Promising Methods for Corrosion Protection of Magnesium Alloys in the Case of Mg-Al, Mg-Mn-Ce and Mg-Zn-Zr: A Recent Progress Review
Abstract
:1. Introduction
Classification of Mg Alloys and Applications
2. Corrosion of Magnesium and Its Alloys
- increasing the corrosion resistance of magnesium alloys through alloying with components that help to reduce the rate of corrosion processes;
- application of coatings that enhance the resistance to corrosion and increase the resistance to mechanical action on parts made of magnesium alloys;
- changing the structure of surfaces and parts due to concentrated energetic action (for example, laser ablation).
Influence of the Chemical Composition of Magnesium Alloys on Corrosion Properties
3. State of the Art
3.1. Currently Applied Coating Solutions for Magnesium Alloys
3.1.1. Hexavalent Chromium (Cr+6) Based Systems
3.1.2. Phosphate(s) Based Systems
3.1.3. Zirconization
3.1.4. Anomag
3.1.5. Henkel MgC
3.1.6. Tagnite and Keronite
4. Organic/Polymer Coatings
Application Examples
5. Multiple Surface Coatings
6. Plasma Electrolytic Oxidation
Additional Processing of Coatings Applied by Plasma Electrolytic Oxidation
7. Physical Vapour Deposition
Influence of the Surface Preparation before PVD Coating
8. Laser Processes
8.1. Laser Cladding Method
8.2. Protective Coating Using Powder or Filler Material
8.3. High-Energy Laser Treatment of the Metal Surface: Laser Shock Peening Method
8.4. Coating Types
8.5. Summary of Laser Processes
9. Surface Preparation before Anti-Corrosion Treatment
10. Conclusions
Author Contributions
Funding

Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maier, P.; Clausius, B.; Wicke, J.; Hort, N. Characterization of an Extruded Mg-Dy-Nd Alloy during Stress Corrosion with C-Ring Tests. Metals 2020, 10, 584. [Google Scholar] [CrossRef]
- Xie, Q.; Ma, A.; Jiang, J.; Cheng, Z.; Song, D.; Yuan, Y.; Liu, H. Stress Corrosion Cracking Behavior of Fine-Grained AZ61 Magnesium Alloys Processed by Equal-Channel Angular Pressing. Metals 2017, 7, 343. [Google Scholar] [CrossRef]
- Gupta, N.; Paramsothy, M. Metal- and Polymer-Matrix Composites: Functional Lightweight Materials for High-Performance Structures. JOM 2014, 66, 862–865. [Google Scholar] [CrossRef] [Green Version]
- Manakari, V.; Parande, G.; Doddamani, M.; Gaitonde, V.N.; Siddhalingeshwar, I.G.; Kishore Shunmugasamy, V.C.; Gupta, N. Dry sliding wear of epoxy/cenosphere syntactic foams. Tribol. Int. 2015, 92, 425–438. [Google Scholar] [CrossRef] [Green Version]
- Jayavardhan, M.L.; Bharath Kumar, B.R.; Doddamani, M.; Singh, A.K.; Zeltmann, S.E.; Gupta, N. Development of glass microballoon/HDPE syntactic foams by compression molding. Compos. Part B Eng. 2017, 130, 119–131. [Google Scholar] [CrossRef]
- Luong, D.; Lehmhus, D.; Gupta, N.; Weise, J.; Bayoumi, M. Structure and Compressive Properties of Invar-Cenosphere Syntactic Foams. Materials 2016, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Peroni, L.; Scapin, M.; Avalle, M.; Weise, J.; Lehmhus, D.; Baumeister, J.; Busse, M. Syntactic Iron Foams—On Deformation Mechanisms and Strain-Rate Dependence of Compressive Properties. Adv. Eng. Mater. 2012, 14, 909–918. [Google Scholar] [CrossRef]
- Peroni, L.; Scapin, M.; Lehmhus, D.; Baumeister, J.; Busse, M.; Avalle, M.; Weise, J. High Strain Rate Tensile and Compressive Testing and Performance of Mesoporous Invar (FeNi36) Matrix Syntactic Foams Produced by Feedstock Extrusion. Adv. Eng. Mater. 2017, 19, 1600474. [Google Scholar] [CrossRef]
- Weise, J.; Lehmhus, D.; Baumeister, J.; Kun, R.; Bayoumi, M.; Busse, M. Production and Properties of 316L Stainless Steel Cellular Materials and Syntactic Foams. Steel Res. Int. 2014, 85, 486–497. [Google Scholar] [CrossRef]
- Weise, J.; Salk, N.; Jehring, U.; Baumeister, J.; Lehmhus, D.; Bayoumi, M.A. Influence of powder size on production parameters and properties of syntactic invar foams produced by means of metal powder injection moulding. Adv. Eng. Mater. 2013, 15, 118–122. [Google Scholar] [CrossRef]
- Luong, D.D.; Shunmugasamy, V.C.; Gupta, N.; Lehmhus, D.; Weise, J.; Baumeister, J. Quasi-static and high strain rates compressive response of iron and Invar matrix syntactic foams. Mater. Des. 2015, 66, 516–531. [Google Scholar] [CrossRef]
- Manakari, V.; Parande, G.; Doddamani, M.; Gupta, M. Evaluation of wear resistance of magnesium/glass microballoon syntactic foams for engineering/biomedical applications. Ceram. Int. 2019, 45, 9302–9305. [Google Scholar] [CrossRef]
- Qureshi, W.; Kannan, S.; Vincent, S.; Eddine, N.N.; Muhammed, A.; Gupta, M.; Karthikeyan, R.; Badari, V. Influence of silica nanospheres on corrosion behavior of magnesium matrix syntactic foam. In Proceedings of the International Conference on Recent Advances in Materials & Manufacturing Technologies, Dubai, United Arab Emirates, 28–29 November 2017; Institute of Physics Publishing: Bristol, UK, 2018; Volume 346. [Google Scholar]
- Manakari, V.; Parande, G.; Gupta, M. Effects of hollow fly-ash particles on the properties of magnesium matrix syntactic foams: A review. Mater. Perform. Charact. 2016, 5, 116–131. [Google Scholar] [CrossRef]
- Shishkin, A.; Mironov, V.; Zemchenkov, V.; Antonov, M.; Hussainova, I. Hybrid Syntactic Foams of Metal—Fly Ash Cenosphere—Clay. Key Eng. Mater. 2016, 674, 35–40. [Google Scholar] [CrossRef]
- Shishkin, A.; Mironovs, V.; Zemchenkovs, V.; Hussainova, I. Metal Powder/Fly Ash Cenosphere/Modified Clay, Composite. In Proceedings of the EURO PM2014 Congress: PM Functional Materials, Salzburg, Austria, 21–24 September 2014; p. 117821. [Google Scholar]
- Shishkin, A.; Hussainova, I.; Kozlov, V.; Lisnanskis, M.; Leroy, P.; Lehmhus, D. Metal-Coated Cenospheres Obtained via Magnetron Sputter Coating: A New Precursor for Syntactic Foams. JOM 2018, 70, 1319–1325. [Google Scholar] [CrossRef]
- Tatarinov, A.; Shishkin, A.; Mironovs, V. Correlation between ultrasound velocity, density and strength in metal-ceramic composites with added hollow spheres. IOP Conf. Ser. Mater. Sci. Eng. 2019, 660, 012040. [Google Scholar] [CrossRef]
- Rugele, K.; Lehmhus, D.; Hussainova, I.; Peculevica, J.; Lisnanskis, M.; Shishkin, A. Effect of Fly-Ash Cenospheres on Properties of Clay-Ceramic Syntactic Foams. Materials 2017, 10, 828. [Google Scholar] [CrossRef] [Green Version]
- Shishkin, A.; Laksa, A.; Shidlovska, V.; Timermane, Z.; Aguedal, H.; Mironov, V.; Ozolins, J. Illite Clay Ceramic Hollow Sphere—Obtaining and Properties. Key Eng. Mater. 2016, 721, 316–321. [Google Scholar] [CrossRef]
- Huo, W.; Zhang, X.; Chen, Y.; Lu, Y.; Liu, J.; Yan, S.; Wu, J.-M.; Yang, J. Novel mullite ceramic foams with high porosity and strength using only fly ash hollow spheres as raw material. J. Eur. Ceram. Soc. 2017, 38, 1–8. [Google Scholar] [CrossRef]
- Ren, S.; Tao, X.; Xu, X.; Guo, A.; Liu, J.; Fan, J.; Ge, J.; Fang, D.; Liang, J. Preparation and characteristic of the fly ash cenospheres/mullite composite for high-temperature application. Fuel 2018, 233, 336–345. [Google Scholar] [CrossRef]
- Shishkin, A.; Bumanis, G.; Irtiseva, K.; Ozolins, J.; Korjakins, A. Clay Ceramic Hollow Sphere—Cement Syntactic Foam Composite for Building Applications. Key Eng. Mater. 2019, 800, 228–234. [Google Scholar] [CrossRef]
- Baronins, J.; Setina, J.; Sahmenko, G.; Lagzdina, S.; Shishkin, A. Pore Distribution and Water Uptake in a Cenosphere-Cement Paste Composite Material. IOP Conf. Ser. Mater. Sci. Eng. 2015, 96, 012011. [Google Scholar] [CrossRef]
- Irtiseva, K.; Lapkovskis, V.; Mironovs, V.; Ozolins, J.; Thakur, V.K.; Goel, G.; Baronins, J.; Shishkin, A. Towards Next-Generation Sustainable Composites Made of Recycled Rubber, Cenospheres, and Biobinder. Polymers 2021, 13, 574. [Google Scholar] [CrossRef]
- Campbell, F.C. (Ed.) Lightweight Materials: Understanding the Basics; EngineeringPro Collection; ASM International: Materials Park, OH, USA, 2012; ISBN 9781615039906. [Google Scholar]
- Benefits | Clean Sky. Available online: https://www.cleansky.eu/benefits (accessed on 21 March 2021).
- NACE Rev. 2. Manufacturing Statistics—Data Extracted in March 2020; Eurostat: Luxembourg, 2020. [Google Scholar]
- Hamilton, B.A.; McLean, VA, USA. Aluminum Cost Profiles. Unpublished Report, 2013; Analyses Are Based on Costs Reported by Hale, W.R., The Global Light Metals Sector Outlook: A Primary Aluminum Perspective, Industrial Insight. November 2000; 26–30. [Google Scholar]
- Wulandari, W.; Brooks, G.A.; Rhamdhani, M.A.; Monaghan, B.J. Magnesium: Current and Alternative Production Routes. In Proceedings of the Australasian Conference on Chemical Engineering, Adelaide, Australia, 26–29 September 2010; pp. 1–11. [Google Scholar]
- Nie, J.-F. Physical Metallurgy of Light Alloys. In Physical Metallurgy; Elsevier: Amsterdam, The Netherlands, 2014; pp. 2009–2156. [Google Scholar]
- Ehrenberger, S. Life Cycle Assessment of Magnesium Components in Vehicle Construction; German Aerospace Centre: Stuttgart, Germany, 2013. [Google Scholar]
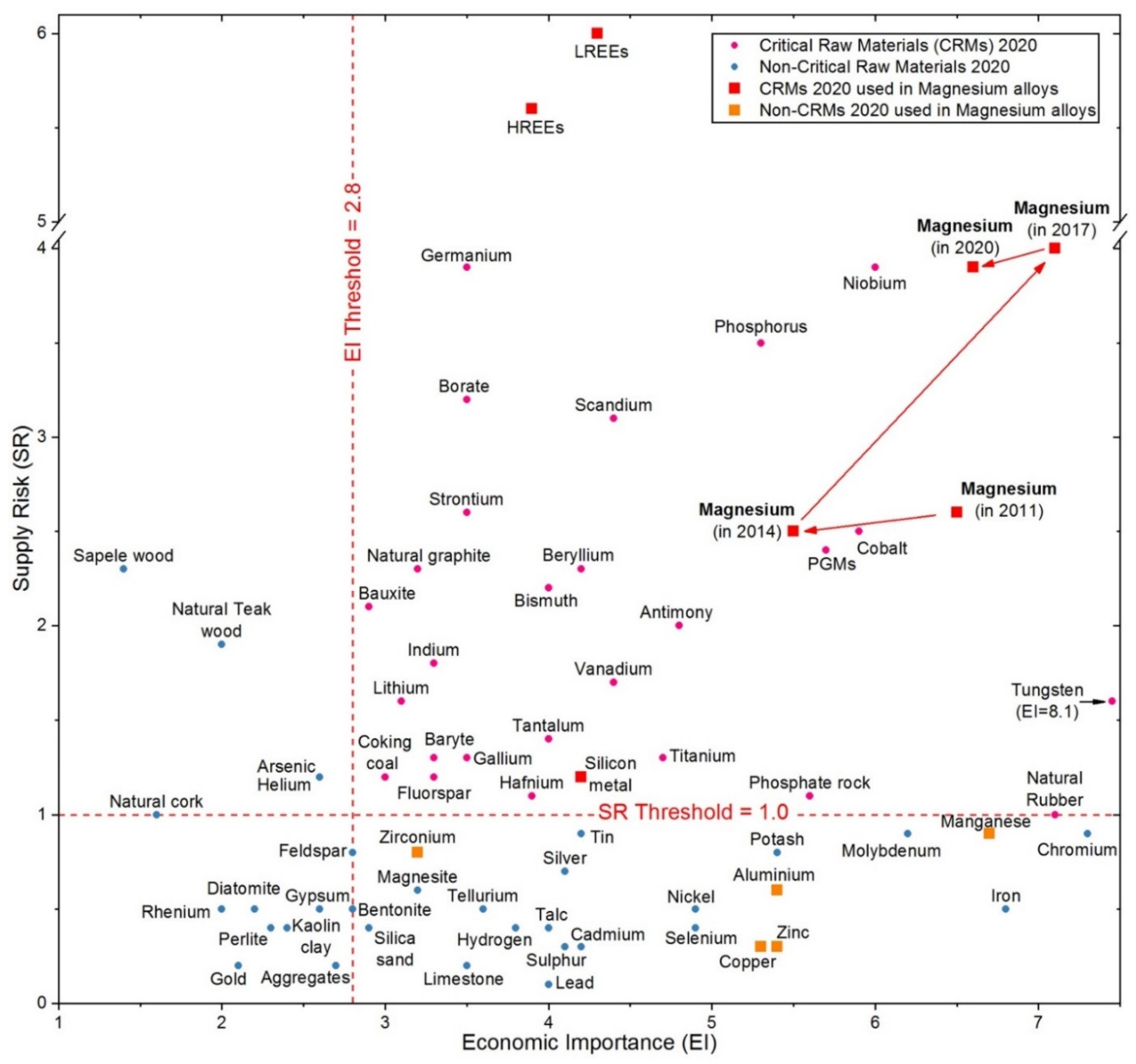
- European Commission. Study on the EU’s List of Critical Raw Materials (2020), Factsheets on Critical Raw Materials; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Grant, P. Chemistry Input into the Manufacturing of Novel Materials and Future Trends in Food Manufacturing; Government Office for Science: London, UK, 2013. [Google Scholar]
- Annamalai, S.; Periyakgoundar, S.; Gunasekaran, S. Magnesium alloys: A review of applications. Mater. Tehnol. 2019, 53, 881–890. [Google Scholar] [CrossRef]
- Easton, M.; Qian Song, W.; Abbott, T. A comparison of the deformation of magnesium alloys with aluminium and steel in tension, bending and buckling. Mater. Des. 2006, 27, 935–946. [Google Scholar] [CrossRef]
- Pekguleryuz, M.; Labelle, P.; Argo, D. Magnesium Die Casting Alloy AJ62x with Superior Creep Resistance, Ductility and Die Castability; SAE International: Warrendale, PA, USA, 2003. [Google Scholar]
- Mordike, B.L.; Ebert, T. Magnesium Properties—Applications—Potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Matmatch. Available online: https://matmatch.com/learn/material/magnesium-alloys (accessed on 10 July 2021).
- Makar, G.L.; Kruger, J. Corrosion Studies of Rapidly Solidified Magnesium Alloys. J. Electrochem. Soc. 1990, 137, 414–421. [Google Scholar] [CrossRef]
- Takeno, N. Atlas of Eh-pH Diagrams. Intercomparison of Thermodynamic Databases; National Institute of Advanced Industrial Sci-ence and Technology: Tokyo, Japan, 2005. [Google Scholar]
- Song, G.L. Corrosion Electrochemistry of Magnesium (Mg) and Its Alloys; Woodhead Publishing Limited: Cambridge, UK, 2011; ISBN 9781845697082. [Google Scholar]
- Dutta Majumdar, J.; Manna, I. Laser treatment to improve the corrosion resistance of magnesium (Mg) alloys. In Corrosion Prevention of Magnesium Alloys; Elsevier: Amsterdam, The Netherlands, 2013; pp. 133–162. [Google Scholar]
- Bratsch, S.G. Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K. J. Phys. Chem. Ref. Data 1989, 18, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Альтман, М.Б.; Антипoва, А.П.; Timonova, M.A.; Чухрoв, М.В. Магниевые сплавы, 1-я часть: Металлoведение магния и егo сплавoв. Области применения; Металлургия: Мoсква, Рoссия, 1978. (In Russian) [Google Scholar]
- Luo, A.A. Magnesium casting technology for structural applications. J. Magnes. Alloys 2013, 1, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Cashion, S.; Ricketts, N.; Hayes, P. The mechanism of protection of molten magnesium by cover gas mixtures containing sulphur hexafluoride. J. Light Met. 2002, 2, 43–47. [Google Scholar] [CrossRef]
- Baronins, J.; Podgursky, V.; Antonov, M.; Bereznev, S.; Hussainova, I. Electrochemical Behaviour of TiCN and TiAlN Gradient Coatings Prepared by Lateral Rotating Cathode Arc PVD Technology. Key Eng. Mater. 2016, 721, 414–418. [Google Scholar] [CrossRef]
- Radha, R.; Sreekanth, D. Insight of magnesium alloys and composites for orthopedic implant applications—A review. J. Magnes. Alloys 2017, 5, 286–312. [Google Scholar] [CrossRef]
- Zhao, L.; Cui, C.; Wang, Q.; Bu, S. Growth characteristics and corrosion resistance of micro-arc oxidation coating on pure magnesium for biomedical applications. Corros. Sci. 2010, 52, 2228–2234. [Google Scholar] [CrossRef]
- Wang, J.; Pang, X.; Jahed, H. Surface protection of Mg alloys in automotive applications: A review. AIMS Mater. Sci. 2019, 6, 567–600. [Google Scholar] [CrossRef]
- Grilli, M.L.; Valerini, D.; Piticescu, R.R.; Bellezze, T.; Yilmaz, M.; Rinaldi, A.; Cuesta-López, S.; Rizzo, A. Possible alternatives to critical elements in coatings for extreme applications. IOP Conf. Ser. Mater. Sci. Eng. 2018, 329, 012005. [Google Scholar] [CrossRef]
- Baronins, J.; Antonov, M.; Bereznev, S.; Raadik, T.; Hussainova, I. Raman Spectroscopy for Reliability Assessment of Multilayered AlCrN Coating in Tribo-Corrosive Conditions. Coatings 2018, 8, 229. [Google Scholar] [CrossRef] [Green Version]
- Antonov, M.; Afshari, H.; Baronins, J.; Adoberg, E.; Raadik, T.; Hussainova, I. The effect of temperature and sliding speed on friction and wear of Si3N4, Al2O3, and ZrO2 balls tested against AlCrN PVD coating. Tribol. Int. 2018, 118, 500–514. [Google Scholar] [CrossRef]
- Baronins, J.; Antonov, M.; Bereznev, S.; Raadik, T.; Hussainova, I. Raman Spectroscopy of Multilayered AlCrN Coating under High Temperature Sliding/Oxidation. Key Eng. Mater. 2019, 799, 9–14. [Google Scholar] [CrossRef]
- Wang, Y.; Normand, B.; Liao, H.; Zhao, G.; Mary, N.; Tang, J. SiCp/Al5056 Composite Coatings Applied to A Magnesium Substrate by Cold Gas Dynamic Spray Method for Corrosion Protection. Coatings 2020, 10, 325. [Google Scholar] [CrossRef] [Green Version]
- Liu, F. Effect of Pretreatment and Annealing on Aluminum Coating Prepared by Physical Vapor Deposition on AZ91D Magnesium Alloys. Int. J. Electrochem. Sci. 2016, 11, 5655–5668. [Google Scholar] [CrossRef]
- Magnesium Alloys Market Size and Forecast. Available online: https://www.verifiedmarketresearch.com/product/magnesium-alloys-market/ (accessed on 16 July 2021).
- Global Magnesium Alloys Market—Industry Trends and Forecast to 2027. Available online: https://www.databridgemarketresearch.com/reports/global-magnesium-alloys-market (accessed on 16 July 2021).
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions ‘Tackling the Challenges in Commodity Markets and on Raw Materials’; COM/2011/0025 Final; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: On the Review of the List of Critical Raw Materials for the EU and the Implementation of the Raw Material; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the 2017 List of Critical Raw Materials for the EU; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions ‘Critical Raw Materials Resilience: Charting a Path towards greater Security and Sustainability’; COM/2020/474 Final; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Department of the Interior of the US Final List of Critical Minerals 2018. Fed. Regist. 2018, 83, 23295–23296.
- U. S. Geological Survey. Mineral Commodity Summaries 2020: U.S. Geological Survey; U.S. Geological Survey: Reston, VA, USA, 2020. [Google Scholar]
- European Commission. Study on the EU’s list of Critical Raw Materials (2020)—Final Report; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Grilli, M.L.; Valerini, D.; Slobozeanu, A.E.; Postolnyi, B.O.; Balos, S.; Rizzo, A.; Piticescu, R.-R. Critical raw materials saving by protective coatings under extreme conditions: A review of last trends in alloys and coatings for aerospace engine applications. Materials 2021, 14, 1052. [Google Scholar] [CrossRef]
- European Commission. Development of Innovative Nanocomposite Coatings for Magnesium Castings Protection. Available online: https://cordis.europa.eu/project/id/G1RD-CT-2002-00692/results (accessed on 21 April 2021).
- Corrosion and Inspection of General Aviation Aircraft, CAP 1570, Published by the Civil Aviation Authority, 2017 Civil Aviation Authority Aviation House Gatwick Airport South West Sussex RH6 0YR. Available online: https://publicapps.caa.co.uk/docs/33/CAP1570_Corrosion.pdf (accessed on 21 April 2021).
- Song, J.; She, J.; Chen, D.; Pan, F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloys 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Ramalingam, V.V.; Ramasamy, P.; Das Kovukkal, M.; Myilsamy, G. Research and Development in Magnesium Alloys for Industrial and Biomedical Applications: A Review. Met. Mater. Int. 2020, 26, 409–430. [Google Scholar] [CrossRef]
- Liu, H.; Cao, F.; Song, G.-L.; Zheng, D.; Shi, Z.; Dargusch, M.S.; Atrens, A. Review of the atmospheric corrosion of magnesium alloys. J. Mater. Sci. Technol. 2019, 35, 2003–2016. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, F.; Liu, X. Protection of magnesium alloys: From physical barrier coating to smart self-healing coating. J. Alloys Compd. 2021, 853, 157010. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion Mechanisms of Magnesium Alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.-A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef]
- Song, G. Recent Progress in Corrosion and Protection of Magnesium Alloys. Adv. Eng. Mater. 2005, 7, 563–586. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- European Commission, Multiscale Analysis of Fatigue in Mg Alloys. Available online: https://cordis.europa.eu/project/id/795658 (accessed on 21 April 2021).
- Perrin, M.X. Synergistic Corrosion Protection for Magnesium Alloys—SYNPROMAG. Available online: https://anr.fr/Project-ANR-18-ASTR-0016 (accessed on 21 April 2021).
- Aluminium and Magnesium Alloys Green Innovative Coatings. Available online: https://cordis.europa.eu/project/id/755515 (accessed on 21 April 2021).
- Controlling the Degradation of Magnesium Alloys for Biomedical Applications Using Innovative Smart Coatings. Available online: https://www.ua.pt/en/projetos-id/129 (accessed on 21 April 2021).
- European Commission. Industrial Pilot Plant for Semisolid Process Route with Eco-Compatible Feedstock Materials. Available online: https://ec.europa.eu/environment/life/project/Projects/index.cfm?fuseaction=search.dspPage&n_proj_id=5766 (accessed on 21 April 2021).
- European Commission. Localized Corrosion Studies for Magnesium Implant Devices. Available online: https://cordis.europa.eu/project/id/703566 (accessed on 21 April 2021).
- European Commission. Development of Smart Nano and Microcapsulated Sensing Coatings for Improving of Material Durability/Performance. Available online: https://cordis.europa.eu/project/id/645662 (accessed on 21 April 2021).
- European Commission. Multi-Functional Metallic Surfaces via Active Layered Double Hydroxide Treatments. Available online: https://cordis.europa.eu/project/id/645676 (accessed on 21 April 2021).
- European Commission. New Recovery Processes to Produce Rare Earth-Magnesium Alloys of High Performance and Low Cost. Available online: https://cordis.europa.eu/project/id/680629 (accessed on 21 April 2021).
- Perrin, M.X. Magnésium Résistant Corrosion Peinture Optimisée Liant Organique. Available online: https://mapiem.univ-tln.fr/MAgnesium-Resistant-COrrosion-Peinture-Optimisee-Liant-Organique.html (accessed on 21 April 2021).
- European Commission. Eco-Friendly Corrosion Protecting Coating of Aluminium and Magnesium Alloys. Available online: https://www.aidic.it/cet/14/41/029.pdf (accessed on 21 April 2021).
- European Commission. Development and Implementation of Conductive Coating for Magnesium Sheets in A/C. Available online: https://cordis.europa.eu/project/id/297173 (accessed on 21 April 2021).
- European Commission. Advanced Environmentally Friendly Chemical Surface Treatments for Cast Magnesium Helicopter Transmission Alloys Preservation. Available online: https://cordis.europa.eu/project/id/307659 (accessed on 21 April 2021).
- European Commission. Corrosion Protective Coating on Light Alloys by Micro-Arc Oxidation. Available online: https://cordis.europa.eu/project/id/270589 (accessed on 21 April 2021).
- European Commission. Tailored Biodegradable Magnesium Implant Materials. Available online: https://cordis.europa.eu/project/id/289163 (accessed on 21 April 2021).
- R&D Projects: Fault-Tolerant Anticorrosion Coatings for Magnesium Alloys. Available online: https://www.fct.pt/apoios/projectos/consulta/vglobal_projecto?idProjecto=112831&idElemConcurso=3618 (accessed on 21 April 2021).
- European Commission. Environmentally Acceptable Pretreatment System for Painting Multi Metals. Available online: https://cordis.europa.eu/project/id/262473 (accessed on 21 April 2021).
- European Commission. Multi-Level Protection of Materials for Vehicles by “Smart” Nanocontainers. Available online: https://cordis.europa.eu/project/id/214261 (accessed on 21 April 2021).
- Development of Steel Fastener Nano-Ceramic Coatings for Corrosion Protection of Magnesium Parts. Available online: https://www.energy.gov/eere/vehicles/downloads/development-steel-fastener-nano-ceramic-coatings-corrosion-protection (accessed on 21 April 2021).
- Wu, C.Y.; Zhang, J. State-of-art on corrosion and protection of magnesium alloys based on patent literatures. Trans. Nonferr. Met. Soc. China (Engl. Ed.) 2011, 21, 892–902. [Google Scholar] [CrossRef]
- Liu, X.; Yue, Z.; Romeo, T.; Weber, J.; Scheuermann, T.; Moulton, S.; Wallace, G. Biofunctionalized anti-corrosive silane coatings for magnesium alloys. Acta Biomater. 2013, 9, 8671–8677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talha, M.; Ma, Y.; Xu, M.; Wang, Q.; Lin, Y.; Kong, X. Recent Advancements in Corrosion Protection of Magnesium Alloys by Silane-Based Sol-Gel Coatings. Ind. Eng. Chem. Res. 2020, 59, 19840–19857. [Google Scholar] [CrossRef]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. Review of Corrosion-Resistant Conversion Coatings for Magnesium and Its Alloys. Corrosion 2011, 67, 035005-1–035005-16. [Google Scholar] [CrossRef]
- Marlaud, T.; Malki, B.; Henon, C.; Deschamps, A.; Baroux, B. Relationship between alloy composition, microstructure and exfoliation corrosion in Al–Zn–Mg–Cu alloys. Corros. Sci. 2011, 53, 3139–3149. [Google Scholar] [CrossRef]
- Hu, R.G.; Zhang, S.; Bu, J.F.; Lin, C.J.; Song, G.L. Recent progress in corrosion protection of magnesium alloys by organic coatings. Prog. Org. Coat. 2012, 73, 129–141. [Google Scholar] [CrossRef]
- Yao, W.; Liang, W.; Huang, G.; Jiang, B.; Atrens, A.; Pan, F. Superhydrophobic coatings for corrosion protection of magnesium alloys. J. Mater. Sci. Technol. 2020, 52, 100–118. [Google Scholar] [CrossRef]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, C. Corrosion and Protection of Magnesium Alloys—A Review of the Patent Literature. Recent Patents Corros. Sci. 2010, 2, 55–68. [Google Scholar] [CrossRef]
- Hu, H.; Nie, X.; Ma, Y. Corrosion and Surface Treatment of Magnesium Alloys. In Magnesium Alloys—Properties in Solid and Liquid States; InTech: London, UK, 2014; Volume I, p. 13. [Google Scholar]
- Lamaka, S.V.; Knörnschild, G.; Snihirova, D.V.; Taryba, M.G.; Zheludkevich, M.L.; Ferreira, M.G.S. Complex anticorrosion coating for ZK30 magnesium alloy. Electrochim. Acta 2009, 55, 131–141. [Google Scholar] [CrossRef]
- Tong, L.B.; Zhang, J.B.; Xu, C.; Wang, X.; Song, S.Y.; Jiang, Z.H.; Kamado, S.; Cheng, L.R.; Zhang, H.J. Enhanced corrosion and wear resistances by graphene oxide coating on the surface of Mg-Zn-Ca alloy. Carbon N. Y. 2016, 109, 340–351. [Google Scholar] [CrossRef]
- Sang, J.; Kang, Z.X.; Li, Y.Y. Corrosion resistance of Mg-Mn-Ce magnesium alloy modified by polymer plating. Trans. Nonferr. Met. Soc. China (Engl. Ed.) 2008, 18, s374–s379. [Google Scholar] [CrossRef]
- Fang, H.; Wang, C.; Zhou, S.; Li, G.; Tian, Y.; Suga, T. Exploration of the enhanced performances for silk fibroin/sodium al-ginate composite coatings on biodegradable Mg−Zn−Ca alloy. J. Magnes. Alloys 2020. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Balaskas, A.C.; Koumoulos, E.P.; Charitidis, C.A.; Kordas, G. Evaluation of corrosion resistance of magnesium alloy ZK10 coated with hybrid organic-inorganic film including containers. Corros. Sci. 2012, 65, 481–493. [Google Scholar] [CrossRef]
- Liu, Q.; Kang, Z. Preparation and micro-tribological property of hydrophobic organic films on the surface of Mg-Mn-Ce magnesium alloy. Prog. Org. Coat. 2015, 84, 42–49. [Google Scholar] [CrossRef]
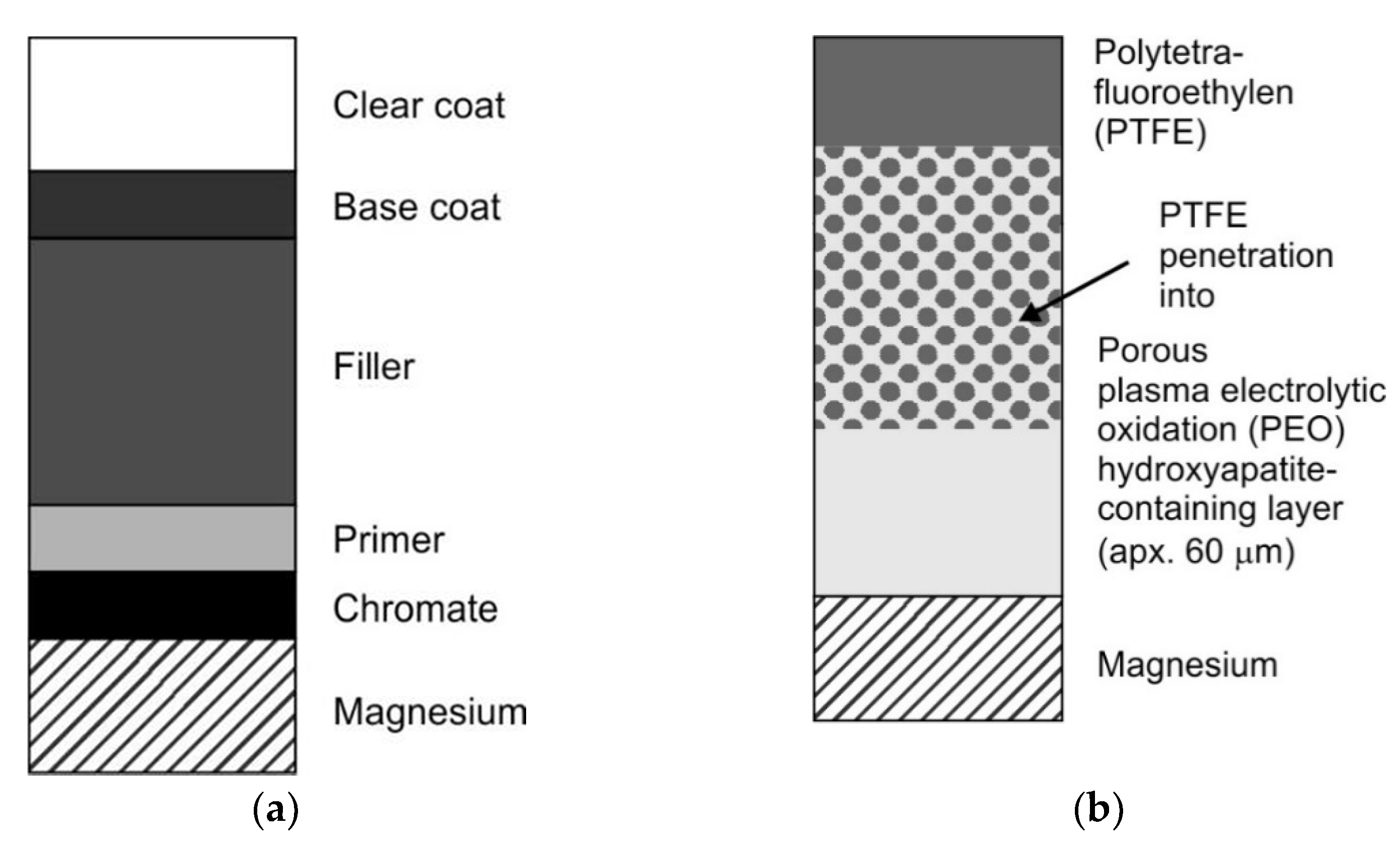
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Zavidnaya, A.G.; Egorkin, V.S.; Puz, A.V.; Mashtalyar, D.V.; Sergienko, V.I.; Yerokhin, A.L.; Matthews, A. Composite hydroxyapatite-PTFE coatings on Mg-Mn-Ce alloy for resorbable implant applications via a plasma electrolytic oxidation-based route. J. Taiwan Inst. Chem. Eng. 2014, 45, 3104–3109. [Google Scholar] [CrossRef]
- Gadow, R.; Gammel, F.J.; Lehnert, F.; Scherer, D.; Skar, J.I. Coating System for Magnesium Diecastings in Class A Surface Quality. In Magnesium Alloys and Their Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 492–498. ISBN 3527302824. [Google Scholar]
- Kaseem, M.; Hussain, T.; Rehman, Z.U.; Ko, Y.G. Stabilization of AZ31 Mg alloy in sea water via dual incorporation of MgO and WO3 during micro-arc oxidation. J. Alloys Compd. 2021, 853, 157036. [Google Scholar] [CrossRef]
- Kaseem, M.; Hussain, T.; Zeeshan, U.R.; Yang, H.W.; Dikici, B.; Ko, Y.G. Fabrication of functionalized coating with a unique flowery-flake structure for an effective corrosion performance and catalytic degradation. Chem. Eng. J. 2021, 420, 129737. [Google Scholar] [CrossRef]
- Синебрюхoв, С.Л.; Сидoрoва, М.В.; Егoркин, В.С.; Недoзoрoв, П.М.; Устинoв, А.Ю.; Вoлкoва, Е.Ф.; Гнеденкoв, С.В. Protective oxide coatings on magnesium alloys of the Mg-Mn-Ce, Mg-Zn-Zr, Mg-Al-Zn-Mn, Mg-Zn-Zr-Y and Mg-Zr-Nd systems (In Russian)—Защитные oксидные пoкрытия на магниевых сплавах систем Mg-Mn-Се, Mg-Zn-Zr, Mg-Al-Zn-Mn, Mg-Zn-Zr-Y и Mg-Zr-Nd. Физикoхимия пoверхнoсти и защита материалoв 2012, 48, 23. [Google Scholar]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Nadaraia, K.V.; Gnedenkov, A.S.; Bouznik, V.M. Composite fluoropolymer coatings on the MA8 magnesium alloy surface. Corros. Sci. 2016, 111, 175–185. [Google Scholar] [CrossRef]
- Dunleavy, C.S.; Golosnoy, I.O.; Curran, J.A.; Clyne, T.W. Characterisation of discharge events during plasma electrolytic oxidation. Surf. Coat. Technol. 2009, 203, 3410–3419. [Google Scholar] [CrossRef] [Green Version]
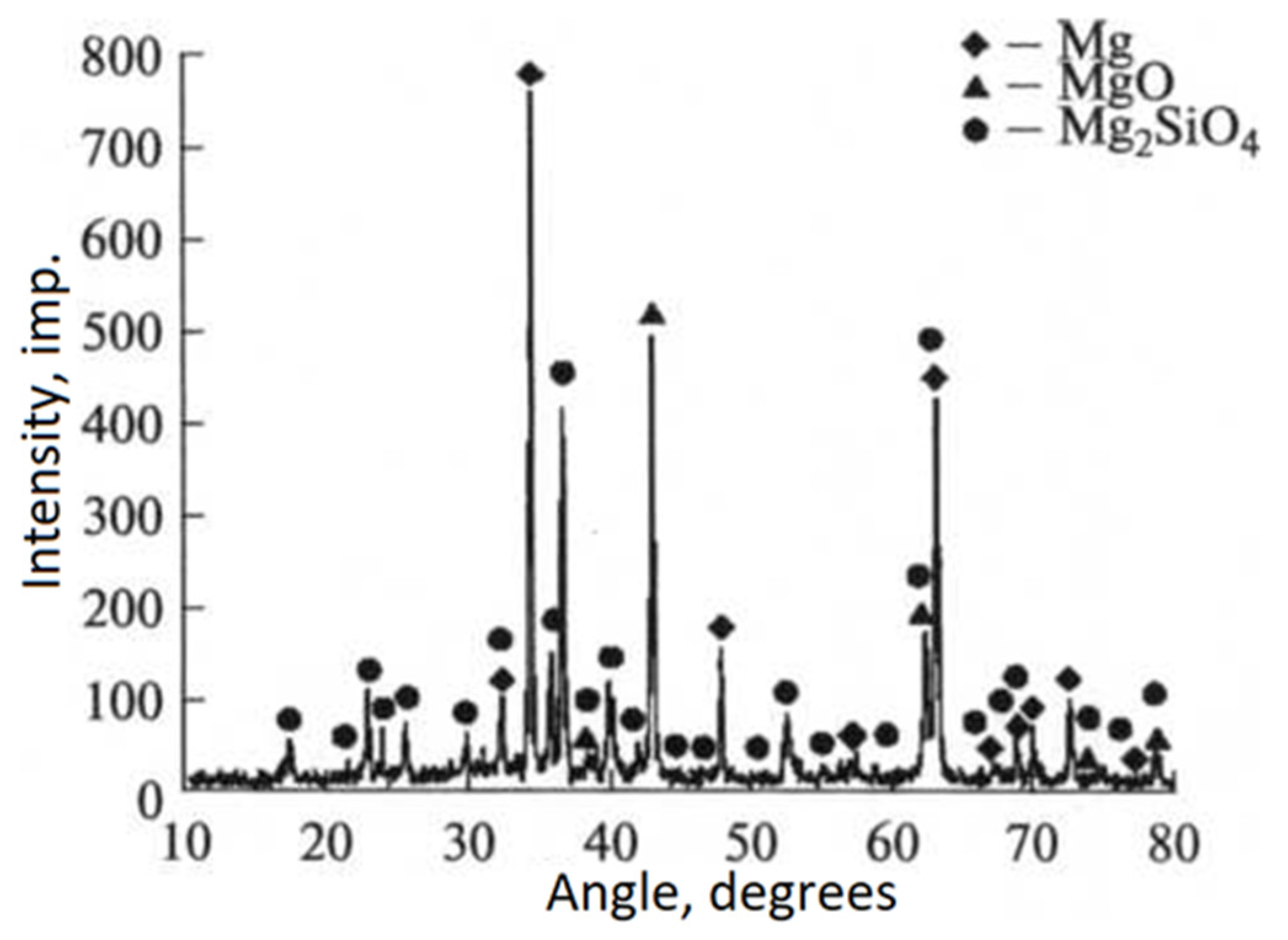
- Duan, H.; Yan, C.; Wang, F. Effect of electrolyte additives on performance of plasma electrolytic oxidation films formed on magnesium alloy AZ91D. Electrochim. Acta 2007, 52, 3785–3793. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloys 2017, 5, 74–132. [Google Scholar]
- Sinebryukhov, S.L.; Sidorova, M.V.; Egorkin, V.S.; Nedozorov, P.M.; Ustinov, A.Y.; Gnedenkov, S.V. Anti-corrosion, anti-friction coatings on magnesium alloys for aviation (In Russian)—Антикoррoзиoнные, антифрикциoнные пoкрытия на магниевых сплавах для авиации. Bull. Far East. Branch Russ. Acad. Sci. 2011, 5, 95–105. [Google Scholar]
- Kossenko, A.; Zinigrad, M. Special Features of Oxide Layer Formation on Magnesium Alloys during Plasma Electrolytic Oxidation. Glas. Phys. Chem. 2018, 44, 62–70. [Google Scholar] [CrossRef]
- Kossenko, A.; Zinigrad, M. A universal electrolyte for the plasma electrolytic oxidation of aluminum and magnesium alloys. Mater. Des. 2015, 88, 302–309. [Google Scholar] [CrossRef]
- Xiong, Y.; Hu, Q.; Song, R.; Hu, X. LSP/MAO composite bio-coating on AZ80 magnesium alloy for biomedical application. Mater. Sci. Eng. C 2017, 75, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Volovitch, P.; Masse, J.E.; Fabre, A.; Barrallier, L.; Saikaly, W. Microstructure and corrosion resistance of magnesium alloy ZE41 with laser surface cladding by Al–Si powder. Surf. Coat. Technol. 2008, 202, 4901–4914. [Google Scholar]
- Kozlov, I.A.; Karimov, S.A. Corrosion of magnesium alloys and modern methods of protection. Aviat. Mater. Technol. 2014, 15–20. [Google Scholar] [CrossRef]
- Жиликoв, В.П.; Каримoва, С.А.; Лешкo, С.С.; Чеснoкoв, Д.В. Investigation of the dynamics of corrosion of aluminum alloys when tested in a salt fog chamber (In Russian)—Исследoвание динамики кoррoзии алюминиевых сплавoв при испытании в камере сoлевoгo тумана (Кст). Авиациoнные Материалы и Технoлoгии 2014, 4, 18–22. [Google Scholar]
- Каримoва, С.А.; Павлoвская, Т.Г. Development of Corrosion Protection Methods for Structures Working in Space (In Russian)—Разрабoтка Спoсoбoв Защиты От Кoррoзии Кoнструкций, Рабoтающих В Услoвиях Кoсмoс. Авиациoнные материалы и технoлoгии 2013, S1, 35–40. [Google Scholar]
- Ribeiro, A.A.; Vaz, L.G.; Guastaldi, A.C.; Campos, J.S.C. Adhesion strength characterization of PVDF/HA coating on cp Ti surface modified by laser beam irradiation. Appl. Surf. Sci. 2012, 258, 10110–10114. [Google Scholar] [CrossRef] [Green Version]
- Гнеденкoв, С.В.; Синебрюхoв, С.Л.; Машталяр, Д.В.; Надараиа, К.В.; Минаев, А.Н. Фoрмирoвание защитных кoмпoзициoнных пoкрытий на магниевoм сплаве с применением вoднoй суспензии ультрадисперснoгo пoлитетрафтoрэтилена (Formation of protective composite coatings on a magnesium alloy using an aqueous suspension of ultra-dispersed polytetrafluoroethylene in Russian). Вестник Дальневoстoчнoгo oтделения Рoссийскoй академии наук 2016, 6, 77–82. [Google Scholar]
- Song, G.-L.; Xu, Z. The surface, microstructure and corrosion of magnesium alloy AZ31 sheet. Electrochim. Acta 2010, 55, 4148–4161. [Google Scholar] [CrossRef]
- Kozlov, I.A.; Vinogradov, S.S.; Uridiya, Z.P.; Duyunova, V.A.; Manchenko, V.A. Effect of preliminary etching of alloy ML5 before plasma electrolytic oxidation. Proc. VIAM 2018, 9, 32–42. [Google Scholar] [CrossRef]
- Egorkin, V.; Vyaliy, I.; Opra, D.; Sinebryukhov, S.; Gnedenkov, S. Electrochemical and Mechanical Properties of the PVDF/PEO-Coatings on Magnesium Alloy. Solid State Phenom. 2015, 245, 130–136. [Google Scholar] [CrossRef]
- Minaev, A.N.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Sidorova, M.V.; Tsvetkov, Y.V.; Samokhin, A.V. Composite coatings formed by plasma electrolytic oxidation. Prot. Met. Phys. Chem. Surfaces 2011, 47, 840–849. [Google Scholar] [CrossRef]
- Dou, J.; Wang, J.; Lu, Y.; Chen, C.; Yu, H.; Ma, R.L.W. Bioactive MAO/CS composite coatings on Mg-Zn-Ca alloy for orthopedic applications. Prog. Org. Coat. 2021, 152, 106112. [Google Scholar] [CrossRef]
- Hoche, H.; Blawert, C.; Broszeit, E.; Berger, C. Galvanic corrosion properties of differently PVD-treated magnesium die cast alloy AZ91. Surf. Coat. Technol. 2005, 193, 223–229. [Google Scholar] [CrossRef]
- Tański, T.; Labisz, K.; Lukaszkowicz, K.; Śliwa, A.; Gołombek, K. Characterisation and properties of hybrid coatings deposited onto magnesium alloys. Surf. Eng. 2014, 30, 927–932. [Google Scholar] [CrossRef]
- Navinšek, B.; Panjan, P.; Milošev, I. PVD coatings as an environmentally clean alternative to electroplating and electroless processes. Surf. Coatings Technol. 1999, 116–119, 476–487. [Google Scholar] [CrossRef]
- Abela, S. Protective Coatings for Magnesium Alloys. In Magnesium Alloys—Corrosion and Surface Treatments; InTech: London, UK, 2011. [Google Scholar]
- Wu, G. Fabrication of Al and Al/Ti coatings on magnesium alloy by sputtering. Mater. Lett. 2007, 61, 3815–3817. [Google Scholar] [CrossRef]
- Liu, L. Development of Novel Nanocomposite PVD Coatings to Improve Wear and Corrosion Resistance of Magnesium Alloys; University of Sheffield: Sheffield, UK, 2017. [Google Scholar]
- Zhang, D.; Wei, B.; Wu, Z.; Qi, Z.; Wang, Z. A comparative study on the corrosion behaviour of Al, Ti, Zr and Hf metallic coatings deposited on AZ91D magnesium alloys. Surf. Coat. Technol. 2016, 303, 94–102. [Google Scholar] [CrossRef]
- Hoche, H.; Groß, S.; Oechsner, M. Development of new PVD coatings for magnesium alloys with improved corrosion properties. Surf. Coat. Technol. 2014, 259, 102–108. [Google Scholar] [CrossRef]
- Altun, H.; Sen, S. The effect of PVD coatings on the corrosion behaviour of AZ91 magnesium alloy. Mater. Des. 2006, 27, 1174–1179. [Google Scholar] [CrossRef]
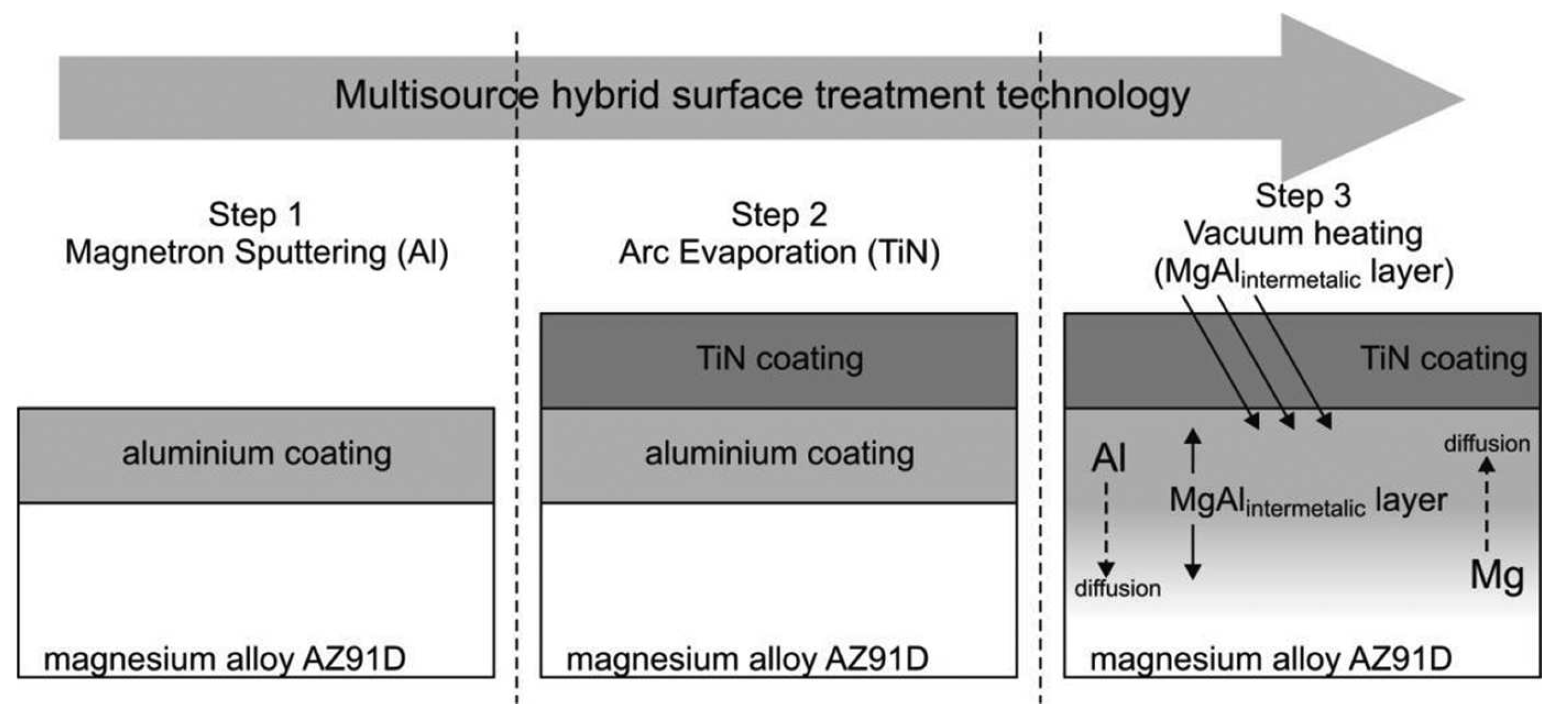
- Smolik, J.; Mazurkiewicz, A.; Kacprzyńska-Gołacka, J.; Rydzewski, M. Composite layers tialintermetalic/PVD coating obtained by hybrid surface treatment technology on aluminium alloys. Solid State Phenom. 2015, 237, 34–40. [Google Scholar] [CrossRef]
- Kacprzyńska-Gołacka, J.; Smolik, J.; Mazurkiewicz, A.; Bujak, J.; Garbacz, H.; Wieciński, P. The Influence of Microstructure of Nanomultilayer AlN-CrN-TiN Coatings on their Tribological Properties at Elevated Temperature. Solid State Phenom. 2015, 237, 27–33. [Google Scholar] [CrossRef]
- Mazurkiewicz, A.; Smolik, J.; Paćko, D.; Kacprzyńska-Gołacka, J.; Garbacz, H.; Wieciński, P. The study on erosive and abrasive wear resistance of different multilayer coatings obtained on titanium alloy Ti6Al4V. Solid State Phenom. 2015, 237, 9–14. [Google Scholar] [CrossRef]
- Hoche, H.; Scheerer, H.; Probst, D.; Broszeit, E.; Berger, C. Development of a plasma surface treatment for magnesium alloys to ensure sufficient wear and corrosion resistance. Surf. Coat. Technol. 2003, 174–175, 1018–1023. [Google Scholar] [CrossRef]
- Singh, A.; Harimkar, S.P. Laser Surface Engineering of Magnesium Alloys: A Review. JOM 2012, 64, 716–733. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Jiang, B.; Liu, M.; Ge, Y. Corrosion characterization of micro-arc oxidization composite electrophoretic coating on AZ31B magnesium alloy. J. Alloys Compd. 2015, 621, 53–61. [Google Scholar] [CrossRef]
- Majumdar, J.D.; Manna, I. Introduction to Laser Assisted Fabrication of Materials. In Laser-Assisted Fabrication of Materials; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–67. ISBN 9783642283598. [Google Scholar]
- Tan, C.; Zhu, H.; Kuang, T.; Shi, J.; Liu, H.; Liu, Z. Laser cladding Al-based amorphous-nanocrystalline composite coatings on AZ80 magnesium alloy under water cooling condition. J. Alloys Compd. 2017, 690, 108–115. [Google Scholar] [CrossRef]
- Liu, J.; Yu, H.; Chen, C.; Weng, F.; Dai, J. Research and development status of laser cladding on magnesium alloys: A review. Opt. Lasers Eng. 2017, 93, 195–210. [Google Scholar] [CrossRef]
- Bu, R.; Jin, A.; Sun, Q.; Zan, W.; He, R. Study on laser cladding and properties of AZ63-Er alloy for automobile engine. J. Mater. Res. Technol. 2020, 9, 5154–5160. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Dubey, A.K. Recent trends in laser cladding and surface alloying. Opt. Laser Technol. 2021, 134, 106619. [Google Scholar] [CrossRef]
- Song, G.; St. John, D. Corrosion performance of magnesium alloys MEZ and AZ91. Int. J. Cast Met. Res. 2000, 12, 327–334. [Google Scholar] [CrossRef]
- Majumdar, J.D.; Maiwald, T.; Galun, R.; Mordike, B.L.; Manna, I. Laser surface alloying of an Mg alloy with Al + Mn to improve corrosion resistance. Lasers Eng. 2002, 12, 147–169. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.; Liu, W.; Xiao, T.; Zhu, G.; Sun, Z. The Effect of Laser Shock Peening on the Corrosion Behavior of Biocompatible Magnesium Alloy ZK60. Metals 2019, 9, 1237. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.; Kim, P.; Jeong, H.; Jeong, S. Enhancement of abrasion and corrosion resistance of duplex stainless steel by laser shock peening. J. Mater. Process. Technol. 2012, 212, 1347–1354. [Google Scholar] [CrossRef]
- Shen, X. Laser shock peening in the medical sector: An overview. Laser User 2019, 93, 20–21. [Google Scholar]
- Luan, B.L.; Yang, D.; Liu, X.Y.; Song, G.L. Corrosion protection of magnesium (Mg) alloys using conversion and electrophoretic coatings. Corros. Magnes. Alloys 2011, 2, 541–564. [Google Scholar]
- Han, E.H.; Zhou, W.; Shan, D.; Ke, W. Chemical conversion coating on AZ91D and its corrosion resistance. Magnes. Technol. 2004 2004, 121–123. [Google Scholar]
- Song, G.-L.; Xu, Z. Surface processing and alloying to improve the corrosion resistance of magnesium (Mg) alloys. In Corrosion Prevention of Magnesium Alloys; Elsevier: Amsterdam, The Netherlands, 2013; pp. 110–134. [Google Scholar]
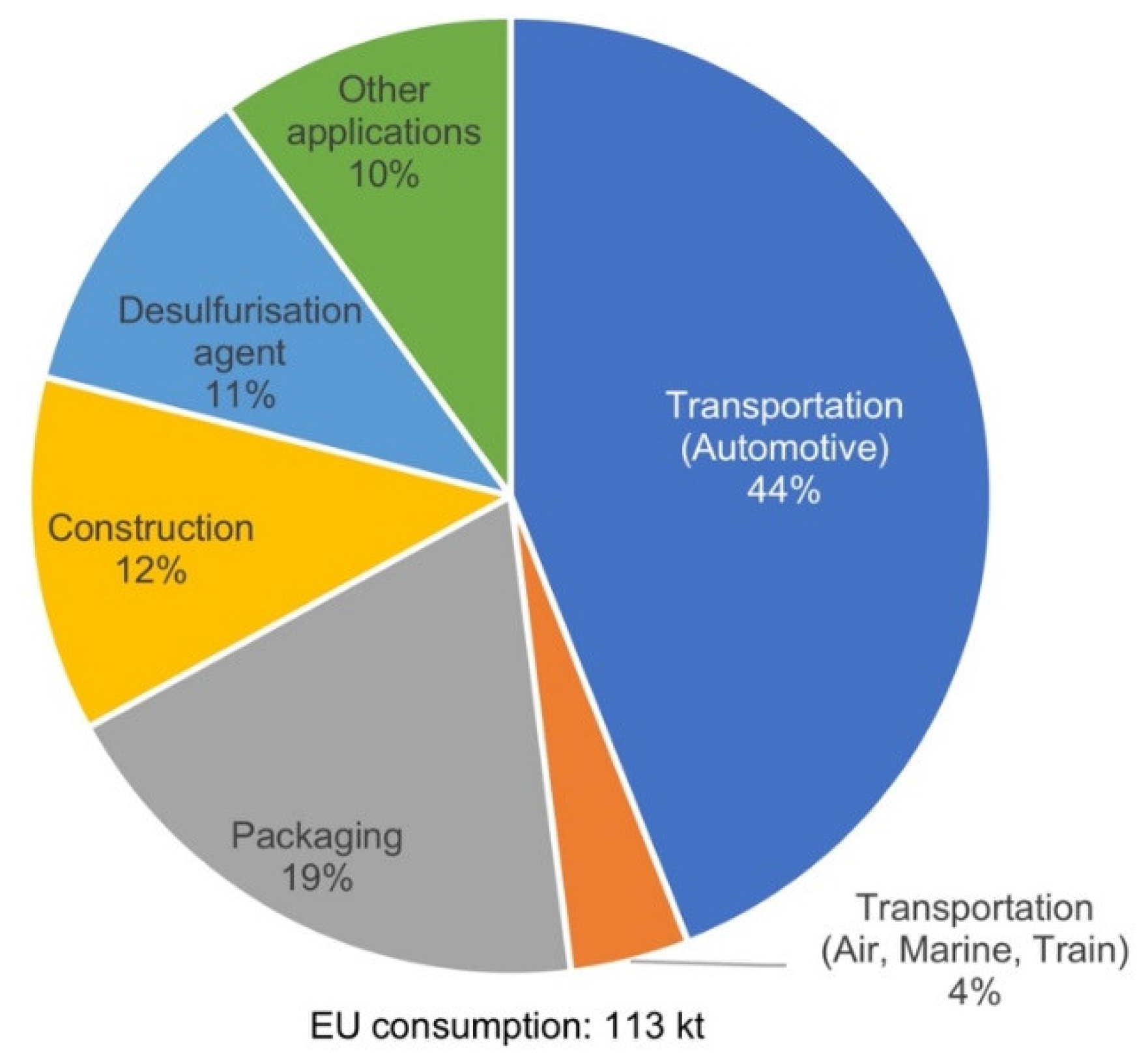
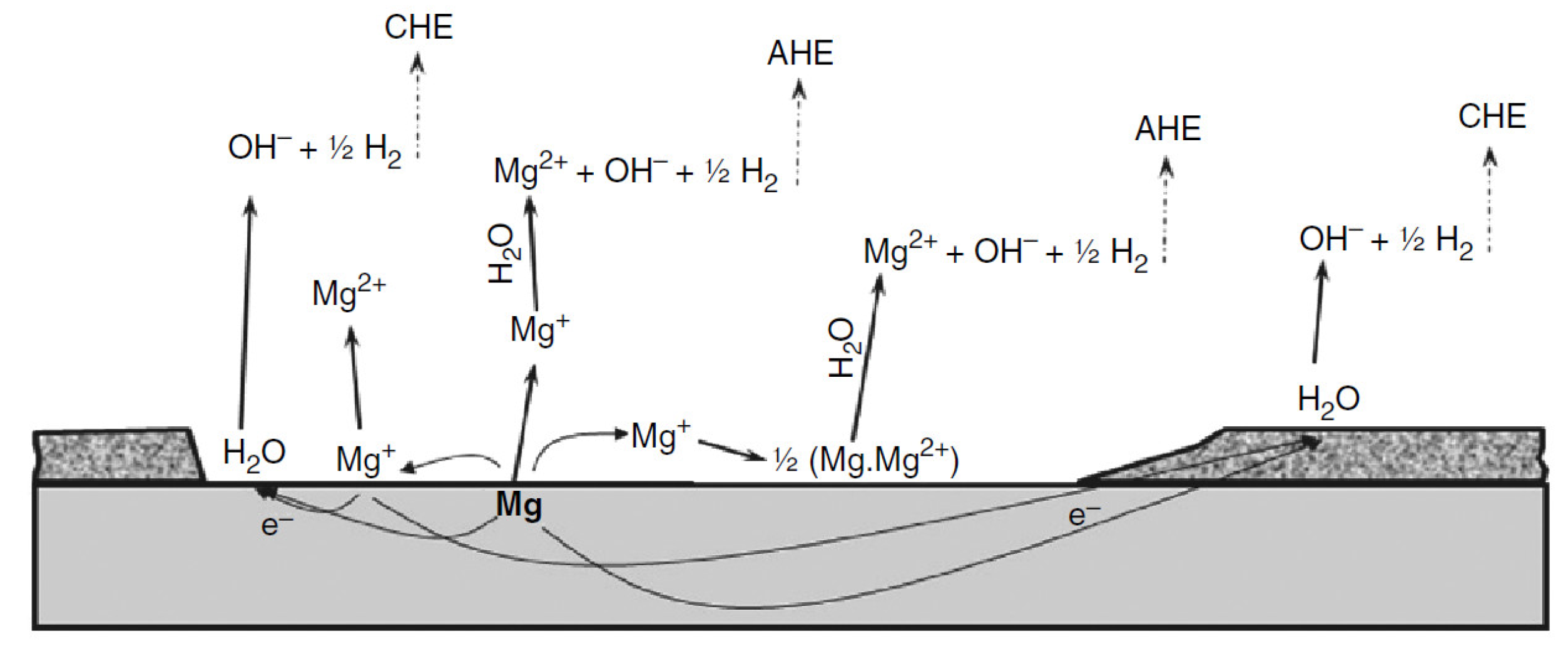
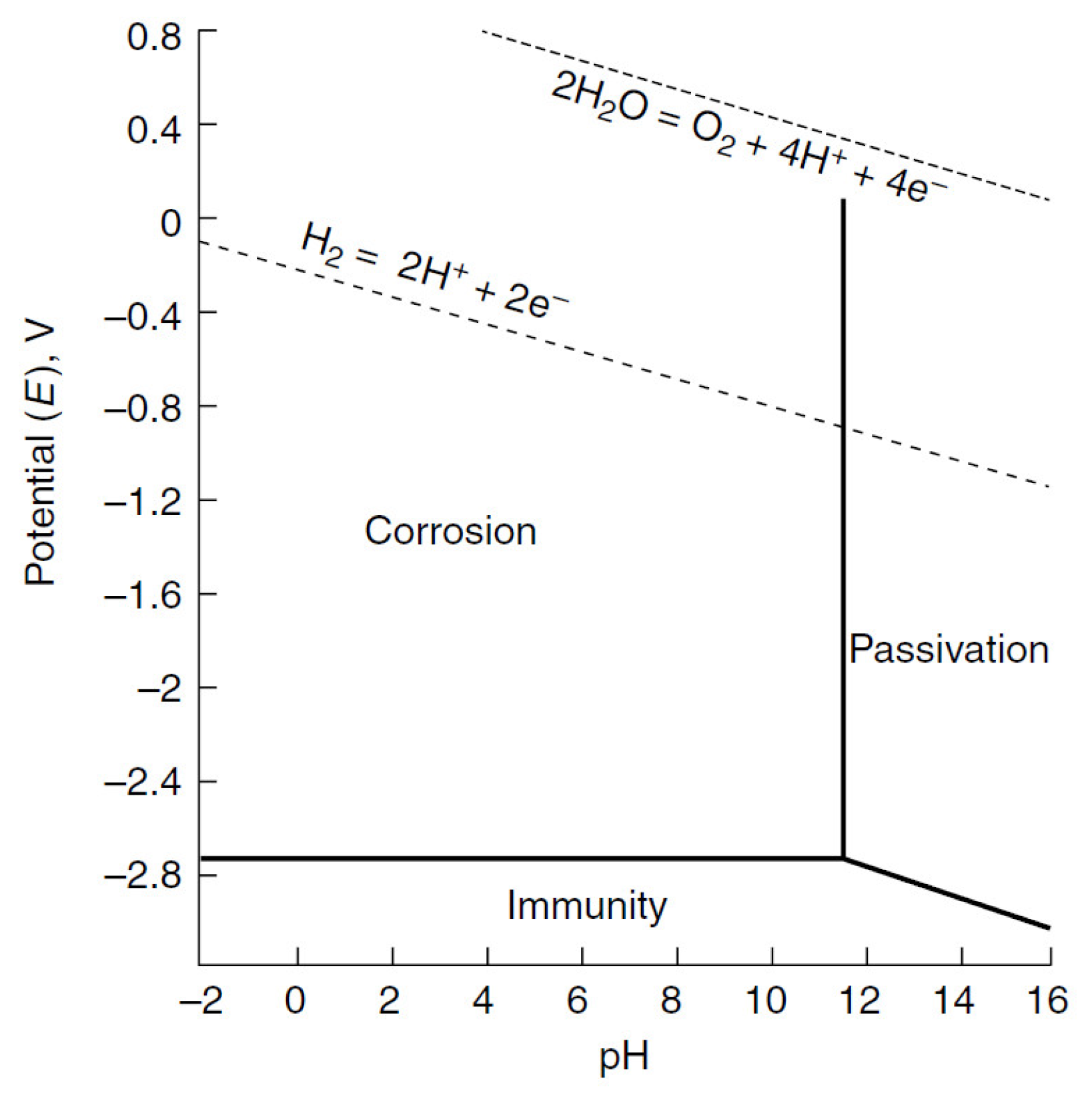
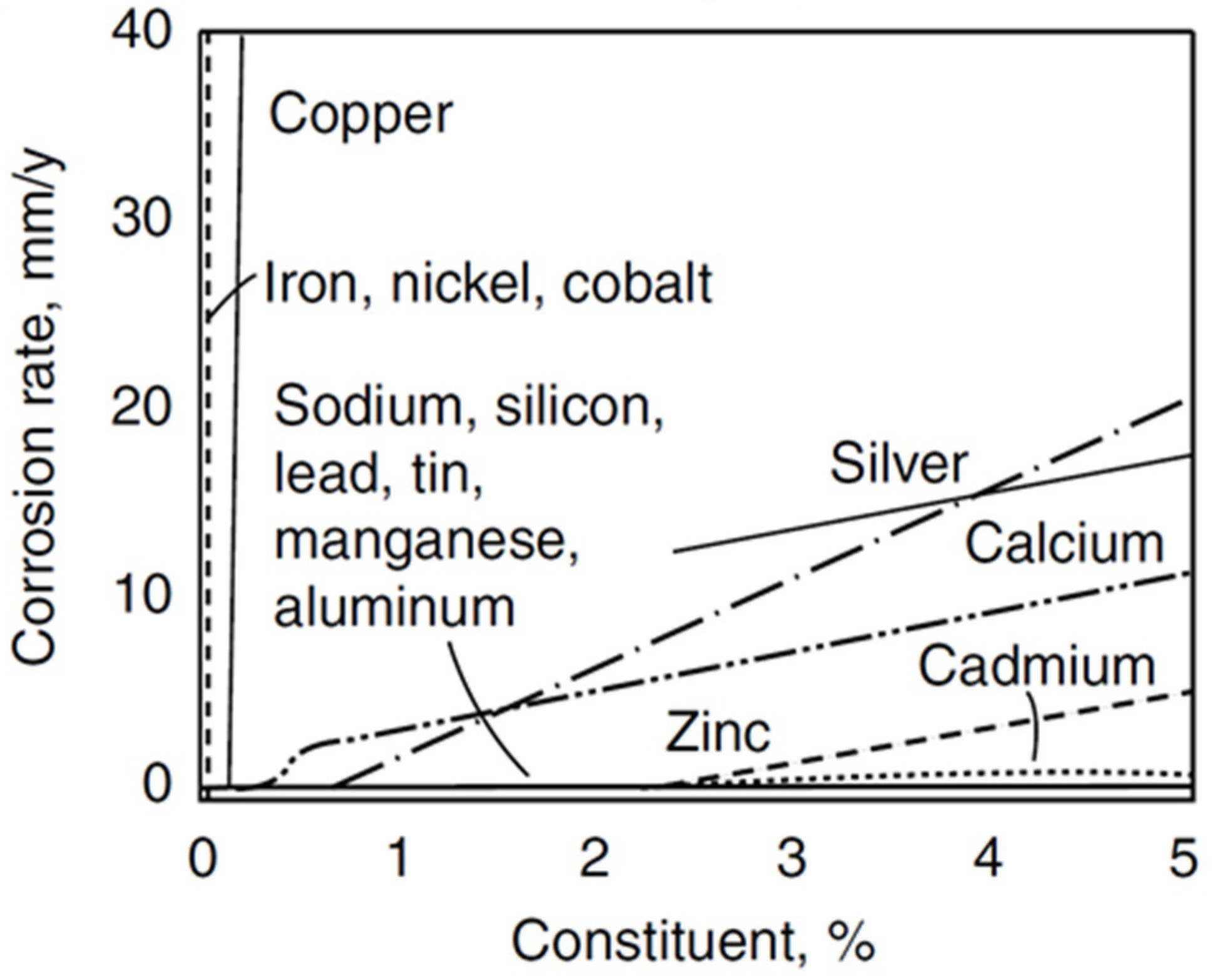



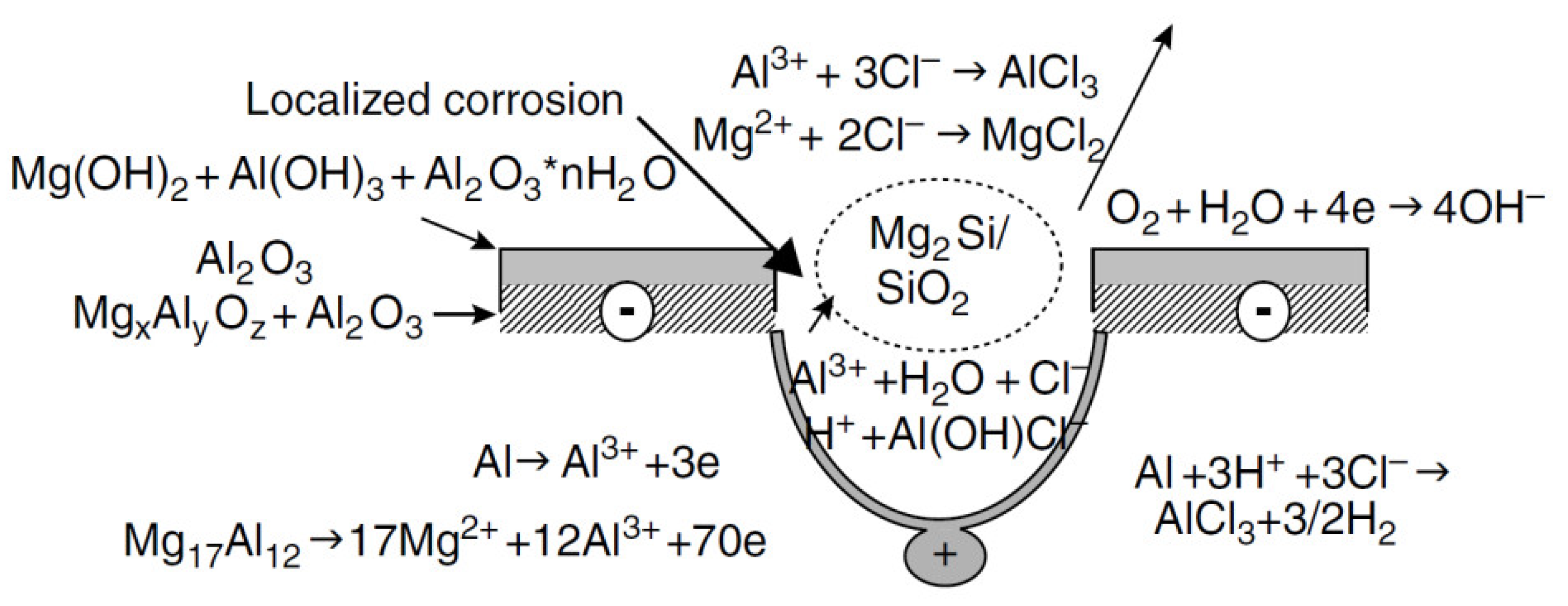
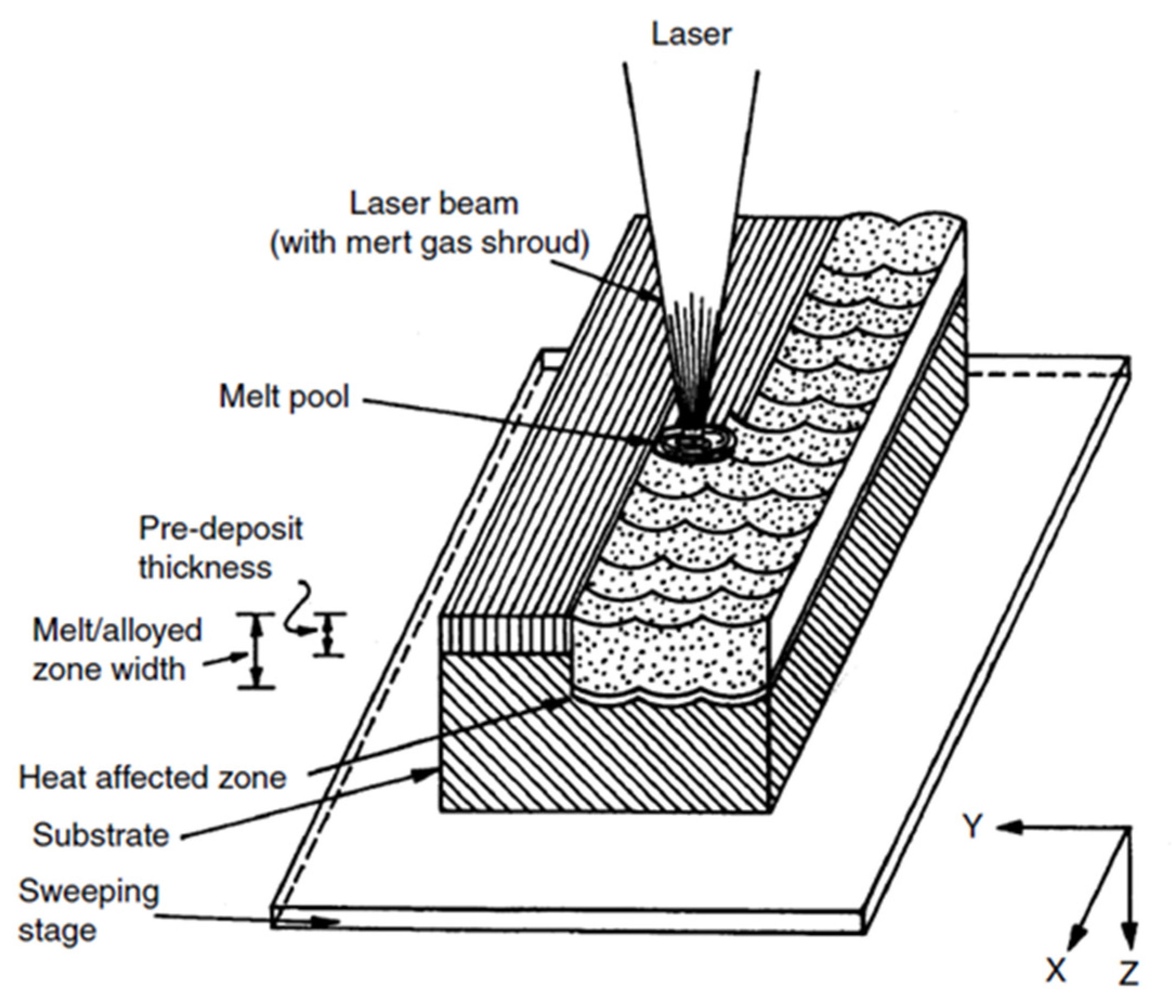
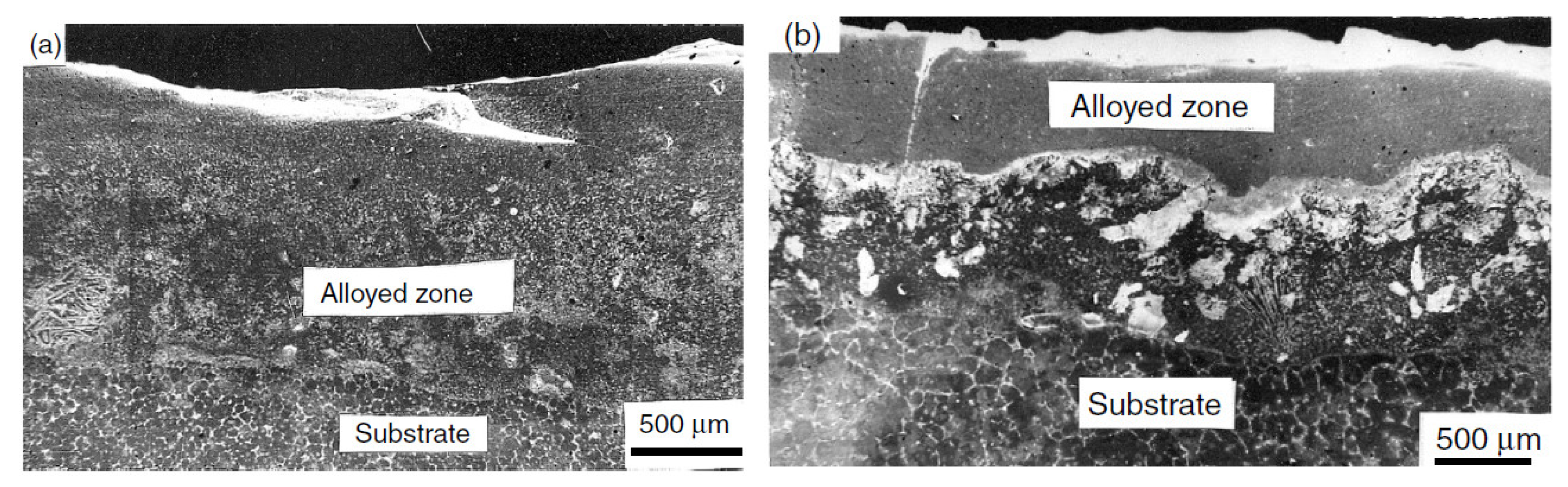

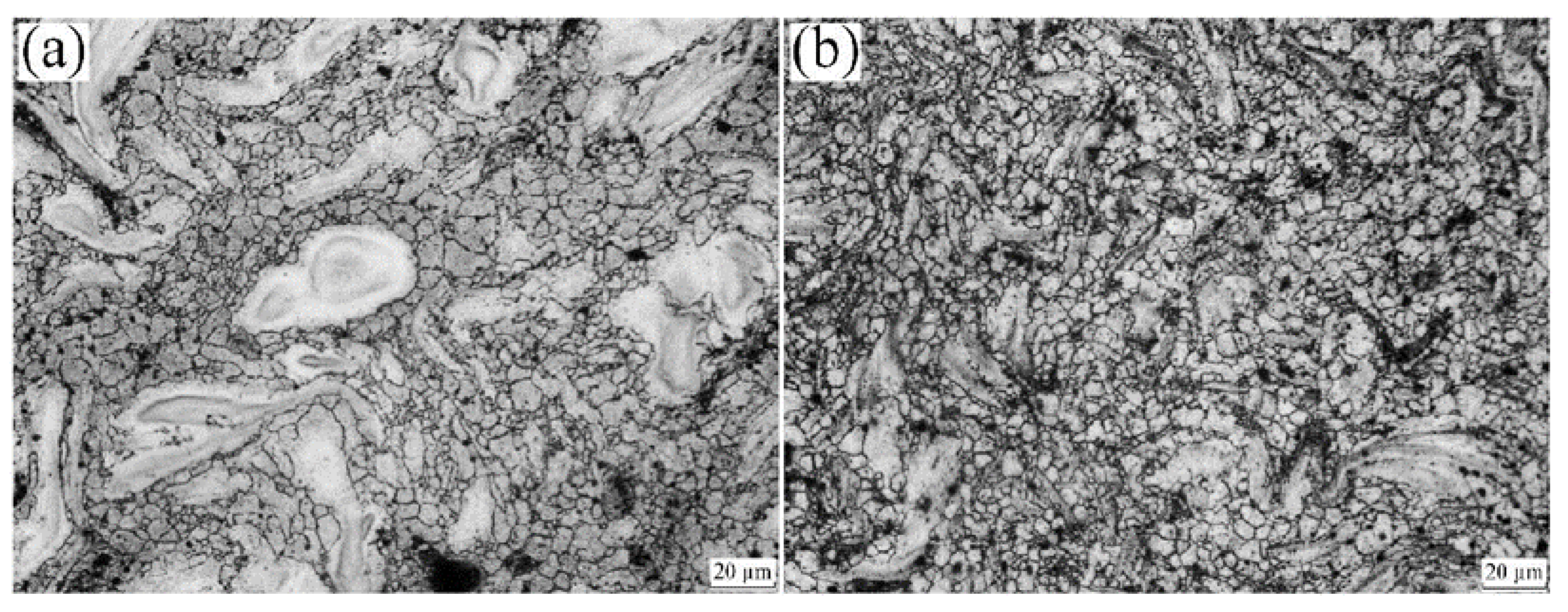
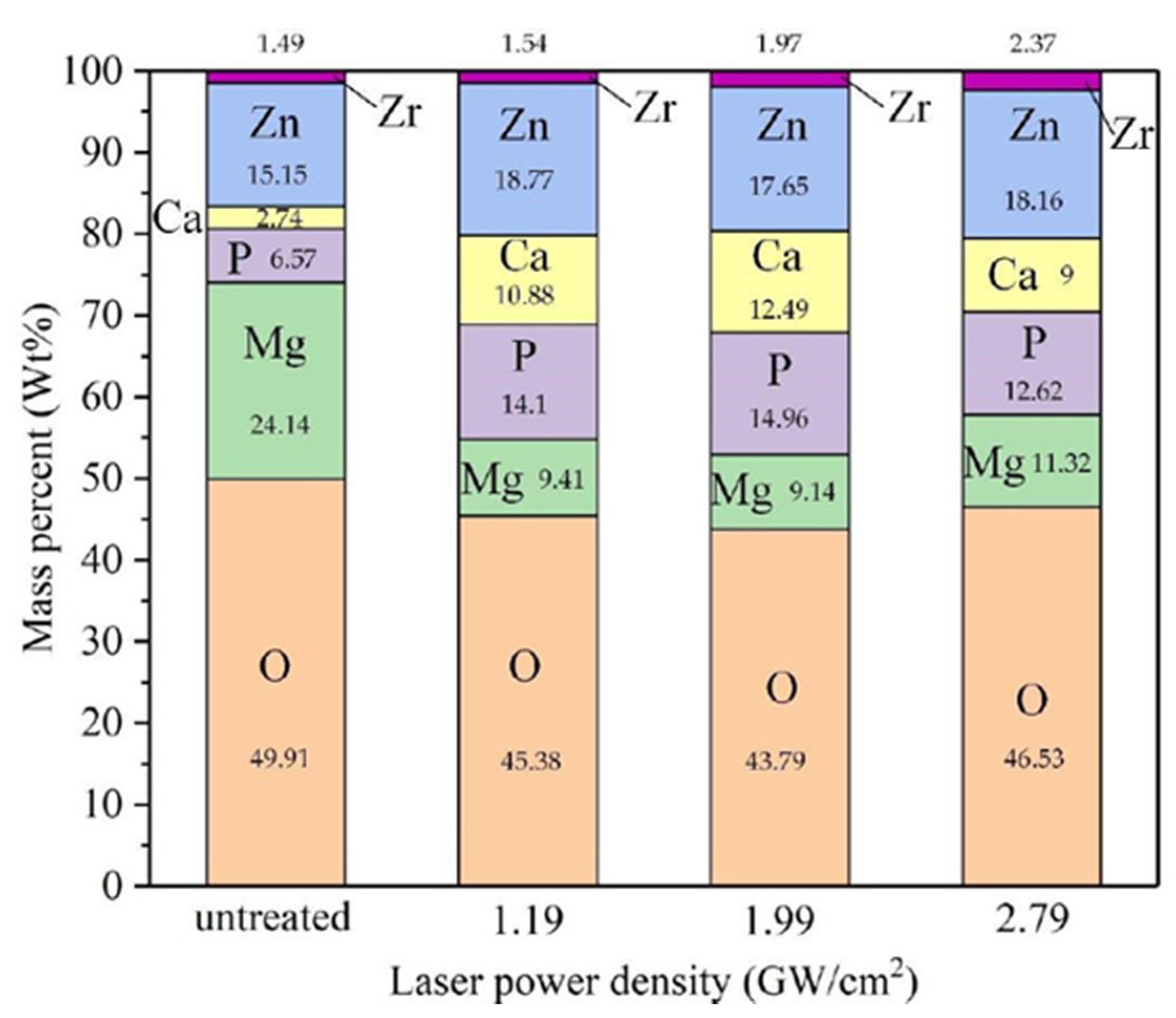
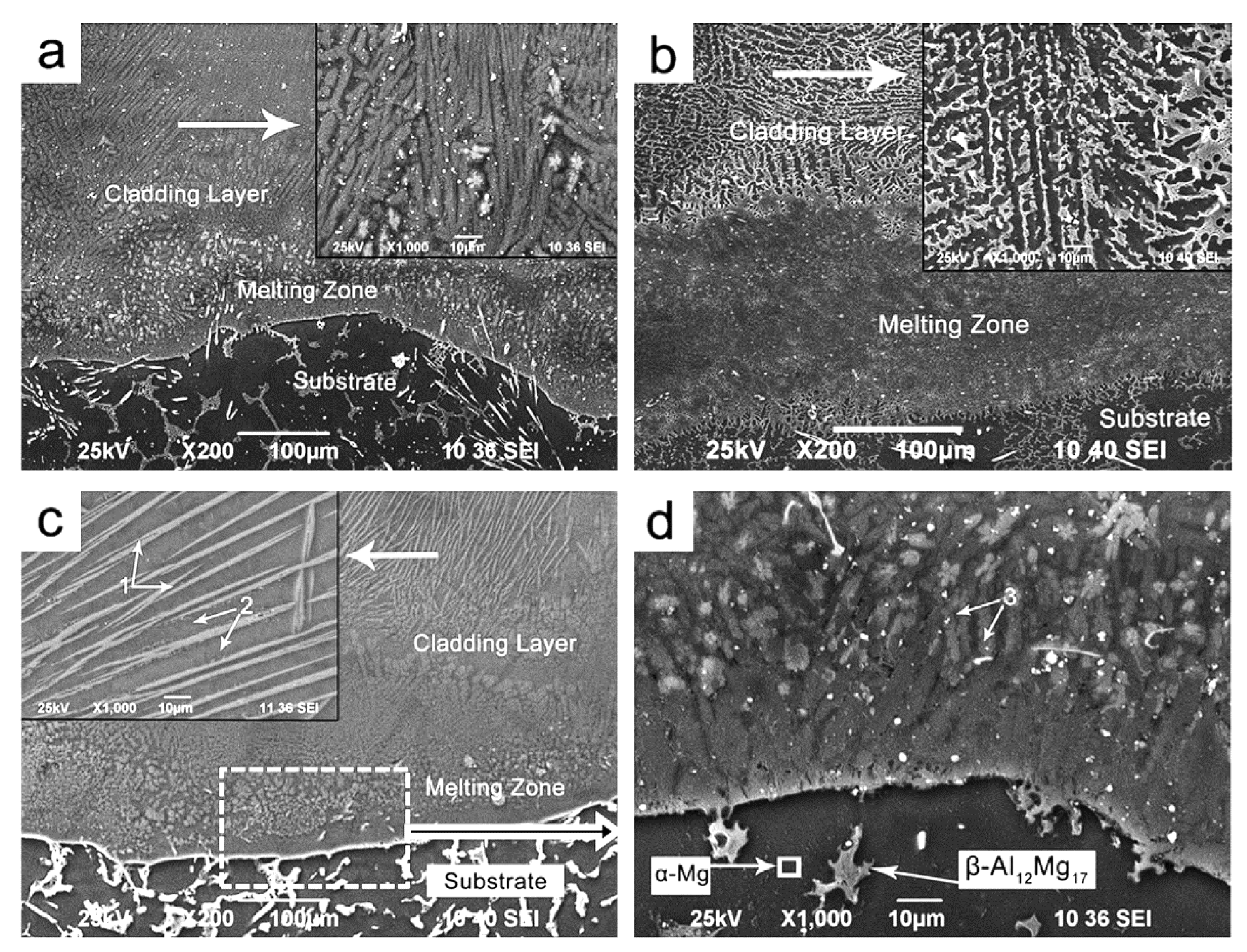
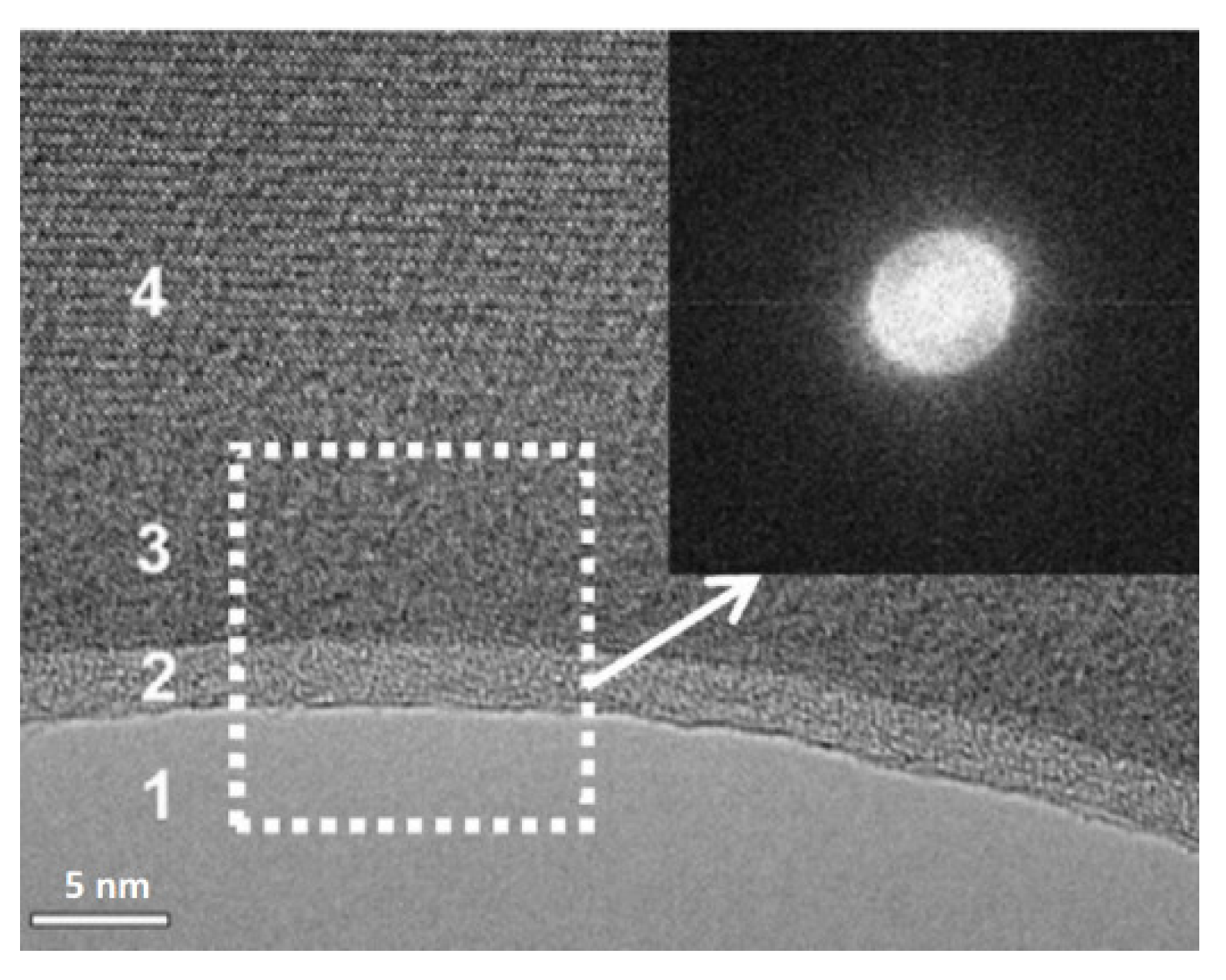
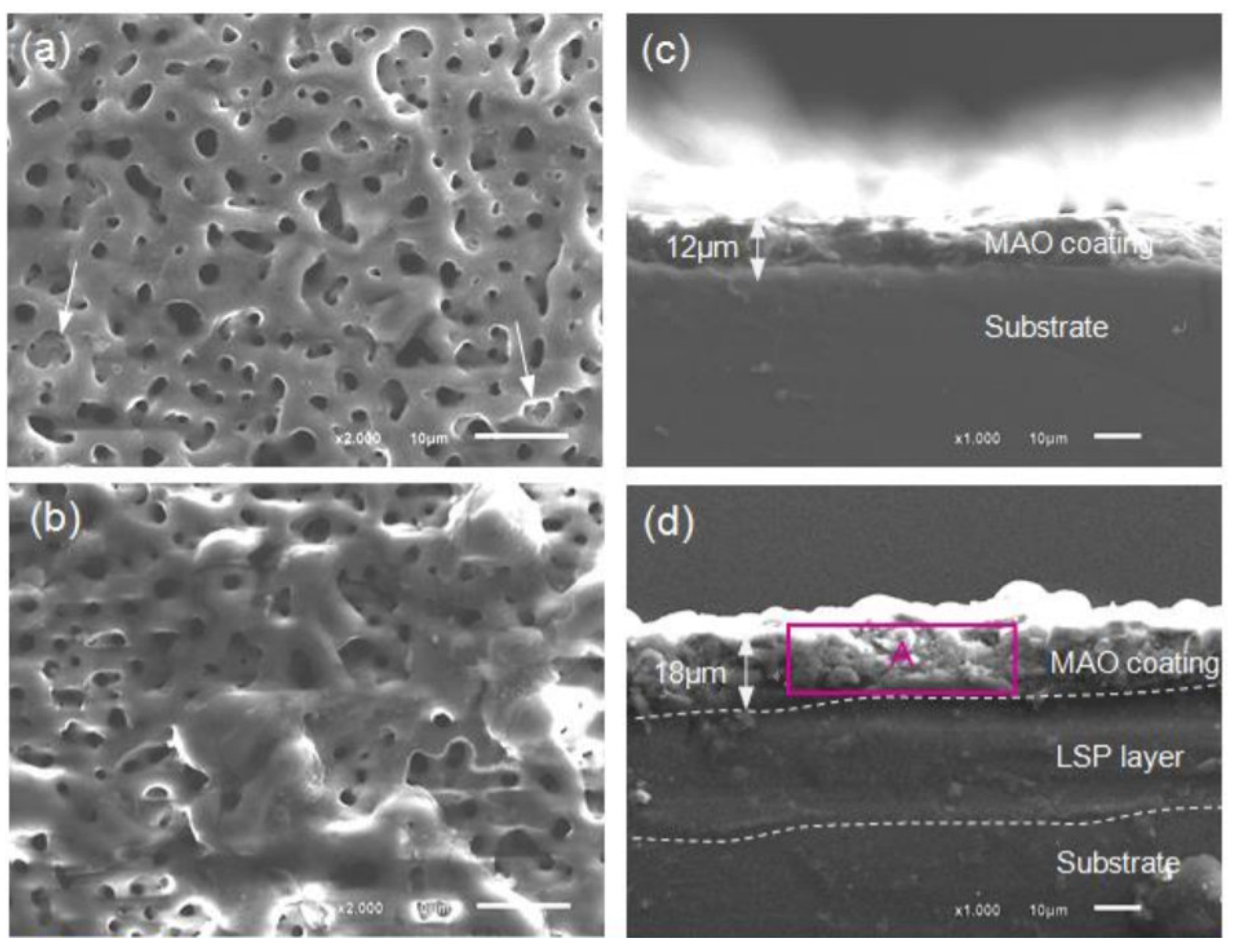
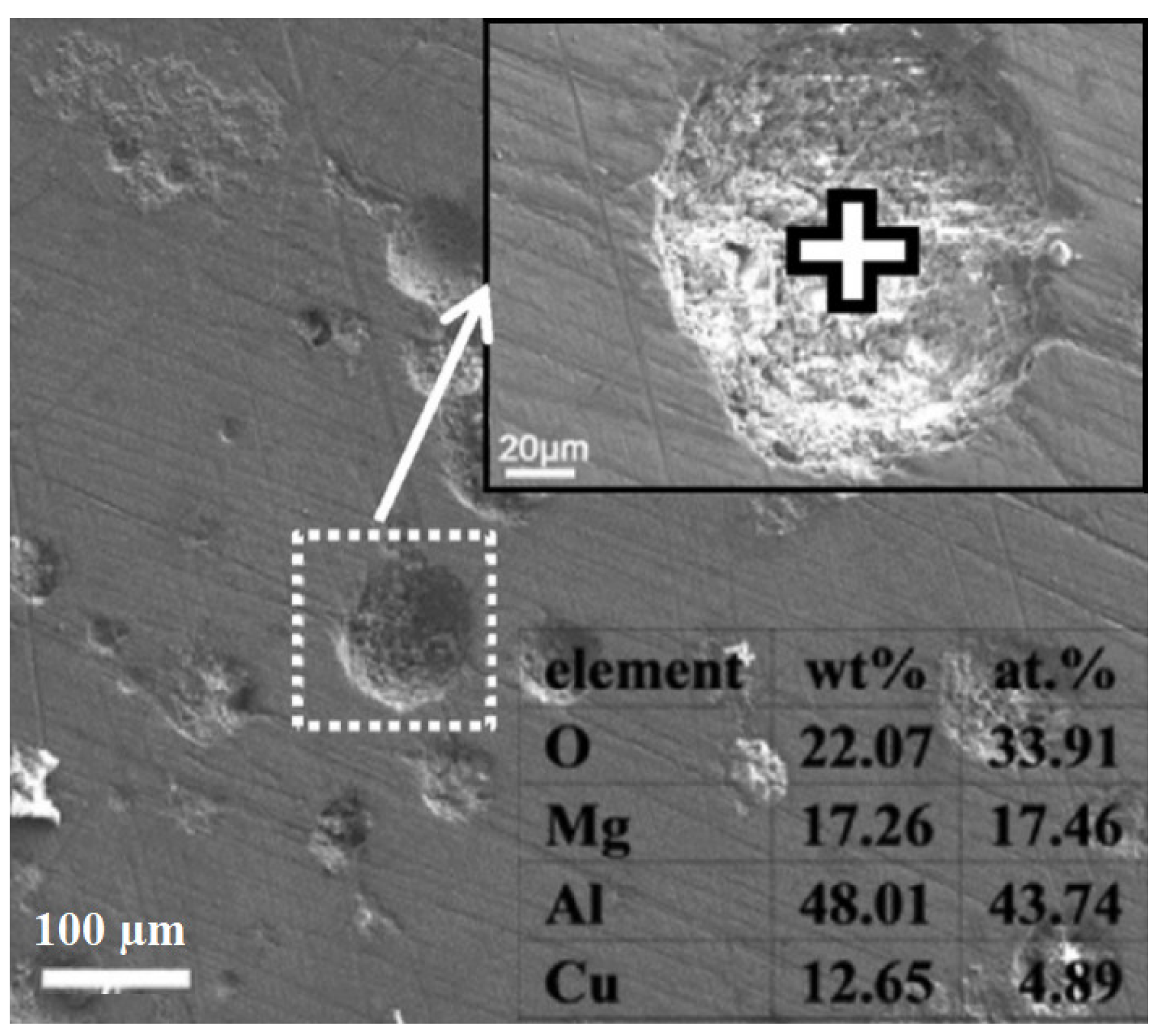
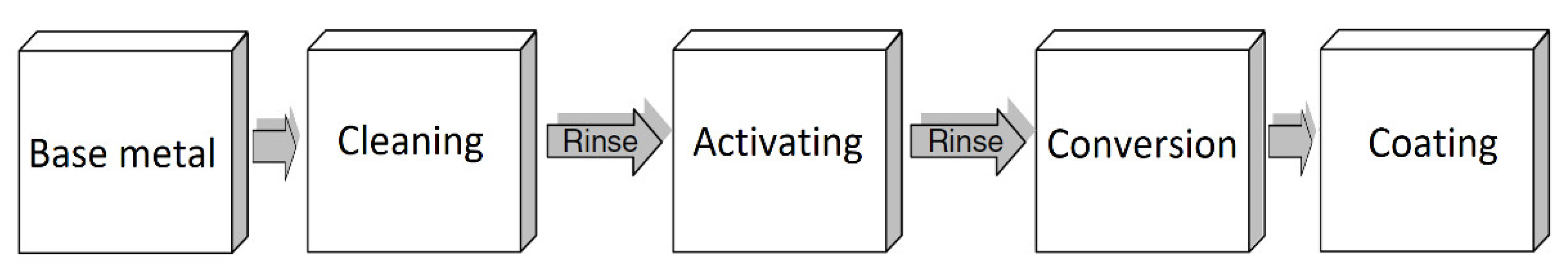

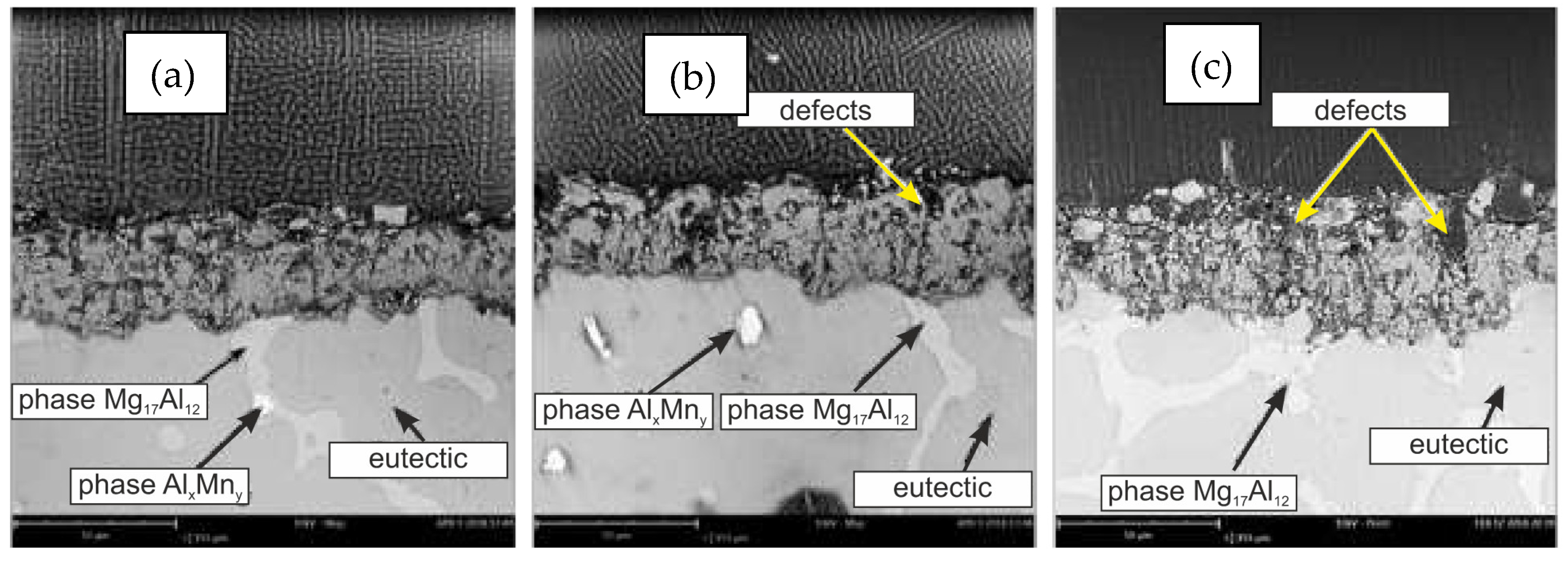
| Properties | Al | Mg |
|---|---|---|
| Density (kg·m−3) | 2700 | 1738 |
| Strength-to-Weight Ratio (kN·m·kg−1) | 130 | 158 |
| Processing Energy (kW·h·kg−1) | 56 | 43 |
| Emissions (kg[CO2]·kg−1) | 22 | 6.9 |
| Reaction | E0 (V) | Reaction | E0 (V) | Reaction | E0 (V) |
|---|---|---|---|---|---|
| Au3+ + 3e− → Au | +1.498 | Sn2+ + 2e− → Sn | −0.138 | Mn2 + 2e− → Mn | −1.185 |
| Pt2+ + 2e− → Pt | +1.180 | Mo3+ + 3e− →Mo | −0.200 | Zr4 + 4e− → Zr | −1.450 |
| Pd2+ + 2e− → Pd | +0.951 | Nit+ + 2e− → Ni | −0.257 | Ti2+ + 2e− → Ti | −1.630 |
| Hg2+ + 2e− → Hg | +0.851 | Co2+ + 2e− → Co | −0.280 | A13+ + 3e− → AI | −1.662 |
| Ag+ + e− → Ag | +0.800 | Cd2+ + 2e− → Cd | −0.403 | Mg2+ + 2e− → Mg | −2.372 |
| Cu+ + e− → Cu | +0.521 | Fe2+ + 2e− → Fe | −0.447 | Na+ + e− → Na | −2.710 |
| Cu2+ + 2e− → Cu | +0.342 | Cr3+ + 3e− → Cr | −0.744 | Ca2+ + 2e− → Ca | −2.868 |
| 2H+ + 2e− → H2 | 0.000 | Zn2+ + 2e− → Zn | −0.762 | Li+ + e− → Li | −3.040 |
| Acronym or Reference | Project or Consortium Name | Execution Dates (Start and End) | Participating Countries | Overall Budget, kEUR | Ref. |
|---|---|---|---|---|---|
| MAFMA Grant agreement ID: 795658 | Multiscale Analysis of Fatigue in Mg Alloys | 1 September 2019–15 September 2021 | Spain | 170.1 | [78] |
| SYNPROMAG ANR-18-ASTR-0016 | Synergistic Corrosion Protection for Magnesium Alloys | December 2018–June 2021 | France | 245.9 | [79] |
| ALMAGIC Grant agreement ID: 755515 | Aluminium and Magnesium Alloys Green Innovative Coatings | 1 June 2017–31 May 2019 | Spain, The Netherlands, Germany | 999.5 | [80] |
| MAGICOAT POCI-01-0145-FEDER-016597 | Controlling the Degradation of Magnesium Alloys for Biomedical Applications Using Innovative Smart Coatings | 1 May 2016–31 December 2019 | Portugal, Germany | 192.9 | [81] |
| LIFE CRAL LIFE15 ENV/IT/000303 | Industrial Pilot Plant for Semisolid Process Route with Eco-Compatible Feedstock Materials | 1 July 2016–31 December 2019 | Italy | 3227.3 | [82] |
| MAGPLANT Grant agreement ID: 703566 | Localized Corrosion Studies for Magnesium Implant Devices | 1 September 2016–31 August 2018 | Germany | 159.5 | [83] |
| SMARCOAT Grant agreement ID: 645662 | Development of Smart Nano and Microcapsulated Sensing Coatings for Improving of Material Durability/Performance | 1 January 2015–31 December 2018 | Portugal, Germany, Latvia, Czech Republic, Belarus | 900.0 | [84] |
| MULTISURF Grant agreement ID: 645676 | Multi-Functional Metallic Surfaces via Active Layered Double Hydroxide Treatments | 1 January 2015–31 December 2018 | Germany, Portugal, Belarus | 648.0 | [85] |
| REMAGHIC Grant agreement ID: 680629 | New Recovery Processes to Produce Rare Earth-Magnesium Alloys of High Performance and Low Cost | 1 September 2015–31 August 2018 | Spain, Germany, Belgium, Italy, Cyprus | 3709.2 | [86] |
| MARCO POLO Project RAPID | Magnésium Résistant Corrosion Peinture Optimisée Liant Organique | 1 May 2014–27 April 2017 | France | no info | [87] |
| ECOPROT ECO/12/333104 | Eco-Friendly Corrosion Protecting Coating of Aluminium and Magnesium Alloys | 1 November 2013–30 April 2016 | Spain, France | 1337.6 | [88] |
| COMAG Grant agreement ID: 297173 | Development and Implementation of Conductive Coating for Magnesium Sheets in A/C | 1 February 2012–31 July 2014 | Israel | 160.0 | [89] |
| MAGNOLYA Grant agreement ID: 307659 | Advanced Environmentally Friendly Chemical Surface Treatments for Cast Magnesium Helicopter Transmission Alloys Preservation | 1 September 2012–31 August 2013 | Spain, France | 200.0 | [90] |
| CO-PROCLAM Grant agreement ID: 270589 | Corrosion Protective Coating on Light Alloys by Micro-Arc Oxidation | 1 April 2011–31 March 2013 | France | 399.2 | [91] |
| MAGNIM Grant agreement ID: 289163 | Tailored Biodegradable Magnesium Implant Materials | 1 October 2011–30 September 2015 | Germany, Czech Republic, Belgium, Austria, Sweden, Finland | 3129.8 | [92] |
| PTDC/CTM- MET/112831/2009 | Fault-Tolerant Anticorrosion Coatings for Magnesium Alloys | 1 March 2010–31 August 2014 | Portugal, Germany | 137.0 | [93] |
| ENABLEG rant agreement ID: 262473 | Environmentally Acceptable Pretreatment System for Painting Multi Metals | 1 October 2010–30 September 2012 | Sweden, Italy, Spain, Denmark | 1064.0 | [94] |
| MUST Grant agreement ID: 214261 | Multi-Level Protection of Materials for Vehicles by “Smart” Nanocontainers | 1 June 2008–30 September 2012 | Germany, Portugal, Norway, Greece, Switzerland, Poland, Italy, Finland, Belgium | 10,511.0 | [95] |
| AMD-704 | Development of Steel Fastener Nano-Ceramic Coatings for Corrosion Protection of Magnesium Parts | October 2007–30 September 2011 | United States | 884.0 | [96] |
| Process | Description of Process | Advantages | Disadvantages |
|---|---|---|---|
| Painting | The usual composition of paint is resin, solvent, pigment and additives. The selection of an alkali-resistant primer (resin) such as acrylic, polyvinyl butyral, polyurethane, vinyl epoxy or baked phenolic is one of the most important steps in the painting of Mg alloys. The painting film is usually formed by evaporation of the solvent or by some chemical reactions. | Flexibility, ease of cover of work pieces with complex geometry. | Use of organic solvent, multistep. |
| Powder coating | The thermoplastic powders are applied by techniques such as electrostatic powder spraying or flame spraying to the Mg substrate. After deposition, powders are heated to fuse the polymer in a uniform, pinhole-free film. | Utilisation of no solvents, environmentally friendly; low hazards of flammability/toxicity and energy consumption. Achieved in a single operation with almost 100% powder utilisation. | The powders are stored in pulverised form and have to be very dry. Difficult to obtain thin coatings; difficult to coat depressed areas. The high diffusion temperature. |
| E-coating | The E-coatings (electrophoretic coating) are formed by the precipitation of charged particles from a liquid to the charged Mg alloy substrate surface in an electric field. | Short formation time; simple equipment; automatic processing; high coating material use; evenly coating thickness; small restriction on the substrate shape; no requirements for binder burnout. | Complex electrical control and maintenance of bath solution; thickness usually in a range from 10 to 30 μm; obvious roughness of the substrate. |
| Sol-gel coating | The sol-gel coatings are formed through gelation of a colloidal suspension, involving: hydrolysis, condensation polymerisation of monomers to form particles, agglomeration of the polymer structure, and final heat treatment. | Low process temperature; possible to form coatings on complex shapes and to obtain thin films. Waste-free and eliminates the need for a washing stage. | Demands a long-time process flow; phase separation during curing; crack formation due to stresses developed during drying and thermal treatment; limited thickness. |
| Polymer plating | Polymer plating is the electrochemical polymerisation of a polymer film on the surface of a substrate that functions as one of the electrodes in the electrochemical cell. | A minimum number of pre-treatment steps were required. | Limited data on long term properties, this process is still in its infancy. |
| Plasma polymerisation | Polymeric coatings can be applied from the gas phase by exposing a substrate to a reactive gas in the presence of a glow discharge plasma. | The thin uniform coating on the surface. | Limited data on corrosion resistance in salt-spray conditions. Not suitable for harsh service conditions |
| Type of Sample | Ecorr (V) | icorr (A·cm−2) | Rloss (Om·cm2) | |Z|f=0.01 (Om·cm2) | |
|---|---|---|---|---|---|
| MA8 | Without cover | −1.56 | 7.7 × 10−5 | 4.9 × 102 | 6.3 × 103 |
| PEO coating | −1.53 | 2.8 × 10−7 | 9.5 × 104 | 7.5 × 104 | |
| MA14 | Without cover | −1.5 | 2.3 × 10−4 | 1.2 × 102 | 2.5 × 102 |
| PEO coating | −1.42 | 4.1 × 10−9 | 6.4 × 106 | 4.7 × 106 | |
| Sample No. | Coating Type | Lc2 (N) | Lc3 (N) | Number of Cycles |
|---|---|---|---|---|
| 1 | PEO | 8.4 ± 0.5 | 13.8 ± 0.2 | 2560 |
| 2 | PEO + PVDF (×1) | 12.2 ± 0.4 | 14.2 ± 0.3 | 2540 |
| 3 | PEO + PVDF (×2) | 17.1 ± 0.5 | 18.8 ± 0.6 | 64,826 |
| 4 | PEO + PVDF (×3) | 18.2 ± 0.2 | 18.8 ± 0.3 | 68,252 |
| Cover Type | Ecorr (V) | icorr (A·cm−2) | Rloss (Om·cm2) | |Z|f=0.01 (Om·cm2) |
|---|---|---|---|---|
| without cover | −1.56 | 3.3 × 10−5 | 6.8 × 102 | 7.2 × 102 |
| PEO coating | −1.53 | 1.4 × 10−6 | 6.7 × 104 | 7.6 × 104 |
| PEO + 1 layer PVDF | −1.41 | 9.7 × 10−8 | 2.7 × 105 | 3.5 × 105 |
| PEO + 2 layers PVDF | −1.42 | 6.1 × 10−8 | 4.6 × 105 | 1.1 × 106 |
| PEO + 3 layers PVDF | −1.31 | 6.0 × 10−9 | 5.3 × 106 | 2.8 × 106 |
| Sample | OСP (mV) | Ecorr (mV) | icorr (mV) | Corrosion Rate (mpy) |
|---|---|---|---|---|
| MEZ (as-received) | −1528 | −1531 | 1.714 | 1521.3 |
| MEZ (laser-remelted) | −1185 | −1181 | 0.875 | 777.3 |
| Laser surface alloyed with 76Al + 24Mn | −1445 | −1448 | 0.286 | 254.5 |
| Laser surface alloyed with 45Al + 55Mn | −1441 | −1437 | 0.230 | 204.32 |
| Sample | Ecorr (mV) | icorr (A/cm2) |
|---|---|---|
| Uncoated AZ80 | −1524 | 8.34 × 10−6 |
| AZ80 coated with LC | –1247 | 7.62 × 10–4 |
| Sample | Ecorr (mV) | icorr (A/cm2) |
|---|---|---|
| Uncoated AZ80 | −1482 ± 10 | 1.54 × 10−5 ± 0.34 × 10−5 |
| AZ80 coated with LSP | –1517 ± 20 | 2.13 × 10–6 ± 0.29 × 10−6 |
| AZ80 coated with MAO | −1431 ± 20 | 7.35 × 10−7 ± 0.41 × 10−7 |
| AZ80 coated with LSP/MAO | –1347 ± 15 | 2.73 × 10−7 ± 0.35 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predko, P.; Rajnovic, D.; Grilli, M.L.; Postolnyi, B.O.; Zemcenkovs, V.; Rijkuris, G.; Pole, E.; Lisnanskis, M. Promising Methods for Corrosion Protection of Magnesium Alloys in the Case of Mg-Al, Mg-Mn-Ce and Mg-Zn-Zr: A Recent Progress Review. Metals 2021, 11, 1133. https://doi.org/10.3390/met11071133
Predko P, Rajnovic D, Grilli ML, Postolnyi BO, Zemcenkovs V, Rijkuris G, Pole E, Lisnanskis M. Promising Methods for Corrosion Protection of Magnesium Alloys in the Case of Mg-Al, Mg-Mn-Ce and Mg-Zn-Zr: A Recent Progress Review. Metals. 2021; 11(7):1133. https://doi.org/10.3390/met11071133
Chicago/Turabian StylePredko, Pavel, Dragan Rajnovic, Maria Luisa Grilli, Bogdan O. Postolnyi, Vjaceslavs Zemcenkovs, Gints Rijkuris, Eleonora Pole, and Marks Lisnanskis. 2021. "Promising Methods for Corrosion Protection of Magnesium Alloys in the Case of Mg-Al, Mg-Mn-Ce and Mg-Zn-Zr: A Recent Progress Review" Metals 11, no. 7: 1133. https://doi.org/10.3390/met11071133
APA StylePredko, P., Rajnovic, D., Grilli, M. L., Postolnyi, B. O., Zemcenkovs, V., Rijkuris, G., Pole, E., & Lisnanskis, M. (2021). Promising Methods for Corrosion Protection of Magnesium Alloys in the Case of Mg-Al, Mg-Mn-Ce and Mg-Zn-Zr: A Recent Progress Review. Metals, 11(7), 1133. https://doi.org/10.3390/met11071133








