Abstract
Acid corrosion is a problem pertaining to corrosion that involves an acid solution. It is important to treat metal to preserve its integrity. Thus, acids are utilized to clean and treat metal surfaces. In return, this may lead to over-etching and metal degradation. Corrosion inhibitors were introduced as a solution for the issue. However, there are some problems associated with the usage of conventional corrosion inhibitors. Traces of nitrites and chromates that are present in the inhibitors may lead to serious health and environmental issues. As a solution, organic green corrosion inhibitors have been studied to replace the conventional corrosion inhibitors. These inhibitor molecules form a protective layer on top of the metal surface to suppress metal dissolution when added to the acid solution. This process prevents direct contact between the metal surfaces and the acid environment. This study explores the usage of natural resources and biomass wastes as the basis for organic green corrosion inhibitors. This study also provides some suggestions for new biomass wastes that can be studied as new organic corrosion inhibitors, and it is aimed at opening the perspective of researchers on exploring new organic inhibitors by using natural resources and biomass wastes.
1. Introduction
Srivastava [1] and Zaher et al. [2] highlighted the profuse usage of mild steel in the industry, especially in the fabrication of machines and structural foundations. Zaher et al. [2] further explained that mild steel is chosen due to its affordability and strength. However, corrosion has always been a challenge faced by the industry players. This is due to the usage of acid as the cleaning medium for mild steel, as explained by Zaher et al. [2] and Sulaiman et al. [3]. This was corroborated by Dehghani et al. [4]. Acid scaling and acid pickling are some examples of such processes. Sulaiman et al. [3] explained that acid erodes the surface of the metal while efficiently cleaning the metal surface. Ikeuba and Okafor [5] further explained that the corrosive nature of acid can lead to metal dissolution. Moreover, Wang et al. [6] stated that this condition can lead to over-etching.
Furthermore, corrosion may have later effects on the industry in terms of money loss, wastage, and engineering disasters. This motion was supported by Koch et al. [7]. The authors reported that the National Association of Corrosion Engineers (NACE) predicted that the loss suffered due to corrosion amounts to 2.5 trillion USD globally. This is equivalent to 3.4% of the global gross domestic product (GDP) in 2013. In addition, Petrovich [8] suggested that engineering disasters that were caused by corrosion totaled 42% worldwide. Koch et al. [7] also agreed that corrosion protection can cut loss by up to 35%. This is equal to savings of 875 billion USD. Ikeuba [5] concluded that corrosion is an unavoidable phenomenon. The author mentioned that, although it cannot be avoided, it can be slowed down by deploying appropriate corrosion protection strategies.
In this context, this study initiated a review of the recent literature concerning corrosion inhibitors in an acidic environment. This study is divided into several sections. Section 1 gives a brief overview of the calamities caused by corrosion. Section 2 presents the fundamental aspects of corrosion. Issues regarding conventional corrosion inhibitors are discussed next. A discussion on the organic green corrosion inhibitors is presented as a solution to problems associated with their conventional counterparts. Lastly, the conclusion and summary are presented in the Section 11.
2. Fundamental Aspects of Corrosion
According to Hassan et al. [9] and Bashir et al. [10], corrosion is the degradation of a metal due to environmental attack through chemical reactions. Furthermore, Bashir et al. [10] explained that a metal continuously undergoes corrosion to reach its stable state once it is extracted from its core underground. Equation (1) can be used to explain the reactions in an electrochemical cell that lead to corrosion. Popoola [11] highlighted the oxidation half reaction of mild steel, as shown in Equation (2).
Popoola [11] also outlined the general reaction that occurs at the cathode, as portrayed in Equation (3). According to the author, a few types of cathodic reactions have been identified including neutral and alkaline solutions, metal ion reduction, metal depositions, and acid solutions. However, a discussion on neutral and alkaline solutions, metal ion reduction, and metal depositions falls outside the scope of this paper. Thus, only cathodic reactions that happen in an acidic environment are portrayed in this paper, as shown in Equations (4) and (5). Lastly, the overall basic equation is displayed in Equation (6).
3. Corrosion in Acidic Environment
Zaher [2] and Bouraoui [12] affirmed that mild steel is very prone to acid attack. The types of acids usually used for acid cleaning and acid pickling are nitric acid , sulfuric acid (), and hydrochloric acid (). This was confirmed by Rodriguez-Torres et al. [13] and El-Haddad et al. [14], who added that the most common types of acids used are and .
Furthermore, Umoren et al. [15] suggested that is more favorable compared to . They stated that there are several reasons that may contribute to this finding, such as HCl needing less pickling time and lower operating temperature. In return, a good surface quality of the metal can be obtained by using less energy. Hence, industry players can operate at a lower budget.
Corrosion of mild steel in the HCl environment has been addressed by El Haddad et al. [14]. The authors found that an acidic environment may accelerate the corrosion of mild steel. Thus, they concluded that the correct selection of corrosion protection method in an acidic environment is very vital. This was corroborated by Ogunleye et al. [16], who mentioned that an understanding of the corrosion behavior is crucial in estimating the corrosion rate. Therefore, corrosion inhibitors have been widely chosen to combat corrosion in an acidic environment.
Javed [17] estimated in his seminal report that the world’s market for corrosion inhibitors will be 8.7 billion USD in the year of 2021. This is a big leap from the amount recorded in 2016, which was 6.9 billion USD. The yearly expansion was calculated to be 4.6%. A similar trend was reported by Grand View Research [18], who stated that the global market for corrosion inhibitors is expected to be 9.9 billion USD by 2027. The annual growth rate is expected to be 3.3% from 2020 through 2027. Likewise, the number of published studies focusing on corrosion inhibitors has also increased. This clearly shows that there is growing interest in corrosion inhibitors as a way to solve corrosion problems among industry players and academicians alike.
Similar findings were reported by Hassan et al. [9], Anupama et al. [19], and Vorobyova et al. [20]. The affordability and capability of the corrosion inhibitors are among the reasons for choosing them. Monticelli [21] further explained that only a small volume of inhibitor is needed to yield the appropriate response (i.e., reducing the corrosion rate), which may also weigh in on the decision made.
4. Corrosion Inhibitors
Monticelli [21] mentioned that the standardized definition of a corrosion inhibitor is a “chemical substance that, when presented in the corrosion system at a suitable concentration, decreases the corrosion rate, without significantly changing the concentration of any corrosive agent”. Abbout [22] called into question the characteristics of effective corrosion inhibitors. Thus, he proposed several characteristics that need to be possessed by an effective corrosion inhibitor, namely, stability at a certain temperature, low cost, and, more importantly, adherence to environmental laws and standards [22]. Furthermore, the corrosion rate needs to decrease once the inhibitors are introduced into the environment. Such is the goal of using corrosion inhibitors. Equation (7) can be used to calculate the efficiency of corrosion inhibitors, where η% is the inhibition efficiency, and and are the corrosion rate without and with corrosion inhibitors, respectively.
In addition, corrosion inhibitors can be divided into three categories: anodic, cathodic, and mixed inhibitors. Monticelli [21] deduced that, regardless of the type of inhibitor, it should form a protective film on top of the metal. In return, the protection layer can prevent the metal from undergoing corrosion. She contemplated that there are three types of film formed: precipitation film, passivating film, and adsorption film. Her assumptions seem to be well founded as there have been numerous authors reporting similar findings. A protective film was discovered in the experiments carried out by K et al. [23], Asadi et al. [24], and Haris et al. [25].
However, considerable attention must be paid when choosing the right inhibitors to be used. It is vital to realize that some inhibitors are specifically designed for specific metals and environments. For instance, inhibitors made of Camilia sinesis extract [26] are designed for mild steel in a 1.0 M HCl environment, whereas Crataegus mexicana extract [13] is designed for AISI 1018 carbon steel in an environment. In conclusion, inhibitors may not work well when they are used for other types of metals and environments outside of their design parameters. Extended research and elaborate testing may be needed to study their efficiency prior to usage.
5. Types of Corrosion Inhibitors
As mentioned previously, there are three types of inhibitors, anodic, cathodic, and mixed, as shown in Figure 1. The mechanisms vary depending on the inhibitor types, as discussed further in this section.
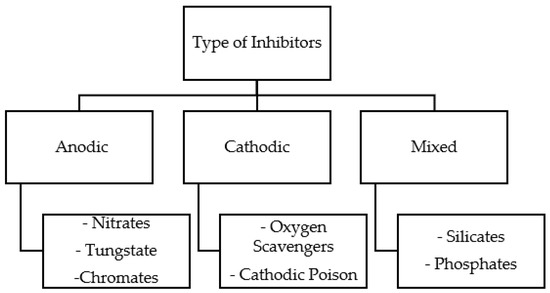
Figure 1.
Types of corrosion inhibitors.
5.1. Anodic Inhibitors
In their analysis, Marzorati et al. [27] drew the attention to the protective film that is formed on the surface of the metal. This finding is in line with Monticelli [21], who also described that the film created by anodic inhibitors is passivating. The film is formed because of the interactions between metal ions and anodic inhibitors. The negative ions move toward the anode and form insoluble hydroxides, as described by Ahmed et al. [28]. This phenomenon can be easily described, as shown in Figure 2. Popoola [11] further explained that the existence of the passivating film prohibits the anodic reaction portrayed in Equation (2). Thus, this action prevents metal degradation.
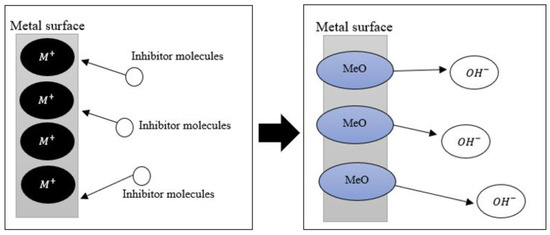
Figure 2.
Mechanism of anodic inhibitors.
Goyal et al. [29] mentioned that anodic passivation films can be further categorized into two types: oxidizing and nonoxidizing films. Examples of oxidizing films are chromates and nitrates, which can be formed with the absence of oxygen. However, Monticelli [21] explained that chromates and nitrates have been linked with issues related to environmental and human health. Recently, doubts about classic anodic oxidizing inhibitors have been raised due to these issues. On the other hand, nonoxidizing types cannot form without the presence of oxygen in the system. Examples of nonoxidizing films are phosphate and molybdate [29].
Furthermore, the effect of anodic inhibitors can be seen by using potentiodynamic polarization (PDP). Analysis done by Anupama et al. [19] proved that corrosion potential () shifts to the anodic region, which conforms to the characteristics of anodic inhibitors. The current after the addition of an inhibitor ( is lower than that without inhibitor (). The reduction in current denotes a reduction in corrosion rate [30].
Moreover, Hedge and Nayak [31] and Seshian et al. [32] suggested that the shift in before and after the addition of inhibitors is ideal in signifying the type of inhibitor. They suggested that, when the displacement of is less than 85 mV, the inhibitor may be categorized as mixed type.
5.2. Cathodic Inhibitors
Ahmed et al. [28] highlighted that cathodic inhibitors have the same principles as anodic inhibitors. They further explained that, while anodic inhibitors involve reactions at the anode, reactions for cathodic inhibitors occur at the cathodic site of the electrochemical cell. Marzorati et al. [27] concluded that cathodic inhibitors act by reducing cathodic reactions. This was supported by Popoola [11], who added that they may also reduce corrosion through cathodic precipitation.
Monticelli [21] added that another major difference between both types of inhibitors is the type of film created. The film made by cathodic inhibitors on the surface of the metal is the passivating type, which is porous. This notion has raised questions regarding the ability of the porous layer to protect the metal from corrosion. However, Monticelli [21] in her paper firmly believed that, despite the porous nature of the film, it can still serve its intended purpose of reducing the corrosion rate.
Furthermore, Ahmed et al. [28] explained that cathodic inhibitors trigger the movement of ions toward the cathodic site. Similar to the analysis of anodic inhibitors, moves to the cathodic region, which is more negative. At the same time, the values of current decrease, suggesting a reduction in corrosion rate. Thus, the claim made by Monticelli [21], which summarized the ability of the porous layer to protect the metal, is fully justified.
5.3. Mixed Inhibitor
According to Marzorati et al. [27] and Ahmed et al. [28], mixed inhibitors are able to act as anodic and cathodic inhibitors at the same time. Umoren [15] explained that mixed inhibitors are able to reduce anodic and cathodic reactions. Various experimental works have been carried out to study the mechanisms of mixed inhibitors. Among the most recent experiments that have been carried out are Şahin et al. [33], Alvarez et al. [34], and Fadhil et al. [35].
Moreover, many attempts have been made by Dehghani et al. [4,36,37] to study a vast number of corrosion inhibitors, many of which are categorized as mixed inhibitors. This was corroborated by Popoola [11], who concluded that approximately 80% of organic green corrosion inhibitors fall in this category. Examples of experiments that have been done include green Eucalyptus leaf extract [4], Citrullus lanatus fruit extract [38], and Peganum harmala seed [39]. The authors have proven that, for all cases, the anodic and cathodic currents are lowered. Furthermore, the displacement of the value of is less than 85 mV. This shows that the inhibitors are mixed type, as described in the previous section.
6. Organic Green Corrosion Inhibitors
A key problem with conventional inhibitors is that they can be potentially harmful and toxic to the environment and humans, as shown in Table 1. This was highlighted by Anupama et al. [40], Fidrusli et al. [41], and Vorobyova et al. [24]. Furthermore, Omotosho et al. [42] concurred and added that the aftermath might be due to the nitrites, chromates, and benzoates. Hassan et al. [9] agreed and explained that most inhibitors have been found to be nondegradable and poisonous. Chromates are categorized as carcinogenic, and extended inhalation can cause serious health issues.

Table 1.
Problem with conventional corrosion inhibitors.
According to El-Haddad [14], fears regarding the practice of using conventional inhibitors began in the year of 1980. Critically, in the year of 1990, the United States of America (USA) introduced The Pollution and Prevention Act to combat this issue. This was reported by Bouraoui et al. [12] and supported by Koch et al. [7]. Koch et al. [7] stressed that strict international law needs to be enforced in order to control the usage of the harmful inhibitors.
In response, Rodriguez-Torres et al. [13] claimed that some industries may design and build residual water treatment methods to abide by the laws and legislations. This approach may incur extra operating cost for the operators and, thus, is deemed financially unfeasible. Accordingly, numerous studies have suggested the usage of organic green corrosion inhibitors. Hassan et al. [9] and Rodriguez-Torres et al. [13] highlighted that organic green corrosion inhibitors must possess the same capabilities as conventional inhibitors, i.e., to reduce corrosion rate and protect the metal.
Rodriguez-Torres et al. [13] claimed that green corrosion inhibitors were discovered long before the concern with conventional inhibitors arose. The first investigation on green corrosion inhibitors was detected in the year of 1930. Interestingly, the study of green corrosion inhibitors is ongoing as shown in Table 2. This shows that the potential of plant-based corrosion inhibitors is limitless.
Table 2 shows the past studies conducted on plant-based organic inhibitors. The maximum inhibition efficiencies of all plant-based organic corrosion inhibitors were recorded to be more than 70%. For instance, the study that done by Xiang et al. [43] on copper in 0.5 M managed to obtain a maximum inhibition efficiency of 97.3%. Moreover, studies by Khadom et al. [44] and Haldhar et al. [45] on low-carbon steel in 1.0 M and 0.5 M successfully achieved maximum inhibition efficiencies of 74.04% and 97.31%, respectively. These results prove that the usage of organic corrosion inhibitors is not only limited to mild steel. Moreover, all the studies listed in Table 2 considered several variables in their experiments, with inhibitor concentration considered in all cases. This shows that the efficiency of an inhibitor is heavily dependent on its concentration. However, interactions between variables have been proven to be as important in achieving high maximum inhibition efficiency, thus resulting in a lower corrosion rate of the metal.

Table 2.
Past studies concerning organic corrosion inhibitors.
Table 2.
Past studies concerning organic corrosion inhibitors.
| Plant Extracts | Type of Metal | Medium | Variables | Maximum Inhibition Efficiency (%) | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Type | Concentration | Inhibitor Concentration | Temperature | Time | ||||
| Xanthium strumarium leaves (XSL) | Low-carbon steel | 1 M | 0.5–1.5 g/L | 40–70 °C | 5–8 h | 74.04 | [44] | |
| Pennisetum purpureum | Mild steel | 3.5% | 0.1–5.0 g/L | 30–60 °C | - | 95.0 | [46] | |
| Portulaca grandiflora leaves (PGL) | N80 carbon steel | 0.5 M | 5–20 mL/L | 30–60 °C | 95.0 | [35] | ||
| Cannabis sativa | Low-carbon steel | 0.5 M | 40–200 mg/L | - | - | 97.31 | [45] | |
| Artichoke extract | Mild steel | 1 M | 200–1000 ppm | 298–328 K | - | 98.7 | [47] | |
| Ixora coccinea extract | Mild steel | 1 M | 1–5% v/v | - | - | 89.38 | [48] | |
| Ixora coccinea extract | Mild steel | 0.5 M | 1–5% v/v | - | - | 77.96 | [48] | |
| Swertia chirata extract | Carbon steel | 0.5 M | 100–500 mg/L | - | - | 92.32 | [30] | |
| Justicia secunda leaf extract | Aluminium | 0.5 M | 50–250 ppm | 30–50 °C | - | 94.3 | [49] | |
| Gongronema latifoliuim | Mild steel | 0.5 M | 0.1–0.5% w/v | 303–323 K | - | 81.69 | [50] | |
| Clinopodium acinos | Mild steel | 1 M | 50–300 ppm | 25–45 °C | - | 89.9 | [51] | |
| Idesia polycarpa Maxim fruit extract | Copper | 0.5 M | 50–300 mg/L | 298–308 K | - | 90.8 | [52] | |
| Saraca ashoka extract | Mild steel | 0.5 M | 25–100 mg/L | - | - | 95.48 | [53] | |
| Tilia cordata | Carbon steel | 1 M | 50–300 mg/L | 30–60 °C | - | 96.0 | [54] | |
| Sunflower seed hull extract | Carbon steel | 1 M | 50–400 ppm | - | - | 98.0 | [55] | |
| Rhus verniciflua | Mild steel | 0.5 M | 100–500 ppm | 303–333 K | 86.0 | [56] | ||
| Ficus religiosa | Mild steel | 0.5 M | 100–500 mg/L | - | - | 92.26 | [57] | |
| Citrus aurantifolia leaves (CAL) | Mild steel | 0.5 M | 50–250 mg/L | - | - | 96.46 | [58] | |
| White tea | Mild steel | 1 M | 20–80 ppm | 25–60 °C | - | 96.0 | [59] | |
| Citrus reticulata leaves (CRLE) | Copper | 0.5 M | 50–500 mg/L | - | 97.3 | [43] | ||
| Glycyrrhiza glabra leaves | Mild steel | 1 M | 200–800 ppm | - | - | 88.0 | [60] | |
| Phoenix dactylifera seed (PDSE) | Mild steel | 1 M | - | - | 1 and 6 h | 97.3 | [33] | |
| Lecaniodiscus cupaniodes | Mild steel | 0.5 M | 1–5 mL/L | - | 5–35 days | 90.0 | [61] | |
| Cleome droserifolia | Mild steel | 1 M | 50–300 ppm | 25–45 °C | - | 92.0 | [62] | |
| Momordica charantia | Carbon steel | 0.5 M | 100–500 mg/L | - | - | 93.51 | [63] | |
| Malva sylvestris | Mild steel | 3.5% | 1000–2000 ppm | - | - | 91.0 | [64] | |
| Lilium brownii leaf extract | X70 Steel | 1 M | 10–200 mg/L | 298–308 K | - | 85.0 | [65] | |
| Pueraria lobata leaf extract | 10# steel | 1 M | 0.1–0.9 g/L | 25–70 °C | - | 94.37 | [66] | |
| Allamanda cathartica | Mild Steel | 1 M | 0.2–1.0% v/v | 303–333 K | - | 72.54 | [67] | |
| Cauliflowers extract | Copper | 0.5 M | 100–400 mg/L | - | 1–48 h | 99.0 | [68] | |
| Peach pomace extract | Mild Steel | 0.5 M | 50–800 ppm | - | 12–265 h | 88.0 | [69] | |
| Bassia muricata extract | Aluminium | 1 M | 50–300 ppm | 298–318 K | - | 90.0 | [70] | |
| Cinnamomum zeylanicum extract | Carbon steel | 1 M | 100–600 ppm | 25–55 °C | - | 81.1 | [71] | |
| Morus alba pendula leaf extract | Carbon steel | 1 M | 0.1–0.4 g/L | 25–60 °C | - | 96.0 | [72] | |
| Bauhinia tomentosa leaf extract | Mild steel | 1 M | 550–700 ppm | 308–333 K | - | 93.47 | [73] | |
| Butea monosperma | Mild steel | 0.5 M | 100–500 ppm | - | - | 98.0 | [74] | |
| Origanum vulgare | Mild steel | 1 M | 400–1200 ppm | - | - | 91.2 | [75] | |
| Canarium schweinfurthii | Mild steel | 0.1 M | 0.1–0.5 g/L | 303–333 K | - | 88.2 | [76] | |
| Ircinia strobilina | Mild steel | 1 M | 0.5–2.0 g/L | - | - | 82.0 | [77] | |
According to Wang et al. [66], inhibition efficiency increases with the increase in inhibitor concentration. The authors also further explained that, at a specific temperature, the efficiency starts to decrease regardless of the increase in inhibitor concentration. This phenomenon is known as extreme concentration [66]. Similar findings were also discussed by Perumal et al. [73]. On the other hand, El-Katori and Al-Mhyawi [70] observed that a rise in temperature can decrease the inhibition efficiency, as shown in Figure 3. Salmasifar et al. [47] agreed and clarified that this scenario is caused by the rate of desorption of inhibitor molecules being higher than the rate of inhibitor adsorption as the temperature increases [50].
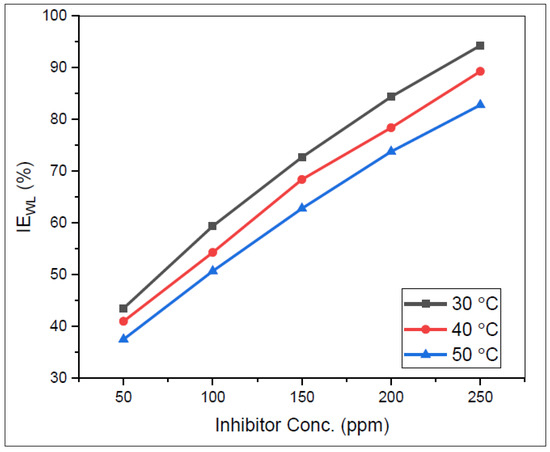
Figure 3.
Relationship between inhibition efficiency () and inhibitor concentration for various temperatures of Justicia secunda leaf extracts. Reprinted from [49].
Moreover, the effect of immersion time was studied by Joseph et al. [61] and Karki et al. [78]. The authors concluded that the inhibition efficiency improves with the increase in concentration time. However, a longer exposure period led to a decrease in the maximum efficiency. This scenario is similar to that of the relationship between temperature and concentration. Nevertheless, organic inhibitors possess large potential. However, careful consideration and planning need to be executed to maintain the quality and efficiency of the corrosion inhibitors. Figure 4, Figure 5 and Figure 6 illustrate the effect of concentration, time, and temperature on the inhibition efficiency, respectively.
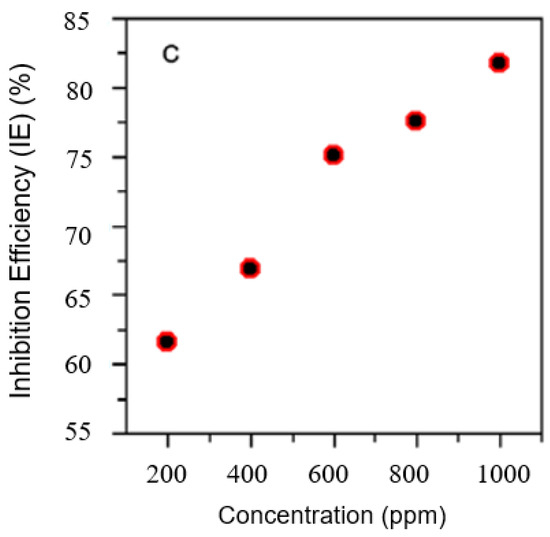
Figure 4.
The relationship between concentration and inhibition efficiency. Reprinted from [78].
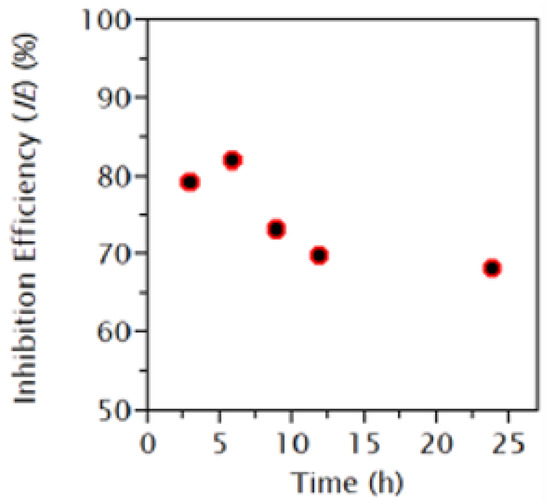
Figure 5.
The relationship between inhibition efficiency and immersion time. Reprinted from [78].
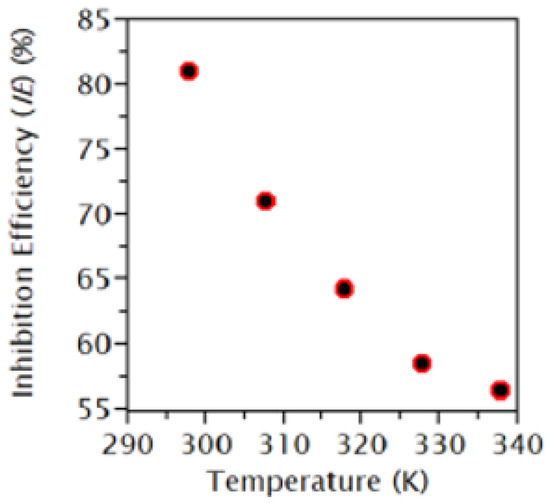
Figure 6.
The relationship between inhibition efficiency and temperature. Reprinted from [78].
7. Inhibition Mechanism
More recently, Vorobyova et al. [20] analyzed the role of the inhibition mechanisms of the corrosion inhibitors. In the paper, the authors listed several types of inhibition mechanisms. In the first type, electronic adsorption, the charged molecules of inhibitors are attracted electrostatically toward the surface of the metal. In the second type, the uncharged electron from the inhibitors is attracted to the charged metal surface. Singh et al. [79] also added that attraction happened via the process of acceptance and donation of ions. Lastly, the third mechanism involves π-bond orbital adsorption. This is defined as the interaction between conjugated molecules of organic inhibitors with the metal surface. It is also possible that the inhibition mechanisms can be a combination of two or more types of the interactions mentioned.
Furthermore, Dariva et al. [26] demonstrated that there are a few criteria regarding effective inhibitors. Chemisorption is one that has been listed by many authors. Several studies, for example, Chaudhari and Patel [80], Salinas-Solano et al. [81], and Idouhli et al. [82], have concluded that chemisorption forms a strong interaction between the metal and inhibitors, as shown in Figure 7. Thus, a film is formed on top of the metal surface. There is still considerable ambiguity regarding chemisorption as one of the criteria. This is because there are still corrosion inhibitors that only undergo physisorption with the metal surface. This was proven by the experiments conducted by Haris et al. [25] and Ituen et al. [46]. They studied the ability of an empty fruit bunch of oil palm and elephant grass as corrosion inhibitors, respectively. Although the inhibitors could only undergo physisorption, which is weaker than chemisorption, the maximum inhibition efficiencies were still considerably high at more than 80%. Subsequently, the corrosion rate was reduced. Accordingly, the existence of a protection film was nominated as the second criterion for an efficient corrosion inhibitor.
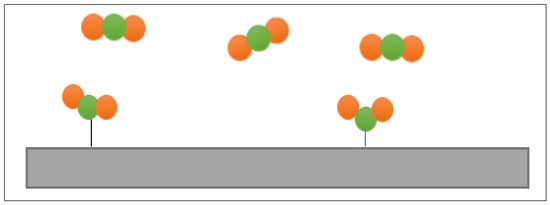
Figure 7.
Illustration of chemisorption.
Chemisorption can be measured by using standard free energy (). It can be easily calculated using Equation (8), where is the adsorption equilibrium constant, R is the universal gas constant, T is the absolute temperature, and 55.5 is the concentration of water.
Chaudhari and Patel [80] suggested that negative values of denote a spontaneous reaction. Ogunleye et al. [16] calculated the values of for Luffa cylindraca leaf extract at 333 K, which ranged between −11.56 and −11.45 kJ/mol. Similar findings were also presented by Nchewi et al. [83] for Tamarindus indica leaf extract tested at numerous temperatures. They obtained values between −1.47 and −1.57 kJ/mol. Fadhil et al. [35] also obtained values of between −17.71 and −22.03 kJ/mol. The authors concluded that the protective layer on the metal surface is stable. The experiments conducted by Nchewi et al. [83] and Fadhil et al. [35] showed that the values of standard free energy are in parallel with the temperature of the acidic environment. This means that increases as the environment becomes hotter.
In addition to the negativity of the standard free energy, the magnitude can be used to recognize the type of sorption of the corrosion inhibitors. Dehghani et al. [37] and Chaudhari and Patel [80] proposed that physisorption occurs when the value of is more than −20 kJ/mol. On the other hand, chemisorption happens when the value of is less than −40 kJ/mol. Idouhli et al. [82] explained that chemisorption involves the sharing of molecules of the inhibitors with the metal surfaces. Chemisorption is a stronger type of adsorption compared to physisorption. Physisorption is an electrostatic interaction between the inhibitor molecules and metal surfaces [82,84]. This is portrayed in Figure 8.
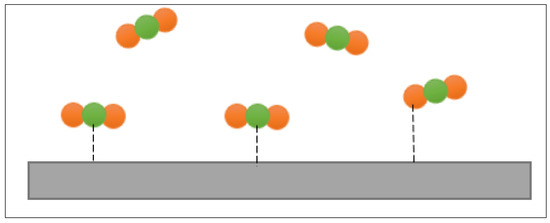
Figure 8.
Illustration of physisorption.
There also exist inhibitors that may undergo both physisorption and chemisorption, which is known as comprehensive adsorption. This type of inhibitor is more desirable as it will create a much stronger interaction compared to just one type of adsorption. Table 3 shows some of the research that has been done that indicates the nature of sorption for various plants-based inhibitors.

Table 3.
Standard free energy for various plants-based inhibitors.
Anupama et al. [19] described that nucleophilic and hydrophobic properties are the criteria of organic corrosion inhibitors. Monticelli [21] cited that hydroxyl is an example of a nucleophilic group. Anupama et al. [19] stated that nucleophilic groups may encourage better coordination between the metal surface and inhibitors, as they support the process of chemisorption. Moreover, hydrophobicity is preferred as it repels water, which causes corrosion. Arkles et al. [86] defined hydrophobicity as the interaction between the metal surface and liquid molecules. This criterion can be measured by using contact-angle tests, as done by Dehghani et al. [38] and Bahlakeh [84]. Asadi et al. [24] mentioned that a larger contact angle is preferred as it shows the hydrophobicity of the inhibitors.
Additionally, the inhibition mechanism of organic green corrosion inhibitors relies heavily on the phytochemical and functional groups of the plants. This was agreed upon by Bashir et al. [10] and Sanaei et al. [87]. Examples of phytochemicals are tannins, flavonoids, and saponins [42]. Figure 9, Figure 10 and Figure 11 show the backbone units of tannins and flavonoids, and the chemical structure of saponins, respectively. Phytochemicals are usually connected with antioxidant properties. Furthermore, Bashir et al. [10] explained that heteroatoms that naturally exist in the plant structure may be major contributors to the ability of plants to be green corrosion inhibitors, i.e., oxygen (O), sulfur (S), and nitrogen (N). Bahlakeh et al. [88] suggested that π electrons in π-bonds are the site for the interactions discussed in the previous section. This was also reported by Goyal et al. [29], who explained that, in most cases, chemisorption happens following physisorption. Ogunleye et al. [16] proposed that OH groups such as OH, –COOC2H5, –COOH, and –OCH3 can also react with metal surfaces.
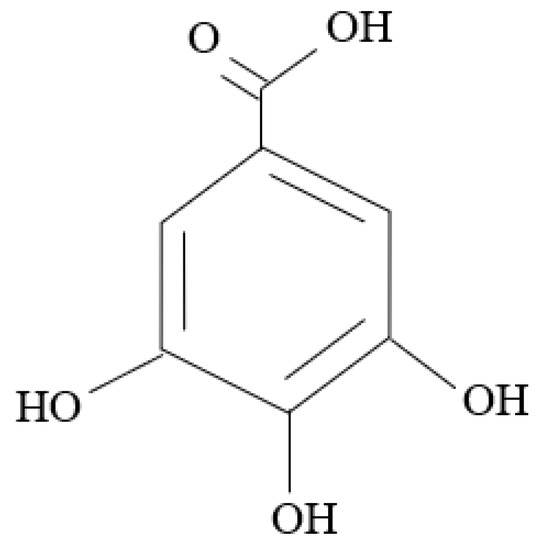
Figure 9.
Backbone unit of tannins.
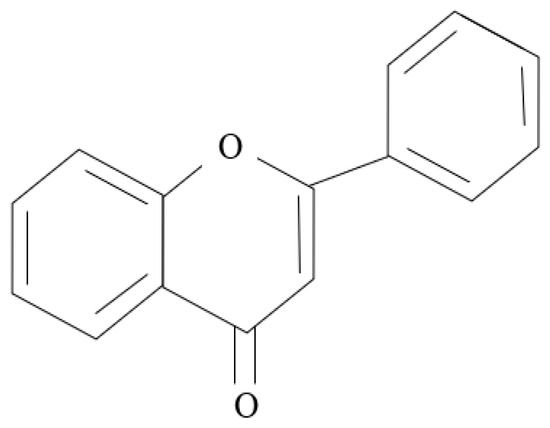
Figure 10.
Backbone unit of flavanoids.
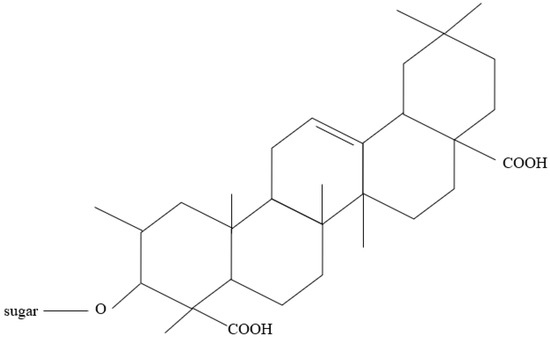
Figure 11.
Chemical structure of saponins.
8. Adsorption Mechanism
An adsorption isotherm can reveal the extent of the interaction between inhibitors and the metal surface, as described by Ogunleye et al. [16]. The authors refuted that the inhibition effect of organic inhibitors can be explained using thermodynamics and adsorption. The usage of empirical equations (e.g., exponential, power, and logarithmic) has not escaped criticism. This is due to the complexities in fitting the data using such an approach. Thus, this issue can be addressed by using equation isotherms such as Langmuir, Temkin, and Freundlich, as shown in Equations (9)–(11). The best type of adsorption can be obtained from the linear correlation coefficient () of the plot, whereby values closer to 1 are more suitable [51], as shown in Figure 12.
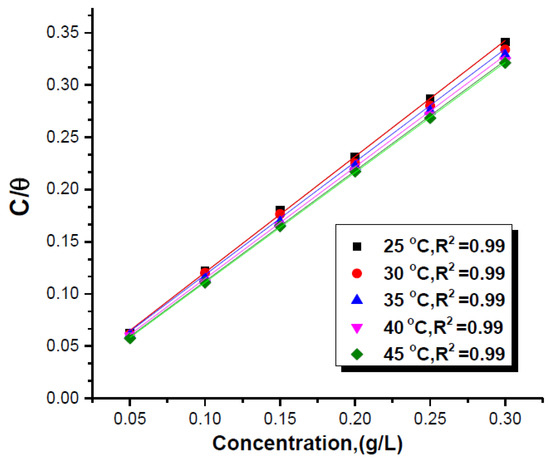
Figure 12.
Fitting of Langmuir adsorption for different concentrations for Clinopodium acinos extract. Reprinted from [88].
The differences among the above isotherms were discussed extensively by Haris et al. [25]. The authors described that monolayer adsorption can be shown using the Langmuir isotherm. The adsorption isotherm signifies the homogeneity of the system. Popoola [11], in his review, highlighted that most organic corrosion inhibitors obey the Langmuir isotherm, such as sweet potato tuber extracts [89], Feronia elephantum leaf [90], and Mangifera Indica [91]. In addition, Anyiam et al. [89] concluded that represents the strength of adsorption, whereby a higher suggests a higher adsorption. The adsorption mechanism can be determined and measured using the fitting of data from the plot of C/θ versus C according to Wang et al. [6].
Moreover, there is a major difference between the Langmuir and Freundlich isotherms, as raised by Haris et al. [25]. As stated, while the Langmuir isotherm is ideal for describing monolayer adsorption, the Freundlich isotherm is suitable for depicting multilayer adsorption. This isotherm is also perfect for representing nonreversible and nonideal adsorption. Another difference is that the Freundlich isotherm can be measured from the data fitting of the plot of log C versus log θ. Peganum harmala seed extract [39] is an example of an inhibitor with this type of adsorption.
Lastly, the Temkin isotherm can be used to categorize the gas phase equilibrium. This is a major pitfall of this adsorption type, as it cannot be used to describe the liquid phase. This paper does not further discuss Temkin adsorption as all the interactions happen in the liquid phase.
9. Biomass Wastes as Organic Green Corrosion Inhibitors
Several studies have focused on organic green corrosion inhibitors; however, their main weakness is that there have been limited attempts to study the potential of biomass wastes as organic corrosion inhibitors, as proven by Marzorati et al. [27].
Table 4 shows the past research concerning corrosion inhibitors derived from biomass wastes. Vorobyova et al. [20] mentioned that organic green corrosion inhibitors are very uncommon. However, their efficiency shows that biomass wastes do have high potential and are worthy of further investigation. According to Table 4, the maximum inhibition efficiencies of all biomass-waste-based inhibitors reported by various authors were reported to be more than 70%. Some of the inhibitors even recorded efficiencies of more than 90%. This shows that there exists a blocking surface on top of the metal surface, which allows reducing the corrosion rate. The efficiency of an inhibitor is inversely proportional to its corrosion rate. In particular, there is one major property that is shared by all biomass wastes listed in the table below. Based on the analysis done using Fourier-transform infrared spectroscopy (FTIR) and ultraviolet/visible light spectroscopy (UV/Vis), there exists absorption bands of O–H, C–H, N–H, C–N, and C=O, as shown in Figure 13 [78]. As mentioned, these heteroatoms can form interactions with the metal through a distinct type of adsorption. This shows that there are still traces of functional groups and plant phytochemicals in biomass wastes.

Table 4.
Past research concerning biomass waste-based organic corrosion inhibitors.
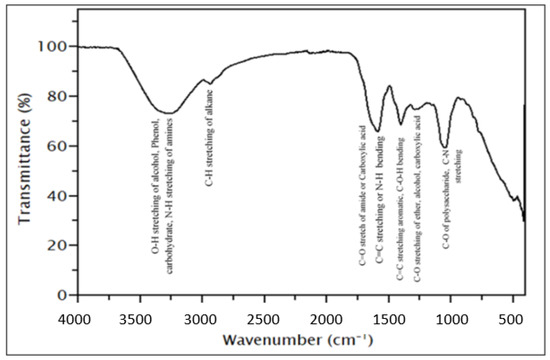
Figure 13.
FTIR analysis for the detection of functional groups. Reprinted from [91].
In addition, Table 4 shows that most inhibitors are categorized as mixed type. According to Seshian et al. [32], mixed inhibitors are responsible for suppressing hydrogen evolution at the cathode and decreasing metal dissolution at the anode. Alvarez et al. [34] highlighted that this is a result of the geometric blocking effect. Anupama et al. [19] corroborated this effect in their paper. In return, a high corrosion efficiency may be achieved due to a decrease in the reaction area, as clearly portrayed in Table 4. However, some past research also found mixed inhibitors with a predominant anodic or cathodic action.
For example, Omothosho et al. [42] studied the effect of organic corrosion inhibitors made from Termanalia catappa on mild steel. The authors discovered that the inhibitor acts as a mixed inhibitor but is predominantly cathodic. Zaher et al. [2] learned that Ammi Visnaga extract acts as a mixed type inhibitor with anodic dominance.
Furthermore, abundant resources of biomass wastes are readily available. On this note, Marzorati [27] concluded that it is sensible to make use of the huge amount of resources. In Malaysia alone, the biomass wastes produced yearly is 160 million metric tons. A vast amount of waste is generated by the oil palm sector. It was estimated that, in 2020, this amount could be as high as 100 million metric tons [107], which is supported by the data generated by Agensi Inovasi Malaysia [108], who also stated that biomass waste will increase yearly. According to the authors, the agriculture sector is the largest contributor to this statistic [108]. In conclusion, it is believed that the principles and mechanisms of biomass waste-based corrosion inhibitors are the same as those of plant-based corrosion inhibitors, as reviewed comprehensively in an earlier section. The assumption is valid as biomass waste originates from plants.
Opportunity to Explore Biomass Wastes
Table 4 shows the past research conducted on biomass waste-based corrosion inhibitors. It is clear that biomass wastes have the potential to achieve high inhibition efficiency. As a result, the corrosion rate may be reduced. According to the World Bioenergy Association [109] in their report titled “Global Bioenergy Statistics 2020”, agricultural biomass wastes encompass about 10% of global wastes. The global supply of crops is massive, which is reflected in the biomass wastes produced by the agricultural sector. Thus, this may provide a great opportunity for the biomass wastes to be studied and commercialized as organic corrosion inhibitors.
For instance, cassava peels and oil palm fronds may be explored as new biomass waste-based corrosion inhibitors. Based on research by Mohd-Asharuddin et al. [110], cassava peels fit the characteristics of plants studied as organic green corrosion inhibitors. The authors stated that cassava peels possess hydroxyl and phenol groups. They also found the existence of C=O carboxyl groups, ionic carboxylic groups, and COOH groups, which were discussed extensively in Section 7.
10. Analysis Techniques for Efficiency
It is vital to measure the effectiveness of organic green corrosion inhibitors. There are several reliable tests that are commonly used such as weight loss balance, electrochemical analysis, and surface morphology, as detailed in this section.
10.1. Weight Loss Balance
The weight loss balance technique is often deployed to test the organic green corrosion inhibitor efficiency, as supported by Haldhar et al. [57], who highlighted that this method is chosen because of its reliability and straightforwardness. Various past studies used this method for Xanthium strumarium leaves [44], tomato peel [92], and Chlorella sorokiniana [111]. According to Khadom et al. [44], the corrosion rate can be calculated from Equation (13). The value calculated from Equation (13) can then be inserted into Equation (7) to get the corrosion inhibitor efficiency.
According to de Oliveira et al. [111], the inhibition efficiency improves with the increase in inhibitor concentration. Similar results were also observed by Zultiniar et al. [112] and Mishurov et al. [113]. According to Karki et al. [78], there is a significant difference in corrosion rate and weight loss between blank solutions and solutions with corrosion inhibitors, as shown in Figure 14. All authors agreed that this phenomenon is due to the increase in coverage of the inhibitor molecules on the metal surface.
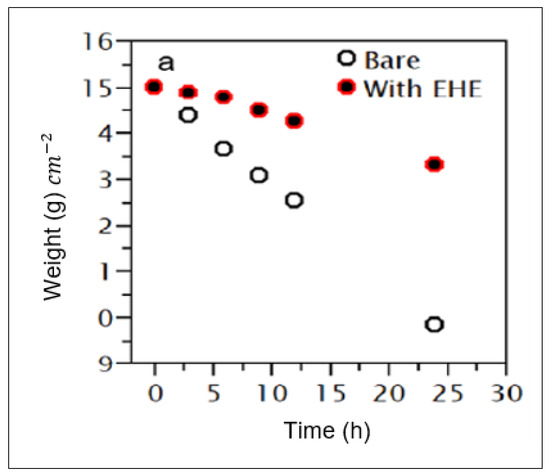
Figure 14.
Comparison of weight loss between blank solution and solution with the presence of Equisetum hyemale extract (EHE). Reprinted from [78].
However, the amount of weight loss increases with rising temperature even in the presence of inhibitors. This trend was observed by Hassan et al. [114], who concluded that this finding is caused by the desorption of inhibitor molecules from the metal surface, as corroborated by Chaudhari and Patel [80].
10.2. Electrochemical Analysis
Papavinasam [115] and Ali et al. [116] highlighted the benefits of using electrochemical analysis in their research. This technique requires a brief testing period and is able to measure a low rate of corrosion. Examples of this technique are potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS). Vorobyova and Skiba [69] and Haldhar et al. [57] stated that electrochemical measurements are usually done by using three-cell systems. The system comprises three electrodes: working electrode (WE), counter electrode (CE), and reference electrode (RE). The working electrode is the metal to be tested, while a platinum electrode and a saturated calomel electrode (SCE) are commonly used as the CE and RE, respectively. According to Haldhar et al. [57] and Elabbasy and Fouda [62], the WE is initially immersed in the test solution for 1 h to achieve a steady state.
10.2.1. Potentiodynamic Polarization (PDP)
Mostafatabar [101] and Dehghani et al. [117] mentioned that PDP can be used to characterize kinetic reactions on the metal surface. The result of PDP is usually presented in the form of a graph, as shown in Figure 15. Furthermore, Tehrani et al. [64] stated that this test can be done using a scanning rate of 1 mV/s with a potential range between −250 mV and +250 mV. Elabbasy and Fouda [118] cited that corrosion current densities () can be obtained as a result. Banu et al. [67] added that Tafel slopes ( and ) may also be measured. The authors also added that corrosion potential () can be determined from extrapolation of the Tafel slopes. The values of may be used to categorize the type of inhibitor, as explained in Section 5. Furthermore, inhibition efficiency can be calculated using the values of the corrosion current densities before () and after () acid immersion, as shown in Equation (14).
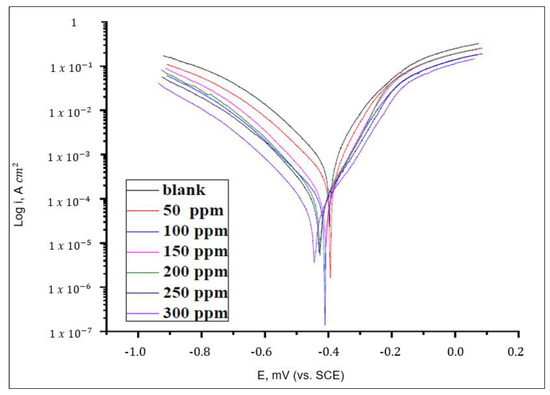
Figure 15.
Potentiodynamic polarization plots for various concentrations of Clinopodium acinos with mild steel in 1 M HCl. Reprinted from [51].
Dehghani et al. [91] stated that it is very important to choose an appropriate scan rate to study the corrosion. The authors explained that the results will be distorted and unreliable if this condition is not met. The authors suggested that, if a high scan rate is used, the corrosion rate will be higher, in response to the higher discharged current. However, if a lower scan rate is utilized, the system will yield unreliable results due to the corrosive acidic environment. In conclusion, the authors suggested that a suitable scan rate of 1 mV·s−1. The same scan rate was also adopted by Mohd et al. [119].
10.2.2. Electrochemical Impedance Spectroscopy (EIS)
Chaudhari and Patel [80] described that EIS is used to provide data for the electrochemical kinetics of the steel surface in an acid solution. This method is also useful to provide information on the electrochemical interfaces, as the adsorption process that occurs is interfacial. Ali et al. [116] further explained that the advantage of using EIS is the absence of scan rate. Thus, it is suitable to test environments with a low conductivity.
Figure 16 shows the circuit diagram commonly used for the test, where represents solution resistance, CPE represents constant phase elements, and is the charge transfer resistance [58,116]. Thomas [48] explained that double-layer capacitance () is abandoned in favor of CPE. This is caused by a divergence from the ideal metal dielectric property due to defects on the metal surface. can be calculated using Equations (15) and (16), where is the CPE constant, is the maximum frequency, is the angular frequency, and n is the phase shift (i.e., −1 ≤ n ≤ 1). In addition, n can also be used to assess the corrosion dissolution mechanism [30]. Furthermore, Tehrani et al. [64] suggested using a frequency range between 0.01 Hz and 10,000 Hz, while Haldhar et al. [30] recommended a frequency range between 0.01 Hz and 100,000 Hz with an amplitude of 5 mV. The results can then be presented in the form of a Nyquist plot, as shown in Figure 17. The inhibition efficiency can be calculated using Equation (17), where is the inhibition efficiency, is the charge transfer resistance without the inhibitors, and is the charge transfer resistance in the presence of inhibitors.
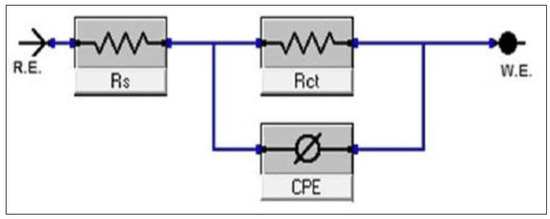
Figure 16.
Circuit diagram for EIS experiment. Reprinted from [116].
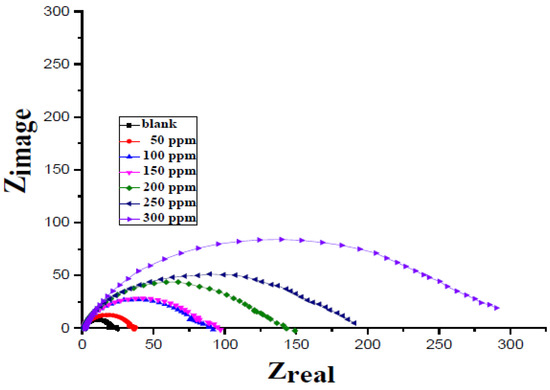
Figure 17.
Nyquist plot for various concentrations of Clinopodium acinos extract. Reprinted from [51].
More importantly, the frequency dispersion can be shown using EIS, which is a major advantage of using this technique. This is because the frequency dispersion is impossible to describe using simple parameters such as capacitance and resistance. According to Anupama et al. [40], frequency dispersion is a feature of solid electrodes, as corroborated by Ece Altunba [33]. The authors identified several factors that may contribute to the phenomenon, such as surface heterogeneity, fractal structures, and impurities [33,40].
10.3. Surface Morphology
Bashir et al. [10] stated that surface morphology can be studied using a scanning electron microscope (SEM). This method has been utilized by a number of researchers such as El-Hadad [14] and Haldhar et al. [58]. According to the authors, SEM can capture detailed images of the metal surfaces at the macroscopic level. The images of the metal surface before and after acid immersion can then be compared and analyzed.
Figure 18 shows SEM images of a metal, presented by Karki et al. [78]. The authors compared the images of metal surfaces immersed in the acid solution with and without the Equisatum hyemale extracts. As shown in Figure 18, the surface of the metal immersed in an acidic environment in the presence of organic inhibitors showed a lesser corrosion effect compared to the metal immersed in a blank solution.
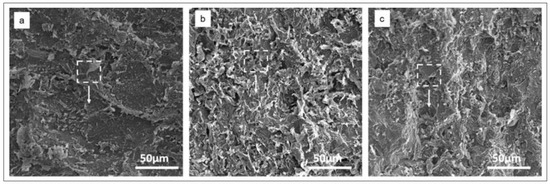
Figure 18.
SEM image of metal (a) without inhibitor, (b) with 400 ppm of Equisetum hyemale extract, and (c) with 1000 ppm of Equisetum hyemale extract. Reprinted from [78].
11. Summary
Corrosion is a global issue that needs to be tackled properly and promptly. There are many considerations that must be taken into account to choose a suitable corrosion protection method. Therefore, corrosion inhibitors are a popular choice compared to other types of prevention methods such as coating and paint. However, conventional inhibitors have been found to be carcinogenic and harmful to the environment. Thus, organic corrosion inhibitors can be used as an alternative. There are a few properties of organic corrosion inhibitors responsible for protection of the metal, such as adsorption type (i.e., physisorption, chemisorption, and comprehensive), the ability to form a protective film on top of the metal surface, and nucleophilic and hydrophobic properties.
In recent years, there has been considerable interest in organic green corrosion inhibitors made from biomass waste extracts such as seed and peels. This is aligned with the concerns regarding the staggering amount of biomass waste produced yearly. Despite the increase in interest, the studies conducted on biomass wastes are far behind the research done on plant-based organic green corrosion inhibitors. As a conclusion, it is recommended to boost the number of studies focusing on biomass wastes. In return, the price of corrosion inhibitors and the operating cost can be reduced, in addition to bringing the plant to its full potential, while substantially decreasing the amount of waste.
Author Contributions
Conceptualization, N.A.R., N.H.A., N.A.F. and M.F.M.T.; investigation, N.A.R. and N.H.A.; writing—original draft preparation, N.A.R.; writing—review and editing, N.H.A., N.A.F. and M.F.M.T. All authors read and agreed to the published version of the manuscript.
Funding
This research was funded by Universiti Teknologi Malaysia (UTM) and the Research Management Center, UTM, under the Fundamental Research Grant Scheme (FRGS), funding number 5F188, the Collaborative Research Grant (CRG), funding numbers 08G22 and 08G32, and the UTM R&D Fund (4J506).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| PDP | Potentiodynamic polarization |
| FTIR | Fourier-transform infrared spectroscopy |
| UV/Vis | Ultraviolet/visible light spectroscopy |
| EIS | Electrochemical impedance spectroscopy |
| SEM | Scanning electron microscope |
| WE | Working electrode |
| RE | Reference electrode |
| CE | Counter electrode |
| SCE | Saturated calomel electrode |
References
- Srivastava, M. Mild Steel Corrosion Inhibition, in 4 N Sulphuric Acid, by a Green Inhibitor. Port. Electrochim. Acta 2020, 38, 99–106. [Google Scholar] [CrossRef]
- Zaher, A.; Chaouiki, A.; Salghi, R.; Boukhraz, A.; Bourkhiss, B.; Ouhssine, M. Inhibition of Mild Steel Corrosion in 1M Hydrochloric Medium by the Methanolic Extract of Ammi visnaga L. Lam Seeds. Int. J. Corros. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Sulaiman, K.O.; Onawole, A.T.; Faye, O.; Shuaib, D.T. Understanding the corrosion inhibition of mild steel by selected green compounds using chemical quantum based assessments and molecular dynamics simulations. J. Mol. Liq. 2019, 279, 342–350. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B. Green Eucalyptus leaf extract: A potent source of bio-active corrosion inhibitors for mild steel. Bioelectrochemistry 2019, 130, 107339. [Google Scholar] [CrossRef] [PubMed]
- Ikeuba, A.I.; Okafor, P.C. Green corrosion protection for mild steel in acidic media: Saponins and crude extracts of Gongronema latifolium. Pigment. Resin Technol. 2019, 48, 57–64. [Google Scholar] [CrossRef]
- Wang, X. Solanum lasiocarpum L. Extract as Green Corrosion Inhibitor for A3 Steel in 1 M HCl Solution. Int. J. Electrochem. Sci. 2019, 14, 1178–1196. [Google Scholar] [CrossRef]
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study; NACE International: Houston, TX, USA, 2016. [Google Scholar]
- Petrović, Z.C. Catastrophes caused by corrosion. Vojn. Glas. 2016, 64, 1048–1064. [Google Scholar] [CrossRef]
- Hassan, N.; Ali, S.M.; Ebrahim, A.; El-Adwy, H.; Rodríguez-Torres, A.; Valladares-Cisneros, M.G. Ginger extract as green corrosion inhibitor of mild steel in hydrochloric acid solution. J. Ind. Eng. Chem. 2019, 12, 1–10. [Google Scholar] [CrossRef]
- Bashir, S.; Singh, G.; Kumar, A. Shatavari (Asparagus Racemosus) as Green Corrosion Inhibitor of Aluminium in Acidic Medium. Port. Electrochim. Acta 2019, 37, 83–91. [Google Scholar] [CrossRef]
- Popoola, L.T. Organic green corrosion inhibitors (OGCIs): A critical review. Corros. Rev. 2019, 37, 71–102. [Google Scholar] [CrossRef]
- Bouraoui, M.M.; Chettouh, S.; Chouchane, T.; Khellaf, N. Inhibition Efficiency of Cinnamon Oil as a Green Corrosion Inhibitor. J. Bio Tribo Corros. 2019, 5, 28. [Google Scholar] [CrossRef]
- Rodríguez-Torres, A.; Valladares-Cisneros, M.G.; Cuevas-Arteaga, C.; Veloz-Rodríguez, M.A. Study of Green Corrosion Inhibition on AISI 1018 Carbon Steel in Sulfuric Acid Using Crataegus mexicana as Eco-Friendly Inhibitor. J. Mater. Env. Sci. 2019, 2508, 101–112. [Google Scholar]
- El-Haddad, M.A.M.; Radwan, A.B.; Sliem, M.H.; Hassan, W.M.I.; Abdullah, A.M. Highly efficient eco-friendly corrosion inhibitor for mild steel in 5 M HCl at elevated temperatures: Experimental & molecular dynamics study. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Obot, I.B.; Suleiman, R.K. A critical review on the recent studies on plant biomaterials as corrosion inhibitors for industrial metals. J. Ind. Eng. Chem. 2019, 76, 91–115. [Google Scholar] [CrossRef]
- Ogunleye, O.; Arinkoola, A.; Eletta, O.; Agbede, O.; Osho, Y.; Morakinyo, A.; Hamed, J. Green corrosion inhibition and adsorption characteristics of Luffa cylindrica leaf extract on mild steel in hydrochloric acid environment. Heliyon 2020, 6, e03205. [Google Scholar] [CrossRef] [PubMed]
- Javed, M. Corrosion Inhibitors. Global Markets; BCC Publishing: Wellesley, MA, USA, 2017. [Google Scholar]
- Grand View Research. Corrosion Inhibitors Market Size, Share & Trends Analysis Report by Product (Organic, Inorganic), by Type (Water Based, Oil Based), by End Use, by Region, And Segment Forecasts, 2020–2027; Grand View Research Inc.: San Francisco, CA, USA, 2020. [Google Scholar]
- Anupama, K.; Ramya, K.; Joseph, A. Electrochemical measurements and theoretical calculations on the inhibitive interaction of Plectranthus amboinicus leaf extract with mild steel in hydrochloric acid. Measurement 2017, 95, 297–305. [Google Scholar] [CrossRef]
- Vorobyova, V.I.; Skiba, M.I.; Shakun, A.S.; Nahirniak, S.V. Relationship between the inhibition and antioxidant properties of the plant and biomass wastes extracts-A Review. Int. J. Corros. Scale. Inhib. 2019, 8, 150–178. [Google Scholar] [CrossRef]
- Monticelli, C. Corrosion Inhibitors; Elsevier: Amsterdam, The Netherlands, 2018; pp. 164–171. [Google Scholar]
- Abbout, S. Green Inhibitors to Reduce the Corrosion Damage; InTech: London, UK, 2020. [Google Scholar] [CrossRef]
- Jain, V.; Rai, B. Thiourea Derivatives as Steel Corrosion Inhibitors: Density Functional Theory and Experimental Studies. In Proceedings of the CORROSION 2019, Nashville, TN, USA, 24–28 March 2019. [Google Scholar]
- Asadi, N.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Utilizing Lemon Balm extract as an effective green corrosion inhibitor for mild steel in 1M HCl solution: A detailed experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Taiwan Inst. Chem. Eng. 2019, 95, 252–272. [Google Scholar] [CrossRef]
- Haris, N.I.N.; Sobri, S.; Kassim, N. Oil palm empty fruit bunch extract as green corrosion inhibitor for mild steel in hydrochloric acid solution: Central composite design optimization. Mater. Corros. 2019, 70, 1111–1119. [Google Scholar] [CrossRef]
- Dariva, C.G.; Galio, A.F. Corrosion inhibitors–principles, mechanisms and applications. Dev. Corros. Prot. 2014, 16, 365–378. [Google Scholar]
- Marzorati, S.; Verotta, L.; Trasatti, S.P. Green Corrosion Inhibitors from Natural Sources and Biomass Wastes. Molecules 2018, 24, 48. [Google Scholar] [CrossRef]
- Al-Mashhadani, M.H.; Ahmed, A.A.; Hussain, Z.; Mohammed, S.A.; Yusop, R.M.; Yousif, E. Inhibition of Corrosion: Mechanisms and Classifications in Overview. Al Qadisiyah J. Pure Sci. 2020, 25, 1–9. [Google Scholar] [CrossRef]
- Goyal, M.; Kumar, S.; Bahadur, I.; Verma, C.; Ebenso, E.E. Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: A review. J. Mol. Liq. 2018, 256, 565–573. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Nguyen, D.L.T.; Kaya, S.; Bahadur, I.; Dagdag, O.; Kim, S.-C. Corrosion inhibition, surface adsorption and computational studies of Swertia chirata extract: A sustainable and green approach. Mater. Chem. Phys. 2021, 267, 124613. [Google Scholar] [CrossRef]
- Hegde, M.; Nayak, S.P. Aqueous extract of Dillenia Pentagyna Fruit as green inhibitor for mild steel corrosion in 0.5 M hydrochloric acid solution. J. Mater. Environ. Sci. 2019, 2508, 22–31. [Google Scholar]
- Bhuvaneshwari, D.S.; Raja, B.; Durai, U. Adina Cordifolia as a corrosion inhibitor—A green approach against mild steel corrosion in 0.5 M sulphuric acid medium. Pigment. Resin Technol. 2019, 49, 63–70. [Google Scholar] [CrossRef]
- Şahin, E.A.; Solmaz, R.; Gecibesler, I.H.; Kardaş, G. Adsorption ability, stability and corrosion inhibition mechanism of phoenix dactylifera extrat on mild steel. Mater. Res. Express 2020, 7, 016585. [Google Scholar] [CrossRef]
- Alvarez, P.E.; Fiori-Bimbi, M.V.; Neske, A.; Brandán, S.A.; Gervasi, C.A. Rollinia occidentalis extract as green corrosion inhibitor for carbon steel in HCl solution. J. Ind. Eng. Chem. 2018, 58, 92–99. [Google Scholar] [CrossRef]
- Fadhil, A.A.; Khadom, A.A.; Ahmed, S.K.; Liu, H.; Fu, C.; Mahood, H.B. Portulaca grandiflora as new green corrosion inhibitor for mild steel protection in hydrochloric acid: Quantitative, electrochemical, surface and spectroscopic investigations. Surf. Interfaces 2020, 20, 100595. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Potential role of a novel green eco-friendly inhibitor in corrosion inhibition of mild steel in HCl solution: Detailed macro/micro-scale experimental and computational explorations. Constr. Build. Mater. 2020, 245, 118464. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B. A detailed electrochemical/theoretical exploration of the aqueous Chinese gooseberry fruit shell extract as a green and cheap corrosion inhibitor for mild steel in acidic solution. J. Mol. Liq. 2019, 282, 366–384. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. A combined experimental and theoretical study of green corrosion inhibition of mild steel in HCl solution by aqueous Citrullus lanatus fruit (CLF) extract. J. Mol. Liq. 2019, 279, 603–624. [Google Scholar] [CrossRef]
- Bahlakeh, G.; Ramezanzadeh, B.; Dehghani, A.; Ramezanzadeh, M. Novel cost-effective and high-performance green inhibitor based on aqueous Peganum harmala seed extract for mild steel corrosion in HCl solution: Detailed experimental and electronic/atomic level computational explorations. J. Mol. Liq. 2019, 283, 174–195. [Google Scholar] [CrossRef]
- Anupama, K.; Ramya, K.; Joseph, A. Electrochemical and computational aspects of surface interaction and corrosion inhibition of mild steel in hydrochloric acid by Phyllanthus amarus leaf extract (PAE). J. Mol. Liq. 2016, 216, 146–155. [Google Scholar] [CrossRef]
- Fidrusli, A.; Suryanto; Mahmood, M. Ginger extract as green corrosion inhibitor of mild steel in hydrochloric acid solution. IOP Conf. Ser. Mater. Sci. Eng. 2018, 290, 12087. [Google Scholar] [CrossRef]
- Omotosho, O.A.; Okeniyi, J.O.; Loto, C.A.; Popoola, A.P.I.; Obi, C.E.; Sonoiki, O.O.O.; Oni, A.B.; Alabi, A.S.; Olarewaju, A.E. Performance of Terminalia catappa on mild steel corrosion in HCl medium. AIP Conf. Proc. 2016, 1758, 030027. [Google Scholar] [CrossRef]
- Xiang, Q.; He, J. Combining theoretical and experimental researches to insight the anti-corrosion nature of Citrus reticulata leaves extract. J. Mol. Liq. 2021, 325, 115218. [Google Scholar] [CrossRef]
- Khadom, A.A.; Abd, A.N.; Ahmed, N.A. Xanthium strumarium leaves extracts as a friendly corrosion inhibitor of low carbon steel in hydrochloric acid: Kinetics and mathematical studies. S. Afr. J. Chem. Eng. 2018, 25, 13–21. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Mandal, N.; Benhiba, F.; Bahadur, I.; Dagdag, O. Anticorrosive properties of a green and sustainable inhibitor from leaves extract of Cannabis sativa plant: Experimental and theoretical approach. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126211. [Google Scholar] [CrossRef]
- Ituen, E.B.; James, A.O.; Akaranta, O. Elephant grass biomass extract as corrosion inhibitor for mild steel in acidic medium. J. Mater. Environ. Sci. 2017, 8, 1498–1507. [Google Scholar]
- Salmasifar, A.; Edraki, M.; Alibakhshi, E.; Ramezanzadeh, B.; Bahlakeh, G. Combined electrochemical/surface investigations and computer modeling of the aquatic Artichoke extract molecules corrosion inhibition properties on the mild steel surface immersed in the acidic medium. J. Mol. Liq. 2021, 327, 114856. [Google Scholar] [CrossRef]
- Vidhya, K.T.; Kakkassery, J.T.; Raphael, V.P.; Ragi, K.; Johnson, R. Ixora coccinea extract as an efficient eco-friendly corrosion inhibitor in acidic media: Experimental and theoretical approach. Curr. Chem. Lett. 2021, 10, 139–150. [Google Scholar] [CrossRef]
- Iroha, N.B.; Maduelosi, N.J. Corrosion inhibitive action and adsorption behaviour of justicia secunda leaves extract as an eco-friendly inhibitor for aluminium in acidic media. Biointerface Res. Appl. Chem. 2021, 11, 13019–13030. [Google Scholar] [CrossRef]
- Aralu, C.C.; Chukwuemeka-Okorie, H.O.; Akpomie, K.G. Inhibition and adsorption potentials of mild steel corrosion using methanol extract of Gongronema latifoliuim. Appl. Water Sci. 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Fouda, A.; El-Gharkawy, E.-S.; Ramadan, H.; El-Hossiany, A. Corrosion resistance of mild steel in hydrochloric acid solutions by clinopodium actions as a green inhibitor. Biointerface Res. Appl. Chem. 2021, 11, 9786–9803. [Google Scholar]
- Zhang, X.; Li, W.; Yu, G.; Zuo, X.; Luo, W.; Zhang, J.; Tan, B.; Fu, A.; Zhang, S. Evaluation of Idesia polycarpa Maxim fruits extract as a natural green corrosion inhibitor for copper in 0.5 M sulfuric acid solution. J. Mol. Liq. 2020, 318, 114080. [Google Scholar] [CrossRef]
- Saxena, A.; Prasad, D.; Haldhar, R.; Singh, G.; Kumar, A. Use of Saraca ashoka extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J. Mol. Liq. 2018, 258, 89–97. [Google Scholar] [CrossRef]
- Fouda, A.S.; Abousalem, A.S.; El-Ewady, G.Y. Mitigation of corrosion of carbon steel in acidic solutions using an aqueous extract of Tilia cordata as green corrosion inhibitor. Int. J. Ind. Chem. 2016, 8, 61–73. [Google Scholar] [CrossRef]
- Hassannejad, H.; Nouri, A. Sunflower seed hull extract as a novel green corrosion inhibitor for mild steel in HCl solution. J. Mol. Liq. 2018, 254, 377–382. [Google Scholar] [CrossRef]
- Prabakaran, M.; Kim, S.-H.; Hemapriya, V.; Gopiraman, M.; Kim, I.S.; Chung, I.-M. Rhus verniciflua as a green corrosion inhibitor for mild steel in 1 M H2SO4. RSC Adv. 2016, 6, 57144–57153. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Saxena, A.; Kumar, R. Experimental and theoretical studies of Ficus religiosa as green corrosion inhibitor for mild steel in 0.5 M H2SO4 solution. Sustain. Chem. Pharm. 2018, 9, 95–105. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Bhardwaj, N. Extraction and experimental studies of Citrus aurantifolia as an economical and green corrosion inhibitor for mild steel in acidic media. J. Adhes. Sci. Technol. 2019, 33, 1169–1183. [Google Scholar] [CrossRef]
- Kaban, A.P.S.; Ridhova, A.; Priyotomo, G.; Elya, B.; Maksum, A.; Sadeli, Y.; Sutopo, S.; Aditiyawarman, T.; Riastuti, R.; Soedarsono, J.W. Development of white tea extract as green corrosion inhibitor in mild steel under 1 M hydrochloric acid solution. East. Eur. J. Enterp. Technol. 2021, 2, 6–20. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M.; Motamedi, M. Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution: Experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Mol. Liq. 2018, 255, 185–198. [Google Scholar] [CrossRef]
- Joseph, O.; Fayomi, O.; Adenigba, O. Effect of lecaniodiscus cupaniodes extract in corrosion inhibition of normalized and annealed mild steels in 0.5 M HCl. Energy Procedia 2017, 119, 845–851. [Google Scholar] [CrossRef]
- Fouda, A.S. Evaluation of Cleome Droserifolia (Samwah) as Green Corrosion Inhibitor for Mild Steel in 1 M HCl Solution. Int. J. Electrochem. Sci. 2018, 13, 7057–7075. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Kamboj, D.; Kaya, S.; Dagdag, O.; Guo, L. Corrosion inhibition, surface adsorption and computational studies of Momordica charantia extract: A sustainable and green approach. SN Appl. Sci. 2021, 3, 1–13. [Google Scholar] [CrossRef]
- Tehrani, M.E.H.N.; Ghahremani, P.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Theoretical and experimental assessment of a green corrosion inhibitor extracted from Malva sylvestris. J. Environ. Chem. Eng. 2021, 9, 105256. [Google Scholar] [CrossRef]
- Zuo, X.; Li, W.; Luo, W.; Zhang, X.; Qiang, Y.; Zhang, J.; Li, H.; Tan, B. Research of Lilium brownii leaves extract as a commendable and green inhibitor for X70 steel corrosion in hydrochloric acid. J. Mol. Liq. 2021, 321, 114914. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Jiang, H.; Gu, Y.; Li, X.; Xu, L.-L. Pueraria lobata leaf extract as green corrosion inhibitor for low carbon steel in 1.0 M HCl solution. Res. Chem. Intermed. 2021, 47, 1051–1069. [Google Scholar] [CrossRef]
- Banu, A.M.; Farzana, B.A.; Ahamed, K.R. Evaluation of inhibitive performance of Allamanda cathartica leaves extract as a green corrosion inhibitor on mild steel in acid medium. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Qiang, Y. Corrosion retardation effect of a green cauliflower extract on copper in H2SO4 solution: Electrochemical and theoretical explorations. J. Mol. Liq. 2021, 321, 114450. [Google Scholar] [CrossRef]
- Vorobyova, V.; Skiba, M. Peach Pomace Extract as Novel Cost-Effective and High-Performance Green Inhibitor for Mild Steel Corrosion in NaCl Solution: Experimental and Theoretical Research. Waste Biomass Valorization 2021, 1–19. [Google Scholar] [CrossRef]
- El-Katori, E.E.; Al-Mhyawi, S. Assessment of the Bassia muricata extract as a green corrosion inhibitor for aluminum in acidic solution. Green Chem. Lett. Rev. 2019, 12, 31–48. [Google Scholar] [CrossRef]
- Foda, A.; Mosallam, H.; El-Khateeb, A.; Fakih, M. Cinnamomum zeylanicum Extract as Green Corrosion Inhibitor for Carbon Steel in Hydrochloric Acid Solutions. Prog. Chem. Biochem. Res. 2019, 2, 120–133. [Google Scholar] [CrossRef][Green Version]
- Jokar, M.; Farahani, T.S.; Ramezanzadeh, B. Electrochemical and surface characterizations of morus alba pendula leaves extract (MAPLE) as a green corrosion inhibitor for steel in 1M HCl. J. Taiwan Inst. Chem. Eng. 2016, 63, 436–452. [Google Scholar] [CrossRef]
- Perumal, S.; Muthumanickam, S.; Elangovan, A.; Karthik, R.; Kannan, R.S.; Mothilal, K.K. Bauhinia tomentosa Leaves Extract as Green Corrosion Inhibitor for Mild Steel in 1M HCl Medium. J. Bio Tribo Corros. 2017, 3, 13. [Google Scholar] [CrossRef]
- Saxena, A.; Prasad, D.; Haldhar, R. Use of Butea monosperma extract as green corrosion inhibitor for mild steel in 0.5 MH2SO4. Int. J. Electrochem. Sci. 2017, 12, 8793–8805. [Google Scholar] [CrossRef]
- Dhaundiyal, P.; Bashir, S.; Sharma, V.; Kumar, A. An investigation on mitigation of corrosion of mildsteel by Origanum vulgare in acidic medium. Bull. Chem. Soc. Ethiop. 2019, 33, 159. [Google Scholar] [CrossRef]
- Ameh, P.O. Electrochemical and computational study of gum exudates from Canarium schweinfurthii as green corrosion inhibitor for mild steel in HCl solution. J. Taibah Univ. Sci. 2018, 12, 783–795. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Fagundes, T.; dos Santos, N.E.; Rocha, T.S.D.M.; Garrett, R.; Borges, R.M.; Muricy, G.; Valverde, A.; Ponzio, E.A. Ircinia strobilina crude extract as corrosion inhibitor for mild steel in acid medium. Electrochim. Acta 2019, 312, 137–148. [Google Scholar] [CrossRef]
- Karki, N.; Neupane, S.; Chaudhary, Y.; Gupta, D.K.; Yadav, A.P. Equisetum hyemale: A new candidate for green corrosion inhibitor family. Int. J. Corros. Scale Inhib. 2021, 10, 206–227. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Quraishi, M.A.; Lin, Y. Investigation of Corrosion Inhibitors Adsorption on Metals Using Density Functional Theory and Molecular Dynamics Simulation. In Corrosion Inhibitors; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Chaudhari, L.P.; Patel, S.N. Corrosion Inhibition Study of Expired Acetazolamide on Mild Steel in Dilute Hydrochloric Acid Solution. J. Bio Tribo Corros. 2019, 5, 20. [Google Scholar] [CrossRef]
- Salinas-Solano, G.; Porcayo-Calderon, J.; de la Escalera, L.M.; Canto, J.; Díaz, M.C.; Sotelo-Mazon, O.; Henao, J.; Martinez-Gomez, L. Development and evaluation of a green corrosion inhibitor based on rice bran oil obtained from agro-industrial waste. Ind. Crop. Prod. 2018, 119, 111–124. [Google Scholar] [CrossRef]
- Idouhli, R.; Koumya, Y.; Khadiri, M.; Aityoub, A.; Abouelfida, A.; Benyaich, A. Inhibitory effect of Senecio anteuphorbium as green corrosion inhibitor for S300 steel. Int. J. Ind. Chem. 2019, 10, 133–143. [Google Scholar] [CrossRef]
- Nchewi, G.; Okoro, L.; Adams, F.; Agboola, B. Corrosion Inhibition Efficiency of Tamarindus Indica Leaves Extracts on Mild Steel in Hydrochloric Acid. J. Phys. Conf. Ser. 2019, 1378, 022051. [Google Scholar] [CrossRef]
- Bahlakeh, G.; Dehghani, A.; Ramezanzadeh, B.; Ramezanzadeh, M. Highly effective mild steel corrosion inhibition in 1 M HCl solution by novel green aqueous Mustard seed extract: Experimental, electronic-scale DFT and atomic-scale MC/MD explorations. J. Mol. Liq. 2019, 293, 111559. [Google Scholar] [CrossRef]
- Saeed, M.; Saleem, M.; Usmani, S.; Malik, I.A.; Al-Shammari, F.A.; Deen, K.M. Corrosion inhibition of mild steel in 1 M HCl by sweet melon peel extract. J. King Saud Univ. Sci. 2019, 31, 1344–1351. [Google Scholar] [CrossRef]
- Arkles, B. Hydrophobicity, Hydrophilicity and Silane Surface Modification; Gelest Inc.: Morrisville, PA, USA, 2011. [Google Scholar]
- Sanaei, Z.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Use of Rosa canina fruit extract as a green corrosion inhibitor for mild steel in 1 M HCl solution: A complementary experimental, molecular dynamics and quantum mechanics investigation. J. Ind. Eng. Chem. 2019, 69, 18–31. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Potential of Borage flower aqueous extract as an environmentally sustainable corrosion inhibitor for acid corrosion of mild steel: Electrochemical and theoretical studies. J. Mol. Liq. 2019, 277, 895–911. [Google Scholar] [CrossRef]
- Anyiam, C.K.; Ogbobe, O.; Oguzie, E.E.; Madufor, I.C.; Nwanonenyi, S.C.; Onuegbu, G.C.; Obasi, H.C.; Chidiebere, M.A. Corrosion inhibition of galvanized steel in hydrochloric acid medium by a physically modified starch. SN Appl. Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Muthukrishnan, P.; Prakash, P.; Ilayaraja, M.; Jeyaprabha, B.; Shankar, K. Effect of Acidified Feronia elephantum Leaf Extract on the Corrosion Behavior of Mild Steel. Met. Mater. Trans. A 2015, 46, 1448–1460. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Bahlakeh, G.; Sanaei, Z.; Ramezanzadeh, B. Corrosion inhibition of mild steel in 1 M HCl solution by ethanolic extract of eco-friendly Mangifera indica (mango) leaves: Electrochemical, molecular dynamics, Monte Carlo and ab initio study. Appl. Surf. Sci. 2019, 463, 1058–1077. [Google Scholar] [CrossRef]
- Halambek, J.; Cindrić, I.; Grassino, A.N. Evaluation of pectin isolated from tomato peel waste as natural tin corrosion inhibitor in sodium chloride/acetic acid solution. Carbohydr. Polym. 2020, 234, 115940. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Yadav, V. Musa acuminata (Green corrosion inhibitor) as anti-pit and anti-cracking agent for mild steel in 5M hydrochloric acid solution. Chem. Data Collect. 2020, 29, 100500. [Google Scholar] [CrossRef]
- Cordeiro, R.F.B. Coffee Husk as Corrosion Inhibitor for Mild Steel in HCl Media. Int. J. Electrochem. Sci. 2018, 13, 12188–12207. [Google Scholar] [CrossRef]
- Abdulmajid, A.; Hamidon, T.S.; Rahim, A.; Hussin, M.H. Tamarind shell tannin extracts as green corrosion inhibitors of mild steel in hydrochloric acid medium Tamarind shell tannin extracts as green corrosion inhibitors of mild steel in hydrochloric acid medium. Mater. Res. Express 2019, 10, 6. [Google Scholar] [CrossRef]
- Odewunmi, N.; Umoren, S.; Gasem, Z. Watermelon waste products as green corrosion inhibitors for mild steel in HCl solution. J. Environ. Chem. Eng. 2015, 3, 286–296. [Google Scholar] [CrossRef]
- Pal, S.; Lgaz, H.; Tiwari, P.; Chung, I.-M.; Ji, G.; Prakash, R. Experimental and theoretical investigation of aqueous and methanolic extracts of Prunus dulcis peels as green corrosion inhibitors of mild steel in aggressive chloride media. J. Mol. Liq. 2019, 276, 347–361. [Google Scholar] [CrossRef]
- Ikpeseni, S.C.; Odu, G.O.; Owamah, H.I.; Onochie, P.U.; Ukala, D.C. Thermodynamic Parameters and Adsorption Mechanism of Corrosion Inhibition in Mild Steel Using Jatropha Leaf Extract in Hydrochloric Acid. Arab. J. Sci. Eng. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Shahini, M.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Superior inhibition action of the Mish Gush (MG) leaves extract toward mild steel corrosion in HCl solution: Theoretical and electrochemical studies. J. Mol. Liq. 2021, 332, 115876. [Google Scholar] [CrossRef]
- da Silva Hernandes, H.; Santana, C.A.; da Santana, J.N.; Aquino Rodrigues, J.G.; D’Elia, E. Application of an Aqueous Extract of Cotton Seed as a Corrosion Inhibitor for Mild Steel in HCl Media. Mater. Res. 2021, 24. [Google Scholar] [CrossRef]
- Mostafatabar, A.H.; Bahlakeh, G.; Ramezanzadeh, B.; Dehghani, A.; Ramezanzadeh, M. A comprehensive electronic-scale DFT modeling, atomic-level MC/MD simulation, and electrochemical/surface exploration of active nature-inspired phytochemicals based on Heracleum persicum seeds phytoextract for effective retardation of the acidic-induced corrosion of mild steel. J. Mol. Liq. 2021, 331, 115764. [Google Scholar] [CrossRef]
- Soltani, N.; Khayatkashani, M. Evaluating performance of malva sylvestris leaf extract for protection of mild steel against corrosion in acidic solution. Asian J. Green Chem. 2021, 5, 39–57. [Google Scholar] [CrossRef]
- Pal, S.; Ji, G.; Lgaz, H.; Chung, I.-M.; Prakash, R. Lemon seeds as green coating material for mitigation of mild steel corrosion in acid media: Molecular dynamics simulations, quantum chemical calculations and electrochemical studies. J. Mol. Liq. 2020, 316, 113797. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Bahadur, I.; Quraishi, M. An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. J. Mol. Liq. 2018, 266, 577–590. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Aloysia citrodora leaves extract corrosion retardation effect on mild-steel in acidic solution: Molecular/atomic scales and electrochemical explorations. J. Mol. Liq. 2020, 310, 113221. [Google Scholar] [CrossRef]
- Yüce, A.O. Corrosion Inhibition Behavior of Robinia pseudoacacia Leaves Extract as a Eco-Friendly Inhibitor on Mild Steel in Acidic Media. Met. Mater. Int. 2020, 26, 456–466. [Google Scholar] [CrossRef]
- Ozturk, M.; Saba, N.; Altay, V.; Iqbal, R.; Hakeem, K.R.; Jawaid, M.; Ibrahim, F.H. Biomass and bioenergy: An overview of the development potential in Turkey and Malaysia. Renew. Sustain. Energy Rev. 2017, 79, 1285–1302. [Google Scholar] [CrossRef]
- Agensi Inovasi Malaysia. National Biomass Strategy 2020: New Wealth Creation for Malaysia’s Palm Oil Industry; Agensi Inovasi Malaysia (Aim): Putrajaya, Malaysia, 2011. [Google Scholar]
- World Bioenergy Association. Global Bioenergy Statistics 2020; World Bioenergy Association: Stockholm, Sweden, 2020. [Google Scholar]
- Mohd-Asharuddin, S.; Othman, N.; Zin, N.S.M.; Tajarudin, H.A. A Chemical and Morphological Study of Cassava Peel: A Potential Waste as Coagulant Aid. MATEC Web Conf. 2017, 103, 6012. [Google Scholar] [CrossRef]
- de Oliveira, G.A.; Teixeira, V.M.; da Cunha, J.N.; Rangel, M.; dos Santos, V.M.P.; Rezende, M.J.C. Biomass of Microalgae Chlorella sorokiniana as Green Corrosion Inhibitor for Mild Steel in HCl Solution. Int. J. Electrochem. Sci. 2021, 16, 210249. [Google Scholar] [CrossRef]
- Komalasari; Zultiniar; Karim, A.R.M.; Susanto, R.; Azhari, I.; Utami, S.P.; Heltina, D. Biomass Extraction as Green Corrosion Inhibitor for Low Carbon Steel in Hydrochloric Acid Solution. J. Phys. Conf. Ser. 2020, 1655. [Google Scholar] [CrossRef]
- Mishurov, V.I.; Shubina, E.N.; Klushin, V.A.; Chizhikova, A.A.; Kashparova, V.P.; Berezhnaya, A.G. Biomass Conversion Products as Steel Corrosion Inhibitors. Russ. J. Appl. Chem. 2019, 92, 620–624. [Google Scholar] [CrossRef]
- Hassan, N.; Ali, S.M.; Ebrahim, A.; El-Adawi, H. Performance evaluation and optimization of Camellia sinensis extract as green corrosion inhibitor for mild steel in acidic medium. Mater. Res. Express 2019, 6, 0865c7. [Google Scholar] [CrossRef]
- Papavinasam, S. Electrochemical polarization techniques for corrosion monitoring. In Techniques for Corrosion Monitoring, 2nd ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 45–77. [Google Scholar]
- Citrus sinensis Extract as a Green Inhibitor for the Corrosion of Carbon Steel in Sulphuric Acid Solution. Biointerface Res. Appl. Chem. 2021, 11, 14007–14020. [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Electronic/atomic level fundamental theoretical evaluations combined with electrochemical/surface examinations of Tamarindus indiaca aqueous extract as a new green inhibitor for mild steel in acidic solution (HCl 1 M). J. Taiwan Inst. Chem. Eng. 2019, 102, 349–377. [Google Scholar] [CrossRef]
- Elabbasy, H.M.; Fouda, A.S. Olive leaf as green corrosion inhibitor for C-steel in Sulfamic acid solution. Green Chem. Lett. Rev. 2019, 12, 332–342. [Google Scholar] [CrossRef]
- Mohd, N.K.; Yeong, S.K.; Ibrahim, N.A.; Nor, S.M.M.; Chan, C.H.; Tang, S.W.; Lim, W.H.; Idris, Z. Corrosion Inhibition, Adsorption Behaviour and Thermodynamic Properties of N-Cinnamalidene Palmitohydrazide on Mild Steel In Hydrochloric Acid Solution. J. Oil Palm Res. 2020, 32, 124–138. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).