On the Unique Microstructure and Properties of Ultra-High Carbon Bearing Steel Alloyed with Different Aluminum Contents
Abstract
:1. Introduction
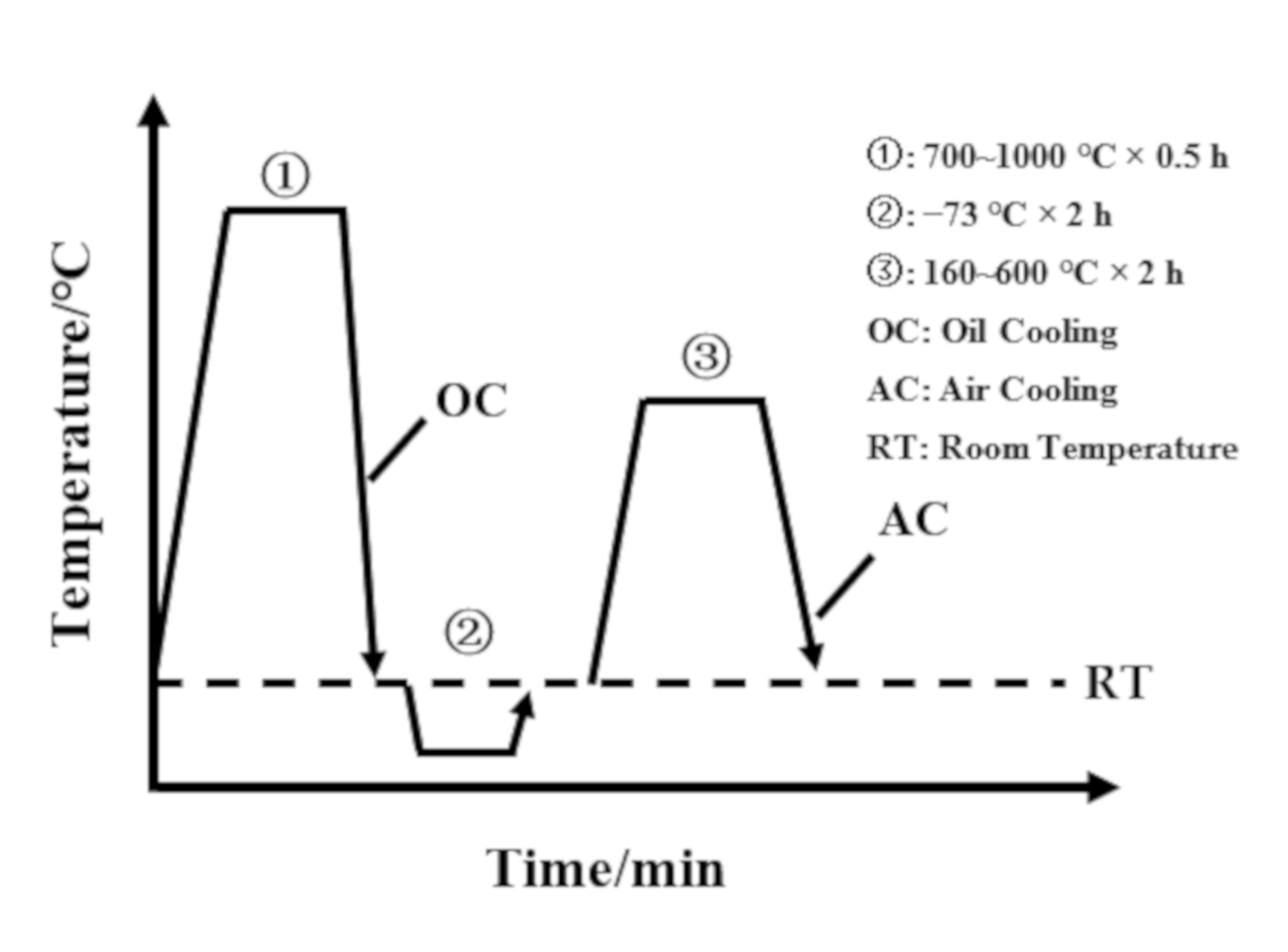
2. Experimental
3. Results and Discussion
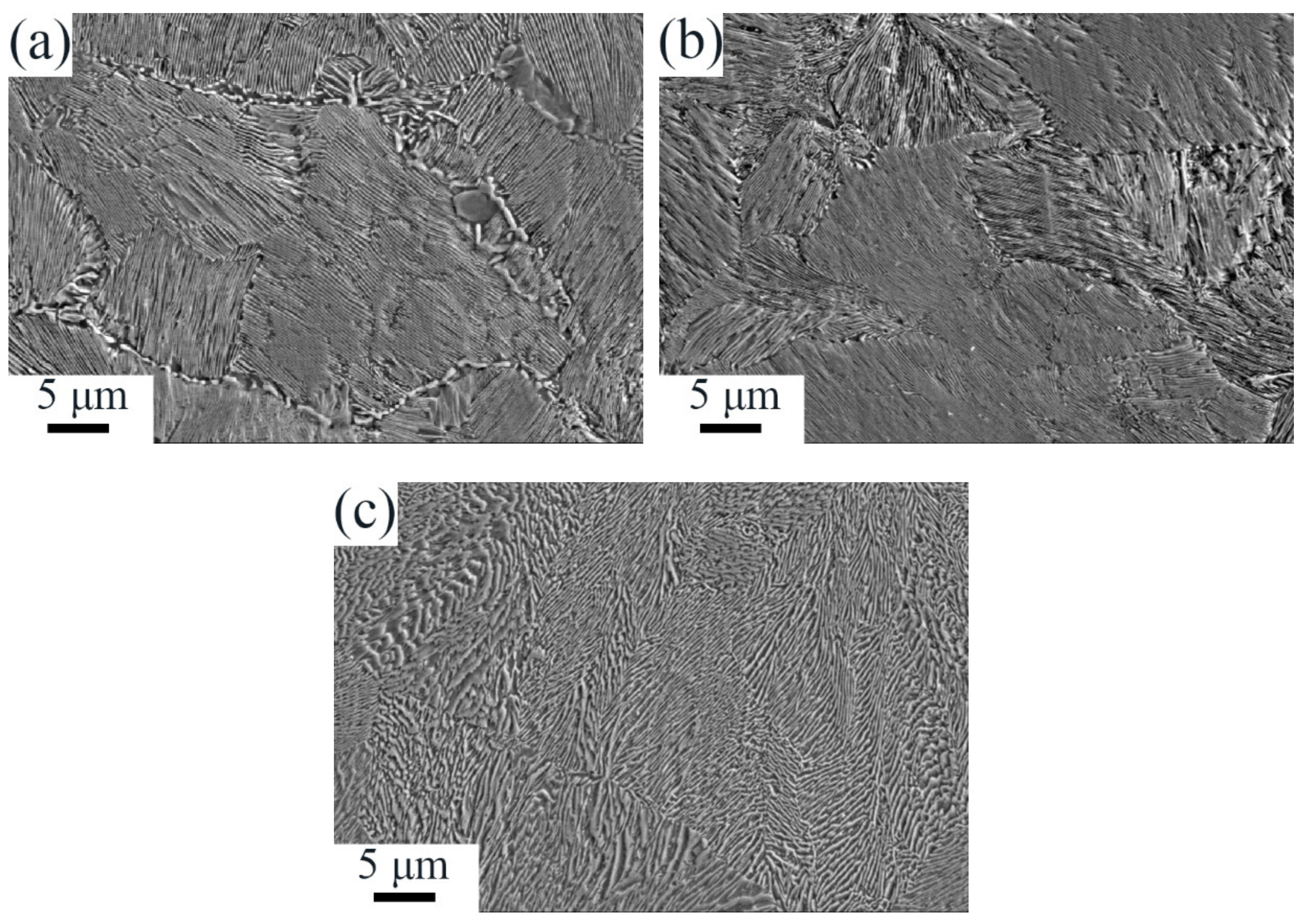
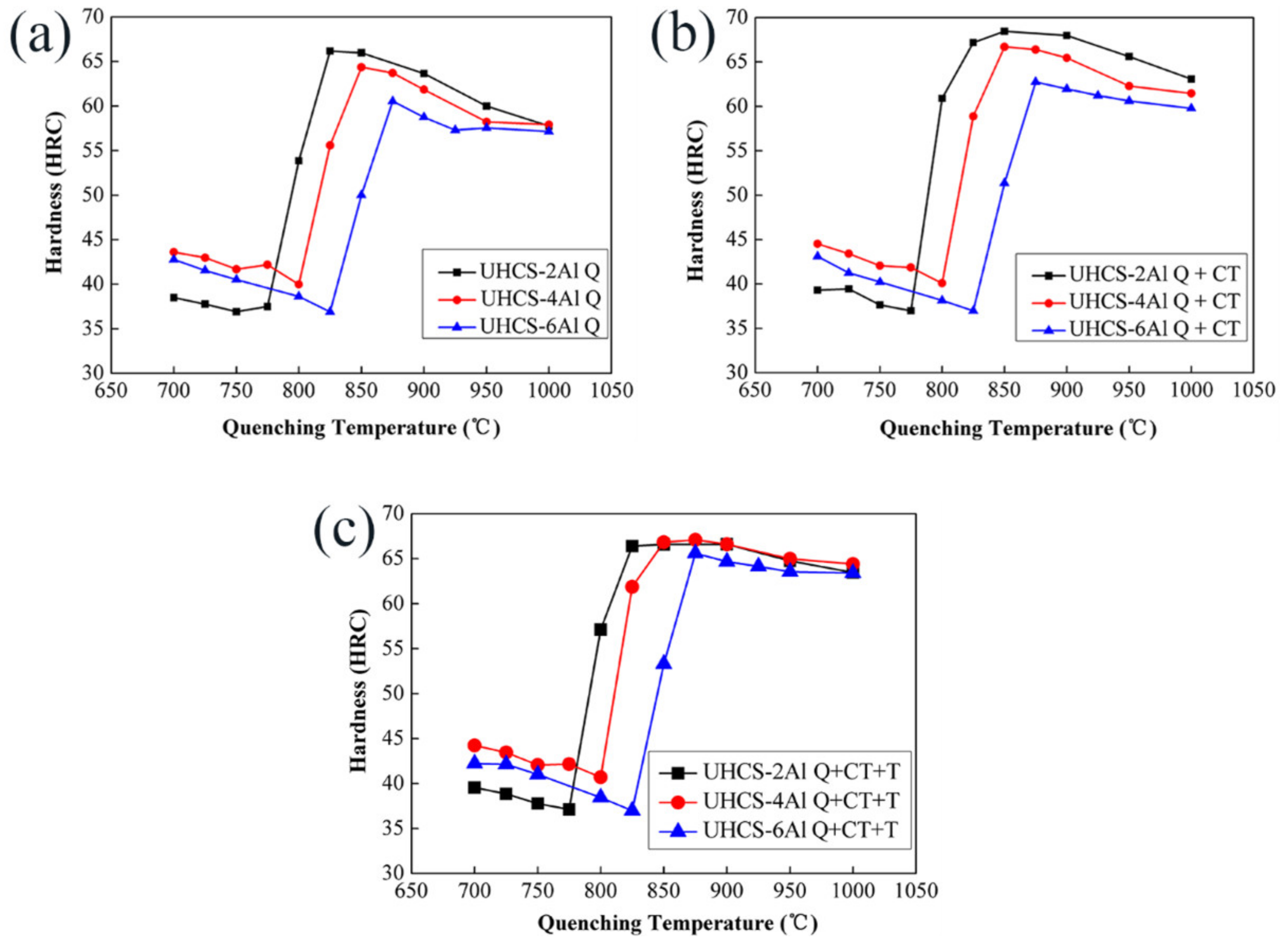
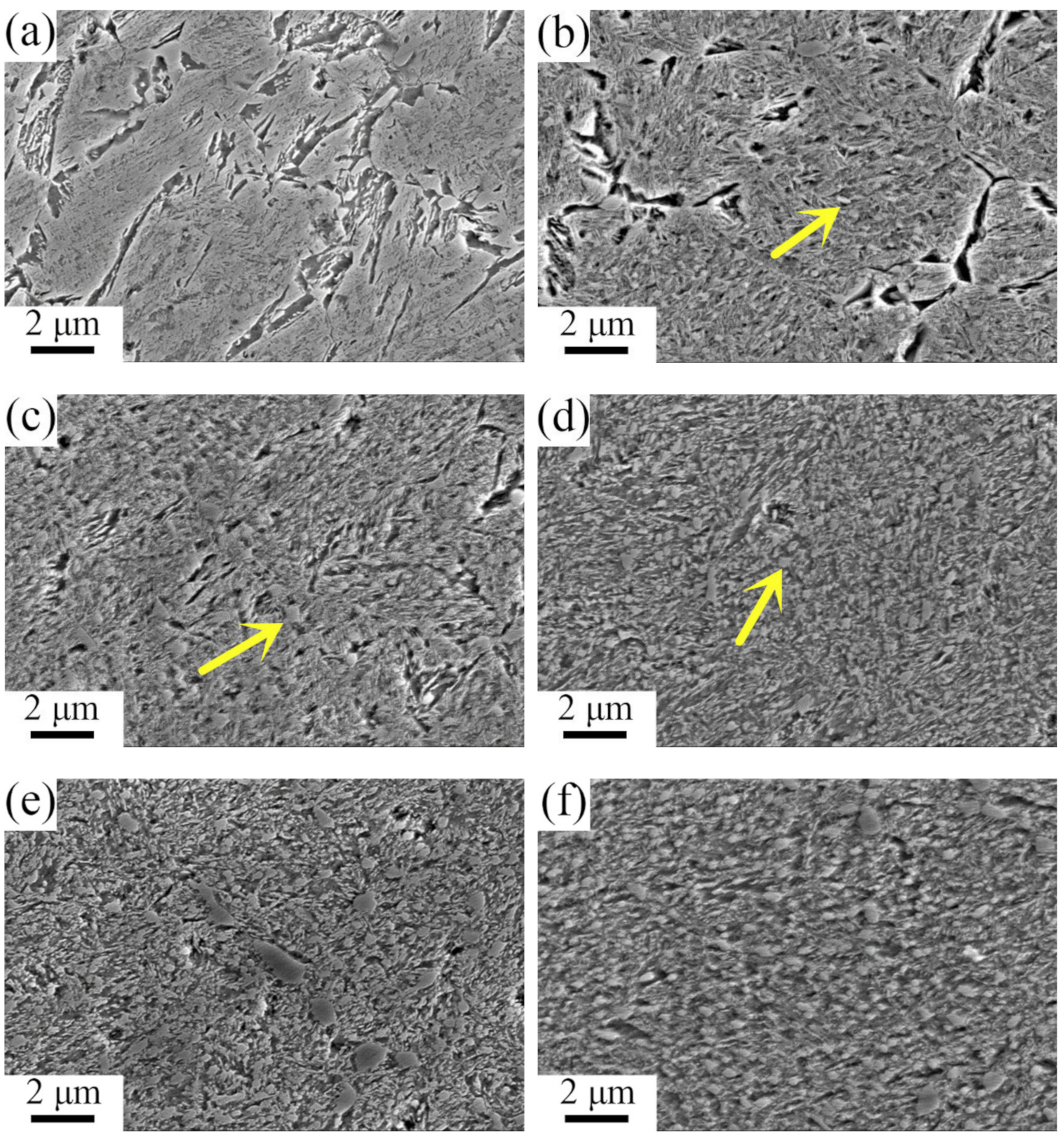
3.1. Microstructures after Forging and Determination of Phase Transition Temperatures
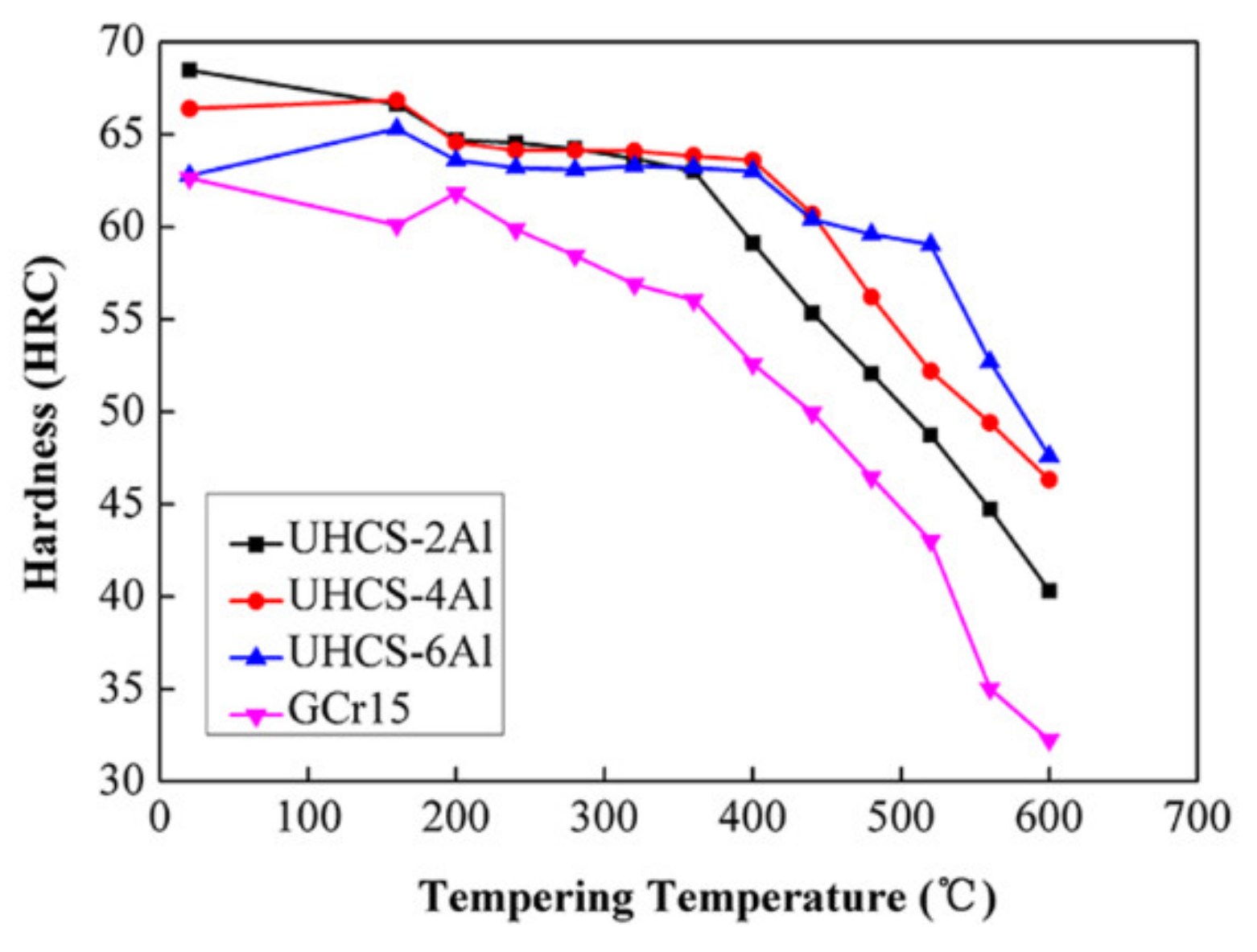
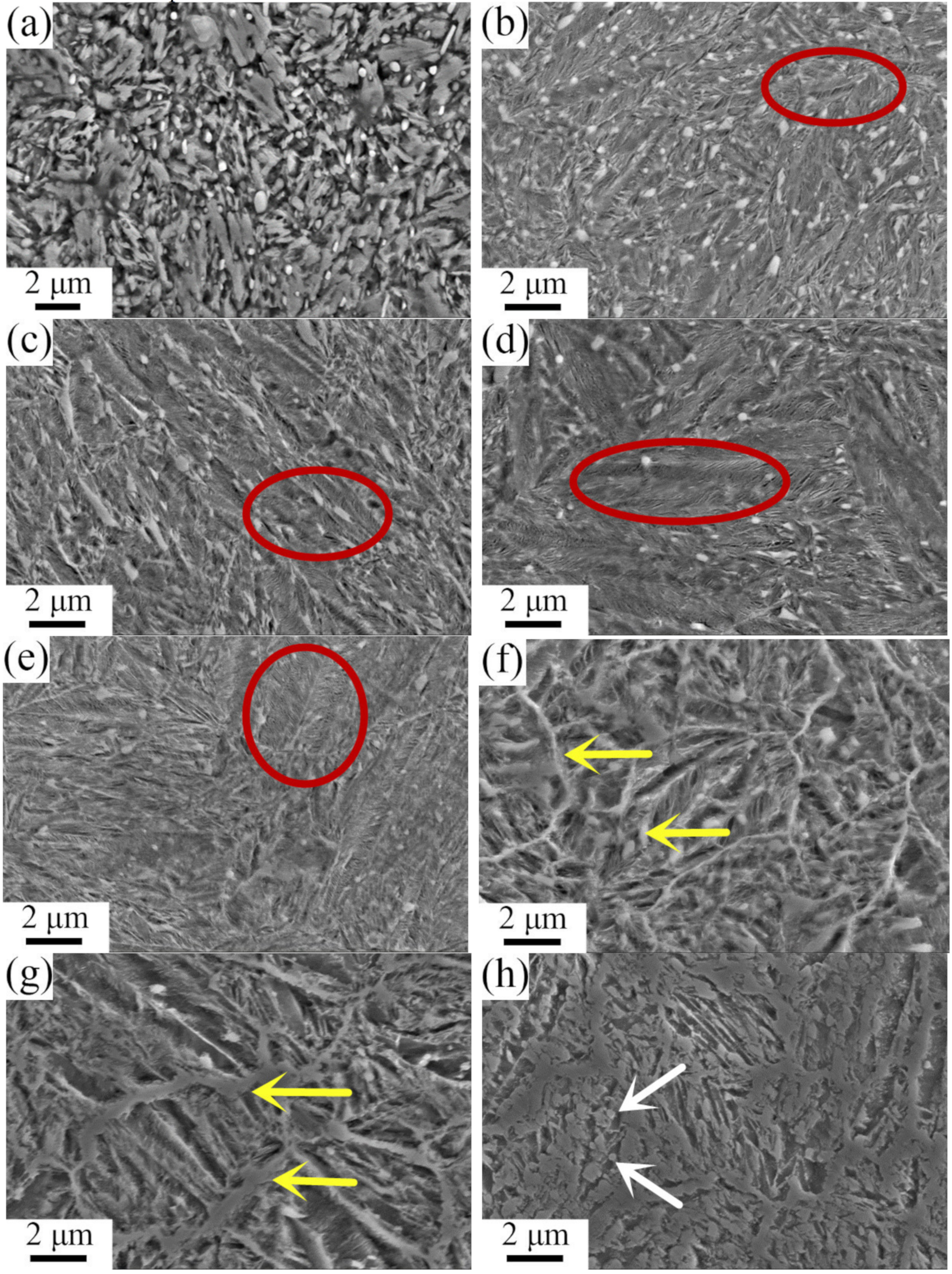
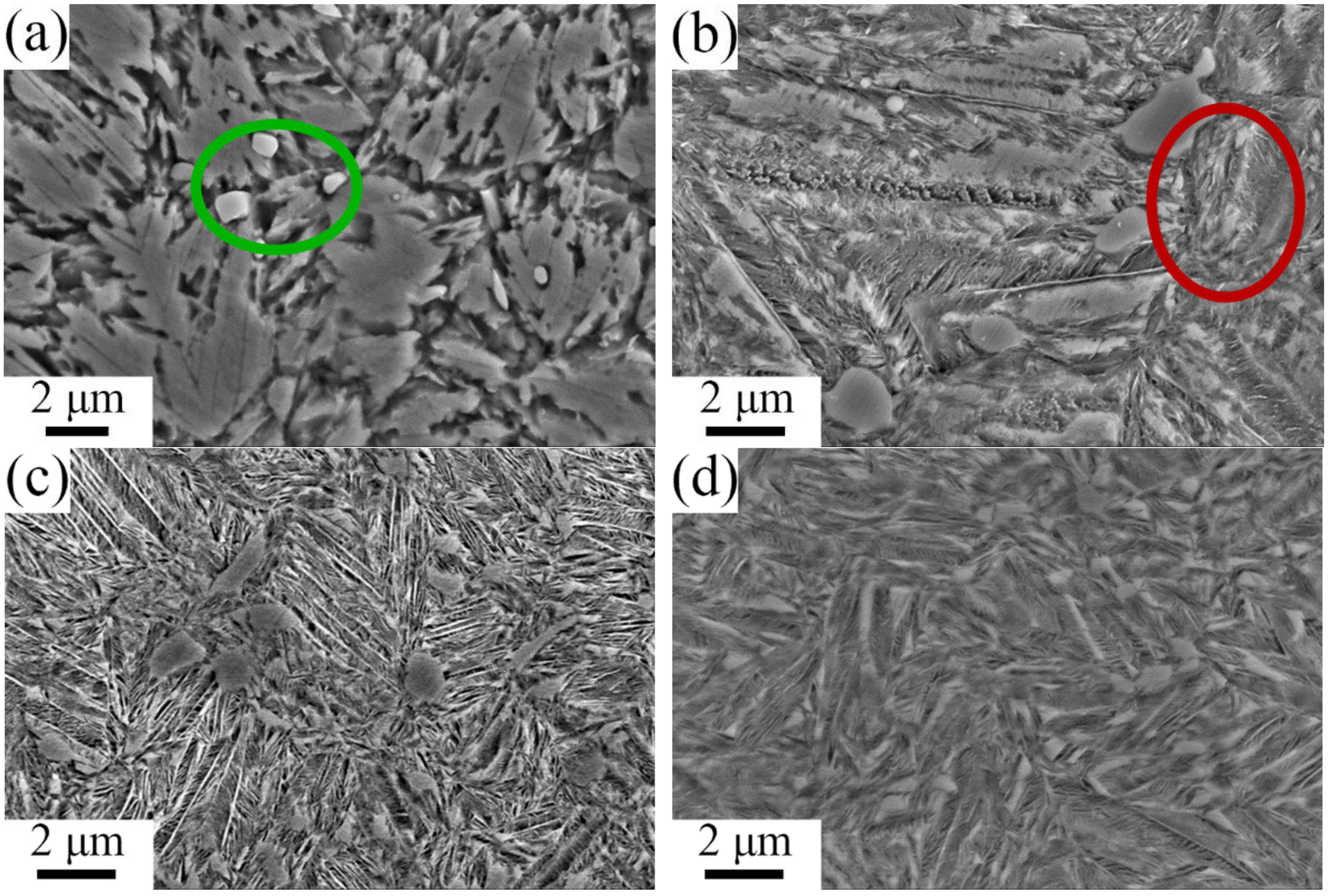
3.2. Tempering Stability and Temperature Resistance
4. Conclusions
- (1)
- The addition of Al significantly reduced the density of steel, inhibited the precipitation of carbide networks and significantly improved the phase transition temperature of ultra-high-carbon steel.
- (2)
- UHCS-2Al, UHCS-4Al and UHCS-6Al austenitized at 825 °C, 850 °C and 875 °C, respectively, and at low-tempering temperature, the hardness peaks were between 65–68 HRC, and such a high hardness was beneficial for the wear-resistance and fatigue properties.
- (3)
- The addition of Al significantly improved the tempering stability and temperature resistance of ultra-high-carbon steel, as the addition of Al inhibited the process of ε-carbide transforming into granular cementite, leading to the failure of martensite to decarbonize to form ferrite. In addition, the greater the amount of added Al, the better the tempering stability and temperature resistance of ultra-high-carbon steel.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sherby, O.D.; Walser, B.; Young, C.M.; Cady, E.M. Superplastic ultra-high carbon steels. Scr. Metall. 1975, 9, 569–573. [Google Scholar] [CrossRef]
- Lesuer, D.R.; Syn, C.K.; Goldberg, A.; Wadsworth, J.; Sherby, O.D. The case for ultrahigh-carbon steels as structural materials. JOM 1993, 45, 40–46. [Google Scholar] [CrossRef]
- Syn, C.K.; Lesuer, D.R.; Sherby, O.D. Enhancing tensile ductility of a particulate-reinforced aluminum metal matrix composite by lamination with Mg-9%Li alloy. Mater. Sci. Eng. A 1996, 206, 201–207. [Google Scholar] [CrossRef]
- Suna, H.; Wadsworth, J.; Lin, J.; Sherby, O.D. Mechanical prperties and microstructure of heat-treated ultrahigh carbon steels. Mater. Sci. Eng. 1979, 38, 35–40. [Google Scholar] [CrossRef]
- Tsuzaki, K.; Sato, E.; Furimoto, S.; Maki, T. Formation of an (α+θ) microduplex structure without thermomechanical processing in superplastic ultrahigh carbon steels. Scr. Mater. 1999, 40, 675–681. [Google Scholar] [CrossRef]
- Özdemir, N.; Orhan, N. Investigation on the superplasticity behavior of ultrahigh carbon steel. Mater. Design 2006, 27, 706–709. [Google Scholar] [CrossRef]
- Hecht, M.D.; Webler, B.A.; Picard, Y.N. Digital image analysis to quantify carbide networks in ultrahigh carbon steels. Mater. Charact. 2016, 117, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Decost, B.L.; Francis, T.; Holm, E.A. Exploring the microstructure manifold: Image texture representations applied to ultrahigh carbon steel microstructures. Acta. Mater. 2017, 133, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Hong-Juan, L.I.; Wang, B.Q.; Song, X.Y.; Guo, S.Z.; Gu, N.J. New spheroidizing technique of ultra-high carbon steel with aluminum addition. J. Iron Steel Res. 2006, 13, 9–13. [Google Scholar]
- Zhang, Z.L.; Liu, Y.N.; Zhu, J.W.; Yu, G. Processing and properties of ultrahigh-carbon (1.6%C) steel. Mater. Sci. Eng. A 2008, 483, 64–66. [Google Scholar] [CrossRef]
- Oyama, T.; Sherby, D.; Wadsworth, J.; Walser, B. Application of the divorced eutectoid transformation to the development of fine-grained, spheroidized structures in ultrahigh carbon steels - Science Direct. Scr. Metall. 1984, 18, 799–804. [Google Scholar] [CrossRef]
- Taleff, E.M.; Syn, C.K.; Lesuer, D.R.; Sherby, O.D. Pearlite in ultrahigh carbon steels: Heat treatments and mechanical properties. Metall. Mater. Trans. A 1996, 27, 111–118. [Google Scholar] [CrossRef]
- Syn, C.K.; Lesuer, D.R.; Sherby, O.D. Influence of microstructure on tensile properties of spheroidized ultrahigh-carbon (1.8 Pct C steel). Metall. Mater. Trans. A 1994, 25, 1481. [Google Scholar] [CrossRef]
- Sherby, O.D.; Oyama, T.; Kum, D.W.; Walser, B.; Wadsworth, J. Ultrahigh carbon steels. JOM 1985, 37, 50–56. [Google Scholar] [CrossRef]
- Hecht, M.D.; Picard, Y.N.; Webler, B.A. Effects of Cr concentration on cementite coarsening in ultrahigh carbon steel. Metall. Mater. Trans. A 2019, 50, 4779–4790. [Google Scholar] [CrossRef]
- Hecht, M.D.; Webler, B.A.; Picard, Y.N. Effects of Nb modification and cooling rate on the microstructure in an ultrahigh carbon steel. Metall. Mater. Trans. A 2018, 49, 2161–2172. [Google Scholar] [CrossRef]
- Chen, X.M.; Liu, Y.N.; Zhu, J.W.; Ge, L.L. Tribological behavior of 1.41 wt% C ultrahigh-carbon steel with quenching and low-temperature tempering treatment. Tribol. Lett. 2010, 38, 79. [Google Scholar] [CrossRef]
- Liu, H.D.; Sun, J.J.; Jiang, T.; Guo, S.W.; Liu, Y.N.; Lin, X. Rolling contact fatigue behavior of an ultrahigh carbon steel. Acta. Metall. Sin. 2014, 12, 1446. [Google Scholar]
- Liu, H.J.; Sun, J.J.; Jiang, T.; Guo, S.W.; Liu, Y.N. Improved rolling contact fatigue life for an ultrahigh-carbon steel with nanobainitic microstructure. Scr. Mater. 2014, 90–91, 17–20. [Google Scholar] [CrossRef]
- Ren, P.; Chen, X.P.; Cao, Z.X.; Mei, L.; Li, W.J.; Cao, W.Q.; Liu, Q. Synergistic strengthening effect induced ultrahigh yield strength in lightweight Fe 30Mn 11Al-1.2C steel. Metall. Mater. Trans. A 2019, 752, 160–166. [Google Scholar] [CrossRef]
- Chen, S.P.; Rana, R.; Haldar, A.; Ray, R.K. Current state of Fe-Mn-Al-C low density steels. Prog. Mater. Sci. 2017, 89, 345–391. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.; Kim, N.J. Brittle intermetallic compound makes ultrastrong low-density steel with large ductility. Nature 2015, 518, 77–79. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.M.; Zhang, W.L.; Wang, C.; Yu, F.; Cao, W.Q. Continuous cooling transformation of an ultrahigh carbon steel. Heat Treat. Metals. 2017, 42, 24–28. [Google Scholar]
- Chen, W.; Li, L.F.; Yang, W.Y.; Sun, Z.Q. Effects of Aluminum on the microstructures of transformation during slow cooling and dynamic phase transformation of undercooled austenite of hypereutectoid steel. Acta Metall. Sin. 2008, 44, 1069–1975. [Google Scholar]










| Materials | C | Si | Mn | Cr | Al | Fe |
|---|---|---|---|---|---|---|
| UHCS-2Al | 1.33 | 0.27 | 0.45 | 1.54 | 2.05 | Bal. |
| UHCS-4Al | 1.40 | 0.33 | 0.47 | 1.68 | 4.7 | Bal. |
| UHCS-6Al | 1.39 | 0.31 | 0.45 | 1.62 | 5.98 | Bal. |
| GCr15 | 1.0 | 0.25 | 0.35 | 1.5 | - | Bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, J.; Zhang, W.; Wang, Y.; Wang, C.; Chen, X.; Shi, Z.; Wang, H.; Cao, W. On the Unique Microstructure and Properties of Ultra-High Carbon Bearing Steel Alloyed with Different Aluminum Contents. Metals 2021, 11, 1116. https://doi.org/10.3390/met11071116
Bai J, Zhang W, Wang Y, Wang C, Chen X, Shi Z, Wang H, Cao W. On the Unique Microstructure and Properties of Ultra-High Carbon Bearing Steel Alloyed with Different Aluminum Contents. Metals. 2021; 11(7):1116. https://doi.org/10.3390/met11071116
Chicago/Turabian StyleBai, Jiaojiao, Wanli Zhang, Yuhui Wang, Cunyu Wang, Xingpin Chen, Zhiyue Shi, Hui Wang, and Wenquan Cao. 2021. "On the Unique Microstructure and Properties of Ultra-High Carbon Bearing Steel Alloyed with Different Aluminum Contents" Metals 11, no. 7: 1116. https://doi.org/10.3390/met11071116
APA StyleBai, J., Zhang, W., Wang, Y., Wang, C., Chen, X., Shi, Z., Wang, H., & Cao, W. (2021). On the Unique Microstructure and Properties of Ultra-High Carbon Bearing Steel Alloyed with Different Aluminum Contents. Metals, 11(7), 1116. https://doi.org/10.3390/met11071116





