [CrossRef]
- El-Mahallawy, N.; Palkowski, H.; Klingner, A.; Diaa, A.; Shoeib, M. Effect of 1.0 wt. % Zn addition on the microstructure, mechanical properties, and bio-corrosion behaviour of micro alloyed Mg-0.24Sn-0.04Mn alloy as biodegradable material. Mater. Today Commun. 2020, 24, 100999. [Google Scholar] [CrossRef]
- Rosalbino, F.; de Negri, S.; Scavino, G.; Saccone, A. Microstructure and in vitro degradation performance of Mg–Zn–Mn alloys for biomedical application. J. Biomed. Mater. Res. Part A 2013, 101, 704–711. [Google Scholar] [CrossRef]
- Hänzi, A.C.; Gerber, I.; Schinhammer, M.; Löffler, J.F.; Uggowitzer, P.J. On the in vitro and in vivo degradation performance and biological response of new biodegradable Mg–Y–Zn alloys. Acta Biomater. 2010, 6, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zheng, Y.; Zhong, S.; Xi, T.; Wang, J.; Wang, W. Corrosion of, and cellular responses to Mg–Zn–Ca bulk metallic glasses. Biomaterials 2010, 31, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; He, Y.; Zhang, H.; Wang, X. Microstructure, mechanical property and in vitro biocorrosion behavior of single-phase biodegradable Mg–1.5Zn–0.6Zr alloy. J. Magnes. Alloys 2014, 2, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Han, E.-H.; Dong, K.; Shan, D.; Yim, C.D.; You, B.S. Effect of hydrogen on the corrosion behavior of the Mg–xZn alloys. J. Magnes. Alloys 2014, 2, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Han, E.-H.; Shan, D.; Yim, C.D.; You, B.S. The effect of Zn concentration on the corrosion behavior of Mg–xZn alloys. Corros. Sci. 2012, 65, 322–330. [Google Scholar] [CrossRef]
- Song, Y.; Han, E.-H.; Shan, D.; Yim, C.D.; You, B.S. The role of second phases in the corrosion behavior of Mg–5Zn alloy. Corros. Sci. 2012, 60, 238–245. [Google Scholar] [CrossRef]
- Zhang, E.; Yang, L.; Xu, J.; Chen, H. Microstructure, mechanical properties and bio-corrosion properties of Mg–Si(–Ca, Zn) alloy for biomedical application. Acta Biomater. 2010, 6, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shan, D.; Song, Y.; Han, E.-H. Influence of yttrium element on the corrosion behaviors of Mg–Y binary magnesium alloy. J. Magnes. Alloys 2017, 5, 26–34. [Google Scholar] [CrossRef]
- Bi, G.; Li, Y.; Zang, S.; Zhang, J.; Ma, Y.; Hao, Y. Microstructure, mechanical and corrosion properties of Mg–2Dy–xZn (x = 0, 0.1, 0.5 and 1 at.%) alloys. J. Magnes. Alloys 2014, 2, 64–71. [Google Scholar] [CrossRef]
- Li, D.; Wang, Q.; Ding, W. Effects of Ho on the microstructure and mechanical properties of Mg-Zn-Ho-Zr magnesium alloys. Rare Met. 2011, 30, 131–136. [Google Scholar] [CrossRef]
- Sajuri, Z.B.; Miyashita, Y.; Hosokai, Y.; Mutoh, Y. Effects of Mn content and texture on fatigue properties of as-cast and extruded AZ61 magnesium alloys. Int. J. Mech. Sci. 2006, 48, 198–209. [Google Scholar] [CrossRef]
- Lugo, M.; Jordon, J.; Solanki, K.; Hector, L.; Bernard, J.; Luo, A.; Horstemeyer, M. Role of different material processing methods on the fatigue behavior of an AZ31 magnesium alloy. Int. J. Fatigue 2013, 52, 131–143. [Google Scholar] [CrossRef]
- Azeem, M.; Tewari, A.; Ramamurty, U. Effect of recrystallization and grain growth on the mechanical properties of an extruded AZ21 Mg alloy. Mater. Sci. Eng. A 2010, 527, 898–903. [Google Scholar] [CrossRef]
- Khan, S.A.; Miyashita, Y.; Mutoh, Y.; Sajuri, Z.B. Influence of Mn content on mechanical properties and fatigue behavior of extruded Mg alloys. Mater. Sci. Eng. A 2006, 420, 315–321. [Google Scholar] [CrossRef]
- Nam, N.D.; Mathesh, M.; Forsyth, M.; Jo, D.S. Effect of manganese additions on the corrosion behavior of an extruded Mg–5Al based alloy. J. Alloy. Compd. 2012, 542, 199–206. [Google Scholar] [CrossRef]
- Crosby, W.H.; Likhite, V.V.; O’Brien, J.E.; David, F. Serum Iron Levels in Ostensibly Normal People. JAMA 1974, 227, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Li, Z.; Pan, Y.; Du, L.; Li, X.; Zheng, Y.; Li, L. In vitro and in vivo studies on biodegradable magnesium alloy. Prog. Nat. Sci. Mater. Int. 2014, 24, 466–471. [Google Scholar] [CrossRef] [Green Version]
- Haghshenas, M. Mechanical characteristics of biodegradable magnesium matrix composites: A review. J. Magnes. Alloys 2017, 5, 189–201. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Zhou, F.; Yang, Y.; Chang, R.; Qiu, K.; Pu, Z.; Li, L.; Zheng, Y. Micro-alloying with Mn in Zn–Mg alloy for future biodegradable metals application. Mater. Des. 2016, 94, 95–104. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Y.; Qin, L. Progress of biodegradable metals. Prog. Nat. Sci. Mater. Int. 2014, 24, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Peron, M.; Torgersen, J.; Berto, F. Mg and Its Alloys for Biomedical Applications: Exploring Corrosion and Its Interplay with Mechanical Failure. Metals 2017, 7, 252. [Google Scholar] [CrossRef] [Green Version]
- Atrens, A.; Liu, M.; Zainal Abidin, N.I. Corrosion mechanism applicable to biodegradable magnesium implants. Mater. Sci. Eng. B 2011, 176, 1609–1636. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, T.; Chu, P. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: A review. Acta Biomater. 2011, 7, 1452–1459. [Google Scholar] [CrossRef]
- Van der Biest, O.; Pelegrin, I.M. Degradation Testing of Magnesium and its Alloys aiming at Biodegradable Implant Applications; Degradatietesten van magnesium en magnesium legeringen voor biologisch afbreekbare implantaten. Dissertation 2016, 19–24. [Google Scholar]
- Pang, S.; Wu, G.; Liu, W.; Sun, M.; Zhang, Y.; Liu, Z.; Ding, W. Effect of cooling rate on the microstructure and mechanical properties of sand-casting Mg–10Gd–3Y–0.5Zr magnesium alloy. Mater. Sci. Eng. A 2013, 562, 152–160. [Google Scholar] [CrossRef]
- Petzow, G. Metallographic Etching: Metallographic and Ceramographic Methods for Revealing Microstructure, 5th ed.; American Society for Metals: Geauga, OH, USA, 1978; pp. 72–74. [Google Scholar]
- Ding, Y.; Wen, C.; Hodgson, P.; Li, Y. Effects of alloying elements on the corrosion behavior and biocompatibility of biodegradable magnesium alloys: A review. J. Mater. Chem. B 2014, 2, 1912–1933. [Google Scholar] [CrossRef] [PubMed]
- Poinern, G.E.J.; Brundavanam, S.; Fawcett, D. Biomedical Magnesium Alloys: A Review of Material Properties, Surface Modifications and Potential as a Biodegradable Orthopaedic Implant. Am. J. Biomed. Eng. 2012, 2, 218–2540. [Google Scholar] [CrossRef] [Green Version]








| Elements | Weight (%) |
|---|---|
| Manganese | <0.05 |
| Silicon | <0.05 |
| Cobalt | <0.01 |
| Aluminum | <0.005 |
| Copper | <0.005 |
| Iron | <0.001 |
| Lead | <0.001 |
| Nickel | <0.001 |
| Zinc | <0.001 |
| Pure Mg | ~Grain Size (µm) |
|---|---|
| LCR-Mg (0.035 °C/s) | 4235 |
| MCR-Mg (0.074 °C/s) | 2252 |
| HCR-Mg (13.5 °C/s) | 6 to 12 |
| Mg Systems | Break Energy (J) | Impact Strength (J/m) |
|---|---|---|
| LCR-Mg | 6.6 | 660 |
| MCR-Mg | 6.54 | 654.2 |
| HCR-Mg | 5.49 | 549.2 |
| Alloying Systems | E (GPa) | YS (MPa) | UTS (MPa) | Ductility (%) |
|---|---|---|---|---|
| LCR-Mg | 4.6 | 15.40 | 48.97 | 10.30 |
| MCR-Mg | 2.6 | 24.19 | 67.4 | 10.29 |
| HCR-Mg | 7.7 | 24.34 | 87.18 | 9.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamel, M.M.; Lopez, H.; Schultz, B.; Otieno, W. The Effect of Solidification Rate on the Microstructure and Mechanical Properties of Pure Magnesium. Metals 2021, 11, 1264. https://doi.org/10.3390/met11081264
Jamel MM, Lopez H, Schultz B, Otieno W. The Effect of Solidification Rate on the Microstructure and Mechanical Properties of Pure Magnesium. Metals. 2021; 11(8):1264. https://doi.org/10.3390/met11081264
Chicago/Turabian StyleJamel, Murtatha M., Hugo Lopez, Benjamin Schultz, and Wilkistar Otieno. 2021. "The Effect of Solidification Rate on the Microstructure and Mechanical Properties of Pure Magnesium" Metals 11, no. 8: 1264. https://doi.org/10.3390/met11081264
APA StyleJamel, M. M., Lopez, H., Schultz, B., & Otieno, W. (2021). The Effect of Solidification Rate on the Microstructure and Mechanical Properties of Pure Magnesium. Metals, 11(8), 1264. https://doi.org/10.3390/met11081264





