Corrosion Fatigue Characteristics of 316L Stainless Steel Fabricated by Laser Powder Bed Fusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Corrosion Testing
2.3. Mechanical and Corrosion Fatigue Testing
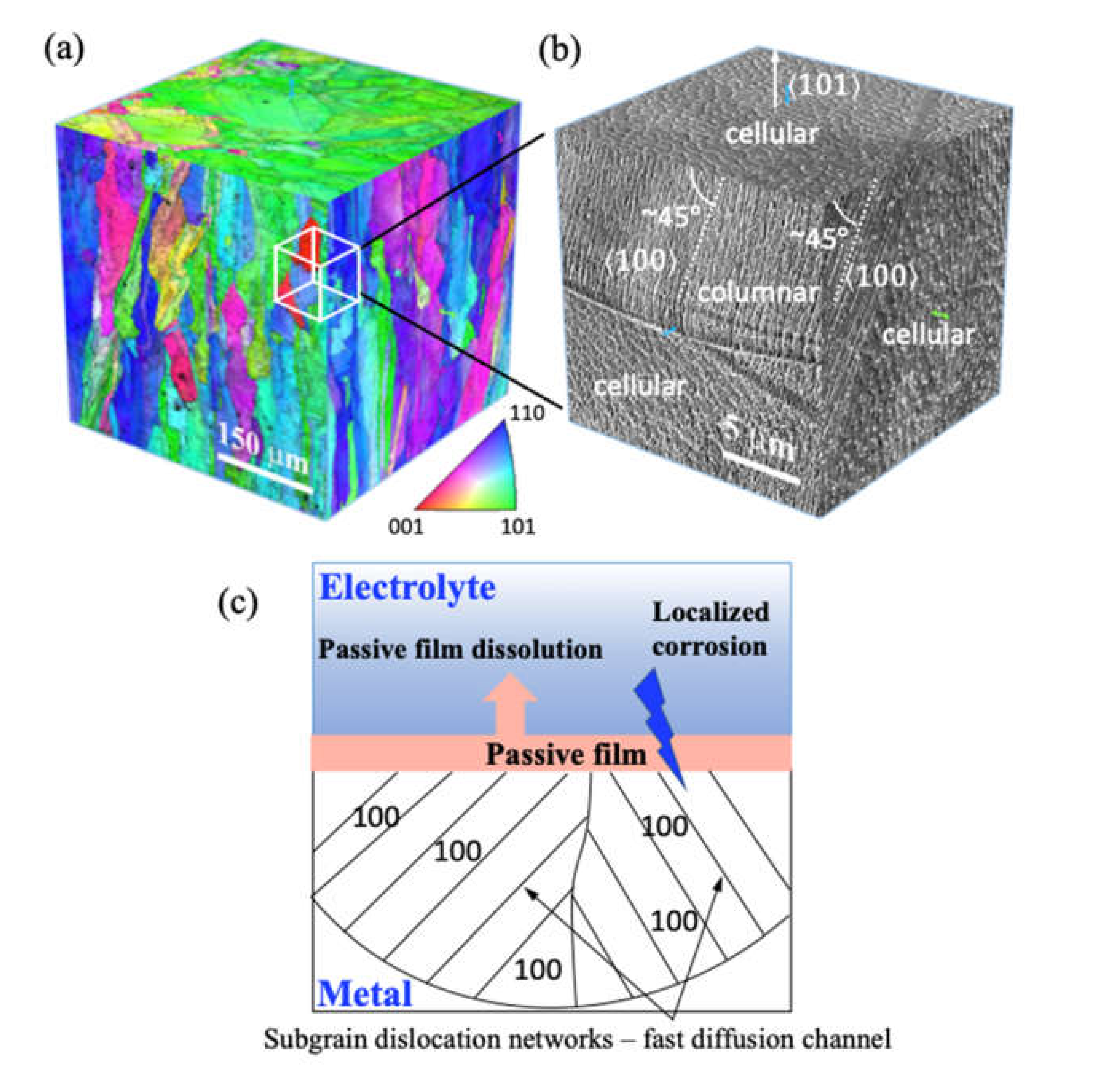
2.4. Microstructural Characterization
3. Results
3.1. Electrochemical Measurement
3.1.1. PP Test for Pitting Corrosion
3.1.2. Cyclic Polarization (CP) for Repassivation
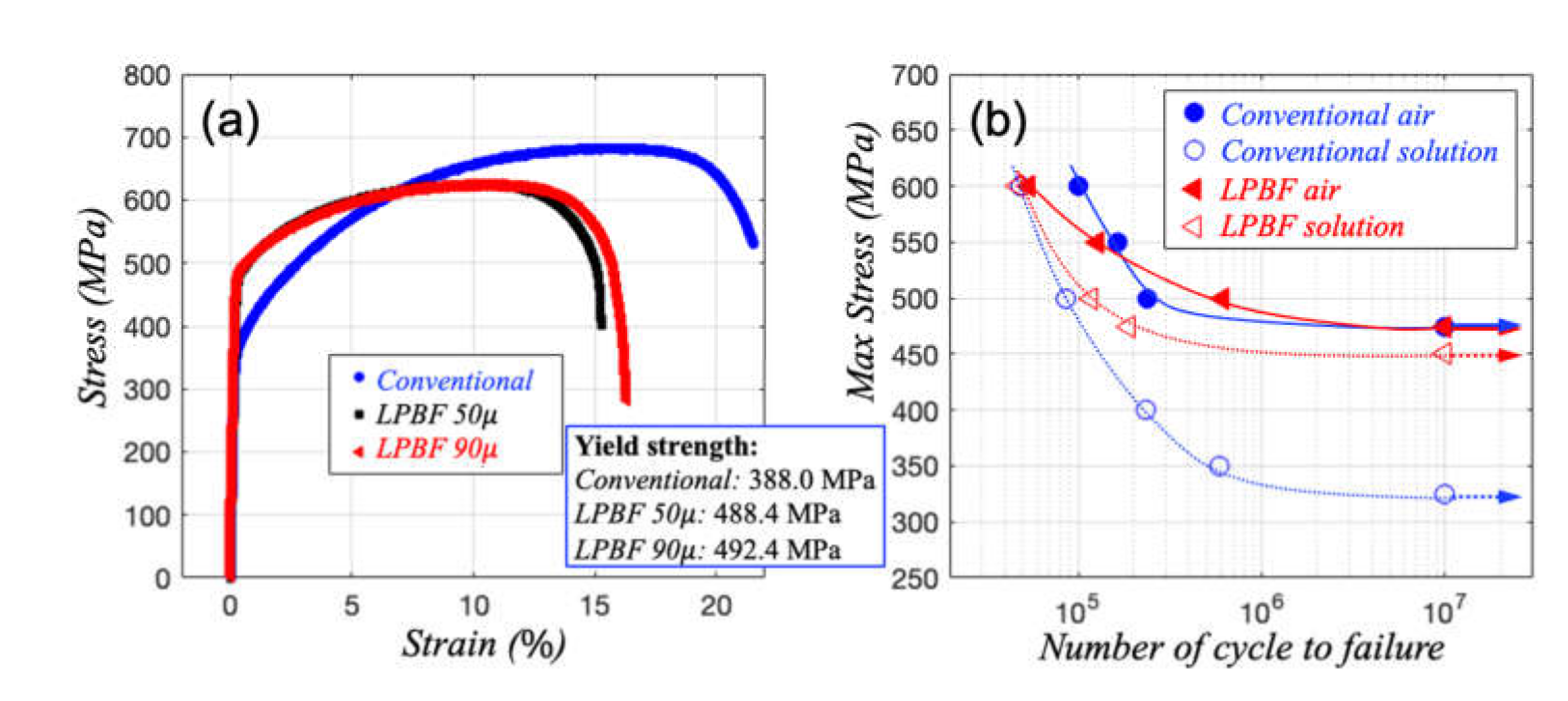
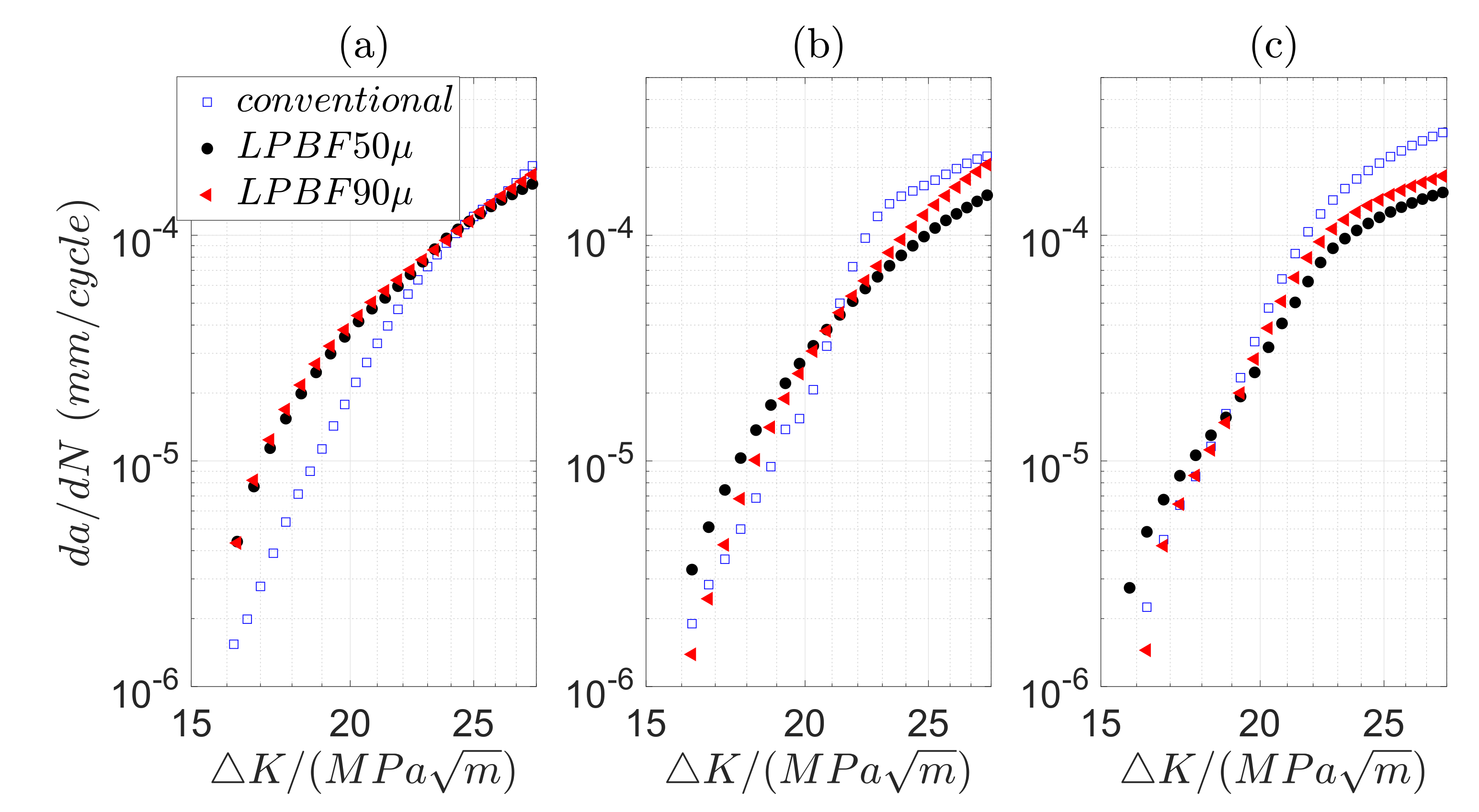
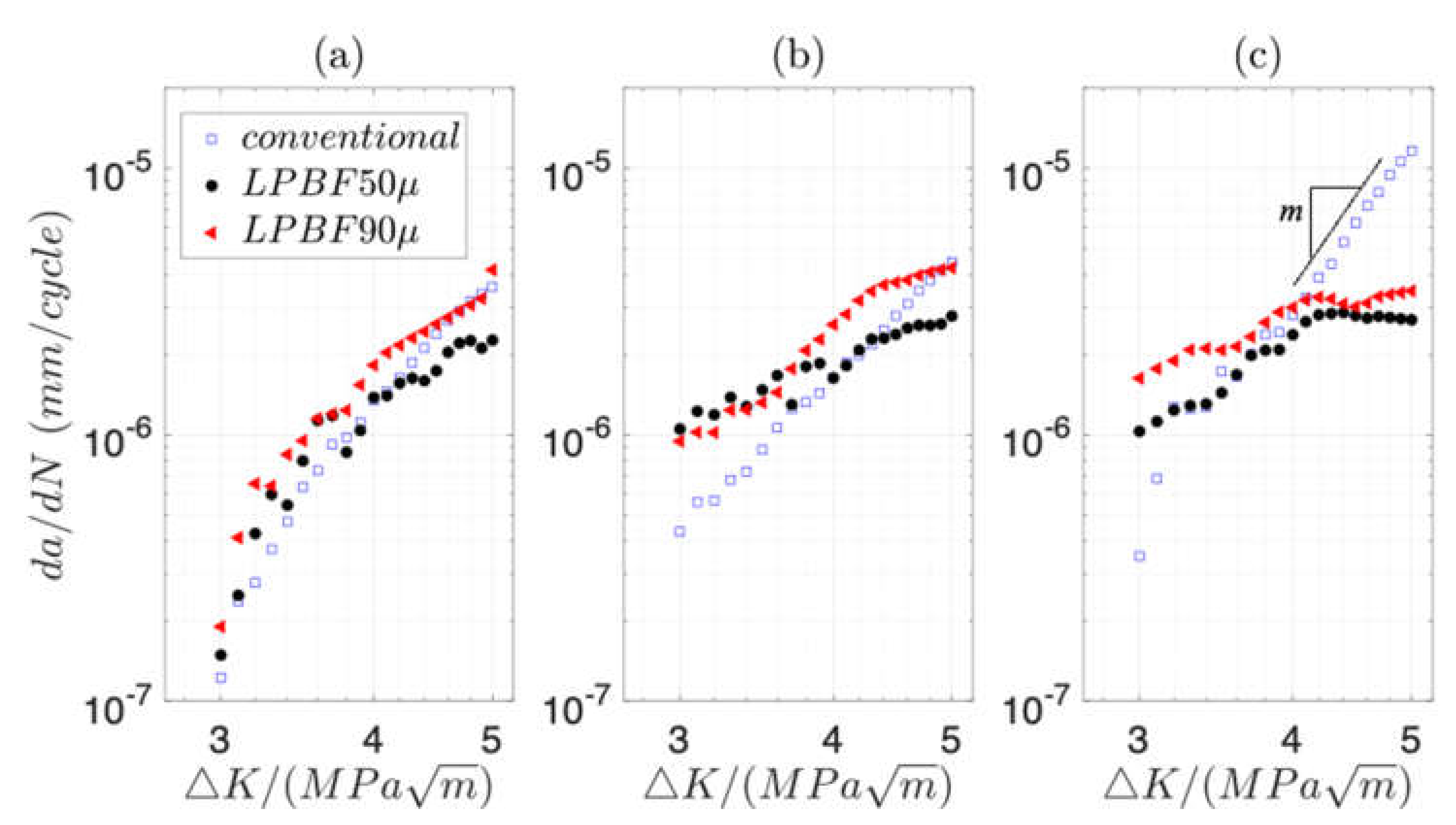
3.2. High Cycle Fatigue (HCF) and Fatigue Crack Propagation (FCP) in Air and 3.5 wt.% NaCl Solution
4. Discussion
5. Conclusions
- The LPBF 316L SSs demonstrate, in general, improved corrosion resistance compared to conventionally manufactured 316L, as reflected by the increased pitting potential and higher repassivation potential.
- While the endurance limit of high cycle fatigue testing is comparable between conventional and LPBF 316L in air, it is significantly higher for LPBF 316L than conventional ones in 3.5 wt.% NaCl solution.
- The crack growth rate in air is, in general, higher in LPBF samples than in conventional ones. However, the growth rate in conventional 316L accelerates in 3.5 wt.% NaCl solution as the crack grows further. At reduced frequency in solution, the crack growth becomes retarded as the crack advances in the LPBF 316L.
- The enhanced pitting corrosion resistance, higher endurance limit, and lower crack growth rate as the crack propagates in 3.5 wt.% NaCl solution of LPBF 316L compared to the conventional ones is likely to relate to the dislocation subgrain structure of the LPBF sample.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrousek, P.; Kvackaj, T.; Kocisko, R.; BIdulska, J.; Luptak, M.; Manfredi, D.; Grande, M.A.; Bidulsky, R. Influence of cryorolling on properties of L-PBF 316l stainless steel tested at 298K and 77K. Acta Metall. Slovaca 2019, 25, 283–290. [Google Scholar] [CrossRef]
- Wang, Y.M.; Voisin, T.; McKeown, J.T.; Ye, J.; Calta, N.P.; Li, Z.; Zeng, Z.; Zhang, Y.; Chen, W.; Roehling, T.T.; et al. Additively manufactured hierarchical stainless steels with high strength and ductility. Nat. Mater. 2017, 17, 63. [Google Scholar] [CrossRef]
- Ziętala, M.; Durejko, T.; Polański, M.; Kunce, I.; Płociński, T.; Zieliński, W.; Łazińska, M.; Stępniowski, W.; Czujko, T.; Kurzydłowski, K.J.; et al. The microstructure, mechanical properties and corrosion resistance of 316L stainless steel fabricated using laser engineered net shaping. Mater. Sci. Eng. A 2016, 677, 1–10. [Google Scholar] [CrossRef]
- Ganesh, P.; Giri, R.; Kaul, R.; Sankar, P.R.; Tiwari, P.; Atulkar, A.; Porwal, R.K.; Dayal, R.K.; Kukreja, L.M. Studies on pitting corrosion and sensitization in laser rapid manufactured specimens of type 316L stainless steel. Mater. Design 2012, 39, 509–521. [Google Scholar] [CrossRef]
- Chao, Q.; Cruz, V.; Thomas, S.; Birbilis, N.; Collins, P.; Taylor, A.; Hodgson, P.D.; Fabijanic, D. On the enhanced corrosion resistance of a selective laser melted austenitic stainless steel. Scr. Mater. 2017, 141, 94–98. [Google Scholar] [CrossRef]
- Lodhi, M.J.K.; Deen, K.M.; Haider, W. Corrosion behavior of additively manufactured 316L stainless steel in acidic media. Materialia 2018, 2, 111–121. [Google Scholar] [CrossRef]
- Andreatta, F.; Lanzutti, A.; Vaglio, E.; Totis, G.; Sortino, M.; Fedrizzi, L. Corrosion behaviour of 316L stainless steel manufactured by selective laser melting. Mater. Corr. 2019, 70, 1633–1645. [Google Scholar] [CrossRef]
- Fazier, W.E. Metal Additive Manufacturing: A Review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Gu, D.; Shen, Y. Balling phenomena in direct laser sintering of stainless steel powder: Metallurgical mechanisms and control methods. Mater. Design 2009, 30, 2903–2910. [Google Scholar] [CrossRef]
- Sander, G.; Thomas, S.; Cruz, V.; Jurg, M.; Birbilis, N.; Gao, X.; Brameld, M.; Hutchinson, C.R. On The Corrosion and Metastable Pitting Characteristics of 316L Stainless Steel Produced by Selective Laser Melting. J. Electrochem. Soc. 2017, 164, C250–C257. [Google Scholar] [CrossRef]
- Kong, D.; Ni, X.; Dong, C.; Zhang, L.; Man, C.; Yao, J.; Xiao, K.; Li, X. Heat treatment effect on the microstructure and corrosion behavior of 316L stainless steel fabricated by selective laser melting for proton exchange membrane fuel cells. Electrochim. Acta 2018, 276, 293–303. [Google Scholar] [CrossRef]
- Lou, X.; Othon, M.A.; Rebak, R.B. Oxide inclusions in laser additive manufactured stainless steel and their effects on impact toughness and stress corrosion cracking behavior. J. Nucl. Mater. 2018, 499, 182–190. [Google Scholar] [CrossRef]
- Lou, X.; Othon, M.A.; Rebak, R.B. Corrosion fatigue crack growth of laser additively-manufactured 316L stainless steel in high temperature water. Corros. Sci. 2017, 127, 120–130. [Google Scholar] [CrossRef]
- Lou, X.; Song, M.; Emigh, P.W.; Othon, M.A.; Andresen, P.L. On the stress corrosion crack growth behaviour in high temperature water of 316L stainless steel made by laser powder bed fusion additive manufacturing. Corros. Sci. 2017, 128, 140–153. [Google Scholar] [CrossRef]
- Sun, S.-H.; Ishimoto, T.; Hagihara, K.; Tsutsumi, Y.; Hanawa, T.; Nakano, T. Excellent mechanical and corrosion properties of austenitic stainless steel with a unique crystallographic lamellar microstructure via selective laser melting. Scr. Mater. 2019, 159, 89–93. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pellegrini, S.; Pavese, M.; Fino, P.; Ambrosio, E.P.; Calignano, F.; Manfredi, D. Corrosion resistance of direct metal laser sintering AlSiMg alloy. Surf. Interface Anal. 2016, 48, 818–826. [Google Scholar] [CrossRef]
- Trelewics, J.; Halada, G.; Donaldson, O.; Manogharan, G. Microstructure and corrosion resistance of laser additively manufactured 316L stainless steel. JOM 2016, 68, 850–859. [Google Scholar] [CrossRef]
- Cruz, V.; Chao, Q.; Birbilis, N.; Fabijanic, D.; Hodgson, P.D.; Thomas, S. Electrochemical studies on the effect of residual stress on the corrosion of 316L manufactured by selective laser melting. Corros. Sci. 2019, 164, 108314. [Google Scholar] [CrossRef]
- Hemmasian Ettefagh, A.; Guo, S. Electrochemical behavior of AISI316L stainless steel parts produced by laser-based powder bed fusion process and the effect of post annealing process. Addit. Manuf. 2018, 22, 153–156. [Google Scholar] [CrossRef]
- Salman, O.O.; Gammer, C.; Chaubey, A.K.; Eckert, J.; Scudino, S. Effect of heat treatment on microstructure and mechanical properties of 316L steel synthesized by selective laser melting. Mater. Sci. Eng. A 2019, 748, 205–212. [Google Scholar] [CrossRef]
- Yusuf, S.M.; Gao, N. Influence of energy density on metallurgy and properties in metal additive manufacturing. Mater. Sci. Technol. 2017, 33, 1269–1289. [Google Scholar] [CrossRef]
- Kluczyński, J.; Śnieżek, L.; Grzelak, K.; Mierzyński, J. The Influence of Exposure Energy Density on Porosity and Microhardness of the SLM Additive Manufactured Elements. Materials 2018, 11, 2304. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.H. Calibrating the Electric Potential Method for Studying Slow Crack Growth. ASTM Mater. Res. Stand. 1965, 5, 442–445. [Google Scholar]
- Gorsse, S.; Hutchinson, C.; Gouné, M.; Banerjee, R. Additive manufacturing of metals: A brief review of the characteristic microstructures and properties of steels, Ti-6Al-4V and high-entropy alloys. Sci. Technol. Adv. Mater. 2017, 18, 584–610. [Google Scholar] [CrossRef] [PubMed]
- Ford, F.P. Environment Induced Cracking of Metals; Gangloff, R.P., Ives, M.B., Eds.; National Association of Corrosion Engineers: Houston, TX, USA, 1990; pp. 139–166. [Google Scholar]
- Sieradzki, K.; Newman, R.C. Brittle behavior of ductile metals during stress-corrosion cracking. Philos. Mag. A 1985, 51, 95–132. [Google Scholar] [CrossRef]
- Edeleanu, C.; Forty, A.J. Some observations on the stress-corrosion cracking of α-brass and similar alloys. Philos. Mag. J. Theor. Exp. Appl. Phys. 1960, 5, 1029–1040. [Google Scholar] [CrossRef]
- Balusamy, T.; Kumar, S.; Sankara Narayanan, T.S.N. Effect of surface nanocrystallization on the corrosion behaviour of AISI 409 stainless steel. Corros. Sci. 2010, 52, 3826–3834. [Google Scholar] [CrossRef]
- Rifai, M.; Miyamoto, H.; Fujiwara, H. Effects of Strain Energy and Grain Size on Corrosion Resistance of Ultrafine Grained Fe-20%Cr Steels with Extremely low C and N Fabricated by ECAP. Int. J. Corros. 2015, 2015, 1–9. [Google Scholar] [CrossRef][Green Version]
- Srinivasan, N.; Kain, V.; Birbilis, N.; Kumar, B.S.; Gandhi, M.N.; Sivaprasad, P.V.; Chai, G.; Lodh, A.; Ahmedabadi, P.; Samajdar, I. Plastic deformation and corrosion in austenitic stainless steel: A novel approach through microtexture and infrared spectroscopy. Corros. Sci. 2016, 111, 404–413. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, X.; Han, E.-H.; Ke, W.; Yang, K.; Jiang, Z. Effects of cold work and sensitization treatment on the corrosion resistance of high nitrogen stainless steel in chloride solutions. Electrochim. Acta 2009, 54, 1618–1629. [Google Scholar] [CrossRef]




| Sample | Power (P) W | Scan Speed (v) mm/s | Hatch Spacing (H) μm | Energy Density (E) J/mm3 |
|---|---|---|---|---|
| S1 | 150 | 1083.0 | 90 | 76.9 |
| S2 | 195 | 1083.0 | 90 | 100.0 |
| S3 | 240 | 1083.0 | 90 | 123.1 |
| S4 | 150 | 1083.0 | 50 | 138.50 |
| S5 | 195 | 1083.0 | 50 | 180.06 |
| S6 | 240 | 1083.0 | 50 | 221.61 |
| S7 | 195 | 1407.9 | 90 | 76.9 |
| Sample Condition | 150 W | 195 W | 240 W | 150 W | 195 W | 240 W | Conventional |
|---|---|---|---|---|---|---|---|
| 90 μm | 50 μm | ||||||
| Ecorr, mV vs. SCE | −159.6 ± 6.3 | −165.4 ± 4.5 | −151.6 ± 7.4 | −154.9 ± 0.4 | −163.2 ± 3.4 | −164.4 ± 6.6 | −164.0 ± 7.9 |
| Icorr, nA/cm2 | 27.9 ± 2.9 | 28.9 ± 11.7 | 28.3 ± 1.2 | 35.7 ± 4.2 | 37.7 ± 4.1 | 28.5 ± 3.4 | 24.9 ± 6.5 |
| Epitt, mV vs. SCE | 635.5 ± 46.5 | 669.5 ± 36.5 | 639.5 ± 47.5 | 692.1 ± 65.2 | 744.5 ± 32.5 | 680.5 ± 0.5 | 458.0 ± 23.3 |
| Conventional | LPBF-XY | LPBF-XZ | |
|---|---|---|---|
| Erepass, mV vs. SCE | 255.5 ± 33.6 | 370.0 ± 20.1 | 410.0 ± 19.5 |
| Epitt-Erepass, mV vs. SCE | 297.4 ± 81.1 | 225.2 ± 38.9 | 257.5 ± 37.0 |
| (a) | Number of Life Cycle to Failure | ||||
|---|---|---|---|---|---|
| Maximum Stress, MPa | Conventional | LPBF50μ | |||
| air | 3.5 wt.% NaCl | air | 3.5 wt.% NaCl | ||
| 600 | 1.01 × 105 | 4.88 × 104 | 5.37 × 104 | 4.68 × 104 | |
| 550 | 1.65 × 105 | X | 1.28 × 105 | X | |
| 500 | 2.41 × 105 | 8.59 × 104 | 6.01 × 104 | 1.18 × 105 | |
| 475 | 1.00 × 107 | X | 1.00 × 107 | 1.87 × 105 | |
| 450 | X | X | X | 1.00 × 107 | |
| 400 | X | 2.33 × 105 | X | X | |
| 350 | X | 5.91 × 105 | X | X | |
| 325 | X | 1.00 × 107 | X | X | |
| (b) | m, Fatigue Crack Propagation at R = 0.833 | ||||
| Conventional | LPBF50μ | ||||
| Air | 3.5 wt.% NaCl, 20 Hz | 3.5 wt.% NaCl, 2 Hz | air | 3.5 wt.% NaCl, 20 Hz | 3.5 wt.% NaCl, 2 Hz |
| 6.02 | 4.54 | 5.84 | 4.43 | 1.88 | 2.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnanasekaran, B.; Song, J.; Vasudevan, V.; Fu, Y. Corrosion Fatigue Characteristics of 316L Stainless Steel Fabricated by Laser Powder Bed Fusion. Metals 2021, 11, 1046. https://doi.org/10.3390/met11071046
Gnanasekaran B, Song J, Vasudevan V, Fu Y. Corrosion Fatigue Characteristics of 316L Stainless Steel Fabricated by Laser Powder Bed Fusion. Metals. 2021; 11(7):1046. https://doi.org/10.3390/met11071046
Chicago/Turabian StyleGnanasekaran, Balachander, Jie Song, Vijay Vasudevan, and Yao Fu. 2021. "Corrosion Fatigue Characteristics of 316L Stainless Steel Fabricated by Laser Powder Bed Fusion" Metals 11, no. 7: 1046. https://doi.org/10.3390/met11071046
APA StyleGnanasekaran, B., Song, J., Vasudevan, V., & Fu, Y. (2021). Corrosion Fatigue Characteristics of 316L Stainless Steel Fabricated by Laser Powder Bed Fusion. Metals, 11(7), 1046. https://doi.org/10.3390/met11071046







