Experimental Phase Equilibria and Isopleth Section of 8Nb-TiAl Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
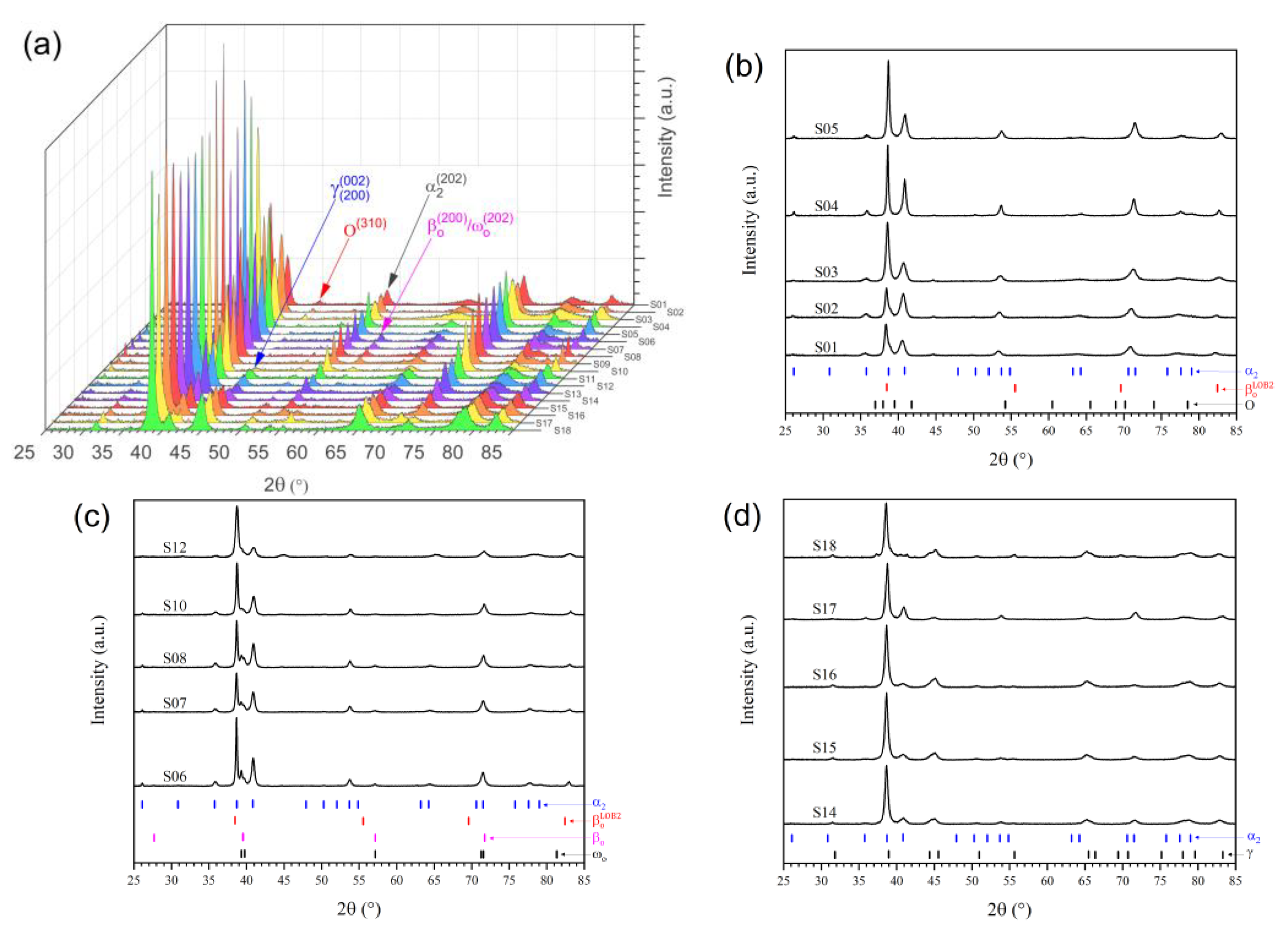
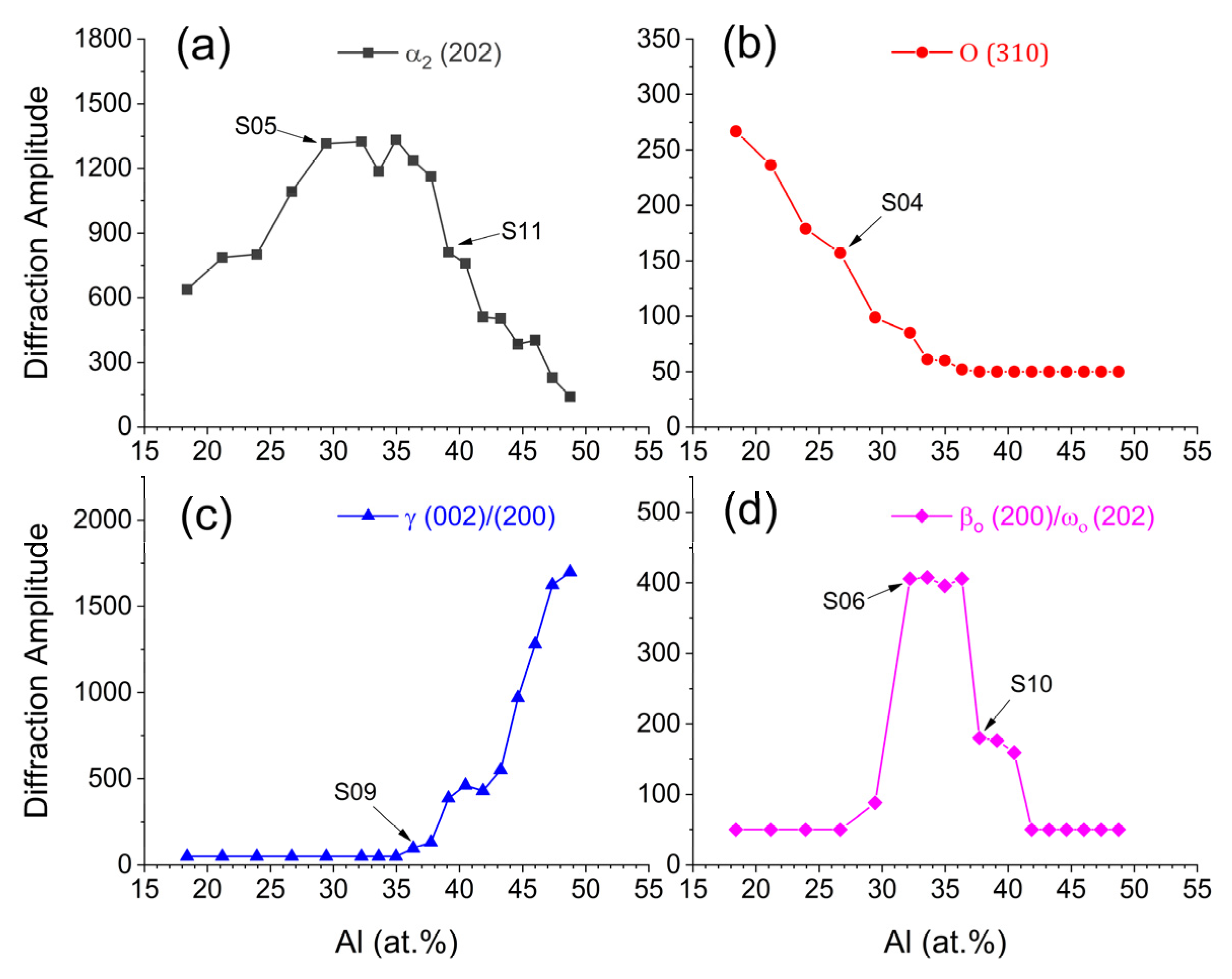
3.1. Phase Evolution
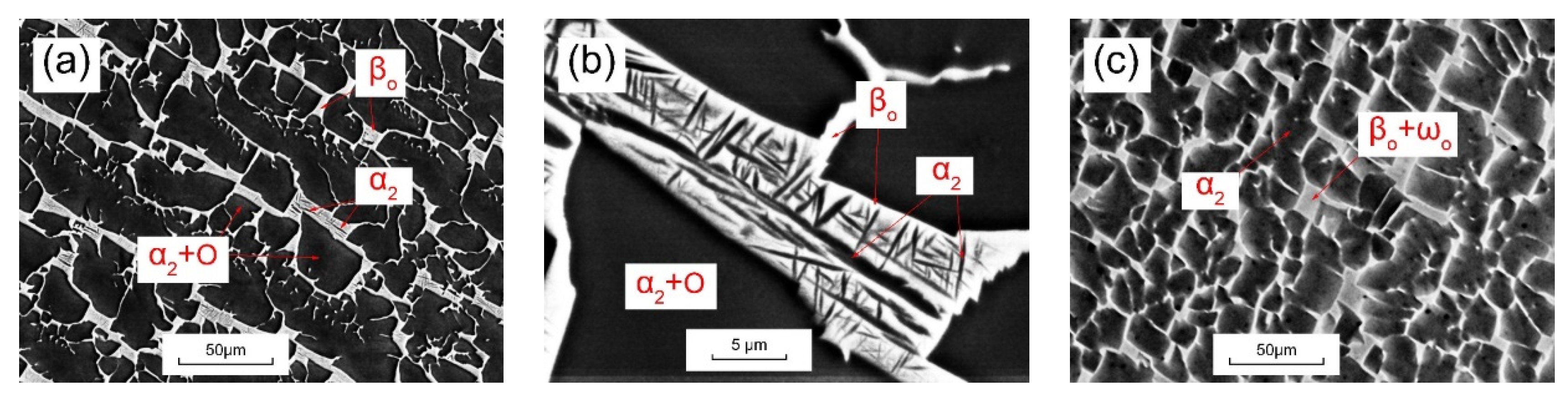
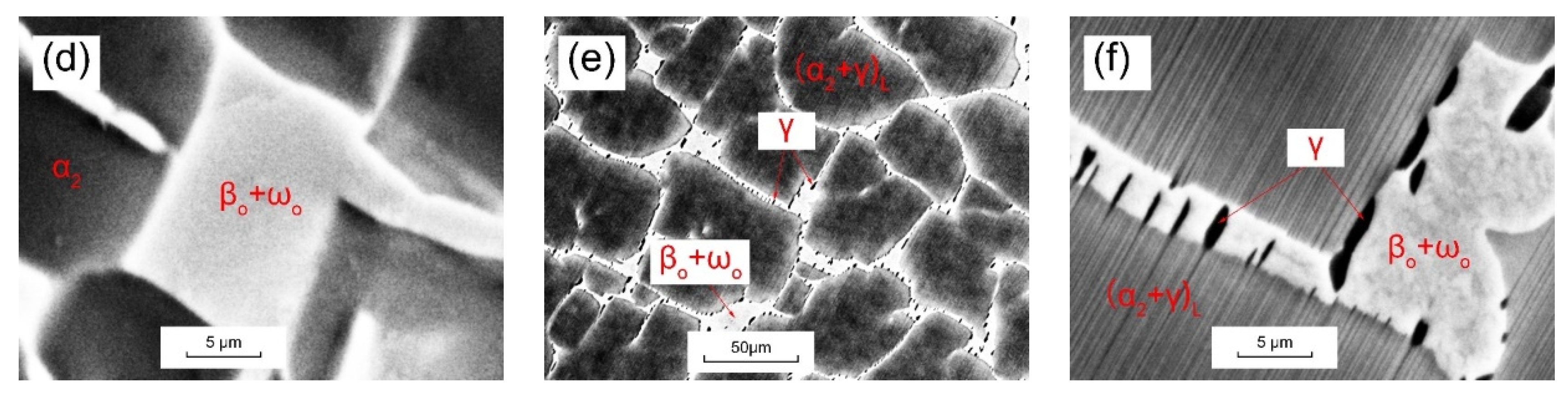
3.2. Morphology
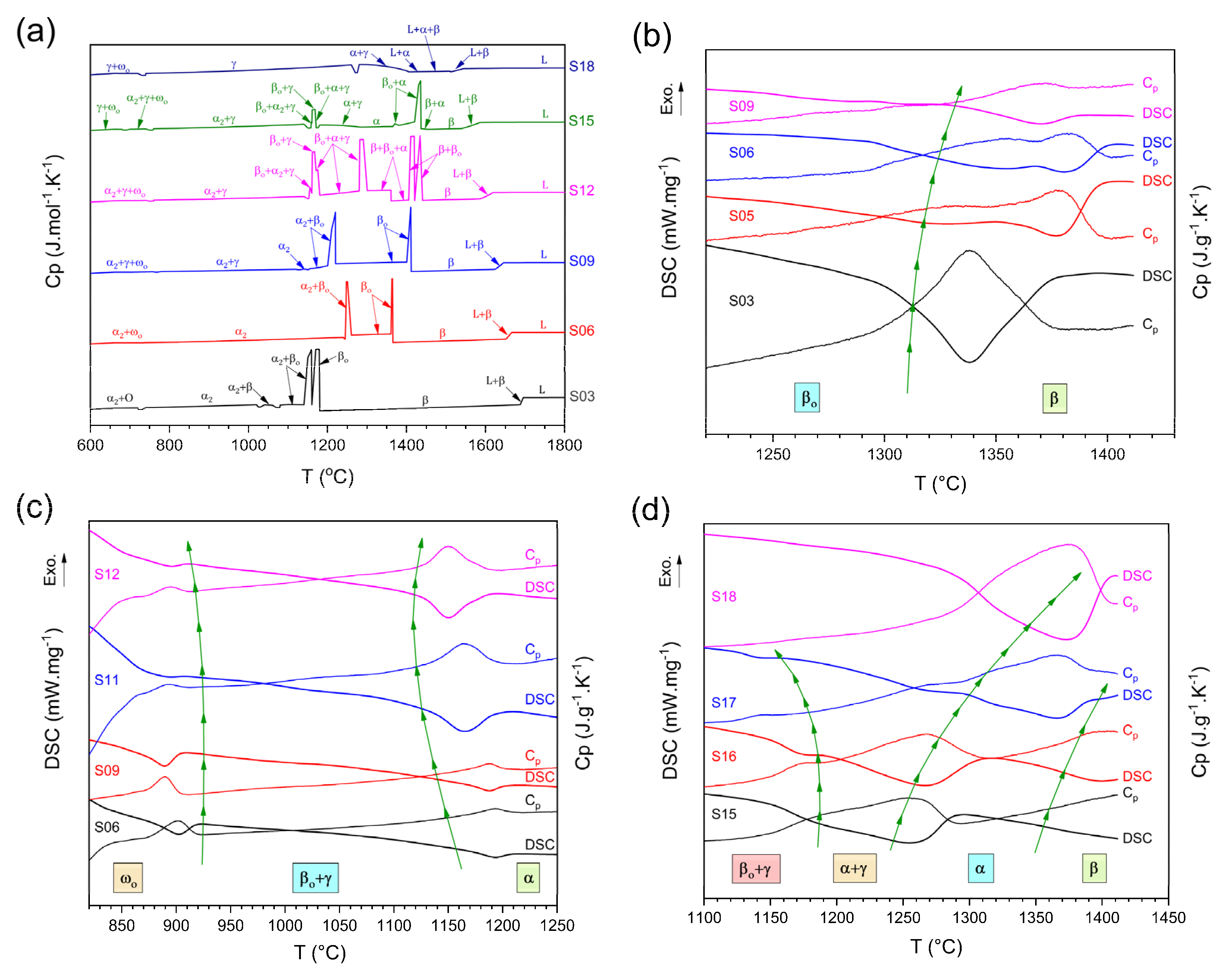
3.3. Thermal Analysis
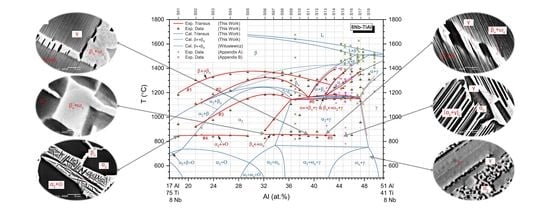
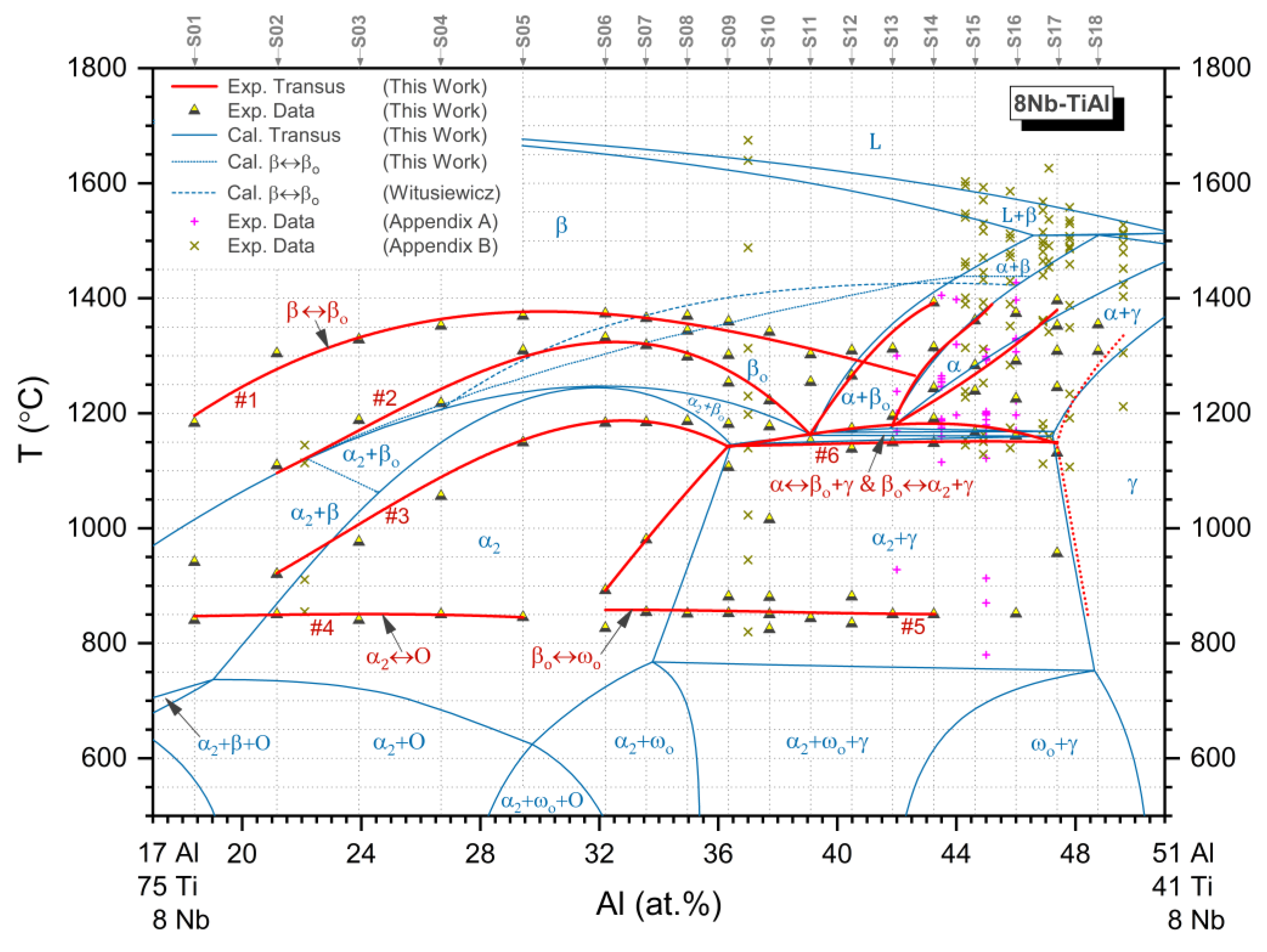
3.4. Isopleth
4. Discussion
4.1. Phase Equilibria
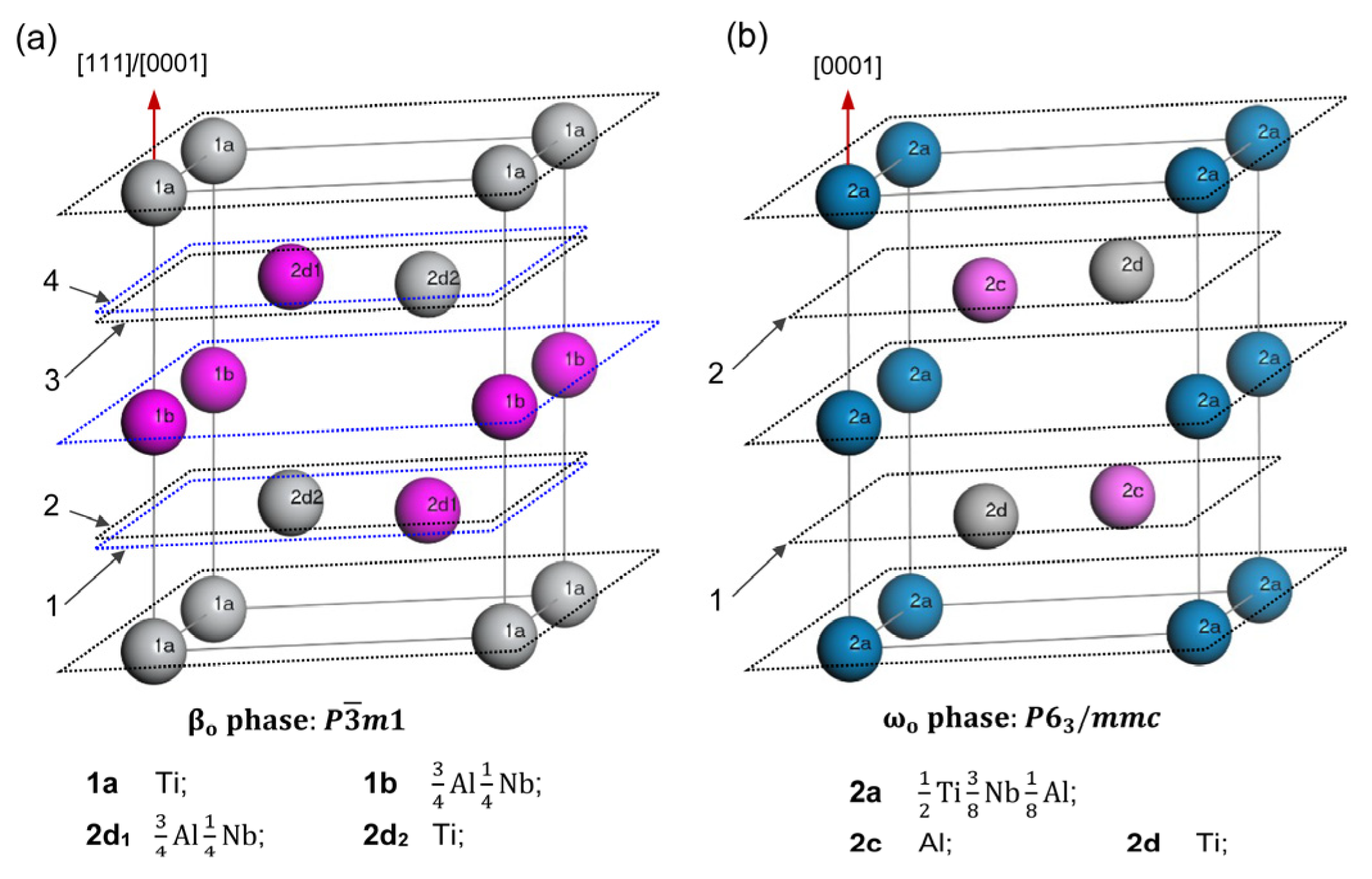
4.2. βo Transformation
4.3. ωo Transformation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Clemens, H.; Kestler, H. Processing and Applications of Intermetallic γ-TiAl-Based Alloys. Adv. Eng. Mater. 2000, 2, 551–570. [Google Scholar] [CrossRef]
- Kim, Y.-W.; Dimiduk, D. Progress in the understanding of gamma titanium aluminides. JOM 1991, 43, 40–47. [Google Scholar] [CrossRef]
- Appel, F.; Oehring, M.; Paul, J.D.H. Nano-Scale Design of TiAl Alloys Based on β-Phase Decomposition. Adv. Eng. Mater. 2006, 8, 371–376. [Google Scholar] [CrossRef]
- Appel, F.; Wagner, R. Microstructure and deformation of two-phase γ-titanium aluminides. Mater. Sci. Eng. R 1998, 22, 187–268. [Google Scholar] [CrossRef]
- Clemens, H.; Wallgram, W.; Kremmer, S.; Güther, V.; Otto, A.; Bartels, A. Design of Novel β-Solidifying TiAl Alloys with Adjustable β/B2-Phase Fraction and Excellent Hot-Workability. Adv. Eng. Mater. 2008, 10, 707–713. [Google Scholar] [CrossRef]
- Hoelzer, D.T.; Ebrahimi, F. Phase Stability of Sigma + Beta Microstructures in the Ternary Nb-Ti-Al System. MRS Proc. 1990, 194, 393. [Google Scholar] [CrossRef]
- Erdely, P.; Werner, R.; Schwaighofer, E.; Clemens, H.; Mayer, S. In-situ study of the time–temperature-transformation behaviour of a multi-phase intermetallic β-stabilised TiAl alloy. Intermetallics 2015, 57, 17–24. [Google Scholar] [CrossRef]
- Wallgram, W.; Schmölzer, T.; Cha, L.; Das, G.; Güther, V.; Clemens, H. Technology and mechanical properties of advanced γ-TiAl based alloys. Int. J. Mater. Res. 2009, 100, 1021–1030. [Google Scholar] [CrossRef]
- Liss, K.-D.; Schmoelzer, T.; Yan, K.; Reid, M.; Peel, M.; Dippenaar, R.; Clemens, H. In situ study of dynamic recrystallization and hot deformation behavior of a multiphase titanium aluminide alloy. J. Appl. Phys. 2009, 106, 113526. [Google Scholar] [CrossRef] [Green Version]
- Tetsui, T.; Shindo, K.; Kaji, S.; Kobayashi, S.; Takeyama, M. Fabrication of TiAl components by means of hot forging and machining. Intermetallics 2005, 13, 971–978. [Google Scholar] [CrossRef]
- Schmoelzer, T.; Liss, K.-D.; Zickler, G.A.; Watson, I.J.; Droessler, L.M.; Wallgram, W.; Buslaps, T.; Studer, A.; Clemens, H. Phase fractions, transition and ordering temperatures in TiAl–Nb–Mo alloys: An in- and ex-situ study. Intermetallics 2010, 18, 1544–1552. [Google Scholar] [CrossRef]
- Cheng, T.T.; Loretto, M.H. The decomposition of the beta phase in Ti–44Al–8Nb and Ti–44Al–4Nb–4Zr–0.2Si alloys. Acta Mater. 1998, 46, 4801–4819. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takeyama, M.; Motegi, T.; Hirota, N.; Matsuo, T. Formation of β-Ti Phase in Multi-component Gamma Alloys. MRS Proc. 2002, 753, BB3.4. [Google Scholar] [CrossRef]
- Takeyama, M.; Kobayashi, S. Physical metallurgy for wrought gamma titanium aluminides: Microstructure control through phase transformations. Intermetallics 2005, 13, 993–999. [Google Scholar] [CrossRef]
- Tetsui, T.; Shindo, K.; Kobayashi, S.; Takeyama, M. A newly developed hot worked TiAl alloy for blades and structural components. Scr. Mater. 2002, 47, 399–403. [Google Scholar] [CrossRef]
- Huber, D.; Clemens, H.; Stockinger, M. Near Conventional Forging of an Advanced TiAl Alloy. MRS Proc. 2012, 1516, 23–28. [Google Scholar] [CrossRef]
- Rackel, M.W.; Stark, A.; Gabrisch, H.; Schell, N.; Schreyer, A.; Pyczak, F. Orthorhombic phase formation in a Nb-rich γ-TiAl based alloy–An in situ synchrotron radiation investigation. Acta Mater. 2016, 121, 343–351. [Google Scholar] [CrossRef]
- Sun, P.; Fang, Z.Z.; Koopman, M.; Paramore, J.; Chandran, K.S.R.; Ren, Y.; Lu, J. An experimental study of the (Ti–6Al–4V)–xH phase diagram using in situ synchrotron XRD and TGA/DSC techniques. Acta Mater. 2015, 84, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Šmilauerová, J.; Harcuba, P.; Kriegner, D.; Janeček, M.; Holý, V. Growth kinetics of ω particles in β-Ti matrix studied by in situ small-angle X-ray scattering. Acta Mater. 2015, 100, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Stark, A.; Oehring, M.; Pyczak, F.; Schreyer, A. In Situ Observation of Various Phase Transformation Paths in Nb-Rich TiAl Alloys during Quenching with Different Rates. Adv. Eng. Mater. 2011, 13, 700–704. [Google Scholar] [CrossRef]
- Clemens, H.; Chladil, H.F.; Wallgram, W.; Zickler, G.A.; Gerling, R.; Liss, K.D.; Kremmer, S.; Güther, V.; Smarsly, W. In and ex situ investigations of the β-phase in a Nb and Mo containing γ-TiAl based alloy. Intermetallics 2008, 16, 827–833. [Google Scholar] [CrossRef] [Green Version]
- Appel, F.; Oehring, M.; Paul, J.D.H. A novel in situ composite structure in TiAl alloys. Mater. Sci. Eng. A 2008, 493, 232–236. [Google Scholar] [CrossRef]
- Liss, K.-D.; Bartels, A.; Clemens, H.; Bystrzanowski, S.; Stark, A.; Buslaps, T.; Schimansky, F.-P.; Gerling, R.; Scheu, C.; Schreyer, A. Recrystallization and phase transitions in a γ-TiAl-based alloy as observed by ex situ and in situ high-energy X-ray diffraction. Acta Mater. 2006, 54, 3721–3735. [Google Scholar] [CrossRef]
- Ohnuma, I.; Fujita, Y.; Mitsui, H.; Ishikawa, K.; Kainuma, R.; Ishida, K. Phase equilibria in the Ti–Al binary system. Acta Mater. 2000, 48, 3113–3123. [Google Scholar] [CrossRef]
- Witusiewicz, V.T.; Bondar, A.A.; Hecht, U.; Velikanova, T.Y. The Al-B-Nb-Ti system: IV. Experimental study and thermodynamic re-evaluation of the binary Al-Nb and ternary Al-Nb-Ti systems. J. Alloy. Compd. 2009, 472, 133–161. [Google Scholar] [CrossRef]
- Witusiewicz, V.T.; Bondar, A.A.; Hecht, U.; Rex, S.; Velikanova, T.Y. The Al-B-Nb-Ti system: II. Thermodynamic description of the constituent ternary system B-Nb-Ti. J. Alloy. Compd. 2008, 456, 143–150. [Google Scholar] [CrossRef]
- Hellwig, A.; Palm, M.; Inden, G. Phase equilibria in the Al-Nb-Ti system at high temperatures. Intermetallics 1998, 6, 79–94. [Google Scholar] [CrossRef]
- Chen, G.L.; Wang, X.T.; Ni, K.Q.; Hao, S.M.; Cao, J.X.; Ding, J.J.; Zhang, X. Investigation on the 1000, 1150 and 1400 °C isothermal section of the Ti-Al-Nb system. Intermetallics 1996, 4, 13–22. [Google Scholar] [CrossRef]
- Ding, J.-J.; Hao, S.-M. Reply to the “comment on ‘investigation on the 1000, 1150 and 1400 °C isothermal section of the Ti-Al-Nb system’ ”—Part II. Modification of 1000 and 1150 °C isothermal sections of the Ti-Al-Nb system. Intermetallics 1998, 6, 329–334. [Google Scholar] [CrossRef]
- Li, L.; Liu, L.; Zhang, L.; Zeng, L.; Zhao, Y.; Bai, W.; Jiang, Y. Phase Equilibria of the Ti-Al-Nb System at 1000, 1100 and 1150 °C. J. Phase Equilibria Diffus. 2018, 39, 549–561. [Google Scholar] [CrossRef] [Green Version]
- Leonard, K.J.; Mishurda, J.C.; Vasudevan, V.K. Phase equilibria at 1100 °C in the Nb-Ti-Al system. Mater. Sci. Eng. A 2002, 329–331, 282–288. [Google Scholar] [CrossRef]
- Jewett, T.J.; Lin, J.C.; Bonda, N.R.; Seitzman, L.E.; Hsieh, K.C.; Chang, Y.A.; Perepezko, J.H. Experimental Determination of the Ti-Nb-Al Phase Diagram At 1200°C. MRS Proc. 1988, 133, 69. [Google Scholar] [CrossRef]
- Xu, S.; Xu, Y.; Liang, Y.F.; Xu, X.J.; Gao, S.B.; Wang, Y.L.; He, J.P.; Lin, J.P. Phase equilibria of the Ti-Al-Nb system at 1300 °C. J. Alloy. Compd. 2017, 724, 339–347. [Google Scholar] [CrossRef]
- Wang, X.T.; Chen, G.L.; Ni, K.Q.; Hao, S.M. The 1400 °C isothermal section of the Ti-Al-Nb ternary system. J. Phase Equilibria 1998, 19, 200–205. [Google Scholar] [CrossRef]
- Xu, S.; Ding, X.F.; Xu, Y.; Liang, Y.F.; Xu, X.J.; Ye, T.; He, J.P.; Lin, J.P. Phase equilibria of the Ti-Al-Nb system at 1400 °C. J. Alloy. Compd. 2018, 730, 270–278. [Google Scholar] [CrossRef]
- Muraleedharan, K.; Nandy, T.K.; Banerjee, D.; Lele, S. Phase stability and ordering behaviour of the O phase in Ti–Al–Nb alloys. Intermetallics 1995, 3, 187–199. [Google Scholar] [CrossRef]
- Boehlert, C.J.; Majumdar, B.S.; Seetharaman, V.; Miracle, D.B. Part I. The microstructural evolution in Ti-Al-Nb O+Bcc orthorhombic alloys. Metall. Mater. Trans. A 1999, 30, 2305–2323. [Google Scholar] [CrossRef]
- Bendersky, L.A.; Boettinger, W.J.; Roytburd, A. Coherent precipitates in the b.c.c./orthorhombic two-phase field of the Ti-Al-Nb system. Acta Metall. Et Mater. 1991, 39, 1959–1969. [Google Scholar] [CrossRef]
- Chen, G.L.; Zhang, W.J.; Liu, Z.C.; Li, S.J.; Kim, Y.-W. Gamma Titanium Aluminides. In Proceedings of the TMS, Warrendale, PA, USA, 28 February–4 March 1999; pp. 371–380. [Google Scholar]
- Kattner, U.R.; Lin, J.-C.; Chang, Y.A. Thermodynamic Assessment and Calculation of the Ti-Al System. Metall. Trans. A 1992, 23, 2081–2090. [Google Scholar] [CrossRef]
- Cupid, D.M. Thermodynamic Assessment of the Ti-Al-Nb, Ti-Al-Cr, and Ti-Al-Mo systems. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2009. [Google Scholar]
- Raghavan, V. Al-Nb-Ti (Aluminum-Niobium-Titanium). J. Phase Equilibria Diffus. 2010, 31, 47–52. [Google Scholar] [CrossRef]
- Paul, J.D.H.; Appel, F.; Wagner, R. The compression behaviour of niobium alloyed γ-titanium alumindies. Acta Mater. 1998, 46, 1075–1085. [Google Scholar] [CrossRef]
- Appel, F.; Oehring, M.; Wagner, R. Novel design concepts for gamma-base titanium aluminide alloys. Intermetallics 2000, 8, 1283–1312. [Google Scholar] [CrossRef]
- Appel, F.; Oehring, M.; Paul, J.D.H.; Klinkenberg, C.; Carneiro, T. Physical aspects of hot-working gamma-based titanium aluminides. Intermetallics 2004, 12, 791–802. [Google Scholar] [CrossRef]
- Appel, F.; Paul, J.D.H.; Oehring, M. Gamma Titanium Aluminide Alloys: Science and Technology; Wiley-VCH: Weinheim, Germany, 2011; p. 745. [Google Scholar]
- Sadi, F.A.; Servant, C.; Cizeron, G. Phase transformations in Ti-29.7Al-21.8Nb and Ti-23.4Al-31.7Nb (at.%) alloys. Mater. Sci. Eng. A 2001, 311, 185–199. [Google Scholar] [CrossRef]
- Cao, W.; Chen, S.L.; Zhang, F.; Wu, K.; Yang, Y.; Chang, Y.A.; Schmid-Fetzer, R.; Oates, W.A. PANDAT software with PanEngine, PanOptimizer and PanPrecipitation for multi-component phase diagram calculation and materials property simulation. Calphad 2009, 33, 328–342. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, L.; Lin, J.P.; Chang, H.; Li, F. Phase transformation behavior and kinetics of high Nb–TiAl alloy during continuous cooling. J. Alloy. Compd. 2016, 668, 22–26. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Zhang, K.; Hu, R.; Song, L.; Fu, H. Atomic-scale observations of B2→ω-related phases transition in high-Nb containing TiAl alloy. Mater. Charact. 2017, 130, 135–138. [Google Scholar] [CrossRef]
- Stark, A.; Bartels, A.; Clemens, H.; Schimansky, F.P. On the Formation of Ordered ω-phase in High Nb Containing γ-TiAl Based Alloys. Adv. Eng. Mater. 2008, 10, 929–934. [Google Scholar] [CrossRef] [Green Version]
- Ye, T.; Song, L.; Gao, S.B.; Liang, Y.F.; Wang, Y.L.; Lin, J.P. Precipitation behavior of the ωo phase in an annealed high Nb-TiAl alloy. J. Alloy. Compd. 2017, 701, 882–891. [Google Scholar] [CrossRef]
- Leonard, K.J.; Tewari, R.; Arya, A.; Mishurda, J.C.; Dey, G.K.; Vasudevan, V.K. Decomposition of the βo phase to the P63/mcm, hP18 structure in Nb–(24–36)Ti–40Al alloys. Acta Mater. 2009, 57, 4440–4453. [Google Scholar] [CrossRef]
- Sadi, F.A.; Servant, C. On the B2→O phase transformation in Ti–Al–Nb alloys. Mater. Sci. Eng. A 2003, 346, 19–28. [Google Scholar] [CrossRef]
- Banerjee, D.; Gogia, A.K.; Nandi, T.K.; Joshi, V.A. A new ordered orthorhombic phase in a Ti3Al-Nb alloy. Acta Metall. 1988, 36, 871–882. [Google Scholar] [CrossRef]
- Gabrisch, H.; Lorenz, U.; Pyczak, F.; Rackel, M.; Stark, A. Morphology and stability of orthorhombic and hexagonal phases in a lamellar γ-Ti-42Al-8.5Nb alloy-A transmission electron microscopy study. Acta Mater. 2017, 135, 304–313. [Google Scholar] [CrossRef]
- Bendersky, L.A.; Boettinger, W.J. Phase transformations in the (Ti, Nb)3Al section of the Ti-Al-Nb system—II. Experimental tem study of microstructures. Acta Metall. Et Mater. 1994, 42, 2337–2352. [Google Scholar] [CrossRef]
- Saunders, N. Phase equilibria in multi-component γ-TiAl based alloys. In Proceedings of the TMS, Warrendale, PA, USA, 28 February–4 March 1999; pp. 183–188. [Google Scholar]
- Holec, D.; Legut, D.; Isaeva, L.; Souvatzis, P.; Clemens, H.; Mayer, S. Interplay between effect of Mo and chemical disorder on the stability of β/βo-TiAl phase. Intermetallics 2015, 61, 85–90. [Google Scholar] [CrossRef]
- Beran, P.; Petrenec, M.; Heczko, M.; Smetana, B.; Žaludová, M.; Šmíd, M.; Kruml, T.; Keller, L. In-situ neutron diffraction study of thermal phase stability in a γ-TiAl based alloy doped with Mo and/or C. Intermetallics 2014, 54, 28–38. [Google Scholar] [CrossRef]
- Leonard, K.J. Phase Equilibria and Solid State Transformations in Niobium-Rich Niobium-Titanium-Aluminum Intermetallic Alloys; University of Cincinnati: Cincinnati, OH, USA, 1999. [Google Scholar]
- Gusev, A.I.; Rempel, A.A.; Magerl, A.J. Disorder and Order in Strongly Nonstoichiometric Compounds: Transition Metal Carbides, Nitrides and Oxides; Springer: Berlin, Germany, 2001; p. 607. [Google Scholar]
- Huang, Z.W. Ordered ω phases in a 4Zr-4Nb-containing TiAl-based alloy. Acta Mater. 2008, 56, 1689–1700. [Google Scholar] [CrossRef]
- Schloffer, M.; Rashkova, B.; Schöberl, T.; Schwaighofer, E.; Zhang, Z.; Clemens, H.; Mayer, S. Evolution of the ωo phase in a β-stabilized multi-phase TiAl alloy and its effect on hardness. Acta Mater. 2014, 64, 241–252. [Google Scholar] [CrossRef]
- Song, L.; Zhang, L.Q.; Xu, X.J.; Sun, J.; Lin, J.P. Omega phase in as-cast high-Nb-containing TiAl alloy. Scr. Mater. 2013, 68, 929–932. [Google Scholar] [CrossRef]
- Song, L.; Xu, X.J.; You, L.; Liang, Y.F.; Wang, Y.L.; Lin, J.P. Ordered α2 to ωo phase transformations in high Nb-containing TiAl alloys. Acta Mater. 2015, 91, 330–339. [Google Scholar] [CrossRef]
- Song, L.; Lin, J.P.; Li, J.S. Phase transformation mechanisms in a quenched Ti-45Al-8.5Nb-0.2W-0.2B-0.02Y alloy after subsequent annealing at 800 °C. J. Alloy. Compd. 2017, 691, 60–66. [Google Scholar] [CrossRef]

| Designation | Pearson | S. G. (#) | Strukt. | Prototype | Lattice Parameters (nm) | ||
|---|---|---|---|---|---|---|---|
| β-Ti(Al,Nb) | cI2 | (229) | A2 | W | a = 0.331 | - | - |
| α-Ti(Al,Nb) | hP2 | (194) | A3 | Mg | a = 0.295 | - | c = 0.468 |
| γ-TiAl | tP4 | (123) | L10 | AuCu | a = 0.283 | - | c = 0.408 |
| α2-Ti3Al | hP8 | (194) | D019 | Ni3Sn | a = 0.577 | - | c = 0.463 |
| βo-TiAl(Nb) | cP2 | (221) | B2 | CsCl | a = 0.322 | - | - |
| ωo-Ti4NbAl3 | hP6 | (194) | B82 | InNi2 | a = 0.458 | - | c = 0.552 |
| O-Ti2NbAl | oC16 | (63) | --- | NaHg | a = 0.616 | b = 0.973 | c = 0.470 |
| Sample | Nominal Composition | Chemical Composition (OES) | ||
|---|---|---|---|---|
| Al | Ti | Nb | ||
| S01 | Ti73.6Al18.4Nb8 | 18.34 | 73.69 | 7.97 |
| S02 | Ti70.8Al21.2Nb8 | 21.13 | 70.89 | 7.98 |
| S03 | Ti68.1Al23.9Nb8 | 23.80 | 68.23 | 7.97 |
| S04 | Ti65.3Al26.7Nb8 | 26.65 | 65.37 | 7.98 |
| S05 | Ti62.6Al29.4Nb8 | 29.37 | 62.66 | 7.97 |
| S06 | Ti59.8Al32.2Nb8 | 32.16 | 59.87 | 7.97 |
| S07 | Ti58.4Al33.6Nb8 | 33.52 | 58.51 | 7.97 |
| S08 | Ti57.0Al35.0Nb8 | 34.89 | 57.11 | 8.00 |
| S09 | Ti55.7Al36.3Nb8 | 36.24 | 55.75 | 8.01 |
| S10 | Ti54.3Al37.7Nb8 | 37.63 | 54.35 | 8.02 |
| S11 | Ti52.9Al39.1Nb8 | 39.01 | 52.96 | 8.03 |
| S12 | Ti51.5Al40.5Nb8 | 40.42 | 51.56 | 8.02 |
| S13 | Ti50.1Al41.9Nb8 | 41.83 | 50.14 | 8.03 |
| S14 | Ti48.8Al43.2Nb8 | 43.13 | 48.84 | 8.03 |
| S15 | Ti47.4Al44.6Nb8 | 44.53 | 47.44 | 8.03 |
| S16 | Ti46.0Al46.0Nb8 | 45.93 | 46.04 | 8.03 |
| S17 | Ti44.6Al47.4Nb8 | 47.35 | 44.63 | 8.02 |
| S18 | Ti43.2Al48.8Nb8 | 48.72 | 43.27 | 8.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Liang, Y.; Song, L.; Hao, G.; Tian, B.; Xu, R.; Lin, J. Experimental Phase Equilibria and Isopleth Section of 8Nb-TiAl Alloys. Metals 2021, 11, 1229. https://doi.org/10.3390/met11081229
Xu Y, Liang Y, Song L, Hao G, Tian B, Xu R, Lin J. Experimental Phase Equilibria and Isopleth Section of 8Nb-TiAl Alloys. Metals. 2021; 11(8):1229. https://doi.org/10.3390/met11081229
Chicago/Turabian StyleXu, Yong, Yongfeng Liang, Lin Song, Guojian Hao, Bin Tian, Rongfu Xu, and Junpin Lin. 2021. "Experimental Phase Equilibria and Isopleth Section of 8Nb-TiAl Alloys" Metals 11, no. 8: 1229. https://doi.org/10.3390/met11081229
APA StyleXu, Y., Liang, Y., Song, L., Hao, G., Tian, B., Xu, R., & Lin, J. (2021). Experimental Phase Equilibria and Isopleth Section of 8Nb-TiAl Alloys. Metals, 11(8), 1229. https://doi.org/10.3390/met11081229