Structure and Properties of Ti-Al-Ta and Ti-Al-Cr Cladding Layers Fabricated on Titanium
Abstract
:1. Introduction
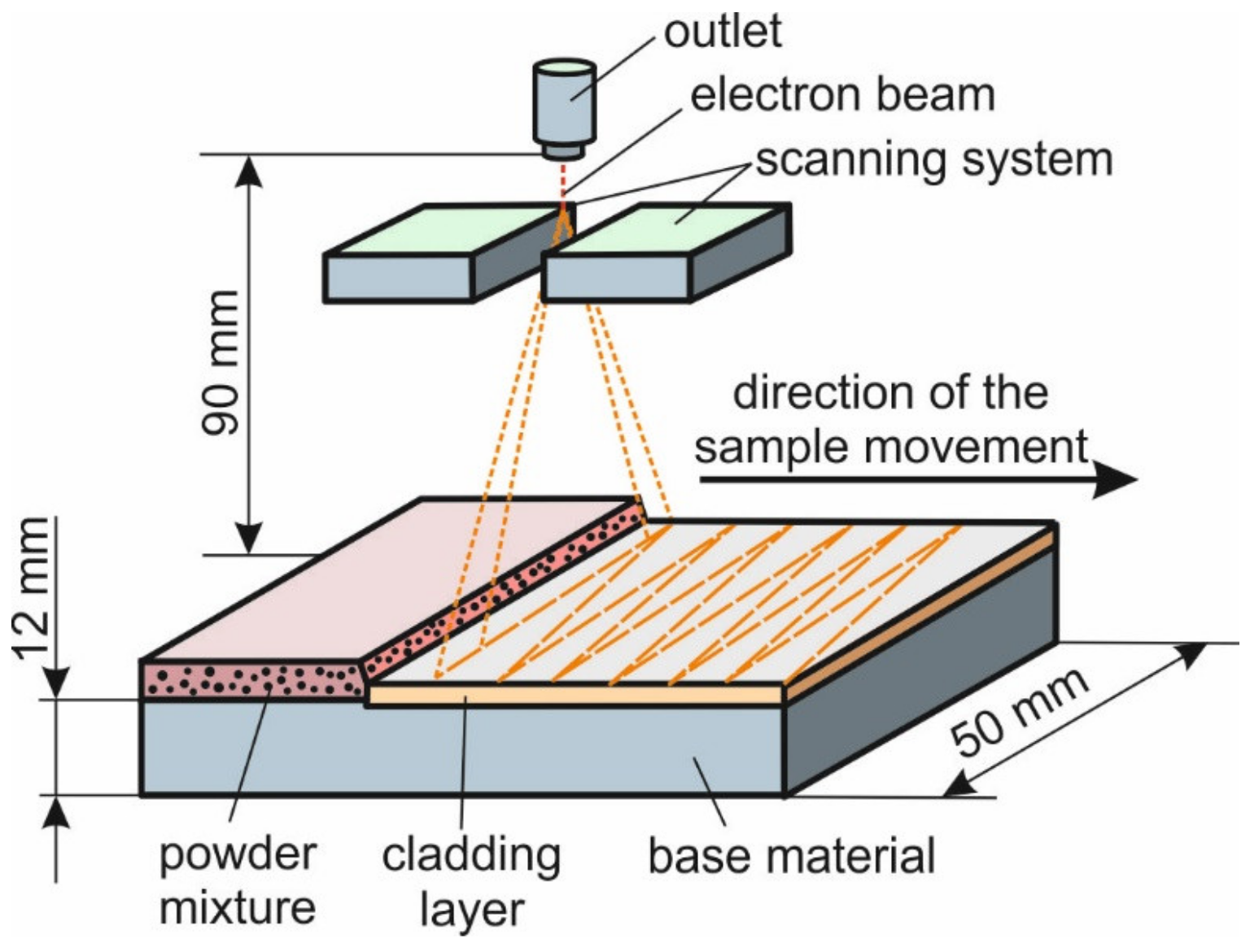
2. Materials and Methods
3. Results
3.1. Microstructure and Phase Compositions
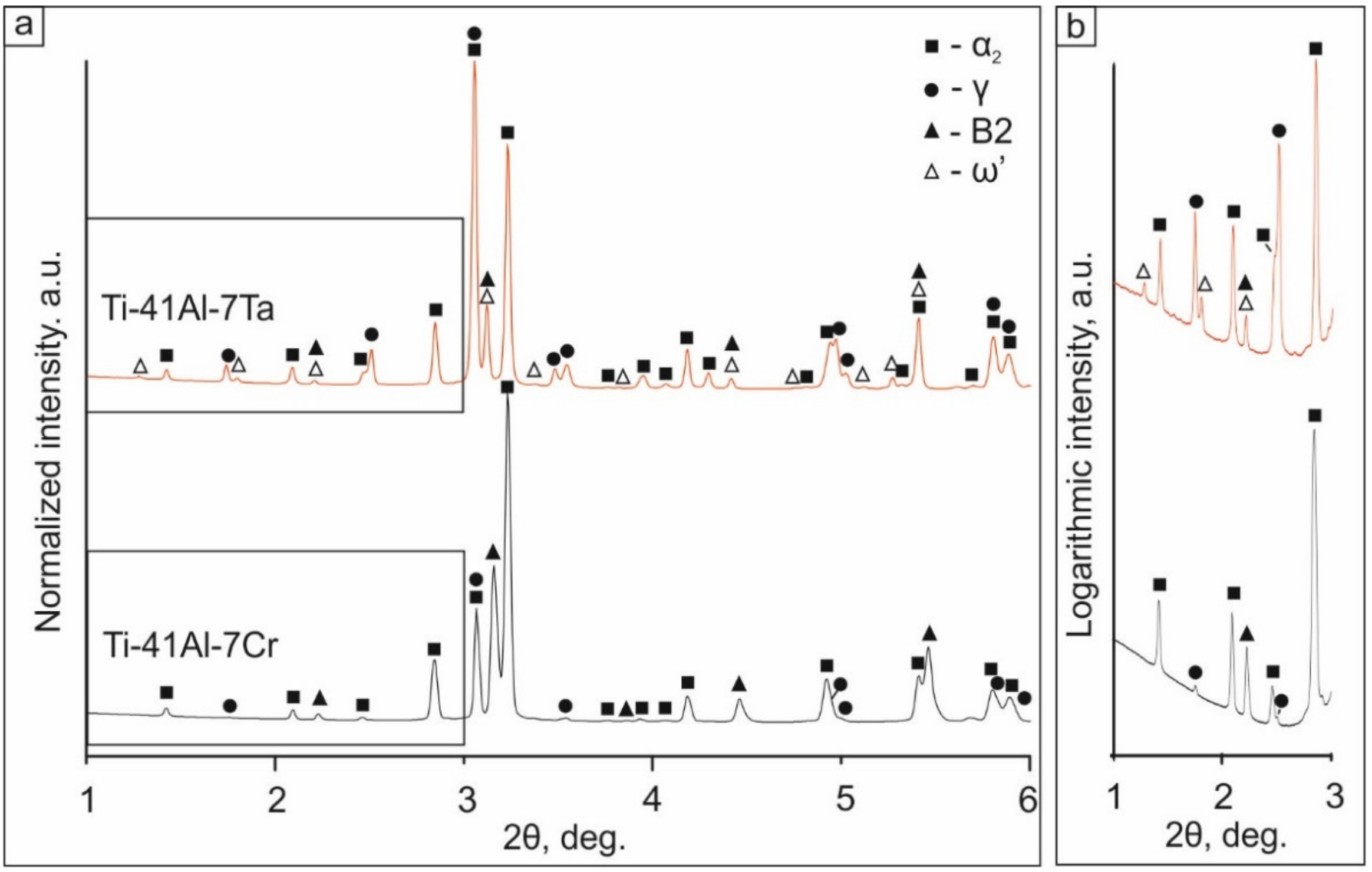
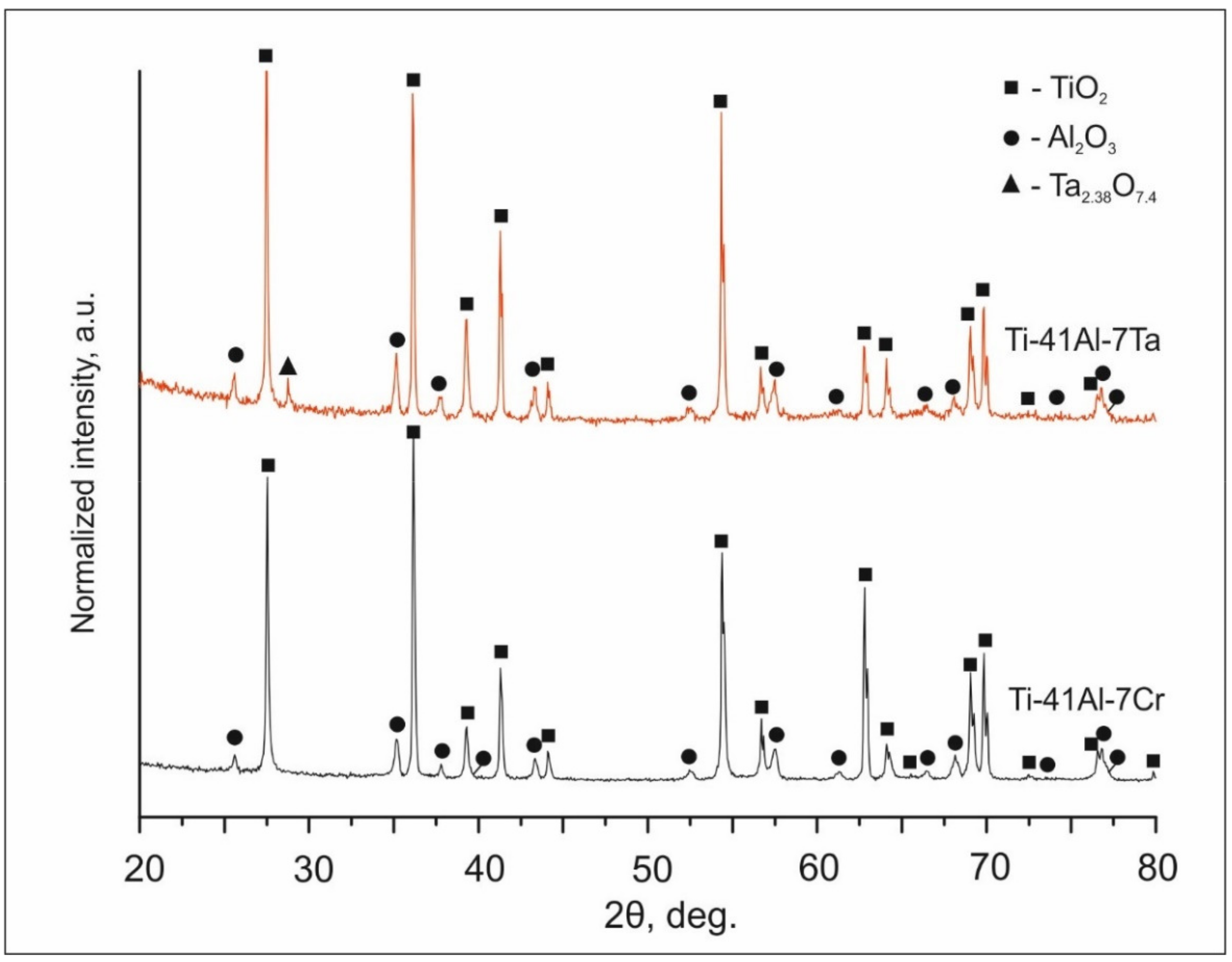
3.1.1. Results of Synchrotron X-ray Diffraction Analysis
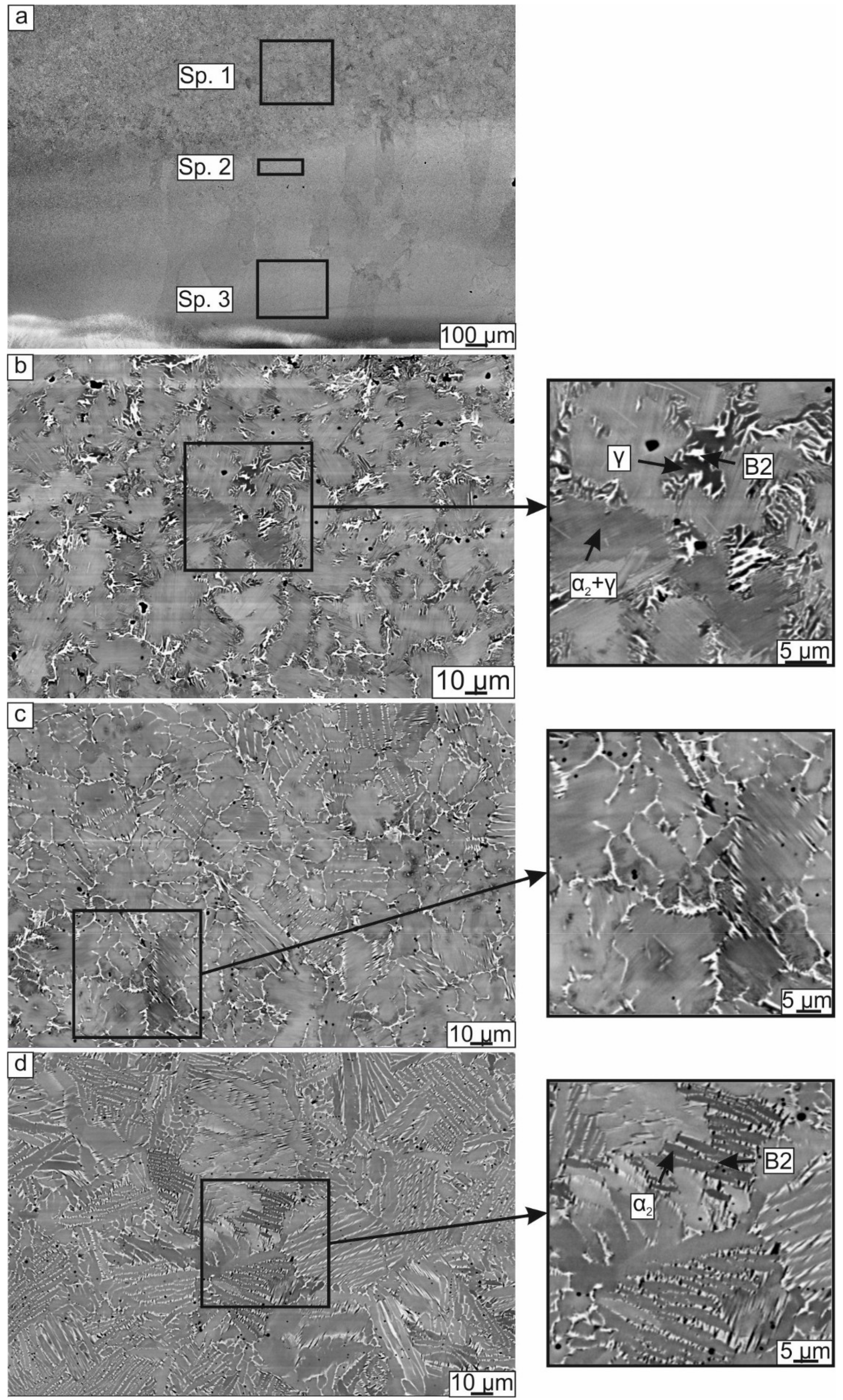
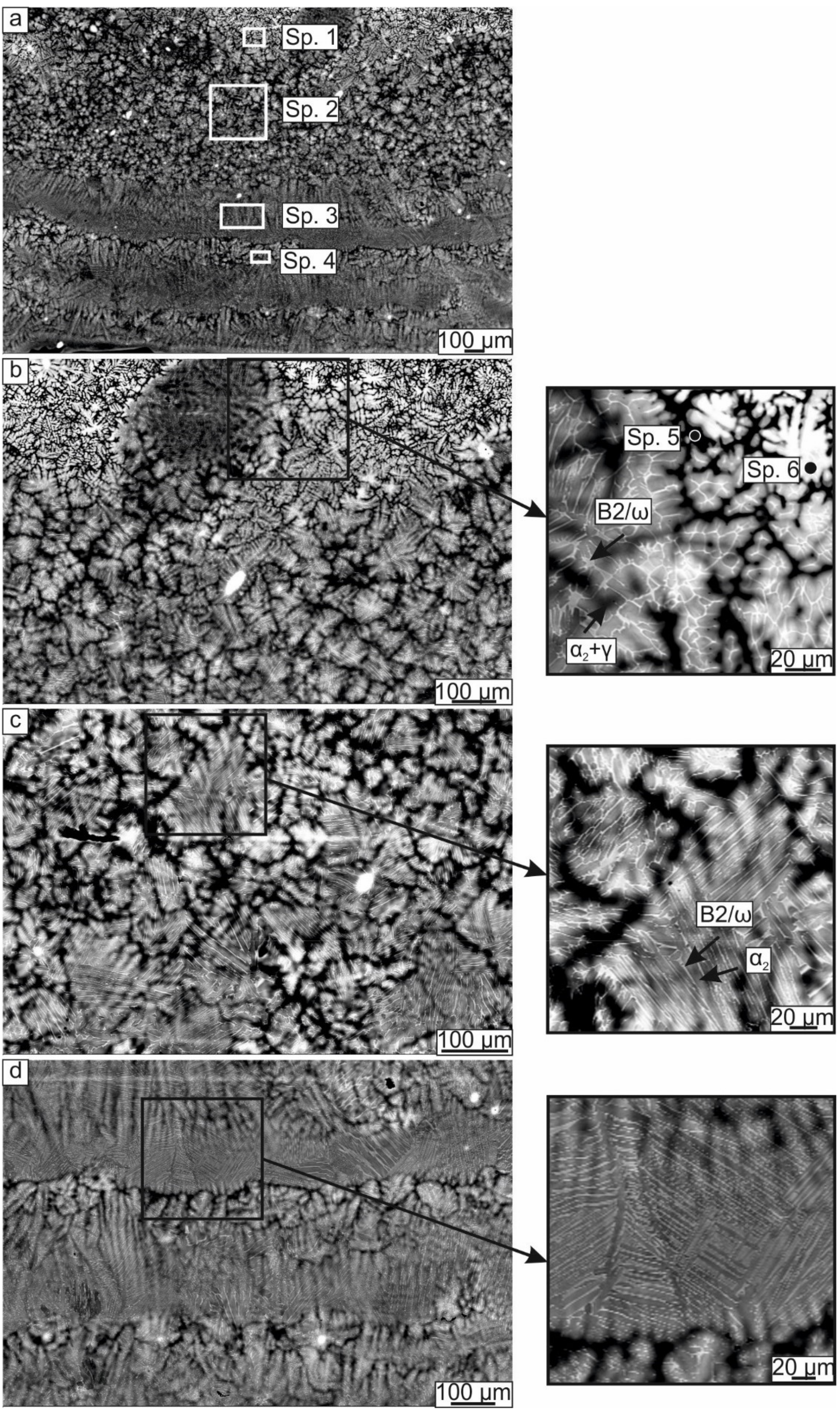
3.1.2. Microstructure of the Samples
3.2. Properties of the Cladding Layers
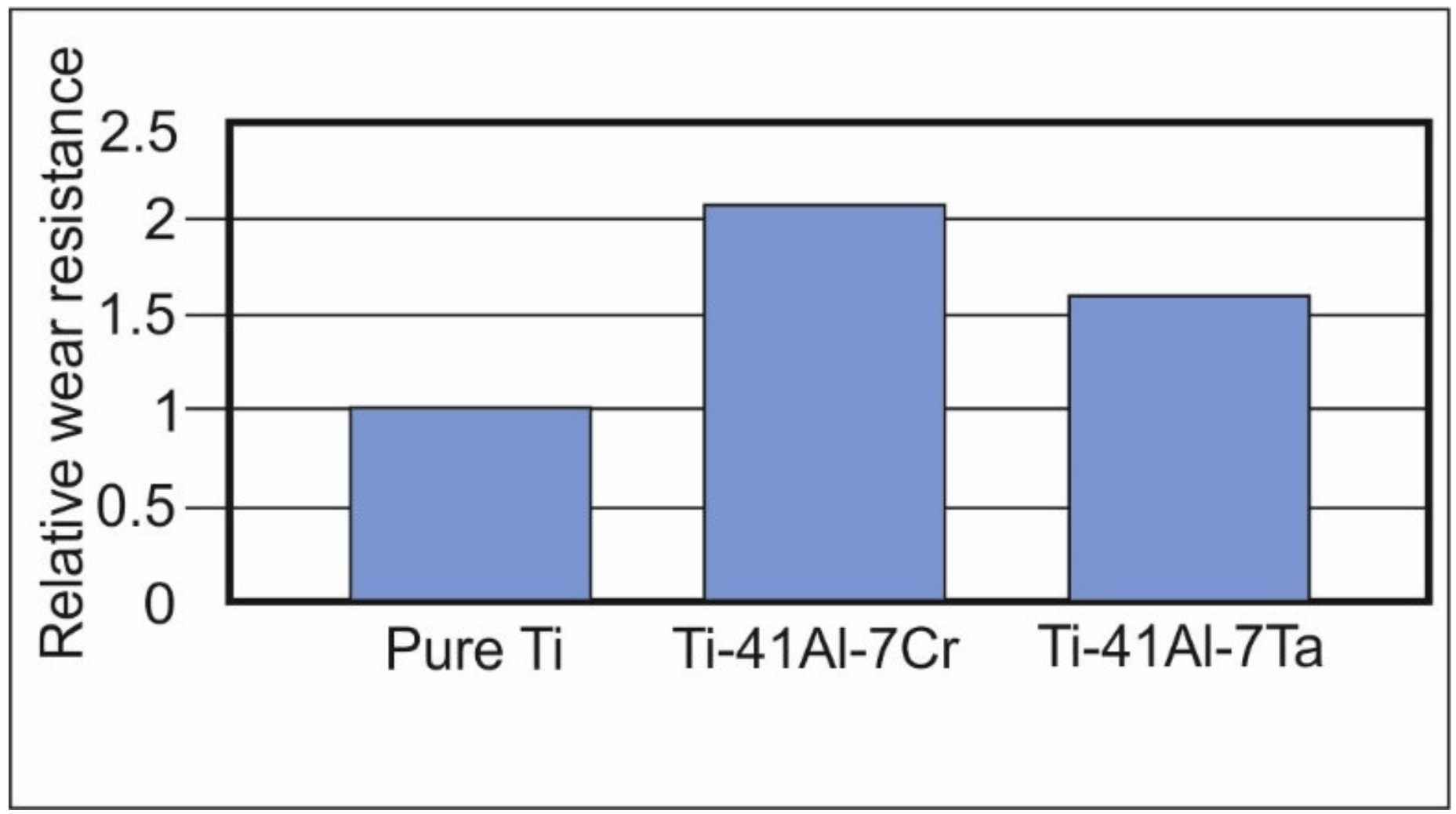
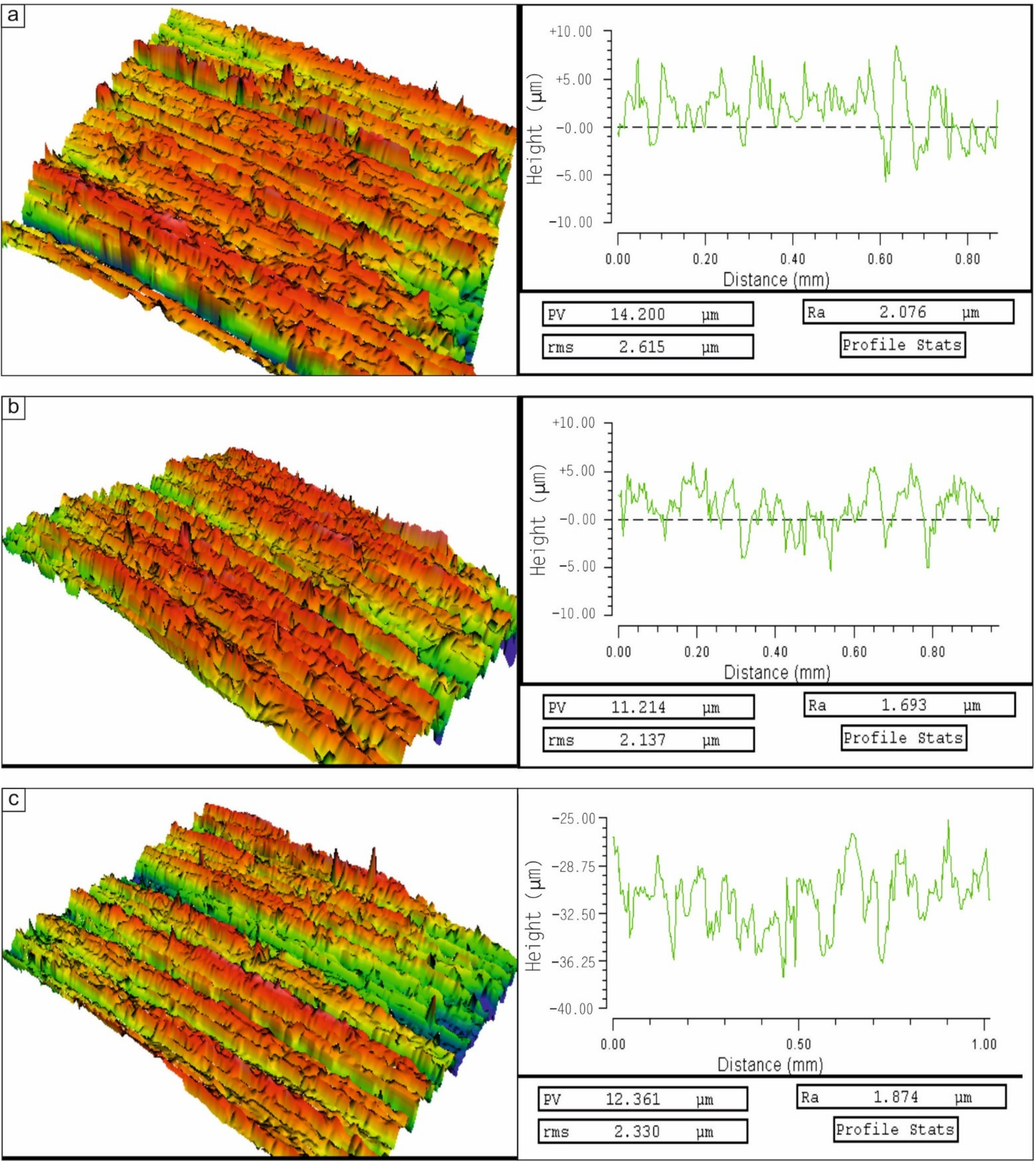
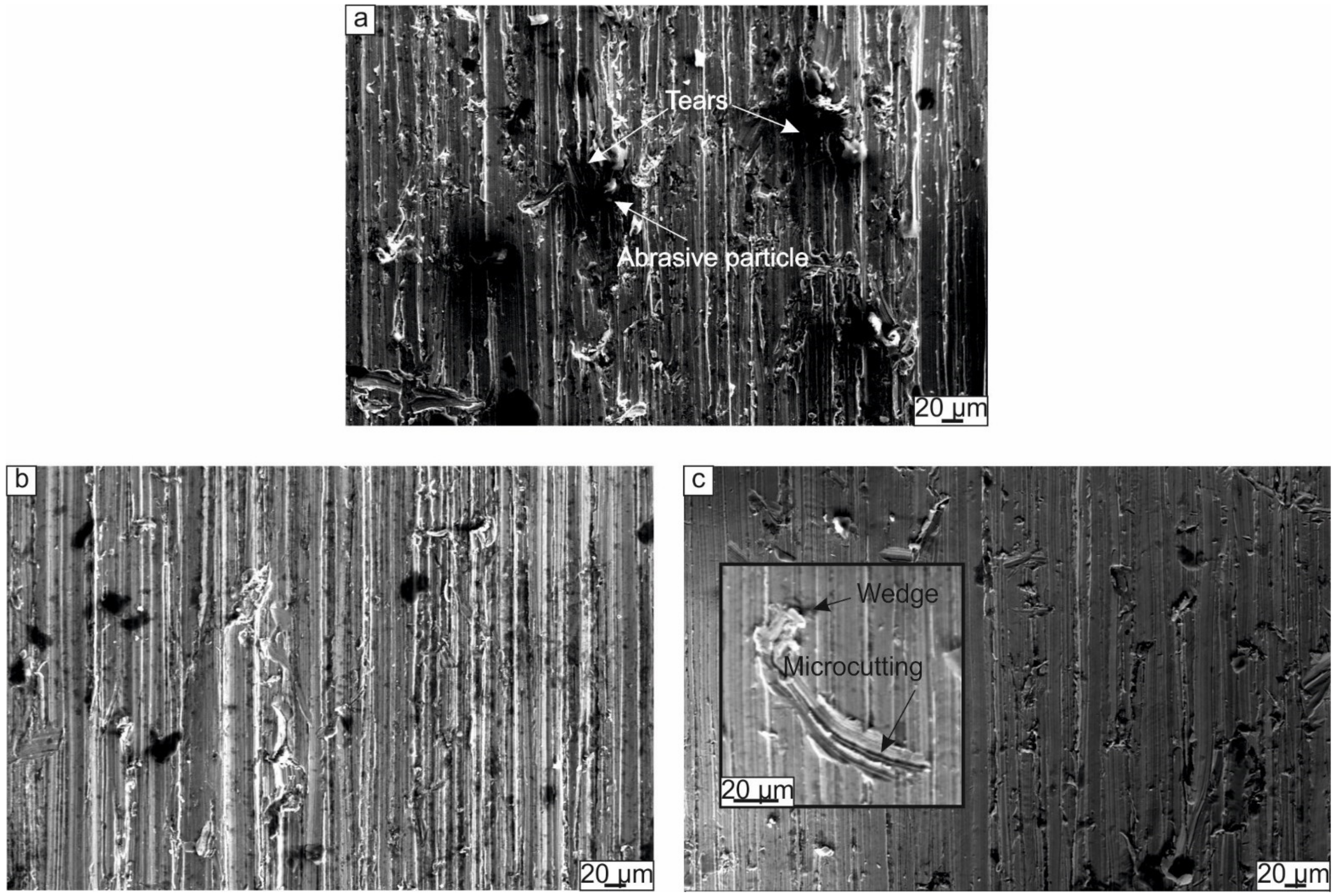
3.2.1. Wear Behavior of the Cladding Layers with Respect to Titanium
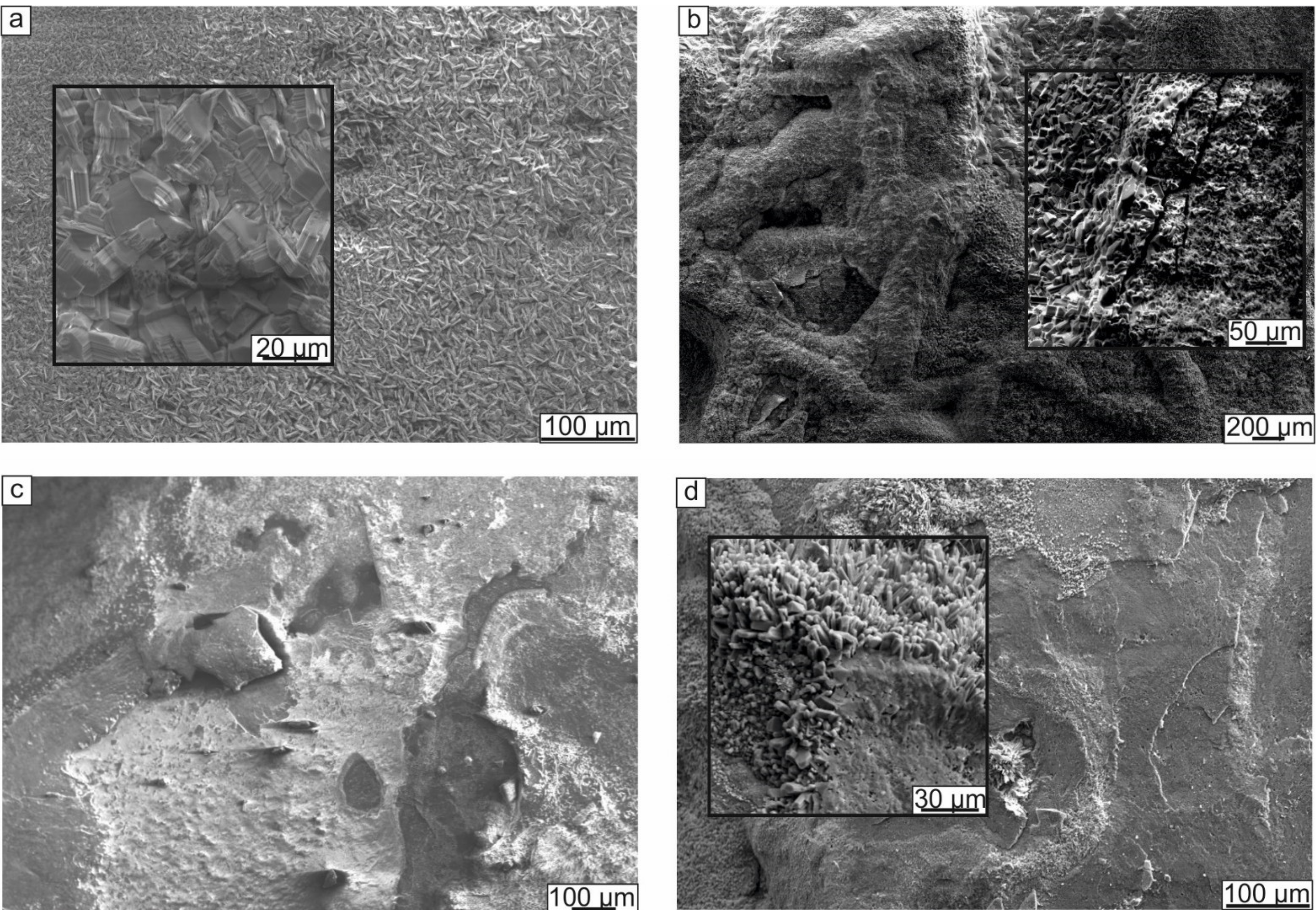
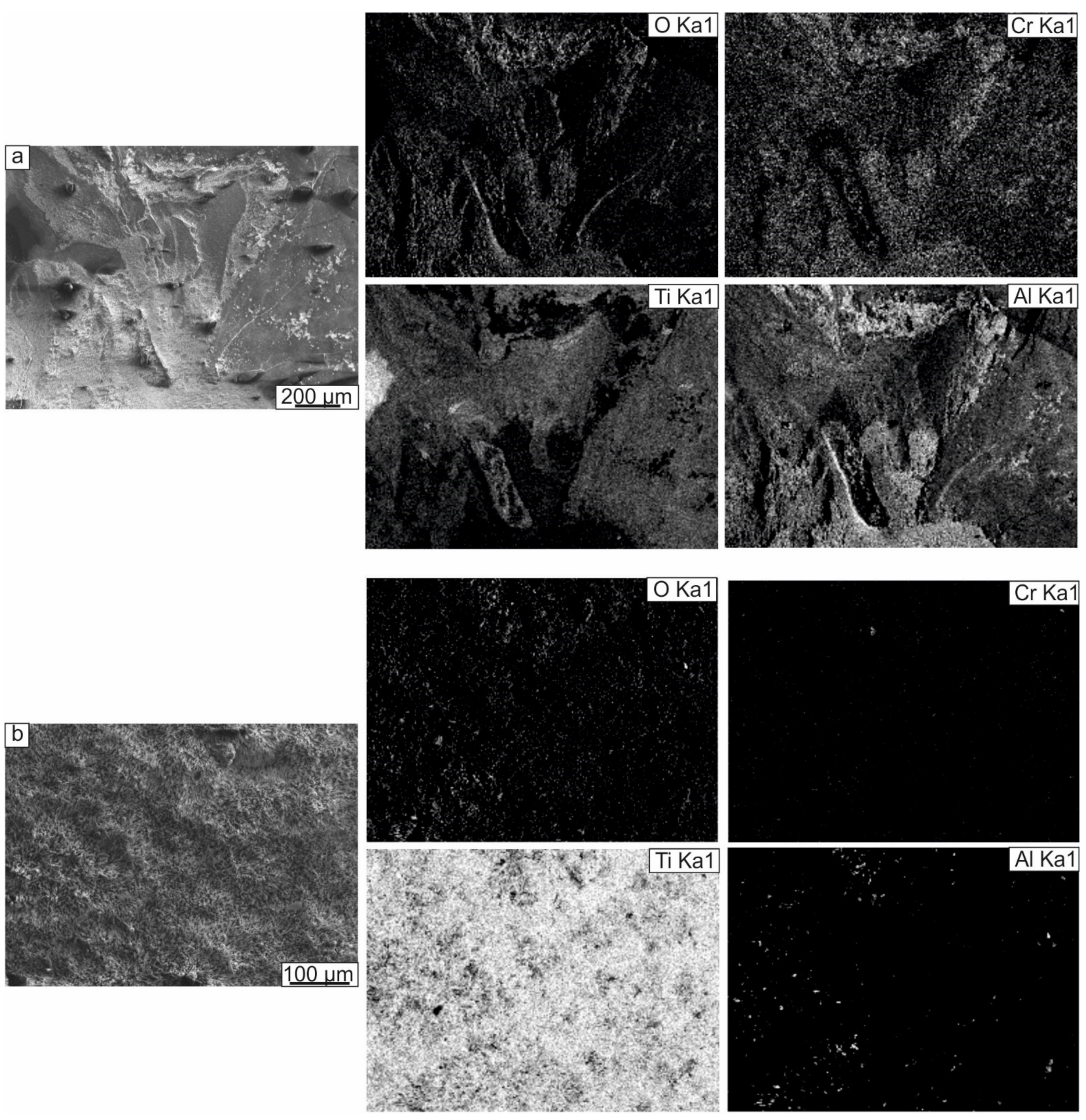
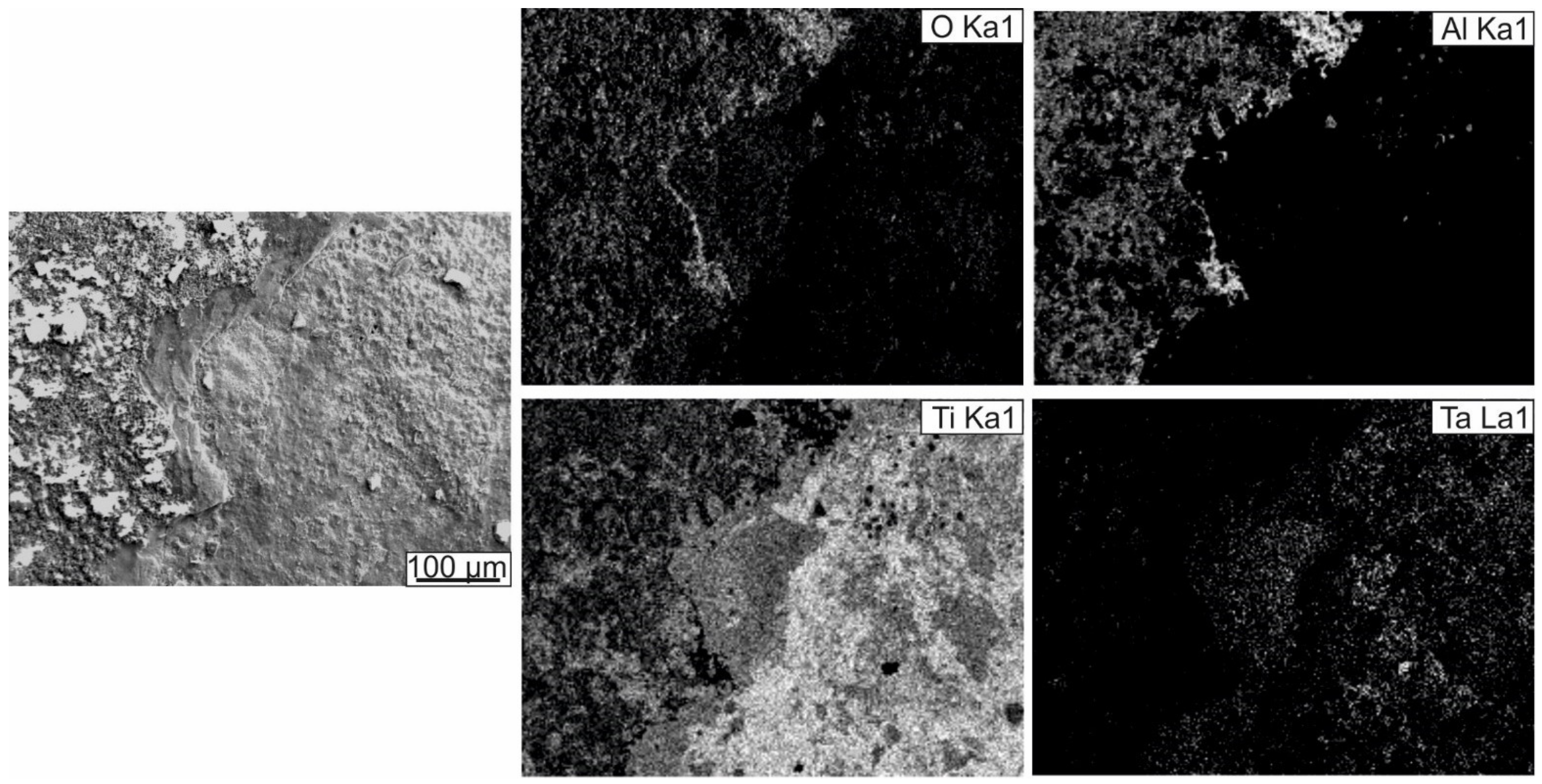
3.2.2. Oxidation Resistance of Cladding Materials
4. Discussion
5. Conclusions
- 1.
- The non-vacuum electron-beam cladding of Ti-Al-Ta and Ti-Al-Cr powder mixtures was used to form protective intermetallic layers with a thickness of up to 2 mm on pure titanium substrates. The cladding layers were defect-free and did not contain cracks or visible voids.
- 2.
- Providing the same atomic concentrations of Ta and Cr in the initial powder mixtures, cladding layers with different phase compositions and different fractions of phases were formed. In the Ti-41Al-7Cr cladding layer, α2, γ, and B2 phases were observed. In addition to the phases mentioned above, the ω′ phase was formed in the layer alloyed with tantalum. The volume fractions of α2, γ, and B2/ω′ phases in the cladding layers depended on the β-stabilizing effect of the alloying element. Being a stronger β-stabilizer, Cr promoted a larger amount of B2 phase in the structure of the cladding layer compared to tantalum (26 vol.% vs. 4.8 vol.%, respectively).
- 3.
- Wear resistance of intermetallic layers depended on the proportion of hard phases in their structure and, consequently, on the type of element that stabilizes certain phases. At abrasive friction, the best results were obtained for the chromium-containing cladding layer characterized by a lower fraction of the soft γ-phase. However, the tantalum-containing cladding layer has also improved the wear resistance of pure titanium. The relative wear resistance of the Ti-41Al-7Ta and Ti-41Al-7Cr was 1.6 and 2.1 times higher than that of pure titanium.
- 4.
- The resistance to oxidation of the cladding layers was determined by the types of phases formed and their fraction and the type of alloying element, and its ability to promote an Al2O3 oxide film formation. Alloying the intermetallic compound with tantalum and chromium contributed to forming a mixed oxide scale on the surfaces of the samples heated at 800 °C for 200 h. However, on the surface of the Ti-41Al-7Cr sample, large areas consisting only of TiO2 were formed. The Ti-41Al-7Ta cladding layer exhibited the best protective properties under high-temperature exposure. Its oxidation resistance was 8 times higher compared to the substrate material.
- 5.
- From the comparison of the layers alloyed with Ta and Cr, it follows that the choice of alloying element depends on the operation condition. While Ta provides better heat resistance, cladding layers alloyed with Cr possess higher wear properties. At the same time, the difference in Ta- and Cr-containing cladding layer properties was not significant. Considering the high price of Ta, the choice of Cr as an alloying element seems to be more rational.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bansal, R.; Singh, J.K.; Singh, V.; Singh, D.D.N.; Das, P. Optimization of Oxidation Temperature for Commercially Pure Titanium to Achieve Improved Corrosion Resistance. J. Mater. Eng. Perform. 2017, 26, 969–977. [Google Scholar] [CrossRef]
- Jamesh, M.; Sankara Narayanan, T.S.N.; Chu, P.K. Thermal Oxidation of Titanium: Evaluation of Corrosion Resistance as a Function of Cooling Rate. Mater. Chem. Phys. 2013, 138, 565–572. [Google Scholar] [CrossRef]
- Leyens, C.; Perters, M. (Eds.) Titanium and Titanium Alloys: Fundamentals and Application; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Lütjering, G.; Williams, J.C. Titanium; Derby, B., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2007; p. 442. [Google Scholar]
- Chechulin, B.B.; Ushakov, S.S.; Razuvaeva, I.N.; Goldfain, V.N. Titanovye Splavy V Mashinostroenii; Mashinostroenie: Leningrad, Russia, 1977; p. 248. [Google Scholar]
- Smialek, J.L. Oxidation Behaviour of TiAl3 Coatings and Alloys. Corros. Sci. 1993, 35, 1199–1208. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Peng, Y.P.; Mao, Y.L.; Pang, C.J.; Lu, L.Y. Effect of Hot-Dip Aluminizing on the Oxidation Resistance of Ti-6Al-4V Alloy at High Temperatures. Corros. Sci. 2012, 55, 187–193. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Peng, Y.P.; Mao, Y.L.; Hou, C.M.; Xu, L. Hot-Dip Aluminizing Fabrication of TiAl 3 Coating on TA15 Alloy and Its High Temperature Oxidation Behaviors. High Temp. Mater. Process. 2012, 30, 519–525. [Google Scholar]
- Leyens, C.; Peters, M.; Kaysser, W.A. Intermetallic Ti-Al Coatings for Protection of Titanium Alloys: Oxidation and Mechanical Behavior. Surf. Coat. Technol. 1997, 94, 34–40. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, J.; Zhao, J.; Wang, L.; Yu, Y.; Chen, J.; Zhou, H. Improvement of the Oxidation and Wear Resistance of Pure Ti by Laser-Cladding Ti 3Al Coating at Elevated Temperature. Tribol. Lett. 2011, 42, 151–159. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Zhang, K. Investigation of the Laser Melting Deposited TiAl Intermetallic Alloy on Titanium Alloy. Adv. Mater. Res. 2011, 146, 1638–1641. [Google Scholar] [CrossRef]
- Guo, B.; Zhou, J.; Zhang, S.; Zhou, H.; Pu, Y.; Chen, J. Phase Composition and Tribological Properties of Ti-Al Coatings Produced on Pure Ti by Laser Cladding. Appl. Surf. Sci. 2007, 253, 9301–9310. [Google Scholar] [CrossRef]
- Guo, B.; Zhou, J.; Zhang, S.; Zhou, H.; Pu, Y.; Chen, J. Tribological Properties of Titanium Aluminides Coatings Produced on Pure Ti by Laser Surface Alloying. Surf. Coat. Technol. 2008, 202, 4121–4129. [Google Scholar] [CrossRef]
- Mizuta, N.; Matsuura, K.; Kirihara, S.; Miyamoto, Y. Titanium Aluminide Coating on Titanium Surface Using Three-Dimensional Microwelder. Mat. Sci. Eng. A 2008, 492, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, T.; Shibutani, T.; Uenishi, K.; Kobayashi, K.F. Fabrication of a Thick Surface Layer of Al3Ti on Ti Substrate by Reactive-Pulsed Electric Current Sintering. Intermetallics 2000, 8, 815–822. [Google Scholar] [CrossRef]
- Lazurenko, D.V.; Bataev, I.A.; Laptev, I.S.; Ruktuev, A.A.; Maliutina, I.N.; Golkovsky, M.G.; Bataev, A.A. Formation of Ti-Al Intermetallics on a Surface of Titanium by Non-Vacuum Electron Beam Treatment. Mater. Charact. 2017, 134, 202–212. [Google Scholar] [CrossRef]
- Appel, F.; Paul, J.D.H.; Oehring, M. Gamma Titanium Aluminide Alloys; Wiley-VCH: Weinheim, Germany; GmbH&Co. KGaA: Weinheim, Germany, 2011; p. 752. [Google Scholar]
- Huang, S.-C.; Hall, E.L. Effects of Cr Additions to Binary TiAl-Base Alloys. Metall. Trans. A 1991, 22, 2619–2627. [Google Scholar] [CrossRef]
- Clemens, H.; Wallgram, W.; Kremmer, S.; Güther, V.; Otto, A.; Bartels, A. Design of Novel β-Solidifying TiAl Alloys with Adjustable β/B2-Phase Fraction and Excellent Hot-Workability. Adv. Eng. Mater. 2008, 10, 707–713. [Google Scholar] [CrossRef]
- Wu, X. Review of Alloy and Process Development of TiAl Alloys. Intermetallics 2006, 14, 1114–1122. [Google Scholar] [CrossRef]
- Vojtěch, D.; Popela, T.; Hamáček, J.; Kützendörfer, J. The Influence of Tantalum on the High Temperature Characteristics of Lamellar Gamma+Alpha 2 Titanium Aluminide. Mater. Sci. Eng. A 2011, 528, 8557–8564. [Google Scholar] [CrossRef]
- Appel, F.; Brossmann, U.; Christopf, U.; Eggert, S.; Janschek, P.; Lorenz, U. Recent Progress in the Development of Gamma titanium Aluminide Alloys. Adv. Eng. Mater. 2000, 2, 699–720. [Google Scholar] [CrossRef]
- Wang, J.N.; Wang, Y. An Investigation of the Origin of the Superplasticity of Cast TiAl Alloys. Int. J. Plast. 2006, 22, 1530–1548. [Google Scholar] [CrossRef]
- Lazurenko, D.V.; Laptev, I.S.; Golkovsky, M.G.; Stark, A.; Paul, J.; Bataev, I.; Ruktuev, A.A.; Song, L.; Gollwitzer, C.; Pyczak, F. Influence of the Ti/Al/Nb Ratio on the Structure and Properties on Intermetallic Layers Obtained on Titanium by Non-vacuum Electron Beam Cladding. Mater. Charact. 2020, 163, 110246. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H.-R. MAUD: A Friendly Java Program for Material Analysis Using Diffraction. In Proceedings of the IUCr: Newsletter of the CPD, McGill University, Montreal, QC, Canada, 9–13 August 1999; pp. 14–15. [Google Scholar]
- Scientific Group Thermodata Europe. Binary Systems. Part 1 _ Elements and Binary Systems from Ag-Al to Au-Tl Al-Mn: Datasheet from Landolt-Börnstein-Group IV Physical Chemistry Volume 19B1: “Binary Systems. Part 1 _ Elements and Binary Systems from Ag-Al to Au-Tl” in SpringerMaterials (10.1007/10655491_41); Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Sauthoff, G. Intermetallics; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Okada, Y.; Taniguchi, S.; Yamagata, R.; Nakashima, H.; Takeyama, M. Nano-Indentation Modulus and Hardness of β-Ti and γ-TiAl Phases in Ti–Al–Cr System. MRS Adv. 2021, 6, 183–186. [Google Scholar] [CrossRef]
- Bendersky, L.A.; Boettinger, W.J.; Burton, B.P.; Biancaniello, F.S.; Shoemaker, C.B. The Formation of Ordered ω-Related Phases in Alloys of Composition Ti 4 Al 3 Nb. Acta Metall. Et Mater. 1990, 38, 931–943. [Google Scholar] [CrossRef]
- Stark, A.; Oehring, M.; Pyczak, F.; Schreyer, A. In Situ Observation of Various Phase Transformation Paths in Nb-Rich TiAl Alloys during Quenching with Different Rates. Adv. Eng. Mater. 2011, 13, 700–704. [Google Scholar] [CrossRef]
- Zhu, B.; Xue, X.; Kou, H.; Li, X.; Li, J. Effect of Microstructure on the Fracture Toughness of Multi-Phase High Nb-Containing TiAl Alloys. Intermetallics 2018, 100, 142–150. [Google Scholar] [CrossRef]
- Stark, A.; Bartels, A.; Clemens, H.; Schimansky, F.P. On the Formation of Ordered ω-phase in High Nb Containing γ-TiAl Based Alloys. Adv. Eng. Mater. 2008, 10, 929–934. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Zhang, L.Q.; Xu, X.J.; Sun, J.; Lin, J.P. Omega Phase in As-Cast High-Nb-Containing TiAl Alloy. Scr. Mater. 2013, 68, 929–932. [Google Scholar] [CrossRef]
- Jewett, T.J.; Ahrens, B.; Dahms, M. Stability of TiAl in the Ti-Al-Cr System. Mat. Sci. Eng. A 1997, 225, 29–37. [Google Scholar] [CrossRef]
- Chen, H.; Weitzer, F.; Krendelsberger, N.; Du, Y.; Schuster, J.C. Reaction Scheme and Liquidus Surface of the Ternary System Aluminum-Chromium-Titanium. Metall. Mater. Trans. A 2009, 40, 2980. [Google Scholar] [CrossRef]
- Ye, L.-H.; Wang, H.; Zhou, G.; Hu, Q.-M.; Yang, R. Phase Stability of TiAl-X (X=V, Nb, Ta, Cr, Mo, W, and Mn) Alloys. J. Alloys Compd. 2020, 819, 153291. [Google Scholar] [CrossRef]
- Strychor, R.; Williams, J.C.; Soffa, W.A. Phase Transformations and Modulated Microstructures in Ti-Al-Nb Alloys. Metall. Trans. A 1988, 19, 225–234. [Google Scholar] [CrossRef]
- Hsiung, L.M.; Cai, W.; Wadley, H.N.G. Microstructure and Phase Evolution in Rapidly-Solidified Ti–24Al–11Nb. In High Temperature Aluminides and Intermetallics; Whang, S.H., Pope, D.P., Liu, C.T., Eds.; Elsevier: Oxford, UK, 1992; pp. 295–303. [Google Scholar]
- Han, T.; Huang, Z.W. Microstructures and Tensile Properties of Ti-46Al-8Ta after Heat Treatment and Thermal Exposure. Cailiao Kexue Yu Gongyi Mater. Sci. Technol. 2013, 21, 90–95. [Google Scholar]
- Buenconsejo, P.J.S.; Kim, H.Y.; Hosoda, H.; Miyazaki, S. Shape Memory Behavior of Ti-Ta and Its Potential as a High-temperature Shape Memory Alloy. Acta Mater. 2009, 57, 1068–1077. [Google Scholar] [CrossRef]
- Ferrari, A.; Paulsen, A.; Frenzel, J.; Rogal, J.; Eggeler, G.; Drautz, R. Unusual Composition Dependence of Transformation Temperatures in Ti-Ta-X Shape Memory Alloys. Phys. Rev. Mater. 2018, 2, 073609. [Google Scholar] [CrossRef] [Green Version]
- Niendorf, T.; Krooß, P.; Batyrsina, E.; Paulsen, A.; Motemani, Y.; Ludwig, A.; Buenconsejo, P.; Frenzel, J.; Eggeler, G.; Maier, H.J. Functional and Structural Fatigue of Titanium Tantalum High Temperature Shape Memory Alloys (HT SMAs). Mater. Sci. Eng. A 2015, 620, 359–366. [Google Scholar] [CrossRef]
- Imayev, V.; Khismatullin, T.; Oleneva, T.; Imayev, R.; Valiev, R.; Wunderlich, R.; Minkow, A.; Hecht, U.; Fecht, H.J. Grain Refinement in Cast Ti-46Al-8Nb AND Ti-46Al-8Ta Alloys Via Massive Transformation. Adv. Eng. Mater. 2008, 10, 1095–1100. [Google Scholar] [CrossRef]
- Xu, X.-J.; Song, L.; Jin, X.-O.; Han, D.-D.; Wang, X.; Lin, J.-P. Microstructure and Microsegregation of Directionally Solidified Ti–45Al–8Nb Alloy with Different Solidification Rates. Rare Met. 2016, 35, 70–76. [Google Scholar] [CrossRef]
- Khanna, A.S. Chapter 6-High-Temperature Oxidation. In Handbook of Environmental Degradation of Materials, 3rd ed.; Kutz, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 117–132. [Google Scholar]
- Dai, J.; Zhu, J.; Chen, C.; Weng, F. High Temperature Oxidation Behavior and Research Status of Modifications on Improving High Temperature Oxidation Resistance of Titanium Alloys and Titanium Aluminides: A Review. J. Alloys Compd. 2016, 685, 784–798. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, Y.; Gong, S.; Xu, H. Effect of Ti–Al–Cr Coatings on the High Temperature Oxidation Behavior of TiAl Alloys. Mater. Sci. Eng. A 2001, 307, 182–187. [Google Scholar] [CrossRef]
- Laska, N.; Braun, R.; Knittel, S. Oxidation Behavior of Protective Ti-Al-Cr Based Coatings Applied on the γ-TiAl Alloys Ti-48-2-2 and TNM-B1. Surf. Coat. Technol. 2018, 349, 347–356. [Google Scholar] [CrossRef]
- Jacobson, N.S.; Brady, M.P.; Mehrotra, G.M. Thermodynamics of Selected Ti-Al and Ti-Al-Cr alloys. Oxid. Met. 1999, 52, 537–556. [Google Scholar] [CrossRef]
- Brady, M.P.; Smialek, J.L.; Smith, J.; Humphrey, D.L. The Role of Cr in Promoting Protective Alumina Scale Formation by γ-Based Ti-Al-Cr alloys—I. Compatibility with Alumina and Oxidation Behavior in Oxygen. Acta Mater. 1997, 45, 2357–2369. [Google Scholar] [CrossRef]
- Wasilewski, R.J. The Solubility of Oxygen in, and the Oxides of, Tantalum. J. Am. Chem. Soc. 1953, 75, 1001–1002. [Google Scholar] [CrossRef]
- Donaldson, O.K.; Hattar, K.; Trelewicz, J.R. Metastable Tantalum Oxide Formation during the Devitrification of Amorphous Tantalum Thin Films. J. Am. Ceram. Soc. 2016, 99, 3775–3783. [Google Scholar] [CrossRef]
- Hashimoto, K.; Seita, K. Formation of Protective Intermediate Phase in Ta Addition TiAl during High Temperature Oxidation. Mater. Sci. Forum 2010, 654-656, 546–549. [Google Scholar] [CrossRef]
- Mitoraj, M.; Godlewska, E.M. Oxidation of Ti-46Al-8Ta in air at 700 °C and 800 °C under Thermal Cycling Conditions. Intermetallics 2013, 34, 112–121. [Google Scholar] [CrossRef]
- Godlewska, E.; Mitoraj, M.; Leszczynska, K. Hot Corrosion of Ti-46Al-8Ta (at.%) Intermetallic Alloy. Corros. Sci. 2014, 78, 63–70. [Google Scholar] [CrossRef]
- Hanrahan, R.J.; Butt, D.P. Oxidation Kinetics and Mechanisms of Ti-Ta Alloys. Oxid. Met. 1997, 47, 317–353. [Google Scholar] [CrossRef]
- Huang, Z.W.; Voice, W.; Bowen, P. Thermal Exposure Induced α2+γ→B2(ω) and α2→B2(ω) Phase Transformations in a High Nb fully Lamellar TiAl Alloy. Scr. Mater. 2003, 48, 79–84. [Google Scholar] [CrossRef]
- Huang, Z.W. Ordered ω Phases in a 4Zr-4Nb-Containing TiAl-Based Alloy. Acta Mater. 2008, 56, 1689–1700. [Google Scholar] [CrossRef]
- Morris, M.A.; Li, Y.G. Deformation Mechanisms and Slip Transfer in a Ti44Al2Mo Alloy. Mat. Sci. Eng. A 1995, 197, 133–145. [Google Scholar] [CrossRef]
- Shao, G.; Miodownik, A.P.; Tsakiropoulos, P. ω-Phase Formation in V‒Al and Ti‒Al‒V Alloys. Philos. Mag. A Phys. Condens. Matter Struct. Defects Mech. Prop. 1995, 71, 1389–1408. [Google Scholar] [CrossRef]
- Shao, G.; Tsakiropoulos, P. On the ω Phase Formation in Cr–Al and Ti–Al–Cr Alloys. Acta Mater. 2000, 48, 3671–3685. [Google Scholar] [CrossRef]
- Shao, G.; Tsakiropoulos, P. Prediction of ω Phase Formation in Ti–Al–X alloys. Mater. Sci. Eng. A 2002, 329–331, 914–919. [Google Scholar] [CrossRef]
- Cheng, T.T.; Loretto, M.H. Preliminary Study on the Decomposition of the Beta Phase in Ti-44Al-8Nb and Ti-44Al-4Ta-4Zr-0.2Si. In Structural Intermetallics; Nathal, M.V., Ed.; Minerals, Metals and Materials Society: Warrendale, PA, USA, 1997; pp. 253–260. [Google Scholar]
- Weiss, I.; Semiatin, S.L. Thermomechanical Processing of Beta Titanium Alloys—An Overview. Mater. Sci. Eng. A 1998, 243, 46–65. [Google Scholar] [CrossRef]
- Blau, P.J. Friction, Lubrication, and Wear Technology, 18th ed.; Harvard: Cambridge, MA, USA, 1992; Volume 18. [Google Scholar]
- Göken, M.; Kempf, M.; Nix, W.D. Hardness and Modulus of the lamellar Microstructure in PST-TiAl Studied by Nanoindentations and AFM. Acta Mater. 2001, 49, 903–911. [Google Scholar] [CrossRef]
- Wang, D.P.; Qi, Z.X.; Zhang, H.T.; Chen, G.; Lu, Y.; Sun, B.A.; Liu, C.T. Microscale Mechanical Properties of Ultra-High-Strength Polysynthetic TiAl-Ti3Al Single Crystals. Mater. Sci. Eng. A 2018, 732, 14–20. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Q.; Qu, S.J.; Xiang, H.P.; Wang, C.; Gao, J.B.; Feng, A.H.; Chen, D.L. Oxidation Mechanisms of an Intermetallic Alloy at High Temperatures. Scr. Mater. 2021, 199, 113852. [Google Scholar] [CrossRef]

| Sample Designation | Ti | Al | Cr | Ta | LiF |
|---|---|---|---|---|---|
| Ti-41Al-7Cr | 1.9 | 6.8 | 2.2 | - | 11.13 |
| Ti-41Al-7Ta | - | 4.4 | - | 7.33 | 10.6 |
| Phase | Space Group | Pearson Symbol | Composition, Homogeneity Range | Reactions | Young’ s Modulus (E), GPa | Yield Strength, MPa | Fracture Toughness (K1c), MPa/m3/2 |
|---|---|---|---|---|---|---|---|
| α2 (Ti3Al) | P63/mmc | hP8 | 22–35 at.% Al | hcp→Ti3Al (congruent) | 100–145 | 700–990 | 13–42 |
| γ (TiAl) | P4/mmm | tP4 | 35–48 at.% Al | L + hcp→AlTi (peritectic) | 160–180 | 400–650 | 10–20 |
| β/B2 | Imm/Pmm | cI2/cP2 | ~39–48 at.% Al, ~47–63 at.% Ti, ~13–22 at.% X | L→bcc (congruent)→B2 (ordering) | 140 | - | - |
| ω/ω′ | P6/mmm/Pm1 | hP3/- | B2→ω′ (diffusionless)→ω (ordering) | - | - |
| Spectrum (Sp.) No | Al | Ti | Cr |
|---|---|---|---|
| Spectrum 1 | 44.2 | 48.7 | 7.1 |
| Spectrum 2 | 40.9 | 52.5 | 6.6 |
| Spectrum 3 | 35.9 | 58.3 | 5.8 |
| Spectrum (Sp.) No | Al | Ti | Ta |
|---|---|---|---|
| Spectrum 1 | 51.0 | 41.4 | 7.6 |
| Spectrum 2 | 38.9 | 54.0 | 6.1 |
| Spectrum 3 | 36.8 | 57.4 | 5.8 |
| Spectrum 4 | 39.8 | 54.0 | 6.2 |
| Spectrum 5 | 42.4 | 52.5 | 5.1 |
| Spectrum 6 | 34.0 | 38.7 | 27.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazurenko, D.V.; Golkovsky, M.G.; Stark, A.; Pyczak, F.; Bataev, I.A.; Ruktuev, A.A.; Petrov, I.Y.; Laptev, I.S. Structure and Properties of Ti-Al-Ta and Ti-Al-Cr Cladding Layers Fabricated on Titanium. Metals 2021, 11, 1139. https://doi.org/10.3390/met11071139
Lazurenko DV, Golkovsky MG, Stark A, Pyczak F, Bataev IA, Ruktuev AA, Petrov IY, Laptev IS. Structure and Properties of Ti-Al-Ta and Ti-Al-Cr Cladding Layers Fabricated on Titanium. Metals. 2021; 11(7):1139. https://doi.org/10.3390/met11071139
Chicago/Turabian StyleLazurenko, Daria V., Mikhail G. Golkovsky, Andreas Stark, Florian Pyczak, Ivan A. Bataev, Alexey A. Ruktuev, Ivan Yu. Petrov, and Ilia S. Laptev. 2021. "Structure and Properties of Ti-Al-Ta and Ti-Al-Cr Cladding Layers Fabricated on Titanium" Metals 11, no. 7: 1139. https://doi.org/10.3390/met11071139
APA StyleLazurenko, D. V., Golkovsky, M. G., Stark, A., Pyczak, F., Bataev, I. A., Ruktuev, A. A., Petrov, I. Y., & Laptev, I. S. (2021). Structure and Properties of Ti-Al-Ta and Ti-Al-Cr Cladding Layers Fabricated on Titanium. Metals, 11(7), 1139. https://doi.org/10.3390/met11071139







